Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fullerenol C60(OH)24 and Aβ42 Oligomers Preparation
2.2. Cell Culture
2.3. Cell Viability and ROS Production Assays
2.4. In Vivo Study with Chronic Dosing of APPswe/PS1dE9 Mice
2.4.1. Animals and Study Flow
2.4.2. Behavioral Assays
Conditioned Taste Aversion
Marble Test
Object Recognition Test
Step-Down Avoidance
O-Maze
Dark-Light Box
Open Field
2.4.3. Euthanasia
2.5. Brain Sectioning for Histological Assays
2.6. Congo Red Staining, Plaque Microscopy, and Scoring of Amyloid Plaque Parameters
2.7. Immunohistochemical Analysis of Astrocyte Activation
2.8. RNA Extraction, cDNA Synthesis, and Real-Time Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
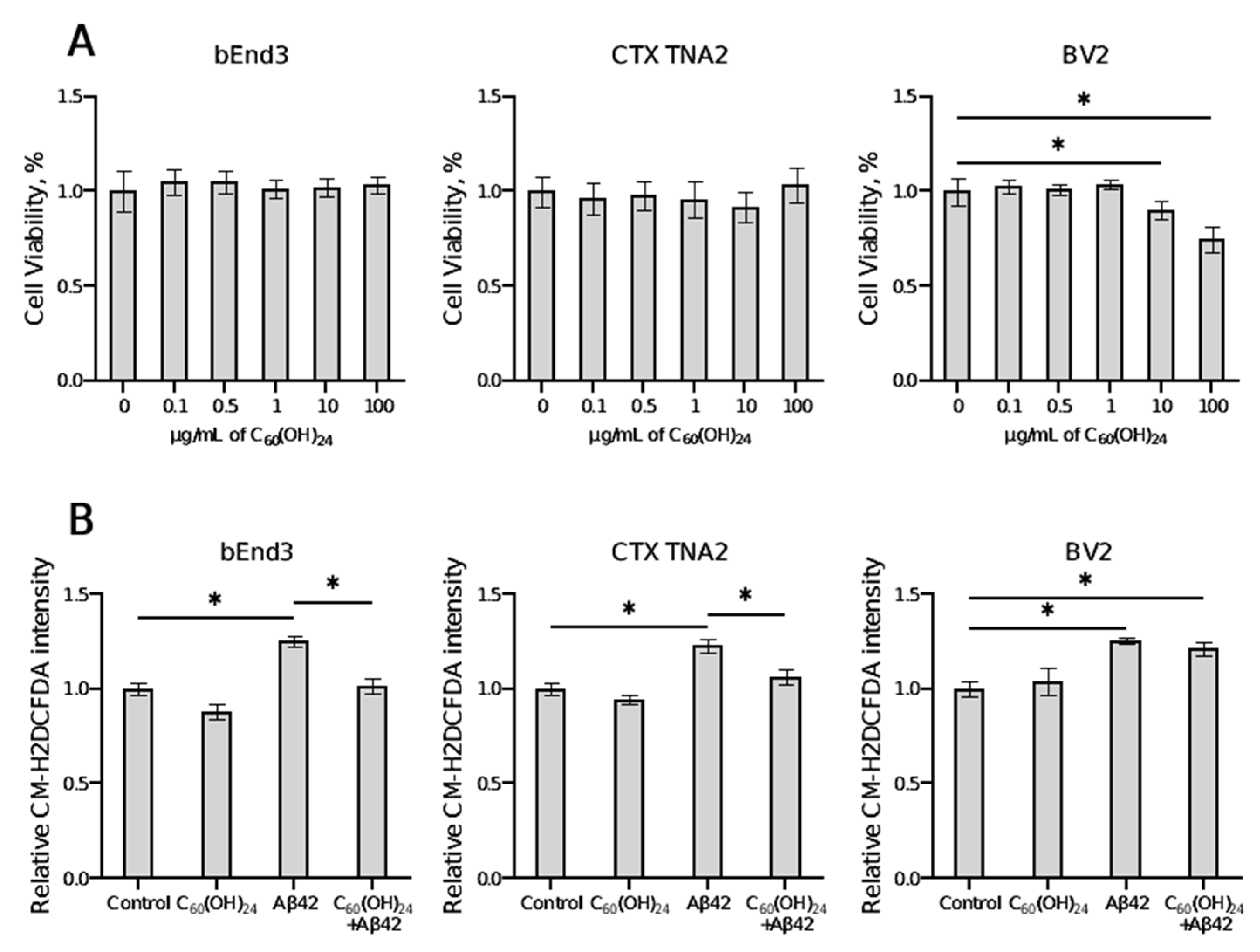
3.1. Effects of Fullerenol on Cell Viability and ROS Production
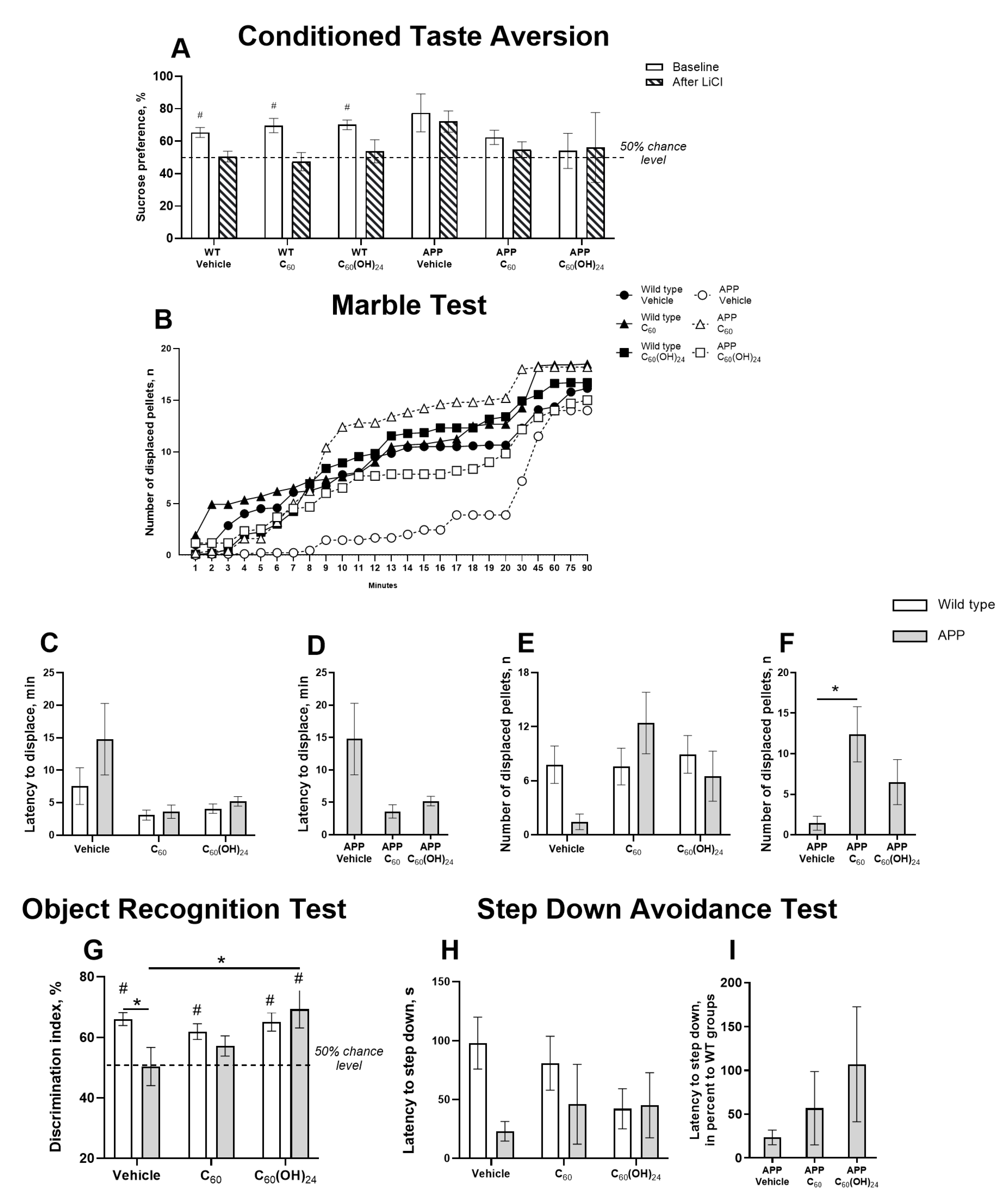
3.2. Differential Effects of Fullerene and Fullerenol on Hippocampus-Related Cognitive Performance in APP/PS1 Mice
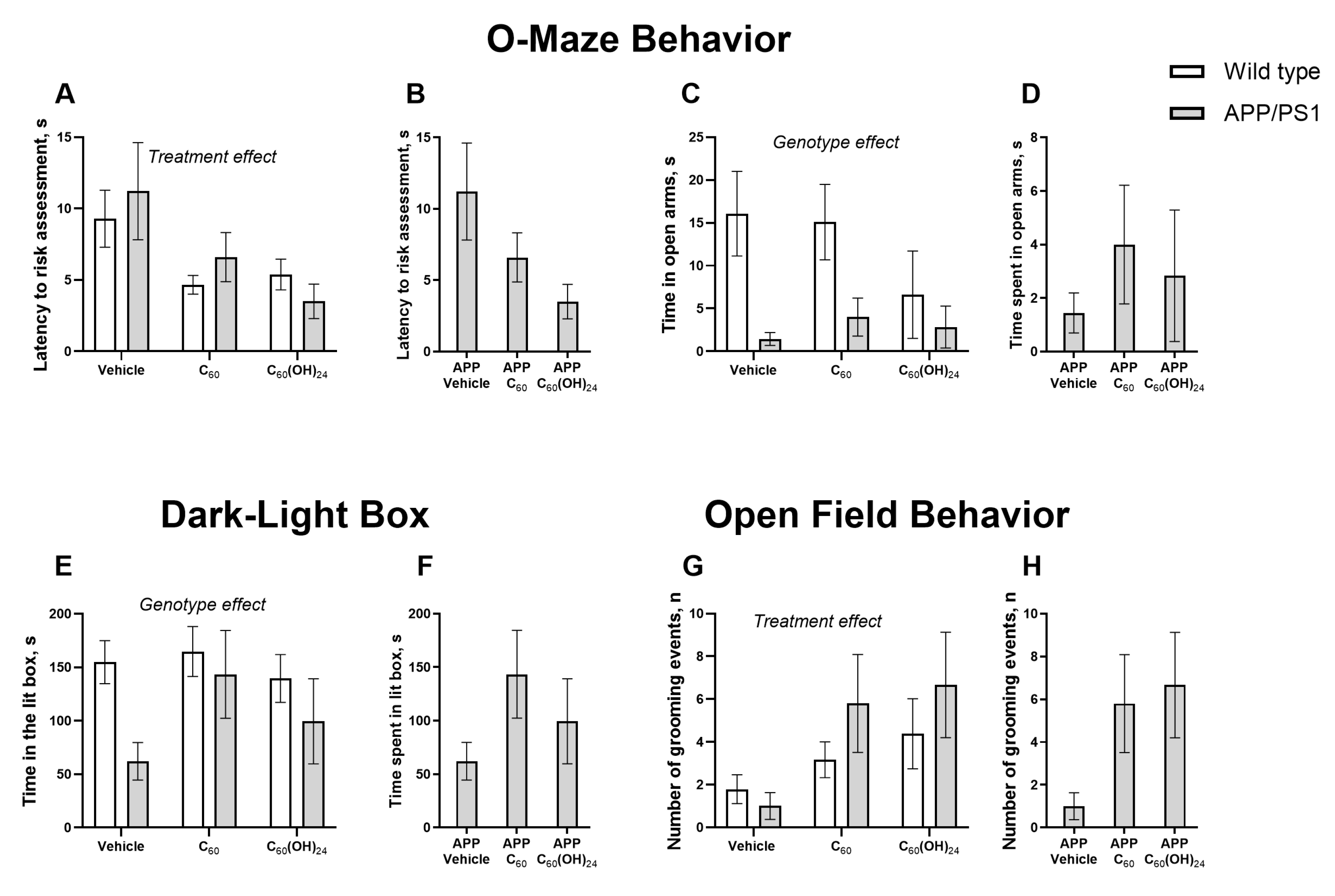
3.3. Chronic Fullerene and Fullerenol Treatment Partially Improves Emotionality in APP/PS1 Mice Without Significantly Affecting Locomotion
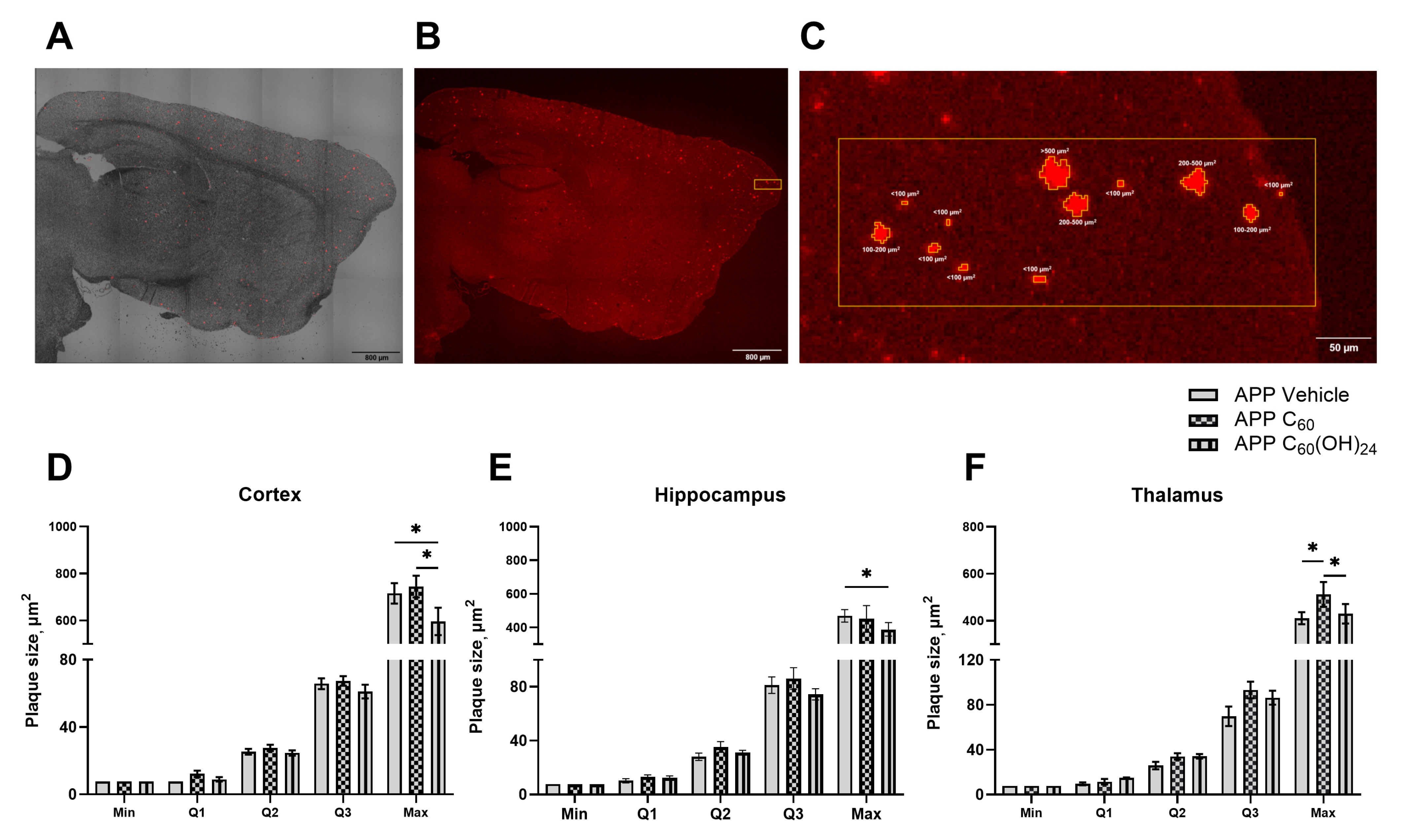
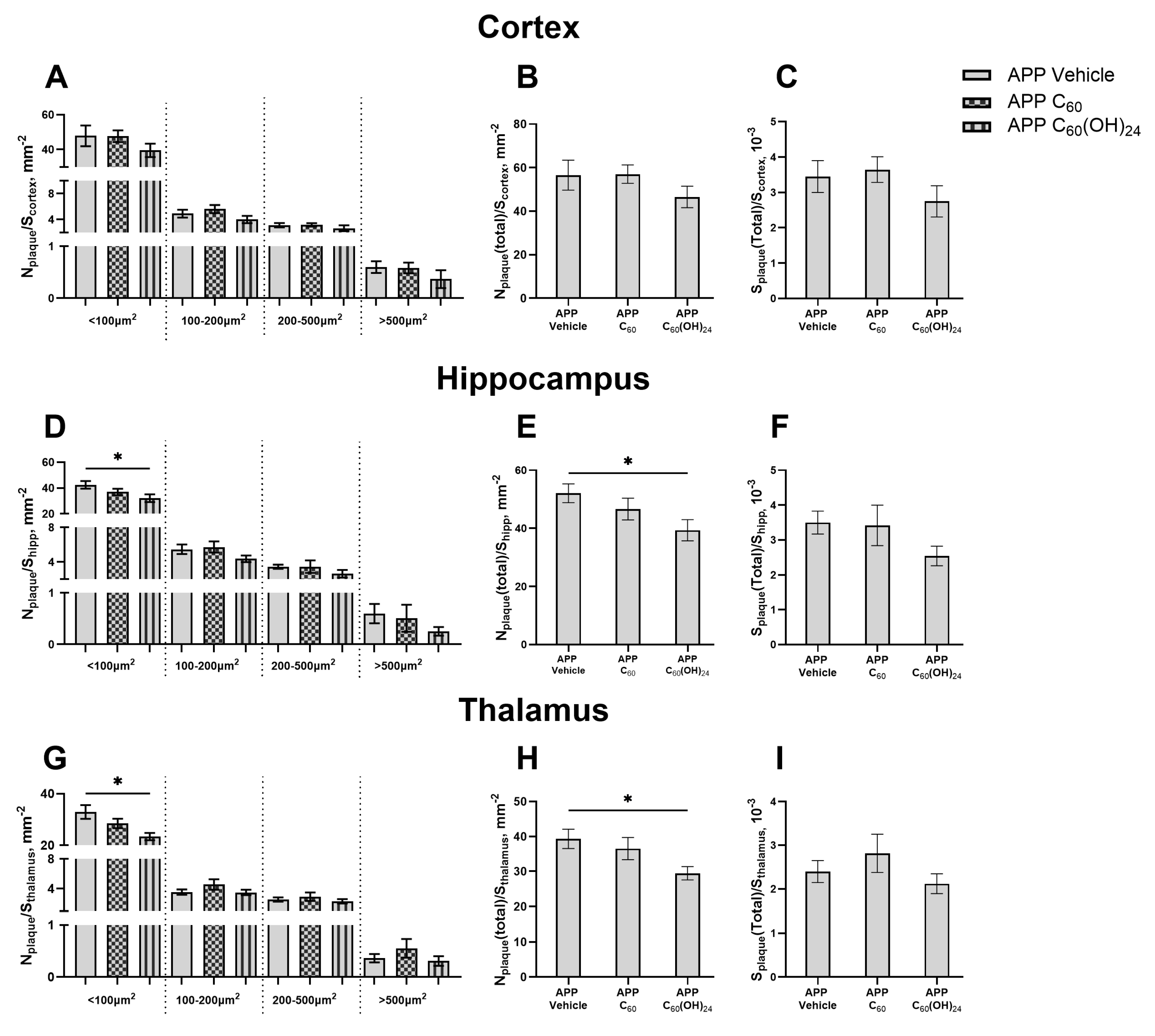
3.4. Distribution of Amyloid Plaques of Various Sizes Across the Cortex, Hippocampus, and Thalamus Under the Influence of Fullerenol
3.5. Beneficial Effects of Treatment with Fullerenol but Not Fullerene on Plaque Deposition in the Brain
3.6. The Impact of the Treatment on the Number of GFAP-Positive Cells in the Brain
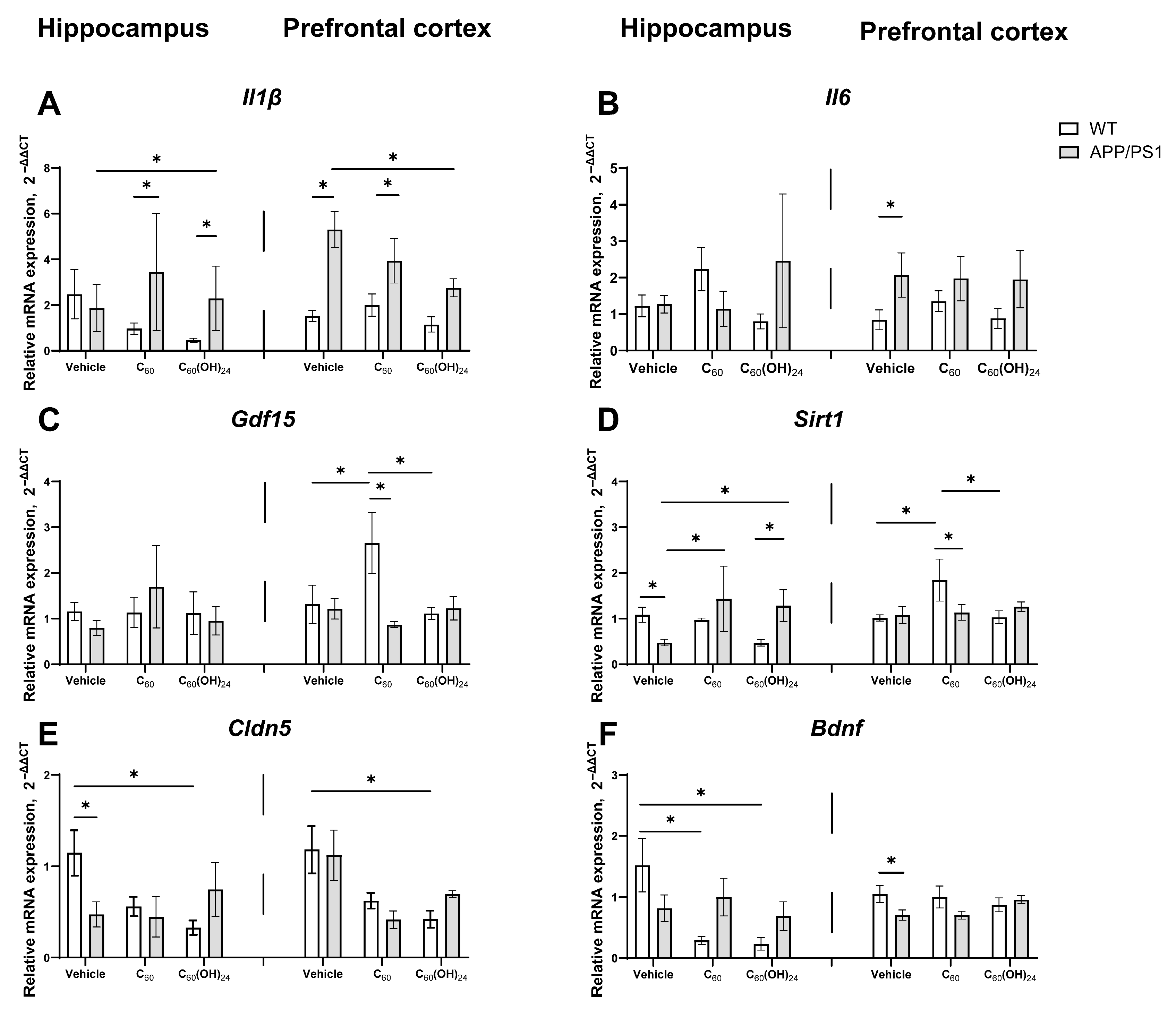
3.7. Region-Specific Modulation of Inflammatory and Plasticity-Related Gene Expression by Fullerene and Fullerenol in APP/PS1 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP/PS1 | APPswe/PS1E9 mice |
| WT | Wild-type |
| ROS | Reactive oxygen species |
| Aβ | Amyloid-beta peptide |
| EEG | Electroencephalogram |
| BBB | Blood-brain barrier |
| PD | Parkinson’s disease |
| DMD | Discrete molecular dynamics |
| ThT | Thioflavin-T |
| TEM | Transmission electron microscopy |
| FTIR | Fourier transform infrared |
| HFIP | 1,1,1,3,3,3-hexafluoro-2-propanol |
| DMSO | Dimethyl sulfoxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBS | Phosphate-buffered saline |
| T-PMT | transmitted light photomultiplier |
| ROI | Region of interest |
| Il1β | Interleukin 1 beta |
| Il6 | Interleukin 6 |
| Gdf15 | Growth Differentiation Factor 15 |
| Sirt1 | Sirtuin 1 |
