Abstract
Characteristic manifestations of skin aging, due to either intrinsic or extrinsic factors, such as ultraviolet (UV) radiation and oxidative stress, include cell senescence, alterations in collagen and elastin networks, and melanogenesis disorders. Natural products are considered a rich source of anti-aging molecules. Accordingly, the screening of a plant extract library from the Greek flora for a panel of biological activities related to skin aging is presented herein. In particular, 52 plant materials extracted using Accelerated Solvent Extraction (ASE) and Supercritical Fluid Extraction (SFE) were assessed for their effects on (1) human skin cell viability, (2) antioxidant activity—using both cell-free and cell-based methods—(3) photoprotective capacity, and (4) interference with collagenase, elastase, and tyrosinase, as well as with proteasomal and lysosomal activities of human skin cells. In vivo phenotypic screens on Danio rerio (zebrafish) embryos were also used for assessing melanogenesis. Many active extracts were identified, some of them for the first time, and others in agreement with previous reports. In general, ASE extracts exhibited higher activities than SFE ones. Seven extracts showed multiple activities, being highly effective in at least four different assays. These data support the potential use of these extracts against skin aging in medicinal and cosmetic applications.
1. Introduction
Skin is the largest organ of the human body and due to its outermost anatomical location assumes—among many other functions—the role of the organism’s boundary and first defense against environmental insults [1]. Skin also usually provides the best representation of organismal aging. Skin aging is affected by a combination of endogenous or intrinsic (genetics, cellular metabolism, hormones, and metabolic processes) and exogenous or extrinsic (light exposure, pollution, ionizing radiation, chemicals, and toxins) parameters [2]. Among the latter, one of the most important factors is ultraviolet (UV) radiation, leading to a characteristic type of skin aging called photoaging [3]. Central features of the aged skin are the progressive loss of cells, as well as alterations in the composition and organization of extracellular matrix (ECM), especially regarding the collagen and elastin networks, leading to the formation of wrinkles [4]. Moreover, discoloration and abnormalities in skin pigmentation (such as senile lentigo and melasma) are quite often observed in the elderly skin [5,6]. Many mechanisms have been reported to underly skin aging manifestations, extremely important ones being oxidative stress and the loss of balance between anabolic and catabolic processes [4,7,8]. Moreover, accumulation of senescent cells has been reported to contribute to skin aging, mainly due to the senescence-associated secretory phenotype (SASP) [9,10] but also through other mechanisms, such as DNA damage-induced melanogenesis [11]. Hence, it is not surprising that a plethora of strategies have been employed against skin aging [2], the most popular among them being the use of medicinal and cosmetic products either to prevent skin damage (e.g., protection from sunlight) or to revert age-related changes (e.g., to enhance collagen production and/or inhibit collagenolytic processes).
The quest of humankind for active molecules in order to enrich our arsenal of pharmaceutics and cosmetics is based both on synthetic approaches and on natural products. The latter—either of plant, marine, or microbial origin—have been the most productive source of leads for the development of drugs, since they exhibit a broad range of structural diversity, being structurally evolved to play specific biological roles [12]. In particular, plants have been used in cosmetic formulations since ancient times, while nowadays, plant-derived ingredients are in increasing demand from the cosmetic and pharmaceutical industries [13]. Greece is known for its unique biodiversity, consisting of approximately 6000 species of higher plants. Of these, ~15% of the plant taxa are endemic, making Greece an area of high conservation priority [14,15]. Due to its wealth, there is an increasing interest in the study of the Greek flora, since a great variety of bioactive compounds can be obtained. Following our previous study on plants endemic in Greece [16], the aim of the present work was to evaluate the chemical content and the biological properties of various plants of the Greek flora for their potential use in cosmetic applications against skin aging. In particular, 52 plant materials from different areas were selected, collected, and extracted using two techniques based on green chemistry principles, i.e., Supercritical Fluid Extraction (SFE-CO2) and Accelerated Solvent Extraction (ASE). The extracts were assessed for their activities regarding a broad spectrum of skin health-related parameters, i.e., viability of human skin cells, antioxidant activity—using both cell-free and cell-based methods—interference with collagenase, elastase, and tyrosinase activity, photoprotection, and proteasomal and lysosomal activities of human skin cells. Beyond these in vitro assays, the extracts were also tested by means of Danio rerio (zebrafish) in vivo phenotypic screens, which are increasingly used to identify novel bioactive compounds, especially in complex samples such as natural product extracts [17,18,19].
2. Materials and Methods
2.1. Plant Material
Samples from 41 genera and 49 different species of Greek flora belonging to 20 different families (Table 1) were collected from various regions across Greece. The botanical identification of the collected plants was performed by Dr. E. Kalpoutzakis from the Department of Pharmacy at the NKUA. Voucher specimens have been deposited at the Herbarium of the Division of Pharmacognosy and Natural Products Chemistry, Department of Pharmacy, NKUA.

Table 1.
Origin of the plant material.
2.2. Extraction
- A.
- Accelerated Solvent Extraction (ASE): Extraction of samples was performed using an ASE 300 apparatus (Dionex, Sunnyvale, CA, USA) with a solvent mixture of distilled water and isopropanol (H2O:iPrOH, 1:1 v/v). A total of 20 g of dried plant material was placed into stainless-steel extraction cells, which were heated and extracted with the following conditions: preheating for 3 min at 40 °C with a pressure of 103 bar, heating cells for 5 min, 3 min static time, flush time 3 min, and purge time 120 s. Obtained extracts were evaporated to dryness using a rotary evaporator and were kept at −20 °C pending analysis. Samples derived from ASE were given the suffix “A” following their number (see Table 1).
- B.
- Supercritical Fluid Extraction-CO2 (SFE-CO2): Extraction of samples was performed using an SFE (Separex, Champigneulles, France) system, using a mixture of carbon dioxide (CO2) and 5% of iPrOH as co-solvent. A total o f20 g of dried plant material was placed into a 100 mL stainless-steel extraction cell. CO2 was pumped (Piston pump P200 LGP50, Separex, France) through the system with a flow rate of 25 g/min, while iPrOH was pumped (Series III P300, Teledyne SSI, State College, PA, USA) with a flow rate of 1.58 mL/min. Oven and basket temperature were set at 40 °C and 35 °C, respectively, with a system pressure of 250 bar. Extracts were collected every 30 min for 2 h, and then were evaporated to dryness using a rotary evaporator. All extracts were kept at −20 °C pending analysis. Samples derived from SFE were given the suffix “B” following their number (see Table 1).
2.3. Chemical Analysis
Liquid Chromatography—High Resolution Mass Spectrometry (LC-HRMS/MS)
LC-MS analysis of obtained extracts both with SFE and ASE was performed using a Velos Pro Ion Trap-Orbitrap Elite Hybrid Mass Spectrometer system (Thermo Scientific, Bremen, Germany), with a heated electrospray ion source (HESI) hyphenated to a Waters Acquity UPLC (Waters Corporation, Milford, MA, USA) system. Separation was carried out on an Ascentis® Express 90Å C18 column (15 cm × 2.1 mm, 2 um), using a mobile phase consisting of H2O with 0.1% v/v formic acid (A) and acetonitrile (B). The gradient method was set as follows: 5% B (0–1 min), 5–100% (1–15 min), 100% B (15–17 min), 100–5% B (17–17.5 min), 5% B (17.5–20 min). The total running time was 20 min. The column was kept at 40 °C during the analysis, and the flow rate was set to 0.3 mL/min. All samples were prepared at a final concentration of 300 μg/mL, and 10 μL was injected into the system. For the HESI source, capillary interface and source heater temperatures were set at 350 °C, while source electrospray voltage was 2.7 kV. S-Lens RF levels were tuned at 45% and 60% for negative and positive polarity, respectively. Data were obtained in negative and positive ionization mode, at a scan range of m/z 114–1000, with a resolution of 120,000. Nitrogen was used as sheath and auxiliary gas with flows set at 45 and 15 arbitrary units, respectively. A data-dependent acquisition (DDA) method was set up involving two scan events: a full MS scan followed by HRMS/MS acquisition. Full-scan spectra were acquired at a high resolution of 60,000 at full width half maximum (FWHM), whereas HRMS/MS acquisition were acquired at a mass resolution of 30,000 at FWHM. Higher-energy collisional dissociation (HCD) resulted in normalized collision energy (NCE) of 65%. System control and data processing were performed using the Xcalibur 2.2 (Thermo Electron, San Jose, CA, USA) software.
