Optimisation of Phenolic Compound Extraction from Agrimonia eupatoria L. Using Response Surface Methodology for Enhanced Yield of Different Phenolics and Maximised Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Plant Material and Preparation of Extracts
2.3. Development of Extraction Process
Experimental Design for Modelling and Optimisation of Extraction Process
2.4. Determination of Individual Phenolic Profile in A. eupatoria
2.5. Determination of Total Phenolic Content and ABTS, and FRAP Activity Assays
2.6. Statistical Analysis
3. Results
3.1. Assessment of Total Phenolic Content
3.2. Assessment of the Amount of Phenolic Acids
3.3. Assessment of the Amount of Flavan-3-ols and Tannins
3.4. Assessment of the Sum of Identified Flavones and Flavonols
3.5. Assessment of the Amount of Agrimoniin
3.6. Assessment of Antioxidant Activity
3.7. Statistical Analysis of Response Models via ANOVA
3.8. Phytochemical Profile and Antioxidant Activity of A. eupatoria Extracts Under Optimised Extraction Conditions
3.9. Correlation Analysis of Phenolic Compounds and Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Snafi, E.A. The Pharmacological and Therapeutic Importance of Agrimonia eupatoria—A Review. Asian J. Pharm. Sci. Technol. 2015, 5, 106–111. [Google Scholar]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 25 May 2025).
- Ciobotaru, L.G.G.; Pavel, A.Z.; Poenaru, R.; Moaca, E.A.; Florescu, R.; Danciu, R.; Dumitrascu, C.; Imbrea, I.; Pop, O. Assessment of the Antioxidant Effect of Ethanolic Extracts Obtained from Agrimonia eupatoria L., Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench Collected from the Eastern Part of Romania. Rev. De Chim. 2018, 69, 2385–2390. [Google Scholar] [CrossRef]
- Bělonožníková, K.; Sladkovská, E.; Kavan, D.; Hýsková, V.; Hodek, P.; Šmíd, D.; Ryšlavá, H. Effect of Agrimonia eupatoria L. and Origanum vulgare L. Leaf, Flower, Stem, and Root Extracts on the Survival of Pseudomonas Aeruginosa. Molecules 2023, 28, 1019. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, O.; Donato, M.M.; Luxo, C.; Almeida, N.; Liberal, J.; Figueirinha, A.; Batista, M.T. Anti-Helicobacter Pylori Potential of Agrimonia eupatoria L. and Fragaria vesca. J. Funct. Foods 2018, 44, 299–303. [Google Scholar] [CrossRef]
- Santos, T.N.; Costa, G.; Ferreira, J.P.; Liberal, J.; Francisco, V.; Paranhos, A.; Cruz, M.T.; Castelo-Branco, M.; Figueiredo, I.V.; Teresa Batista, M. Antioxidant, Anti-Inflammatory, and Analgesic Activities of Agrimonia eupatoria L. Infusion. Evid.-Based Complement. Altern. Med. 2017, 2017, 8309894. [Google Scholar] [CrossRef]