| Cldn5 | Claudin 5 |
| Bdnf | Brain-Derived Neurotrophic Factor |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor Necrosis Factor alpha |
| PFC | Prefrontal cortex |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| SCFA | Short-chain fatty acids |
References
- Prince, M.; Ali, G.-C.; Guerchet, M.; Prina, M.; Albanese, E.; Wu, Y.-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2013, 9, 208–245. [CrossRef] [PubMed]
- Galvan, Y.P.; Alperovich, I.; Zolotukhin, P.; Prazdnova, E.; Mazanko, M.; Belanova, A.; Chistyakov, V. Fullerenes as Anti-Aging Antioxidants. Curr. Aging Sci. 2017, 10, 56–67. [Google Scholar] [CrossRef]
- Baati, T.; Bourasset, F.; Gharbi, N.; Njim, L.; Abderrabba, M.; Kerkeni, A.; Szwarc, H.; Moussa, F. The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene. Biomaterials 2012, 33, 4936–4946. [Google Scholar] [CrossRef]
- Xie, L.; Luo, Y.; Lin, D.; Xi, W.; Yang, X.; Wei, G. The molecular mechanism of fullerene-inhibited aggregation of Alzheimer’s beta-amyloid peptide fragment. Nanoscale 2014, 6, 9752–9762. [Google Scholar] [CrossRef]
- Andalib, S.; Nikpour, M.; Hamidi, M.; Javadi, R.; Mohammadpour, H. Evaluation and Comparison of Fullerene (C60) Aqueous Suspension Administration Effects with Memantine HCL in Rat Model of Alzheimer’s Disease Considering Behavioral Patterns and Spatial Memory. J. Microbiota 2024, 1, e143061. [Google Scholar] [CrossRef]
- Dai, W.; Zhao, M.; Chen, C.; Zhou, C.; Wang, P.; Yang, Z.; Gao, S.; Lu, Y.; Zhang, J.; Liu, X. Nano C60 Promotes Synaptic Distribution of Phosphorylated CaMKIIα and Improves Cognitive Function in APP/PS1 Transgenic Mice. ACS Chem. Neurosci. 2022, 13, 3534–3543. [Google Scholar] [CrossRef]
- Kotelnikova, R.; Smolina, A.; Grigoryev, V.; Faingold, I.; Mischenko, D.; Rybkin, A.; Poletayeva, D.; Vankin, G.; Zamoyski, V.; Voronov, I.; et al. Influence of water-soluble derivatives of [60]fullerene on therapeutically important targets related to neurodegenerative diseases. Med. Chem. Commun. 2014, 5, 1664–1668. [Google Scholar] [CrossRef]
- Vorobyov, V.; Kaptsov, V.; Gordon, R.; Makarova, E.; Podolski, I.; Sengpiel, F. Neuroprotective Effects of Hydrated Fullerene C60: Cortical and Hippocampal EEG Interplay in an Amyloid-Infused Rat Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- Gordon, R.; Podolski, I.; Makarova, E.; Deev, A.; Mugantseva, E.; Khutsyan, S.; Sengpiel, F.; Murashev, A.; Vorobyov, V.; Pennington, C. Intrahippocampal Pathways Involved in Learning/Memory Mechanisms are Affected by Intracerebral Infusions of Amyloid-β25-35 Peptide and Hydrated Fullerene C60 in Rats. J. Alzheimer’s Dis. 2017, 58, 711–724. [Google Scholar] [CrossRef]