2.4. Cells and Culture Conditions
The following cell strains were used in this study: human neonatal foreskin fibroblasts AG01523 (obtained from Coriell Institute for Medical Research, Camden, NJ, USA), human neonatal foreskin fibroblasts BJ (obtained from the American Tissue Culture Collection ATCC, Rockville, MD, USA), human adult primary dermal fibroblasts DSF (from the pre-existing cell bank of the Laboratory of Cell Proliferation and Ageing, NCSR “Demokritos” [20]), human keratinocytes HaCaT (kindly provided by Prof. Evangelos Kolettas), and mouse melanoma B16-F10 cells (obtained from ATCC). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with penicillin (100 U/mL)/streptomycin (100 mg/mL) (obtained from Biosera, Nuaillé, France) and 10% (v/v) FBS (from Life Technologies Europe BV Thessaloniki, Greece) in a humidified atmosphere of 5% CO2 at 37 °C and they were subcultured using a trypsin/citrate (0.25%:0.30% w/v) solution. Cell culture samples were periodically tested and found to be free of mycoplasma.
2.5. Assessment of Cell Viability
Cells (human skin fibroblasts and human keratinocytes) were plated in 96-well flat-bottomed transparent microplates (Corning Inc., Corning, NY, USA) at a subculture split ratio in DMEM 10% FBS. Following overnight incubation to ensure proper cell attachment, additional medium was added containing serial dilutions of the test extracts. Since the test extracts were dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA), corresponding dilutions of DMSO were used as controls. After incubation for 72 h, the medium was replaced by serum-free, phenol-red-free DMEM (PAN Biotech GmbH, Aidenbach, Germany) containing 1 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma), and incubation was continued for 4 h. The medium was then discarded, the insoluble purple formazan crystals were solubilized in 2-propanol, and the absorbance was measured using a Spark multimode microplate reader equipped with plate stacker (Tecan Group Ltd., Männedorf, Switzerland) at a wavelength of 550 nm (reference wavelength 690 nm). Each experiment was performed in quadruplicate, and the results are the mean of three independent experiments. The results are presented in the form of the highest noncytotoxic concentrations detected, in order to be used in the subsequent cell-based assays.
2.6. Free-Radical Scavenging Assay
The ability of the extracts to scavenge the free radical 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) was assessed as previously described [21]. Briefly, the extracts diluted in DMSO at their highest noncytotoxic concentration (see above Section 2.5) were thoroughly mixed with an equal volume of a fresh DPPH (Sigma) ethanolic solution (1 mM). The absorbance at 520 nm was measured at regular time intervals in a Tecan Spark multimode microplate reader. DMSO served as negative control, while the water-soluble vitamin E analogue 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox; Sigma) at 100 μM was used as positive control.
2.7. Assessment of Intracellular Reactive Oxygen Species (ROS)
Cells (human skin fibroblasts and human keratinocytes) were plated in clear-bottomed black 96-well microplates (Porvair Ltd., Fareham, UK) at a 1:2 split ratio density in the presence of FBS. When the cells reached confluency, the test extracts were added, dissolved in serum-free medium at the indicated concentrations. DMSO at corresponding dilutions served as negative control, with Trolox (100 μM) and N-acetyl-cysteine (NAC; 5 mM; Sigma) as positive control for fibroblasts and keratinocytes, respectively. Following an overnight incubation, dichloro-dihydro-fluorescein diacetate (DCFH-DA; Sigma) was added at a final concentration of 10 μM in serum free medium and fluorescence emission at 520 nm (excitation wavelength: 485 nm) was measured after 24 h, using a Spark (Tecan) microplate reader. The experiments were repeated three times. Each time, the samples were added in quadruplicates. Furthermore, to assess the impact of selected test extracts on stimulated intracellular ROS levels, following overnight incubation with the extract, 200 μM H2O2 was added along with 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 10 μM final concentration. After incubation for 30 min in the dark (37 °C), cells were lysed [150 mM NaCl, 1% (v/v) NP-40, 50 mM Tris, pH 8.0] and their protein content was measured using the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The produced fluorescence was measured in a Spark multimode microplate reader (Tecan) (490 nm excitation and 540 nm emission wavelengths).
2.8. Assessment of Photoprotective Activity
Cells (human skin fibroblasts and human keratinocytes) were plated in 96-well microplates at a density of 8000 cells/well in DMEM containing 10% (v/v) FBS. When the cells reached confluency, the test extracts diluted in DMEM 10% FBS were added at their highest non-cytotoxic concentration and at a 5-fold lower concentration. Following incubation for 5 h, the medium was discarded and replaced by PBS containing the same extract concentrations and the cells were placed in a UV box, as reported previously [22], under a UVB lamp (Sankyo Denki Co., Hiratsuka, Japan) with an emission spectrum of 280–360 nm and a peak at 306 nm. Fibroblasts and keratinocytes were irradiated for the appropriate time interval so as to receive a total energy of 420 mJ/cm2 and 700 mJ/cm2, respectively. After irradiation, medium was changed once more to DMEM 10% FBS containing the same extract concentrations, and the cells were incubated for 72 h. Cells treated with the corresponding DMSO concentrations and subjected to the same procedure but not exposed to the UV lamp served as controls. Cell viability at the end of the incubation time was assessed using the Neutral Red method, i.e., culture medium was replaced with serum-free, phenol-red-free DMEM containing neutral red (Sigma) at a concentration of 0.0075% (w/v). The uptake of the neutral red dye by viable cells was determined 4 h later, after washing with PBS and dissolving with a solution of ethanol and water (1:1) acidified with 1% acetic acid. The neutral red fluorescence emission was measured at 645 nm following excitation at 530 nm using a Spark multimode microplate reader (Tecan). The results are presented as percent inhibition of the cytotoxic activity exerted on DMSO-treated, UVB-treated cultures based on the following formula:
where S: UVB-treated sample, NC: non-UVB-treated control, and UC: UVB-treated control. Experiments were performed in quadruplicate, and the results presented are the mean of three independent experiments.
%Inhibition = (1 − ((NC − S)/(NC − UC)) × 100,
2.9. Inhibition of Enzymatic Activities
2.9.1. Collagenase
To determine the effect of the extracts on collagenase activity, the fluorogenic substrate Dabcyl-Gaba-Pro-Gln-Gly-Leu-Glu (EDANS)-Ala-Lys-NH2 (TNO211; Cayman Chemical, Ann Arbor, MI, USA) was used [23]. Extracts at their highest non-cytotoxic concentration were mixed with Clostridium histolyticum-derived collagenase (Sigma) diluted in Tris-HCl buffer supplemented with Ca2+ (pH: 7.5) at a concentration of 50 μg/mL and incubated for 15 min, at 37 °C, in black 96-well plates (Greiner Bio-One GmbH, Kremsmünster, Austria). TNO211 was then added at a final concentration of 10 μM and the fluorescence emission was monitored at 480 nm, following excitation at 340 nm, using a Tecan Spark multimode microplate reader. Vehicle (DMSO) and epigallocatechin gallate (EGCG) were used as negative and positive control, respectively [24]. The fluorescence of a blank sample, containing the buffer and the substrate but no enzyme, was also measured. The percentage of collagenase activity was calculated with the following equation:
where S: sample, B: blank, and C: control. The experiment was conducted twice, and each time the samples were added in duplicates. The results are demonstrated as mean ± SD.