- Pour, M.G.; Mirazi, N.; Moradkhani, S.; Rafieian-Kopaei, M.; Rahimi-Madiseh, M. A Comprehensive Review on Phytochemical, Pharmacological and Therapeutic Properties of Agrimonia eupatoria L. J. Herbmed Pharmacol. 2020, 10, 14–30. [Google Scholar] [CrossRef]
- Karlińska, E.; Romanowska, B.; Kosmala, M. The Aerial Parts of Agrimonia procera Wallr. and Agrimonia eupatoria L. as a Source of Polyphenols, and Especially Agrimoniin and Flavonoids. Molecules 2021, 26, 7706. [Google Scholar] [CrossRef]
- Kuczmannová, A.; Balažová, A.; Račanská, E.; Kameníková, M.; Fialová, S.; Majerník, J.; Nagy, M.; Gál, P.; Mučaji, P. Agrimonia eupatoria L. and Cynara cardunculus L. Water Infusions: Comparison of Anti-Diabetic Activities. Molecules 2016, 21, 564–576. [Google Scholar] [CrossRef]
- Iguchi, T.; Kuroda, M.; Kan, C.; Fujii, T.; Mimaki, Y. Chemical Constituents in the Whole-Plant Extract of Agrimonia eupatoria and Their Aldose Reductase Inhibitory Activities. Shoyakugaku Zasshi 2020, 74, 60–61. [Google Scholar] [CrossRef]
- Lee, K.H.; Rhee, K.H. Anti-nociceptive effect of Agrimonia eupatoria extract on a cisplatin-induced neuropathic model. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 139–144. [Google Scholar] [CrossRef]
- Muruzovic, M.; Mladenovic, K.; Stefanovic, O.; Zugic-Petrovic, T.; Comic, L. In Vitro Interaction between Agrimonia eupatoria L.: Extracts and Antibiotic. Kragujev. J. Sci. 2017, 39, 157–164. [Google Scholar] [CrossRef]
- Kaňuchová, M.; Brindza Lachová, V.; Bogdanová, K.; Sabová, J.; Bonová, P.; Vasilenko, T.; Kováč, I.; Novotný, M.; Mitrengová, P.; Sahatsapan, N.; et al. Assessment of Agrimonia eupatoria L. and Lipophosphonoxin (DR-6180) Combination for Wound Repair: Bridging the Gap Between Phytomedicine and Organic Chemistry. Biomolecules 2024, 14, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Muruzović, M.; Mladenović, K.G.; Stefanović, O.D.; Vasić, S.M.; Čomić, L.R. Extracts of Agrimonia eupatoria L. as Sources of Biologically Active Compounds and Evaluation of Their Antioxidant, Antimicrobial, and Antibiofilm Activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Mortazavi, S.A.; Sharifi, A. Extraction of Phenolic Compounds from Agrimonia eupatoria Using Microwave and Ultrasound-Assisted Extraction Methods. J. Food Bioprocess Eng. 2022, 5, 1–8. [Google Scholar] [CrossRef]
- Miranda, A.R.; Albrecht, C.; Cortez, M.V.; Soria, E.A. Pharmacology and Toxicology of Polyphenols with Potential As Neurotropic Agents in Non-Communicable Diseases. Curr. Drug Targets 2018, 19, 97–110. [Google Scholar] [CrossRef]