- Malm, T.; Koistinaho, J.; Kanninen, K. Utilization of APPswe/PS1dE9 Transgenic Mice in Research of Alzheimer’s Disease: Focus on Gene Therapy and Cell-Based Therapy Applications. Int. J. Alzheimer’s Dis. 2011, 2011, 517160. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, M. Fullerene inhibits β-amyloid peptide aggregation. Biochem. Biophys. Res. Commun. 2003, 303, 576–579. [Google Scholar] [CrossRef]
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of α-tocopherol. Free Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef]
- Kong, A.; Liu, T.; Deng, S.; Xu, S.; Luo, Y.; Li, J.; Du, Z.; Wang, L.; Xu, X.; Fan, X. Novel antidepressant-like properties of the fullerenol in an LPS-induced depressive mouse model. Int. Immunopharmacol. 2023, 116, 109792. [Google Scholar] [CrossRef] [PubMed]
- Seke, M.; Stankovic, A.; Zivkovic, M. Capacity of fullerenols to modulate neurodegeneration induced by ferroptosis: Focus on multiple sclerosis. Mult. Scler. Relat. Disord. 2025, 97, 106378. [Google Scholar] [CrossRef]
- Li, X.; Deng, R.; Li, J.; Li, H.; Xu, Z.; Zhang, L.; Feng, L.; Shu, C.; Zhen, M.; Wang, C. Oral [60]fullerene reduces neuroinflammation to alleviate Parkinson’s disease via regulating gut microbiome. Theranostics 2023, 13, 4936–4951. [Google Scholar] [CrossRef]
- Semenov, K.N.; Ivanova, D.A.; Ageev, S.V.; Petrov, A.V.; Podolsky, N.E.; Volochaeva, E.M.; Fedorova, E.M.; Meshcheriakov, A.A.; Zakharov, E.E.; Murin, I.V.; et al. Evaluation of the C60 biodistribution in mice in a micellar ExtraOx form and in an oil solution. Sci. Rep. 2021, 11, 8362. [Google Scholar] [CrossRef]
- Sun, Y.; Kakinen, A.; Zhang, C.; Yang, Y.; Faridi, A.; Davis, T.P.; Cao, W.; Ke, P.C.; Ding, F. Amphiphilic surface chemistry of fullerenols is necessary for inhibiting the amyloid aggregation of alpha-synuclein NACore. Nanoscale 2019, 11, 11933–11945. [Google Scholar] [CrossRef]
- Shi, Q.; Fang, C.; Zhang, Z.; Yan, C.; Zhang, X. Visualization of the tissue distribution of fullerenols in zebrafish (Danio rerio) using imaging mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 7649–7658. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Perovic, M.; Ciric, J.; Matovic, V.; Srbovan, M.; Koruga, D.; Kanazir, S.; Ivkovic, S. The presymptomatic treatment with 3HFWC nanosubstance decreased plaque load in 5XFAD mouse model of Alzheimer’s disease. CNS Neurosci. Ther. 2024, 30, e14188. [Google Scholar] [CrossRef]