%Collagenase activity = ((S − B)/(C − B)) × 100,
The above method was also adapted to measure matrix metalloproteinase (MMP) activity secreted by human skin fibroblasts. Cells were plated in 12-well tissue cultures plates and cultured until confluency. Then, the culture medium was replaced by serum-free DMEM containing the test extracts, each at its highest non-cytotoxic concentration. After incubation for 24 h, all wells received serum-free DMEM and were further incubated for 24 h. Conditioned media (CM) were collected from all wells, clarified by centrifugation (10,000× g, 10 min, 4 °C), and incubated with TNO211 (10 μM). Fluorescence was measured at 480 nm (excitation wavelength 340 nm), as described above. Cultures treated with the corresponding DMSO concentration yielded the control CM.
2.9.2. Elastase
To determine the effect of the extracts on elastase activity, the substrate N-(methoxysuccinyl)-L-alanyl-L-alanyl-L-prolyl-L-valine 4-nitroanilide (Sigma) was used. The test extracts at their highest non-cytotoxic concentration were pre-incubated with 5 mM of the above elastase substrate in a Tris-HCl buffer (0.2 M, pH: 8.0) for 15 min at 37 °C. Then, elastase from porcine pancreas (Sigma; 0.3 U/mL) was added and the mixture was further incubated for 15 min at 37 °C, before measurement of the absorbance at 495 nm in a Tecan Spark multimode microplate reader. Vehicle (DMSO) and N-(methoxysuccinyl)-L-alanyl-L-alanyl-L-prolyl-L-valine chloromethylketone (Sigma) at 750 μM were used as negative and positive control, respectively. The absorbance of a blank sample, containing the buffer and the substrate but no enzyme, was also measured. Percentage of the enzyme inhibition was calculated according to the following equation:
where C: control, Cb: blank control, S: sample, Sb: blank sample. Each sample was tested in duplicate, and the results are presented as mean ± SD of two independent experiments.
%Inhibition = (((C − Cb) − (S − Sb))/(C − Cb)) × 100,
The above method was also adapted to measure elastase activity in cell lysates of human skin fibroblasts. Confluent cell cultures were incubated with selected extracts at the indicated concentrations for 24 h. Then, the cells were trypsinized, washed in PBS, collected by centrifugation (500× g, 5 min, room temperature), and lysed in 20 mM Tris HCl (pH 7.6) containing 20 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.2% (v/v) Nonidet P 40, 5 mM ATP, and 10% (v/v) glycerol. Protein content of the lysates was assessed using the Bradford method, and 30 μg protein were used to assess the ability of each lysate to inhibit porcine pancreas elastase activity, as described above.
2.9.3. Tyrosinase
Inhibition of mushroom tyrosinase activity was assessed using L-DOPA as substrate based on the absorbance of the dopachrome formed [16]. The tested extracts diluted in phosphate buffer (0.1 M KH2PO4/K2HPO4, pH 6.5) were incubated with a mushroom tyrosinase (Sigma) solution (100 U/mL) in a 96-well microplate at room temperature for 10 min in the dark. Then, L-DOPA (Merck KGaA, Darmstadt, Germany) was added at a concentration of 2.5 mM, and absorbance at 475 nm was recorded using a Tecan Spark multimode microplate reader. Each extract was used at its highest non-cytotoxic concentration. Vehicle (DMSO) and kojic acid (Apollo Scientific, Stockport, UK) served as negative and positive control, respectively. A blank for each extract (including controls) was studied in parallel, in the absence of tyrosinase. Percentage of the enzyme inhibition was calculated according to the following equation:
where C: control, Cb: blank control, S: sample, Sb: blank sample. Each sample was tested in duplicate, and the results are presented as mean ± SD of two independent experiments.
%Inhibition = (((C − Cb) − (S − Sb))/(C − Cb)) × 100,
In order to assess tyrosinase activity in cell lysates, B16F10 cells were plated in 6-well plates for 24 h before their incubation with the indicated concentrations of the tested extracts for another 24 h. Then, the cells were trypsinized, washed in PBS, collected by centrifugation (500× g, 5 min, room temperature), and lysed with 1% NP-40 lysis buffer (pH 6.8). An amount of 20 μg of total protein was diluted in PBS and mixed with 5 mM L-DOPA (Merck KGaA, Darmstadt, Germany). Samples were set in triplicate in a 96-well plate and were incubated at 37 °C for 1 h. The absorbance was measured at 492 nm using a Tecan Spark microplate reader. Kojic acid was used as positive control.
2.10. Inhibition of Melanogenesis in Zebrafish
2.10.1. Zebrafish Maintenance and Breeding
Zebrafish embryos were raised under standard laboratory conditions at 28 °C [25]. The genetic backgrounds used were wild-type AB strains for all the screenings. Zebrafish were maintained in accordance with the European Directive 2010/63 for the protection of animals used for scientific purposes and the Recommended Guidelines for Zebrafish Husbandry Conditions. The experimental protocols described in this study were carried out with zebrafish larvae up to 96 h post-fertilization (hpf), and therefore these experiments are not considered animal experiments and do not fall under the protection guidelines of the directive 2010/63/EU revising directive 86/609/EEC on the protection of animals used for scientific purposes as adopted on 22 September 2010.
2.10.2. Compound Treatment and Phenotype-Based Evaluation of Melanogenesis
For the calculation of LC50, the OECD guidelines for Fish Embryo Acute Toxicity Test (TG 236) were used [26]. In brief, newly fertilized zebrafish eggs were exposed to the test compounds at 3 hpf for a period of 96 h. Every 24 h, lethal effects were assessed and compared to the control values. At the end of the exposure period, acute toxicity was determined and LC50 was calculated. For monitoring the melanogenic inhibitory activity, synchronized and dechorionated 24 hpf embryos were treated with various concentrations of the extracts (below the LC50) in 12-well plates containing 2 mL of embryo medium (0.3 g/L “Instant Ocean” Sea Salts and 0.08 g/L CaSO4⋅2H2O). Test compounds were dissolved in DMSO that was always used as vehicle control up to 0.1% (v/v) final concentration. In all experiments, 0.2 mM 1-phenyl-2-thiourea (PTU), a known TYR inhibitor, was considered as a standard positive control. The testing period varied between 24 and 48 h and the effects on the pigmentation were observed under a stereomicroscope.
2.11. Effects on Proteasomal and Lysosomal Activities
2.11.1. Proteasomal Activity
For measuring proteasome peptidase activity, human skin fibroblasts and human keratinocytes, treated with the tested extracts at the indicated concentrations for 24 h, were lysed on ice in a buffer suitable for the isolation of intact 26S proteasomes (0.2% NP-40, 5 mM ATP, 10% glycerol, 20 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 20 mM Tris, pH 7.6). Lysates were cleared by centrifugation at 19,000× g (4 °C) and protein content was estimated with the Bradford assay (Bio-Rad, Hercules CA, USA). Cleared supernatants were immediately used to determine the chymotrypsin-like (CT-L/LLVY) proteasome proteolytic activity by a 30 min incubation with the fluorogenic peptide Suc-Leu-Leu-Val-Tyr-AMC, (Enzo Life Sciences Inc., Farmingdale, NY, USA) at 37 °C. Fluorescence was recorded in a Tecan Spark microplate reader at excitation and emission wavelengths of 350 nm and 440 nm, respectively.
2.11.2. Lysosomal Activity
For measuring cathepsins’ activity, human skin fibroblasts and human keratino-cytes, treated with the tested extracts at the indicated concentrations for 24 h, were lysed in an extraction buffer (1 mM dithiothreitol and Tris 50 mM, pH 4.0) and the lysates were cleared at 17,000× g for 20 min at 4 °C. Following protein content measurement with the Bradford assay, 5 μg of protein was incubated in the reaction buffer (50 mM sodium acetate, 8 mM cysteine-hydrochloride, 1 mM EDTA, pH 4.0) containing the fluorogenic substrate Z-FR-AMC (Enzo Life Sciences Inc.) for 30 min at 37 °C. Fluorescence was measured in a Spark multimode microplate reader (Tecan) at excitation and emission wavelengths of 350 and 440 nm, respectively.