- Elnour, A.A.M.; Mohamed, E.S.M.; Musa, K.H.; Kabbashi, N.A.; Alam, M.Z. Challenges of Extraction Techniques of Natural Antioxidants and Their Potential Application Opportunities as Anti-Cancer Agents. Health Sci. J. 2018, 12, 596. [Google Scholar] [CrossRef]
- Khademi, F. Beyond the Present: Future Research Directions on the Extraction and Fractionation of Bioactives, with the Focus on Phytochemicals. IOSR J. Biotechnol. Biochem. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Subramani, V.; Tomer, V.; Balamurali, G.; Mansingh, P. Artificial Neural Network in Optimization of Bioactive Compound Extraction: Recent Trends and Performance Comparison with Response Surface Methodology. Anal. Sci. 2025, 41, 101–117. [Google Scholar] [CrossRef]
- Ba, D.; Boyaci, I.H. Modeling and Optimization I: Usability of Response Surface Methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Serna-Cock, L.; Belmares-Cerda, R.; Aguilar, C.N. Extraction of Antioxidants from Mango Seed Kernel: Optimization Assisted by Microwave. Food Bioprod. Process. 2017, 105, 188–196. [Google Scholar] [CrossRef]
- Malheiros, J.; Simões, D.M.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Agrimonia eupatoria L.: An Integrative Perspective on Ethnomedicinal Use, Phenolic Composition and Pharmacological Activity. J. Ethnopharmacol. 2022, 296, 115498. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines & Health Care (EDQM). European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023; Volume 11.8. [Google Scholar]
- Kaunaite, V.; Vilkickyte, G.; Raudone, L. Phytochemical Diversity and Antioxidant Potential of Wild Heather (Calluna vulgaris L.) Aboveground Parts. Plants 2022, 11, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844–864. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ucar, Y.; Tadesse, E.E.; Rathod, N.; Kulawik, P.; Trif, M.; Esatbeyoglu, T.; Ozogul, F. Tannins for food preservation and human health: A review of current knowledge. Appl. Food Res. 2025, 5, 100738. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Lin, J.T.; Wang, C.K.; Chen, H.Y.; Yang, D.J. Antioxidant Properties of Various Solvent Extracts from Lychee (Litchi chinenesis Sonn.) Flowers. Food Chem 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520–4543. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of Solvent Effect on the Extraction of Phenolic Compounds and Antioxidant Capacities from the Berries: Application of Principal Component Analysis. Chem. Cent. J. 2014, 8, 1–9. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioproc. Tech. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Skalicka-Woźniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Piwowarski, J.P.; Granica, S.; Tomczyk, M. A Comprehensive Review of Agrimoniin. Ann. N. Y. Acad. Sci. 2017, 1401, 166–180. [Google Scholar] [CrossRef]