- Gelderman, M.P.; Simakova, O.; Clogston, J.D.; Patri, A.K.; Siddiqui, S.F.; Vostal, A.C.; Simak, J. Adverse effects of fullerenes on endothelial cells: Fullerenol C60(OH)24 induced tissue factor and ICAM-I membrane expression and apoptosis in vitro. Int. J. Nanomed. 2008, 3, 59–68. [Google Scholar] [CrossRef]
- Yamawaki, H.; Iwai, N. Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1495–C1502. [Google Scholar] [CrossRef]
- Yamago, S.; Tokuyama, H.; Nakamura, E.; Kikuchi, K.; Kananishi, S.; Sueki, K.; Nakahara, H.; Enomoto, S.; Ambe, F. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem. Biol. 1995, 2, 385–389. [Google Scholar] [CrossRef]
- Tsoy, A.; Askarova, S.; Shalakhmetova, T.; Umbayev, B.; Adambekov, S.; Zhumadilov, Z. Role of ROS in Aβ42 mediated cell surface P-selectin expression and actin polymerization. Mol. Neurodegener. 2013, 8, P44. [Google Scholar] [CrossRef]
- Dahlgren, K.N.; Manelli, A.M.; Stine, W.B.; Baker, L.K.; Krafft, G.A.; LaDu, M.J. Oligomeric and Fibrillar Species of Amyloid-β Peptides Differentially Affect Neuronal Viability*. J. Biol. Chem. 2002, 277, 32046–32053. [Google Scholar] [CrossRef]
- Costa-Nunes, J.P.; Cline, B.H.; Araújo-Correia, M.; Valença, A.; Markova, N.; Dolgov, O.; Kubatiev, A.; Yeritsyan, N.; Steinbusch, H.W.; Strekalova, T. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. Biomed. Res. Int. 2015, 2015, 596126. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Veniaminova, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Walitza, S.; Anthony, D.C.; Lim, L.W.; et al. ASD-like behaviors, a dysregulated inflammatory response and decreased expression of PLP1 characterize mice deficient for sialyltransferase ST3GAL5. Brain Behav. Immun.-Health 2021, 16, 100306. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Steinbusch, H.W.M. Measuring behavior in mice with chronic stress depression paradigm. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Veniaminova, E.; Oplatchikova, M.; Bettendorff, L.; Kotenkova, E.; Lysko, A.; Vasilevskaya, E.; Kalueff, A.V.; Fedulova, L.; Umriukhin, A.; Lesch, K.-P.; et al. Prefrontal cortex inflammation and liver pathologies accompany cognitive and motor deficits following Western diet consumption in non-obese female mice. Life Sci. 2020, 241, 117163. [Google Scholar] [CrossRef]