2.12. Assessment of Gene Expression
Confluent cultures of human skin fibroblasts were incubated with selected extracts at the indicated concentrations for 24 h. Then, total RNA was extracted using RNAiso plus reagent (DSS Takara Bio India LTD, New Delhi, India) and quantified in a BioSpec nano spectrophotometer (Shimadzu, Kyoto, Japan). cDNA was produced from 1 μg of RNA using the FastGene Scriptase II cDNA Kit (NIPPON Genetics, Düren, Germany), according to the manufacturer’s instructions, and subjected to qPCR reactions using the HOT FIREPol® EvaGreen® qPCR Supermix (Solis BioDyne, Tartu, Estonia) in a PikoRealTM Real-Time PCR System (Thermo Fisher Scientific Inc., Waltham, MA, USA). mRNA levels were quantified in comparison to the untreated control using the 2−ΔΔCT method [27] and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene. Sequences of the primers used in the current study are presented in Supplementary Table S1.
3. Results
3.1. Plant Material and Assessment of Cytotoxicity
Greece is widely acknowledged as a biodiversity hotspot, home to numerous native plant species with potent therapeutic properties. These species hold significant value for industries such as cosmetics, pharmaceuticals, and food [16]. Therefore, based on prior research and ethnobotanical knowledge, the plant species studied were selected from families including, among others, Asteraceae, Fabaceae, Lamiaceae, Ericaceae, and Onagraceae (Table 1). The selection of plants was based on previous knowledge related to cosmetic applications, as well as literature data. To comprehensively analyze the composition of the collected plants, each sample was dried, pulverized, and extracted using two environmentally sustainable methods: SFE-CO2 and ASE. These methods are known for their efficiency in recovering a wide range of metabolites with different polarities, making the extracts suitable for diverse industrial applications [28]. ASE predominantly yielded medium- to high-polarity compounds, with extraction efficiencies ranging from 53.5% to 5.54% (w/w), while SFE was more effective for non-polar compounds, with yields ranging from 16.6% to 0.63% (w/w).
All extracts were subjected to cytotoxicity assessment, both on human skin fibroblast cultures and keratinocytes, using the MTT method. Concentrations tested were from 0.032 μg/mL to 100 μg/mL. In Table 2, the highest non-cytotoxic concentration for each extract is depicted. This concentration was further used in all experiments.

Table 2.
Highest non-cytotoxic extract concentration for human skin fibroblasts (DSF) and keratinocytes (HaCaT).
3.2. Antioxidant Activity
3.2.1. Radical Scavenging Activity
The ability of the extracts at their highest non-cytotoxic concentration to scavenge the free radical DPPH is presented in Table 3. The most active extract was found to be 7A achieving a DPPH inhibition similar to that of the positive control. In general, extracts obtained through ASE (with the suffix A) were found to be more active than the ones obtained through SFE (with the suffix B).

Table 3.
Scavenging of DPPH by the test extracts.
3.2.2. Attenuation of ROS
Beyond the assessment of free radical scavenging in a cell-free system (see above DPPH), the ability of the extracts to suppress ROS intracellularly was evaluated in human skin fibroblast and human keratinocyte cultures (Table 4A and 4B, respectively). A total of 34 extracts were found to be equally or more potent than the positive control (Trolox at 100 μM) in fibroblast cultures. In general, the extracts seemed to be less efficient in attenuating ROS levels in keratinocyte cultures.

Table 4.
Suppression of ROS levels by the extracts (A) in human skin fibroblast cultures (B) in human keratinocyte cultures.
Results presented in Table 4 refer to the basal intracellular ROS levels. An extract (19B) was randomly selected among the active ones for testing its capacity to counteract the effect of the oxidative stress-inducing factor, H2O2 (200 μM), in human skin fibroblasts. Indeed, 19B at 10 μg/mL was found capable to suppress not only basal intracellular ROS levels but also intracellular ROS stimulated by H2O2 (Figure 1A). Moreover, incubation of fibroblasts with the (randomly selected) extract 47B at 10 μg/mL concentration led to a mild up-regulation of the NRF2 gene, as well as its downstream targets NQO1 and TXNRD1 (Figure 1B).
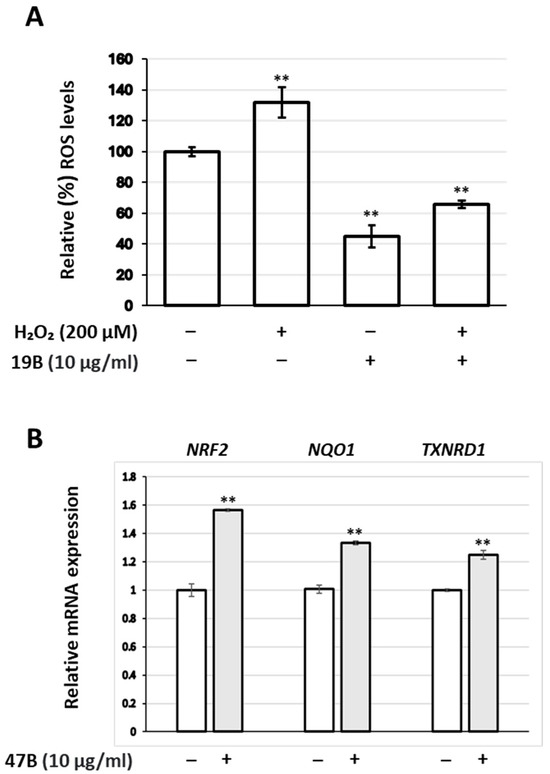
Figure 1.
(A) Relative (%) ROS levels of human skin fibroblasts treated with 19B at 10 μg/mL and/or H2O2 (200 μM) for 24 h. (B) Relative expression levels of the NRF2 gene, as well as its downstream targets (NQO1 and TXNRD1), in human skin fibroblasts treated with the extract 47B at 10 μg/mL for 24 h. Values in control samples were set to 100% (A) or 1 (B); GAPDH gene expression was used as a reference for total RNA input. Bars, ±SD; ** p < 0.01.
3.3. Photoprotection
Photoprotective abilities of the extracts were assessed by exposure of both human skin fibroblast (DSF) and keratinocyte (HaCaT) cultures to the extracts’ highest non-cytotoxic concentrations prior to, during, and post irradiation with UVB doses known to lead to cell death at approx. 50% compared to the non-irradiated control. As shown in Table 5, the highest photoprotection to DSF cells was conferred by extracts 8A and 40A, while 3A, 5A, 11A, and 21A were also active to a lesser extent. In general, the extracts were found to be less active on HaCaT cell cultures.

Table 5.
Percent inhibition of UVB-induced cytotoxicity.
3.4. Inhibition of Enzymatic Activities Related to Skin Health
3.4.1. Collagenase Inhibition
The ability of the extracts to inhibit enzyme activities with crucial roles for skin health, i.e., collagenase, elastase, and tyrosinase was assessed in cell-free systems, and the results are shown in Table 5, Table 6, and Table 7, respectively. A total of 17 extracts, all belonging to the ASE group, were found to inhibit collagenase activity, the most active (inhibition over 25%) being 7A, 20A, 21A, 3A, 5A, 8A, and 51A (Table 6).

Table 6.
Inhibition of collagenase activity by active a extracts.

Table 7.
Inhibition of elastase activity by active a extracts.
To test whether this activity is retained also against human skin cell-produced collagenases, MMP activity secreted by human skin fibroblasts was also measured following incubation for 24 h with selected extracts. As shown in Figure 2, the activity of these extracts against fibroblast-secreted MMPs was confirmed.
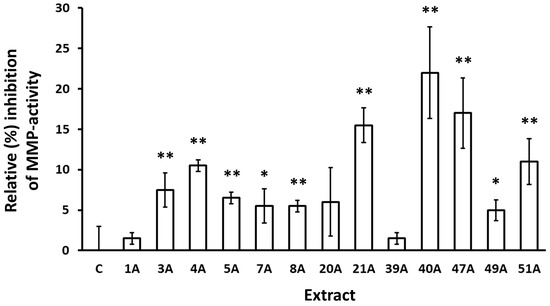
Figure 2.