- Granica, S.; Kluge, H.; Horn, G.; Matkowski, A.; Kiss, A.K. The Phytochemical Investigation of Agrimonia eupatoria L. and Agrimonia Procera Wallr. as Valid Sources of Agrimoniae herba—The Pharmacopoeial Plant Material. J. Pharm. Biomed. Anal. 2015, 114, 272–279. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin Sensitizes Pancreatic Cancer to Apoptosis through ROS-Mediated Energy Metabolism Dysfunction. Phytomedicine 2022, 96, 153807. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Sheichenko, O.P.; Fedotcheva, N.I. New Properties and Mitochondrial Targets of Polyphenol Agrimoniin as a Natural Anticancer and Preventive Agent. Pharmaceutics 2021, 13, 2089–2105. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Chirikova, N.K.; Olennikov, D.N. Agrimoniin, an Active Ellagitannin from Comarum palustre Herb with Anti-α-Glucosidase and Antidiabetic Potential in Streptozotocin-Induced Diabetic Rats. Molecules 2017, 22, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Casetti, F.; Bullerkotte, U.; Haarhaus, B.; Vagedes, J.; Schempp, C.M.; Wölfle, U. Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla Erecta. Molecules 2016, 21, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Agrimonia eupatoria L., Herba (Agrimoniae herba); EMA/HMPC/680597/2013; European Medicines Agency: London, UK, 2015; Available online: https://www.ema.europa.eu/en/medicines/herbal/agrimoniae-herba (accessed on 27 June 2025).
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological Actions and Underlying Mechanisms of Catechin: A Review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and Bioavailability of Quercetin Glycosides in Humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Liancai, Z.; Jun, T.; Bochu, W.; Rui, H.; Yuping, L.; Chao, Z. Antioxidant Activities of Aqueous Extract from Agrimonia Pilosa Ledeb and Its Fractions. Chem. Biodivers. 2009, 6, 1716–1726. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A Review of the Antioxidant Potential of Medicinal Plant Species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Amarowicz, R. Tannins: The New Natural Antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 549–551. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity; Woodhead Publishing: Sawston, Cambridge, UK, 2019; ISBN 9780128147740. [Google Scholar]