- Cline, B.H.; Steinbusch, H.W.M.; Malin, D.; Revishchin, A.V.; Pavlova, G.V.; Cespuglio, R.; Strekalova, T. The neuronal insulin sensitizer dicholine succinate reduces stress-induced depressive traits and memory deficit: Possible role of insulin-like growth factor 2. BMC Neurosci. 2012, 13, 110. [Google Scholar] [CrossRef]
- Strekalova, T.; Costa-Nunes, J.P.; Veniaminova, E.; Kubatiev, A.; Lesch, K.-P.; Chekhonin, V.P.; Evans, M.C.; Steinbusch, H.W.M. Insulin receptor sensitizer, dicholine succinate, prevents both Toll-like receptor 4 (TLR4) upregulation and affective changes induced by a high-cholesterol diet in mice. J. Affect. Disord. 2016, 196, 109–116. [Google Scholar] [CrossRef]
- Malatynska, E.; Steinbusch, H.W.M.; Redkozubova, O.; Bolkunov, A.; Kubatiev, A.; Yeritsyan, N.B.; Vignisse, J.; Bachurin, S.; Strekalova, T. Anhedonic-like traits and lack of affective deficits in 18-month-old C57BL/6 mice: Implications for modeling elderly depression. Exp. Gerontol. 2012, 47, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, Y.; Munkuev, A.; Mozhaitsev, E.; Suslov, E.; Tsypyshev, D.; Chaprov, K.; Begunov, R.; Volcho, K.; Salakhutdinov, N.; Neganova, M. Elaboration of the Effective Multi-Target Therapeutic Platform for the Treatment of Alzheimer’s Disease Based on Novel Monoterpene-Derived Hydroxamic Acids. Int. J. Mol. Sci. 2023, 24, 9743. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Semakov, A.; Tsypyshev, D.; Chaprov, K.; Klochkov, S.; Neganova, M. Neuroprotective Effects and Cognitive Enhancement of Allomargaritarine in 5xFAD Alzheimer’s Disease Mice Model. OBM Neurobiol. 2024, 8, 207. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Vorobyov, V.; Deev, A.; Morozova, O.; Oganesyan, Z.; Krayushkina, A.M.; Ivanova, T.A.; Chaprov, K. Early Effects of Alpha-Synuclein Depletion by Pan-Neuronal Inactivation of Encoding Gene on Electroencephalogram Coherence between Different Brain Regions in Mice. Biomedicines 2023, 11, 3282. [Google Scholar] [CrossRef]
- Phillips, J.M.; Winfree, R.L.; Seto, M.; Schneider, J.A.; Bennett, D.A.; Dumitrescu, L.C.; Hohman, T.J. Pathologic and clinical correlates of region-specific brain GFAP in Alzheimer’s disease. Acta Neuropathol. 2024, 148, 69. [Google Scholar] [CrossRef]
- Rodríguez-Giraldo, M.; González-Reyes, R.E.; Ramírez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a Therapeutic Target in Alzheimer’s Disease-Comprehensive Review and Recent Developments. Int. J. Mol. Sci. 2022, 23, 3630. [Google Scholar] [CrossRef]