Inhibition of MMP activity secreted by human skin fibroblasts following incubation with the indicated extracts for 24 h. Control (conditioned medium from cultures treated with vehicle) was set to 0%. Bars, ±SD; * p < 0.05; ** p < 0.01.
3.4.2. Elastase Inhibition
Moreover, 27 extracts were found to inhibit elastase activity (Table 7), the most active being 20A, 21A, 5A, 22A, 28, and 19A. The most promising extracts were then examined for their effect on elastase activity in human skin fibroblast cultures. As observed in Figure 3, the extracts 5A and 20A were found to exhibit significant anti-elastase activity.
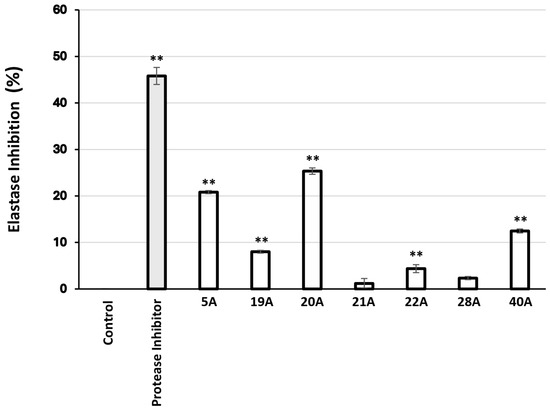
Figure 3.
Relative (%) elastase inhibitory activity in human skin fibroblast cultures after 24 h of treatment with 10 μg/mL of the extracts. Protease inhibitor at 10 μL/mL was used as a positive control. Bars, ± SD; ** p < 0.01.
3.4.3. Tyrosinase Inhibition
Finally, 93 extracts were found to inhibit mushroom tyrosinase activity (Table 8), the most active (inhibition > 50%) being 10B, 20A, 30A, 22A, 21A, 37A, 1A, 37B, 5A, 40A, and 21B.

Table 8.
Inhibition of tyrosinase activity by the extracts.
These most active extracts identified in vitro were subsequently evaluated for their biological activity in B16F10 melanocytes to confirm their efficacy (Figure 4).
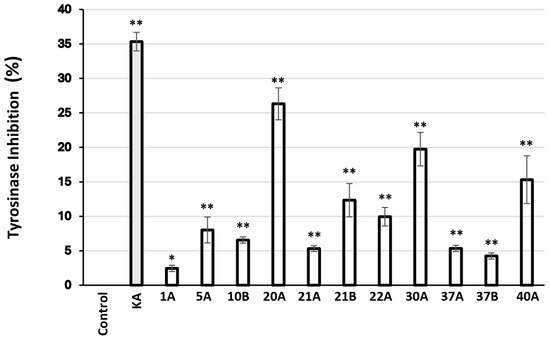
Figure 4.
Relative (%) tyrosinase inhibitory activity in murine B16F10 melanocytes after 24 h of treatment with 10 μg/mL of the extracts. Kojic acid (KA) at 2 mM was used as a positive control. Bars, ± SD; * p < 0.05; ** p < 0.01.
3.4.4. In Vivo Evaluation of Tyrosinase Inhibition Using a Zebrafish Embryo Melanogenesis Assay
Extracts were tested in vivo by adding them in the water of developing zebrafish embryos. According to the guidelines for fish embryo toxicity test (OECD 236), we estimated the LC50 and then went on and retested for more specific phenotypes at different (including sublethal) concentrations. Several morphological phenotypes were noted but focusing on the inhibition of melanocyte differentiation or melanin synthesis assay [29], we identified the following extracts that were also active in the in vitro tyrosinase inhibition assay: 10B (Figure 5D), 20A (Figure 5E), and 22A (Figure 5F) were the most potent, followed by 21A (Figure 5G). In addition, 1A, 30A, 37A, and 37B also inhibited melanogenesis but showed less activity in vivo (Figure 5). As a positive control, in this assay, the commonly used 1-phenyl 2-thiourea (PTU) was employed. In this figure, the extracts were added 24 h post-fertilization (hpf) and embryos were dechorionated by pronase treatment. This methodology was utilized in the whole extract samples in order to bypass early developmental defects and putative difficulties of the relevant bioactive compounds to penetrate the chorion of zebrafish embryos but still use the highest possible concentration.
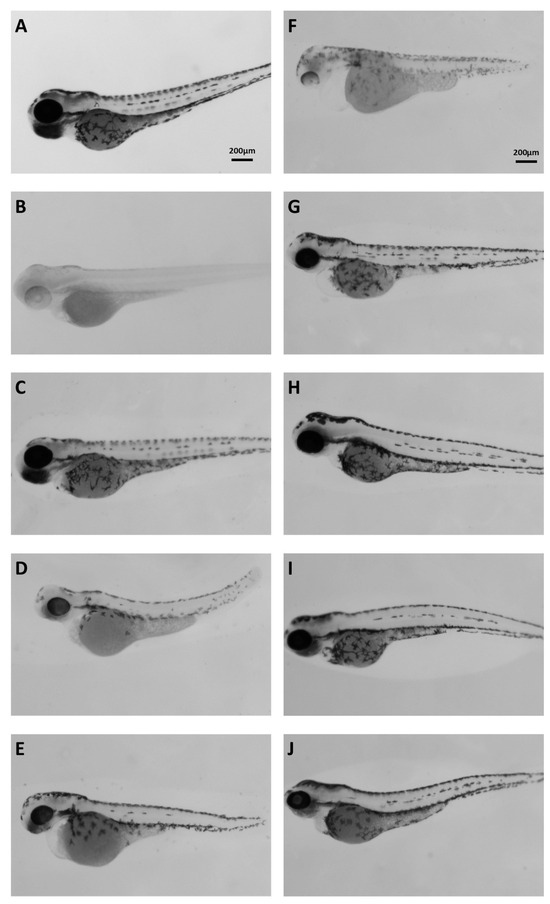
Figure 5.
Brightfield images of 72 hpf embryos. (A) Control; (B) PTU-treated embryos; (C) 1A 200 μg/mL; (D) 10B 20 μg/mL; (E) 20A 20 μg/mL; (F) 22A 100 μg/mL; (G) 21A 100 μg/mL; (H) 30A 100 μg/mL; (I) 37A 200 μg/mL; (J) 37B 100 μg/mL.
3.5. Effect of the Extracts on the Main Proteostatic Pathways
The most promising extracts were further examined for their capacity to activate cellular protective mechanisms, which ensure proteome stability and integrity. Specifically, their effects on the main proteostatic pathways were investigated to assess their potential cytoprotective role. We observed that the extracts 20A and 21A increased the main proteasome peptidase activity, i.e., the chymotrypsin-like activity (CT-L/β5) in human skin fibroblasts (Figure 6A). Furthermore, cell exposure to the extract 21A at the concentration of 10 μg/mL led to significant induction of cathepsins enzymatic activity (Figure 6B).
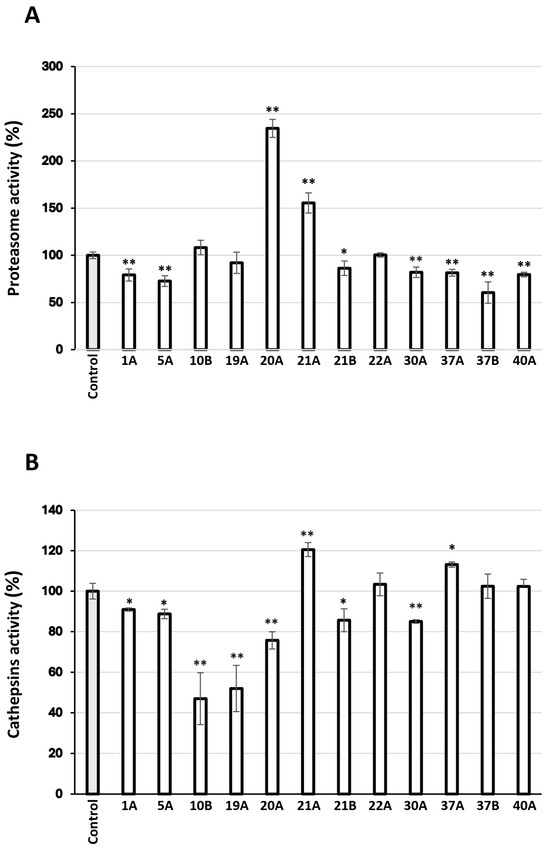
Figure 6.