- Arvind, N.; Kiran, D.; Pallavi, G.; Amit, S. Antioxidant Potential and Effect of Extraction Solvent on Total Phenol Content, Flavonoids Content and Tannin Content of Ficus Palmata Forssk. Int. J. Pharm. Sci. Rev. Res. 2018, 49, 19–24. [Google Scholar]
- Surendar, K.; Neha; Sharma, N.; Akshay, T. A Review on Medicinal Plants Having Antioxidant Potential with Market Value of Ginger | Enhanced Reader. Int. J. Pharmacogn. Pharm. Res. 2023, 5, 30–34. [Google Scholar]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.K.; Ayadi, M.T. Total Phenolic, Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5, 52–57. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 25, 3380. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.M.A.N.; Nadeem, M.; Safdar, M.N.; Jabbar, S.; Mahmood, S.; Murtaza, M.A.; Ameer, K.; Ibrahim, S.A. Antioxidant Activity and Phenolic Content of Sonication-and Maceration-Assisted Ethanol and Acetone Extracts of Cymbopogon citratus Leaves. Separations 2022, 9, 244. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]



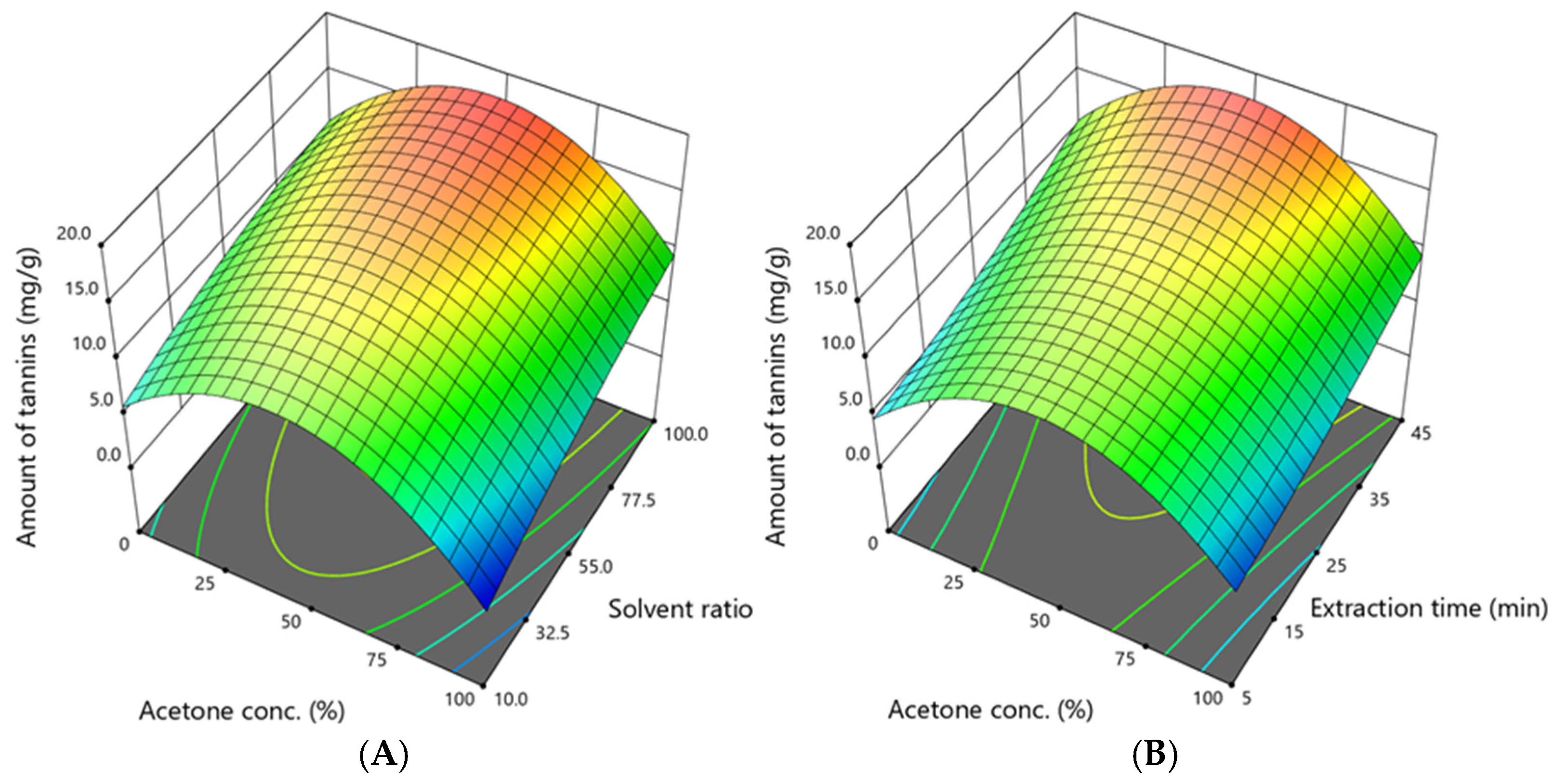




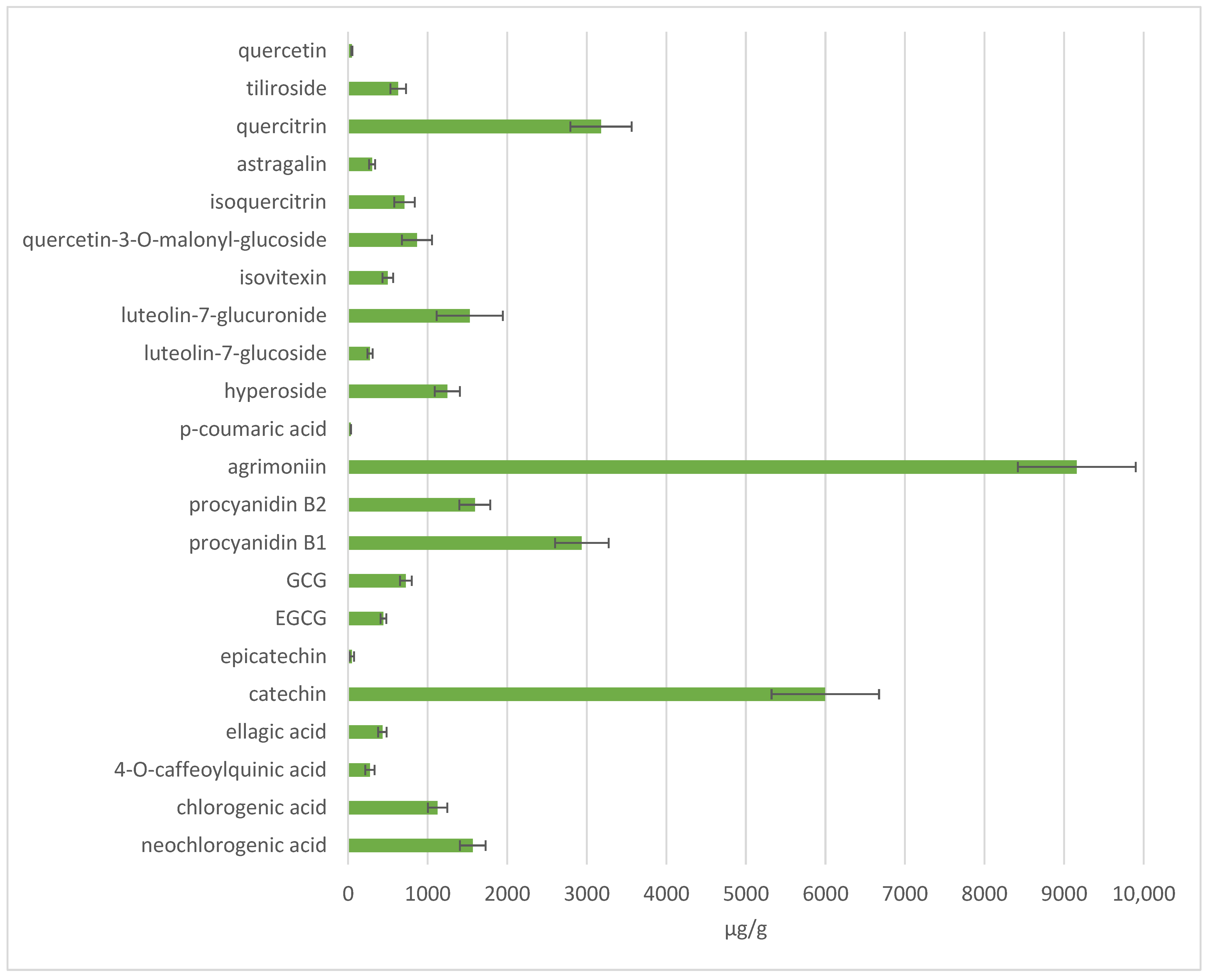

| Extraction No. | Factor A | Factor B | Factor C |
|---|---|---|---|
| Acetone Concentration (%) | Solvent Ratio | Extraction Time (min) | |
| E-01 | 50 | 55 | 25 |
| E-02 | 100 | 100 | 5 |
| E-03 | 0 | 100 | 5 |
| E-04 | 50 | 55 | 45 |
| E-05 | 50 | 100 | 25 |
| E-06 | 50 | 10 | 25 |
| E-07 | 0 | 100 | 45 |
| E-08 | 50 | 55 | 5 |
| E-09 | 0 | 10 | 45 |
| E-10 | 100 | 100 | 45 |
| E-11 | 100 | 55 | 25 |
| E-12 | 0 | 10 | 5 |
| E-13 | 100 | 10 | 5 |
| E-14 | 0 | 55 | 25 |
| E-15 | 100 | 10 | 45 |
| Response | Model | p-Value |
|---|---|---|
| Total phenolic content | quadratic | 0.0503 |
| Cumulative amount of phenolic acids | linear | 0.0382 |
| Cumulative amount of tannins | quadratic | 0.1560 |
| Cumulative amount of flavonoids | quadratic | 0.1725 |
| Amount of agrimoniin | quadratic | 0.1824 |
| Radical scavenging activity | quadratic | 0.0789 |
| Reducing activity | quadratic | 0.0060 |
| Response | ||
|---|---|---|
| Factors | Cumulative Amount of Phenolic Acids | Reducing Activity |
| A (acetone concentration) | p = 0.0126 | p = 0.0023 |
| B (solvent ratio) | p = 0.2082 | p = 0.2283 |
| C (extraction time) | p = 0.2821 | p = 0.1084 |
| AB | – | p = 0.9401 |
| AC | – | p = 0.2089 |
| BC | – | p = 0.8997 |
| A2 | – | p = 0.0008 |
| B2 | – | p = 0.6955 |
| C2 | – | p = 0.3675 |