- Shaftel, S.S.; Kyrkanides, S.; Olschowka, J.A.; Miller, J.N.; Johnson, R.E.; O’Banion, M.K. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Investig. 2007, 117, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Di Bona, D.; Plaia, A.; Vasto, S.; Cavallone, L.; Lescai, F.; Franceschi, C.; Licastro, F.; Colonna-Romano, G.; Lio, D.; Candore, G.; et al. Association between the interleukin-1beta polymorphisms and Alzheimer’s disease: A systematic review and meta-analysis. Brain Res. Rev. 2008, 59, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-L.; Sun, H.-Y.; Wang, H.-Q. Therapeutic potential of targeting SIRT1 for the treatment of Alzheimer’s disease. Sheng Li Xue Bao [Acta Physiol. Sin.] 2023, 75, 99–107. [Google Scholar] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Schuhmann, M.K.; Fluri, F. Effects of Fullerenols on Mouse Brain Microvascular Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 1783. [Google Scholar] [CrossRef]
- Yuan, Q.; Bao, B.; Li, M.; Tang, Y. Bioactive Composite Nanoparticles for Effective Microenvironment Regulation, Neuroprotection, and Cell Differentiation. ACS Appl. Mater. Interfaces 2022, 14, 15623–15631. [Google Scholar] [CrossRef]
- Bolshakova, O.; Borisenkova, A.; Suyasova, M.; Sedov, V.; Slobodina, A.; Timoshenko, S.; Varfolomeeva, E.; Golomidov, I.; Lebedev, V.; Aksenov, V.; et al. In vitro and in vivo study of the toxicity of fullerenols C60, C70 and C120O obtained by an original two step method. Mater. Sci. Eng. C 2019, 104, 109945. [Google Scholar] [CrossRef]
- Wei, J.; Wang, H.; Jiang, H.; Peng, H.; Gu, C.; Zhou, Y.; Liao, Y.; Wang, D.; Yang, Q.; Ren, G.; et al. Fullerenol reduces vascular injury caused by ischemia-reperfusion. Carbon 2025, 234, 119949. [Google Scholar] [CrossRef]
- Andrievsky, G.V.; Bruskov, V.I.; Tykhomyrov, A.A.; Gudkov, S.V. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic. Biol. Med. 2009, 47, 786–793. [Google Scholar] [CrossRef]
- Dugan, L.L.; Gabrielsen, J.K.; Yu, S.P.; Lin, T.-S.; Choi, D.W. Buckminsterfullerenol Free Radical Scavengers Reduce Excitotoxic and Apoptotic Death of Cultured Cortical Neurons. Neurobiol. Dis. 1996, 3, 129–135. [Google Scholar] [CrossRef]
- Ye, S.; Chen, M.; Jiang, Y.; Chen, M.; Zhou, T.; Wang, Y.; Hou, Z.; Ren, L. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defence system. Int. J. Nanomed. 2014, 9, 2073–2087. [Google Scholar] [CrossRef]