(A) Relative (%) chymotrypsin-like proteasome peptidase activity in human skin fibroblasts treated with 10 μg/mL of the extracts for 24 h. (B) Relative (%) lysosomal-cathepsins activity in human skin fibroblasts treated with 10 μg/mL of the extracts for 24 h. Control samples values were set to 100%. Bars, ± SD; * p < 0.05; ** p < 0.01.
4. Discussion
The aim of the present work was to evaluate the chemical content and the biological properties of a great variety of Greek plants towards their potential use in skin protection applications. The plants belonged to 20 different families, i.e., Adoxaceae, Anacardiaceae, Apiaceae, Asteraceae, Boraginaceae, Cactaceae, Cistaceae, Crassulaceae, Cupressaceae, Ericaceae, Fabaceae, Hypericaceae, Lamiaceae, Malvaceae, Onagraceae, Paeoniaceae, Pinaceae, Plumbaginaceae, Polygonaceae, and Rutaceae. Extraction of each plant material was performed using two different techniques, i.e., ASE and SFE-CO2, both being environmentally friendly, fast, cost-effective, and providing fully controllable conditions and hence extracts with consistent compositions [30]. The initial step of the extracts’ biological assessment was the evaluation of their cytotoxicity on human skin fibroblasts and keratinocytes. As shown in Table 2, most of the extracts were not cytotoxic at the highest concentration tested (100 μg/mL) following a 72-hr incubation (61 extracts for fibroblasts and 70 extracts for keratinocytes). The highest non-cytotoxic concentration of 20 μg/mL was determined for 30 extracts regarding fibroblasts and for 23 extracts regarding keratinocytes, while 12 extracts (regarding fibroblasts) and 11 extracts (regarding keratinocytes) exhibited lower limits of cytotoxicity. All subsequent in vitro assays (even cell-free ones) were performed at these highest non-cytotoxic concentrations for each extract, ensuring the applicability of our findings on living skin cells.
All extracts were assessed for their antioxidant capacity by means of (a) a cell-free assay (DPPH) and (b) their ability to suppress intracellular ROS levels in human skin fibroblast and keratinocyte cultures. The most active extracts in the cell-free assay were those obtained through ASE from Cistus creticus ssp. eriocephalus and Polygonum idaeum, suppressing DPPH absorbance to 13.2% and 15.1% of the vehicle absorbance, respectively (Table 3), hence being more active than Trolox (100 μM), which was used as a positive control. An additional 19 extracts were also active to a lesser extent (between 20% and 50% of the vehicle absorbance), 54 were less active yet showed DPPH absorbance below that of the vehicle, and the remaining 29 were inactive. Interestingly, the extracts produced through ASE (which yields extracts with more polar constituents [31]) were more active, in agreement with previous studies showing higher antioxidant capacity in polar than non-polar extracts [32,33,34]. The existing literature supports our findings for many of the plants identified as possessing significant antioxidant activity, such as C. creticus, C. salvifolius, C. parviflorus [35], E. dodonaei [36], S. pomifera [37], S. sediforme [38], E. parviflorum [39], S. officinalis [40,41], C. siliqua [21], H. perforatum [42], J. oxycedrus [43], S. Sclarea [41], J. turbinata [44], C. siliquastrum [45], C. coggygria [46], A. unedo [47], and P. terebinthus [48], while for some other plants, e.g., P. idaeum, A. graecum, A. cephalonica, and A. canencens, an antioxidant activity is reported here for the first time.
Antioxidant activities of the extracts as estimated by the reduction of intracellular ROS levels using the DCFH-DA assay were generally qualitatively different from those obtained through the DPPH assay (compare Table 3 and Table 4; Figure 7). In most of the cases, the extracts exhibited higher activity in the DCFH-DA assay than in the DPPH one (72 out of 104 extracts for fibroblast cultures; 60 out of 104 for keratinocytes). Moreover, 90% of the 32 extracts exhibiting higher DPPH inhibition than ROS suppression in fibroblast cultures was obtained through ASE, similarly to 75% of the 44 extracts showing higher activity in the DPPH assay than ROS suppression in keratinocytes. This could be the outcome of the extracts’ different capacity for cell internalization, which may depend on their polarity, among others. On the other hand, such differences may stem from the inherent limitations of each assay [49]. In any case, live cell assays for determining the antioxidant activity of plant extracts are considered to be a closer simulation of the in vivo responses [50]. Moreover, ROS suppression was more effective in fibroblast cultures than in keratinocytes (80 out of 104 extracts were more potent for fibroblasts), which may be the outcome of different basal ROS levels between the two cell types. Nevertheless, extract 19B from Origanum vulgare ssp. hirtum was found to also be able to suppress external induction of ROS levels by hydrogen peroxide treatment (Figure 1A). In agreement with this observation, an ethanolic O. vulgare extract has been reported to inhibit hydrogen peroxide cytotoxicity against human A549 cells [51]. Assessment of the extract 47B (from Citrus medica) led to the observation that ROS suppression in human skin fibroblasts is accompanied by activation of the NRF2 transcription factor, as well as its downstream targets NQO1 and TXNRD1 (Figure 1B). These results agree with reports on NRF2 activation by various components of Citrus plants [52,53,54,55,56,57].
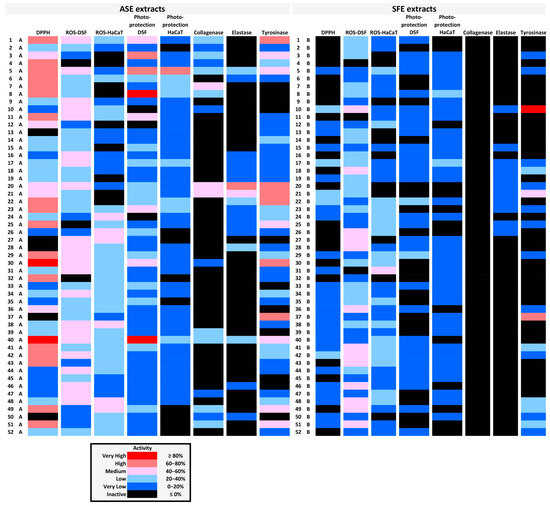
Figure 7.
Graphic representation of the various biological activities of each extract in the form of a heatmap. Inhibition of the activities presented in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 (see above) is depicted here in different colors depending on its degree. The bioactivities of ASE vs. SFE extracts of each plant material are shown in each horizontal line.
Regarding photoprotection of human skin fibroblasts, the most active extracts were those obtained through ASE from Epilobium parviflorum and Polygonum idaeum (exhibiting inhibition of UVB-induced cytotoxicity by 88% and 86%, respectively), followed by Cistus parviflorus (64%), Arbutus unedo (62%), Hypericum perforatum (56%), Cistus creticus ssp. eriocephalus (54%), Acantholimon graecum (48%), Pistacia terebinthus (43%), Abies cephalonica (41%), and others with less than 40% inhibitory activity (Table 5). A previous study assessing photoprotection based on UV-induced hydroxyl radical generation failed to detect the photoprotective activity of E. parviflorum [58], while the protective activity of P. idaeum has also not been reported elsewhere. Besides the already known protection conferred by A. unedo against the cytotoxic effects of UVB irradiation [59] and similar reports on other subspecies of the genera Cistus, Hypericum, and Pistacia [60,61,62], extracts identified as photoprotective through the present screening represent novel findings. Interestingly, the photoprotection of human keratinocytes by these extracts was generally less pronounced (Table 5), with the exception of the ASE extract from C. parviflorus (65% inhibition of UVB-induced cytotoxicity), a fact that could be related to the higher survival rates of keratinocyte monolayers compared to fibroblasts following their exposure to UV radiation [63]. Moreover, for most of the plants studied here, ASE extracts exhibited more potent photoprotective activity than SPE ones, possibly indicating that this activity may be conferred by polar molecules [64].