| Response | Model p-Value | Factor A p-Value | Factor A2 p-Value |
|---|---|---|---|
| Total phenolic content | <0.0001 | 0.0009 | <0.0001 |
| Cumulative amount of phenolic acids | 0.0133 | 0.0133 | – |
| Cumulative amount of tannins | 0.0045 | 0.1850 | 0.0020 |
| Cumulative amount of flavonoids | 0.0056 | 0.2128 | 0.0024 |
| Amount of agrimoniin | 0.0017 | 0.2232 | 0.0006 |
| Radical scavenging activity | <0.0001 | 0.0014 | <0.0001 |
| Reducing activity | <0.0001 | 0.0002 | <0.0001 |
| Response | Adjusted R2 | Predicted R2 | Δ R2 | Adeq Precision | The Equations (Actual Factors 1) |
|---|---|---|---|---|---|
| Total phenolic content | 0.8100 | 0.7456 | 0.06 | 11.1 | Y = 66.75 + 2.026X − 0.025X2 |
| Cumulative amount of phenolic acids | 0.3395 | 0.1948 | 0.14 | 4.9 | Y = 2.798 − 0.018X |
| Cumulative amount of tannins | 0.5257 | 0.3647 | 0.16 | 5.8 | Y = 6.74 + 0.276X − 0.003X2 |
| Cumulative amount of flavonoids | 0.5078 | 0.3408 | 0.17 | 5.6 | Y = 4.24 + 0.173X − 0.002X2 |
| Amount of agrimoniin | 0.5982 | 0.4619 | 0.14 | 6.5 | Y = 2.03 + 0.145X − 0.002X2 |
| Radical scavenging activity | 0.7881 | 0.7162 | 0.07 | 10.4 | Y = 871.16 + 28.31X − 0.35X2 |
| Reducing activity | 0.8650 | 0.8191 | 0.05 | 13.5 | Y = 345.05 + 11.72X − 0.15X2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukackas, J.; Žilius, M.; Šaltytė, G.; Raudonė, L. Optimisation of Phenolic Compound Extraction from Agrimonia eupatoria L. Using Response Surface Methodology for Enhanced Yield of Different Phenolics and Maximised Antioxidant Activity. Antioxidants 2025, 14, 831. https://doi.org/10.3390/antiox14070831
Sukackas J, Žilius M, Šaltytė G, Raudonė L. Optimisation of Phenolic Compound Extraction from Agrimonia eupatoria L. Using Response Surface Methodology for Enhanced Yield of Different Phenolics and Maximised Antioxidant Activity. Antioxidants. 2025; 14(7):831. https://doi.org/10.3390/antiox14070831
Chicago/Turabian StyleSukackas, Justinas, Modestas Žilius, Gerda Šaltytė, and Lina Raudonė. 2025. "Optimisation of Phenolic Compound Extraction from Agrimonia eupatoria L. Using Response Surface Methodology for Enhanced Yield of Different Phenolics and Maximised Antioxidant Activity" Antioxidants 14, no. 7: 831. https://doi.org/10.3390/antiox14070831
APA StyleSukackas, J., Žilius, M., Šaltytė, G., & Raudonė, L. (2025). Optimisation of Phenolic Compound Extraction from Agrimonia eupatoria L. Using Response Surface Methodology for Enhanced Yield of Different Phenolics and Maximised Antioxidant Activity. Antioxidants, 14(7), 831. https://doi.org/10.3390/antiox14070831