- Maarouf, C.L.; Walker, J.E.; Sue, L.I.; Dugger, B.N.; Beach, T.G.; Serrano, G.E. Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS ONE 2018, 13, e0203659. [Google Scholar] [CrossRef]
- Tsoy, A.; Umbayev, B.; Kassenova, A.; Kaupbayeva, B.; Askarova, S. Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 964. [Google Scholar] [CrossRef]
- Kozhakhmetov, S.; Kaiyrlykyzy, A.; Jarmukhanov, Z.; Vinogradova, E.; Zholdasbekova, G.; Alzhanova, D.; Kunz, J.; Kushugulova, A.; Askarova, S. Inflammatory Manifestations Associated With Gut Dysbiosis in Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2024, 2024, 9741811. [Google Scholar] [CrossRef]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef]
- Ji, Z.Q.; Sun, H.; Wang, H.; Xie, Q.; Liu, Y.; Wang, Z. Biodistribution and tumor uptake of C60(OH)xin mice. J. Nanopart. Res. 2006, 8, 53–63. [Google Scholar] [CrossRef]
- Maksin, T.; Djokic, D.; Jankovic, D.; Djordjevic, A.; Neskovic, O. Comparison of some physico-chemical parameters and biological behaviour of fullerenol labelled with technetium-99m. J. Optoelectron. Adv. Mater. 2007, 9, 2571–2577. [Google Scholar]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Gonçalves, E.; Johnson, T.I.; Zecchini, V.R.; da Costa, A.S.H.; Gaude, E.; Drubbel, A.V.; Theobald, S.J.; Abbo, S.R.; Tran, M.G.B.; et al. Correction: Corrigendum: Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 2016, 540, 150. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Chandrashekar, D.V.; Roules, G.C.; Jagadeesan, N.; Panchal, U.R.; Oyegbesan, A.; Imiruaye, O.E.; Zhang, H.; Garcia, J.; Kaur, K.; Win, S.; et al. Hepatic LRP-1 plays an important role in amyloidosis in Alzheimer’s disease mice: Potential role in chronic heavy alcohol feeding. Neurobiol. Dis. 2024, 199, 106570. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, D.-Y.; Wang, Y.-J. Peripheral clearance of brain-derived Aβ in Alzheimer’s disease: Pathophysiology and therapeutic perspectives. Transl. Neurodegener. 2020, 9, 16. [Google Scholar] [CrossRef]
- Chapanian, R.; Tse, M.Y.; Pang, S.C.; Amsden, B.G. The role of oxidation and enzymatic hydrolysis on the in vivo degradation of trimethylene carbonate based photocrosslinkable elastomers. Biomaterials 2009, 30, 295–306. [Google Scholar] [CrossRef]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.F.; Luh, T.-Y.; Choi, D.W.; Lin, T.-S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef] [PubMed]