During the assessment of the putative inhibitory capacity of the extracts against collagenase enzymatic activity, 17 out of 104 extracts were found to be active (Table 6). Interestingly, all of them were produced through ASE, while none of the SFE-derived extracts were active. The highest inhibition of collagenase activity was achieved by Epilobium dodonaei extract, followed by Pistacia lentiscus, Pistacia terebinthus, Arbutus unedo, Cistus parviflorus, Epilobium parviflorum, Juniperus turbinata, Abies cephalonica, Paeonia mascula ssp. hellenica, Juniperus oxycedrus ssp. deltoides, Polygonum idaeum, Ceratonia siliqua, and Citrus medica, and Achillea millefolium. E. dodonei, and E. parviflorum anti-collagenase activities are reported here for the first time, though there are similar reports in the literature regarding Epilobium augustifolium [65,66]. Regarding the extracts from the leaves and branches of the two Pistacia species, this is also a novel report, though we have previously shown the anti-collagenase activity of a P. lentiscus gum extract [67], and other teams have shown such an activity for other species of the Pistacia genus [68,69]. Inhibition of collagenase activity has also been reported previously for A. unedo [69] and C. siliqua [21]. All other extracts have not been tested previously for anti-collagenase activity, though data for other species of certain genera can be found in the literature, such as for Paeonia lactiflora [70], Polygonum cuspidatum [71], and Achillea sintenisii [72]. Beyond the inhibitory activity of the extracts against a collagenase of exogenous origin (from Clostridium histolyticum), their activity against MMPs produced by human skin fibroblast cultures was also studied and verified. All extracts tested were also found to inhibit fibroblast-secreted MMPs, although at different percentages from those observed against C. histolyticum collagenase (Figure 2). This may be due to differences among the activities of the different enzymes, but it may also reflect possible effects of the test extracts on MMP expression and secretion by the fibroblast cultures. More detailed analysis for each extract would be necessary to delineate these mechanisms. The most potent inhibition of fibroblast-MMP activity was achieved by Polygonum idaeum extract, followed by Citrus medica and Pistacia terebinthus. Notably, the inhibition of MMPs by C. medica extract aligns perfectly with published observations showing inhibition of MMP expression and/or activity by extracts from various Citrus species and isolated constituents in vitro and in vivo [73,74,75].
Assessment of the extracts’ ability to inhibit elastase activity yielded 27 active extracts out of 104 (Table 7). Again, ASE extracts exhibited higher activities than SFE ones, though—in contrast to collagenase inhibitory capacity—some elastase inhibitory activity could be recovered in SFE extracts in certain cases (e.g., P. lentiscus and P. terebinthus). The most active extracts in terms of elastase inhibition were those from Pistacia lentiscus and Pistacia terebinthus, followed by Cistus parviflorus, Sedum sediforme, Polygonum idaeum, Atractylis gummifera B, and Origanum vulgare ssp. hirtum. Our data agree with previous studies on extracts from plants of the Pistacia genus [68,76,77,78]. This is the first time that anti-elastase activity is shown in extracts from C. parviflorus, S. sediforme, P. idaeum, A. gummifera, and O. vulgare ssp. hirtum, although reports on other species of these genera exist in the literature, e.g., for a resin from Cistus ladanifer L. [79], for a leaf extract from Sedum dendroideum [80], for flavonol glucuronides from Polygonum aviculare L. [81], and for an Origanum vulgare L.-derived essential oil [82]. Beyond inhibition of porcine pancreas elastase in a cell-free assay system, the most active extracts were also used to treat human skin fibroblast cultures, and then the elastase-inhibitory activity of the cell lysates was determined (Figure 3). The results from the cell-based assessment are in line with those from the cell-free assay, confirming that the extracts are capable to exert their anti-elastase activity in the cellular environment.
Regarding inhibition of mushroom tyrosinase activity, the most active extracts were found to be those from Glycyrrhiza glabra (SFE), Pistacia lentiscus (ASE), Cistus creticus ssp. eriocephalus (ASE), Sedum sediforme (ASE), Pistacia terebinthus (ASE), Hippocrepis emerus (ASE), Abies cephalonica (ASE), Hippocrepis emerus (SFE), Cistus parviflorus (ASE), Polygonum idaeum (ASE), Pistacia terebinthus (SFE), Juniperus turbinata (ASE), Juniperus oxycedrus ssp. deltoides (ASE), and Arbutus unedo (ASE), in order of decreasing activity (Table 8). Most of these extracts were also found to inhibit statistically significantly melanogenesis in murine melanocytes in vitro (Figure 4). Moreover, we have used the zebrafish embryo melanocyte pattern and melanin quantification in order to identify and functionally verify the above tyrosinase-inhibiting extracts in vivo (Figure 5). Despite the complexity of the whole extracts, several active ones were identified and a phenotype-driven sub-fractionation would be necessary to enrich for the relevant bioactive compound. G. glabra extracts have been reported to inhibit tyrosinase activity and melanogenesis [83,84,85], most probably due to the presence of glabridin [83,86]. Interestingly, G. glabra extracts were also reported to contain other molecules capable of stimulating melanogenesis [87]; hence, the extraction method may play a decisive role for the finally assessed activity. In this vein, we have previously shown that G. glabra ethyl acetate and methanol extracts (using ASE) were able to inhibit tyrosinase activity and melanogenesis [88]. On the other hand, in the present study, the anti-tyrosinase activity was extracted with the SFE method, while the ASE extract (using water and isopropanol as solvent) proved to be inactive (Table 8). Regarding the two extracts from plants of the genus Pistacia (P. lentiscus and P. terebinthus), there are also reports in the literature supporting their tyrosinase inhibitory activity [77,89]. Notably, this is the first report regarding the tyrosinase inhibitory activity of a C. creticus ssp. eriocephalus extract, although there are publications regarding other subspecies of the genus Cistus [90,91], including C. parviflorus [92]. Similarly, the ability of A. cephalonica and P. idaeum extracts to suppress tyrosinase activity is shown for the first time, since there were reports in the literature only for other subspecies of the Abies [93,94] and Polygonum [95,96] genera. The antimelanogenic activities of S. sediforme, H. emerus, and A. unedo extracts are also in agreement with previous reports by us and others [16,88,97,98,99]. Essential oils from J. oxycedrus have been shown to possess tyrosinase inhibitory activity [43], as well as extracts from other subspecies of the Juniperus genus [100,101,102], though—to our knowledge—there are no such reports regarding J. turbinata.