- Marecki, J.C.; Cota-Gomez, A.; Vaitaitis, G.M.; Honda, J.R.; Porntadavity, S.; St. Clair, D.K.; Flores, S.C. HIV-1 Tat regulates the SOD2 basal promoter by altering Sp1/Sp3 binding activity. Free Radic. Biol. Med. 2004, 37, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Melchor, M.H.; Susana, F.G.; Francisco, G.S.; Hiram, I.B.; Norma, R.F.; Jorge, A.L.; Perla, Y.L.; Gustavo, B.I. Fullerenemalonates inhibit amyloid beta aggregation, in vitro and in silico evaluation. RSC Adv. 2018, 8, 39667–39677. [Google Scholar] [CrossRef]
- da Silva Gonçalves, A.; França, T.C.; Vital de Oliveira, O. Computational studies of acetylcholinesterase complexed with fullerene derivatives: A new insight for Alzheimer disease treatment. J. Biomol. Struct. Dyn. 2016, 34, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Podol’skiĭ, I.; Poddubnaia, Z.A.; Godukhin, O.V. Fullerenes C60, antiamyloid action, the brain and cognitive processes. Biofizika 2010, 55, 88–94. [Google Scholar] [CrossRef]
- Faiyaz, M.; Ganayee, M.A.; Akhtar, S.; Krishnan, S.; Flora, B.; Dogra, D.; Jha, N.K.; Chellappan, D.K.; Negi, P.; Dua, K.; et al. Nanomaterials in Alzheimer’s disease treatment: A comprehensive review. Front. Biosci. Landmark Ed. 2021, 26, 851–865. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askarova, S.; Sitdikova, K.; Kassenova, A.; Chaprov, K.; Svirin, E.; Tsoy, A.; de Munter, J.; Gorlova, A.; Litavrin, A.; Deikin, A.; et al. Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice. Antioxidants 2025, 14, 834. https://doi.org/10.3390/antiox14070834
Askarova S, Sitdikova K, Kassenova A, Chaprov K, Svirin E, Tsoy A, de Munter J, Gorlova A, Litavrin A, Deikin A, et al. Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice. Antioxidants. 2025; 14(7):834. https://doi.org/10.3390/antiox14070834
Chicago/Turabian StyleAskarova, Sholpan, Kseniia Sitdikova, Aliya Kassenova, Kirill Chaprov, Evgeniy Svirin, Andrey Tsoy, Johannes de Munter, Anna Gorlova, Aleksandr Litavrin, Aleksei Deikin, and et al. 2025. "Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice" Antioxidants 14, no. 7: 834. https://doi.org/10.3390/antiox14070834
APA StyleAskarova, S., Sitdikova, K., Kassenova, A., Chaprov, K., Svirin, E., Tsoy, A., de Munter, J., Gorlova, A., Litavrin, A., Deikin, A., Nedorubov, A., Appazov, N., Kalueff, A., Chernopiatko, A., & Strekalova, T. (2025). Distinctive Effects of Fullerene C60 and Fullerenol C60(OH)24 Nanoparticles on Histological, Molecular and Behavioral Hallmarks of Alzheimer’s Disease in APPswe/PS1E9 Mice. Antioxidants, 14(7), 834. https://doi.org/10.3390/antiox14070834