Targeting cellular mechanisms responsible for proteome quality control by natural products is a promising approach to delay cellular senescence and/or in vivo aging [103]. Thus, we examined the activity of the two main proteolytic pathways of the cell, the Ubiquitin-Proteasome Pathway (UPP) and the Autophagy-Lysosome Pathway (ALP) [104]. Specifically, we assessed the main proteasome peptidase activity, the chymotrypsin–like activity (CT-L/β5), and the most abundant lysosomal proteases’ activity, that of cathepsins. Regarding the proteasomal activity, 12 extracts (9 ASE and 3 SFE) were tested, 75% of which were found to decrease peptidase action, while only two of them had a positive effect. Proteasome is considered a crucial target for therapeutic intervention; both its downregulation and upregulation can be beneficial for the progression delay of age-related diseases like cancer and neurodegeneration [105]. Therefore, proteasome inhibitors have gained a lot of interest in their efficacy against inflammation and cancer [106,107,108]. Extracts isolated using the ASE technique from Origanum vulgare and Sedum sediforme have been tested for their effect on proteasome activity in our previous study [16], where they were found not to change significantly or to mildly decrease the activity; these findings agree with the results of the present study. In addition, proteasome inhibition can lead to activation of the ALP pathway, indirectly mediating the maintenance of protein homeostasis [109]. Extracts obtained with the ASE technique from Pistacia lentiscus and Pistacia terebinthus were found here, for the first time, to activate the proteasome. Regarding autophagy modulation, it is known to be inhibited in some pathological conditions, contributing to the disease deterioration, while it may be permissive in others, towards pathogenesis [110]. From the extracts examined in the present study, two were found to activate cathepsins’ activity, one isolated from Pistachia terebinthus and one from Hippocrepis emerus, both obtained with the ASE technique. Suppression of cathepsins’ activity was observed after treatment with the majority of the extracts. Autophagy has been suggested to delay the skin photoaging process caused by UV irradiation through degradation of oxidized lipids and metabolic wastes; thus, natural products inducing the activity of this pathway may be promising agents for the cosmeceutical and medicinal industries [111,112]. However, some cathepsins can be secreted and remain functionally active outside of the lysosome. Specifically, extracellular cathepsins contribute to the degradation of many extracellular matrix components, like elastin, collagen, proteoglycans, and fibronectins, and participate in physiological processes, like wound healing and bone remodeling. They have been reported to be upregulated in pathological states, where uncontrolled cell proliferation, invasion, and differentiation or elastin depletion, caused by aberrant ECM dynamics, are observed [113,114,115]. Therefore, a number of natural products have been reported as selective inhibitors of some cathepsins [116,117].
Activities of all extracts assessed in the present study are summarized in a heatmap (Figure 7), which could prove valuable for the selection of specific targets for more detailed analysis. Moreover, certain extracts seem to combine multiple activities very useful for the cosmetic industry. In particular, extract 5A and extract 40A scored high in six of the bioactivity assays performed, extract 1A scored high in five of them, and extracts 8A, 21A, 22A, and 30A scored high in four assays (Figure 7).
To correlate chemical composition with biological activity, extracts found to exhibit multiple bioactivities were analyzed using LC-HRMS/MS for qualitative identification of the derived compounds. To that end, dereplication strategies were employed, i.e., spectrometric and chromatographic features, HRMS/MS fragmentation patterns, and in-house and open databases, as well as literature information. Compounds were tentatively identified based on accurate mass (Δm < 5 ppm), RDBeq., and chromatographic and spectrometric characteristics. Twelve (12) metabolites were annotated in extract 1A from Abies cephalonica, including dicarboxylic acids, flavonoids, abeosides and abietane-type diterpenes (Supplementary Table S2A). Notably, compounds such as 15-hydroxy-7-oxodehydroabietic acid ([M+H-H2O]+, m/z = 313.1796), 15-hydroxydehydroabietic acid ([M+H-H2O]+, m/z = 299.2003), and 7-oxodehydroabietic acid ([M-H]−, m/z = 313.1808), have been previously reported within the Abies genus [118,119]. Several of these diterpenes are known to possess moderate antibacterial and anti-inflammatory properties [120]. Additionally, abeoside D ([M-H+FA]−, m/z = 507.1715) and abeoside G ([M-H+FA]−, m/z = 519.2081) have also been previously identified and isolated from the trunk of Abies species. Abeoside G, along with abeosides A and B, have demonstrated strong anti-neuroinflammatory activity [121].
In extract 5A (Cistus parviflorus), 19 metabolites were tentatively identified, including quinic acid ([M-H]−, m/z = 191.0563), epigallocatechin ([M-H]−, m/z = 305.0670), quercetin-3-glucoside ([M-H]−, m/z = 463.0883), and tiliroside ([M-H]−, m/z = 593.1296), among others (Supplementary Table S2B). Overall, the available literature on C. parviflorus is significantly more limited compared to other Cistus species. Nevertheless, it has been reported that C. parviflorus exhibits stronger tyrosinase inhibition than other species [92].
As for extract 8A (E. parviflorum), 20 metabolites were annotated. Derivatives of phenolic acids like gallic acid ([M-H]−, m/z = 169.0141), gentisic acid ([M-H]−, m/z = 153.0194), ellatannins such as oenothein B ([M-2H]2−, m/z = 1567/783.0679), flavonoids (e.g., quercetin [M+H]+, m/z = 303.0496, kaempferol [M+H]+, m/z = 287.0549) and flavonoid glycosides (e.g., myricetin 3-O-rhamnoside [M-H]−, m/z = 463.0874, astragalin [M-H]−, m/z = 447.0923) were tentatively identified (Supplementary Table S2C). These compounds have previously been reported within the Epilobium genus and in general in the Onagraceae family [122,123]. Notably, several studies have suggested that oenothein B may play a key role in the pharmacological activities attributed to Epilobium species [124].
Twelve metabolites were annotated in extract 21A (Pistacia terebinthus), including hydrolysable tannins (3,5-Di-O-galloylquinic acid, [M-H]−, m/z = 495.0779), flavonoids (Myricetin-O-glucuronide, [M-H]−, m/z = 493.0618 and Myricitrin, [M-H]−, m/z = 463.0877), and fatty acids ((9Z)-5,8,11-trihydroxyoctadec-9-enoic acid, [M-H]−, m/z = 327.2178) (Supplementary Table S2D). These compounds have been previously identified in this species [89]. Traditionally, several parts of the tree have been used in folk medicine. Previous studies have shown that methanolic extracts of P. terebinthus exhibit strong antioxidant activity and high metal-chelating capacity. Moreover, the extracts inhibited key metabolic and neurological enzymes (AChE, BChE, α-amylase, α-glucosidase, tyrosinase), highlighting their potential for neuroprotective, metabolic, and cosmetic applications [125].
In extract 22A (Sedum sediforme), 17 metabolites were annotated, most of which belong to the class of flavonoid glycosides (Supplementary Table S2E). Notably, compounds identified include gentisic acid 5-O-glucoside ([M-H]−, m/z = 315.0722), quercitrin ([M-H]−, m/z = 447.0930), eriodictyol-4′-O-glucoside ([M-H]−, m/z = 449.1088), and tricin 7-glucoside ([M-H]−, m/z = 491.1196), all of which have previously been reported in methanolic extracts of S. sediforme [126]. Furthermore, earlier studies have shown that methanolic extracts from the aerial parts of S. sediforme exhibit strong inhibitory effects on 5-lipoxygenase, suggesting an anti-inflammatory potential, along with notable antioxidant activity [127,128].
Regarding C. creticus ssp. eriocephalus extract 30A, 23 metabolites were annotated (Supplementary Table S2F). The majority belong to the flavonoid class, including tiliroside ([M-H]−, m/z = 593.1299), acacetin ([M-H]−, m/z = 283.0615), luteolin ([M-H]−, m/z = 285.0406), and kaempferol ([M-H]−, m/z = 285.0406). Additionally, phenolic acids such as protocatechuic acid ([M-H]−, m/z = 153.0195) and hydroxybenzoate esters like urlanneoside ([M-H]−, m/z = 285.0619) were detected. These metabolites are commonly found within the Cistus genus [129]. However, similar to C. parviflorus, the literature on C. creticus ssp. eriocephalus remains limited, with most existing studies focusing primarily on its essential oil composition. In traditional medicine, various Cistus species have been widely used for their anti-inflammatory, hemostatic, tonic, anti-diabetic, and antispasmodic properties [130,131].
Literature on Polygonum idaeum remains limited, which complicates compound identification. Nevertheless, 21 compounds were annotated in the extract 40A (Supplementary Table S2G), primarily belonging to the class of benzoic acid derivatives (e.g., gentisic acid [M-H]−, m/z = 153.0194, 4-hydroxybenzoic acid [M-H]−, m/z = 137.0245), polyphenols (e.g., gallic acid [M-H]−, m/z = 169.0143 and rosmarinic acid ([M-H]−, m/z = 359.0771), flavonoid glycosides (e.g., avicularin [M-H]−, m/z = 433.0778), and fatty acids (e.g., stearidonic acid [M+H]+, m/z = 277.2157). These metabolites have been previously reported in other Polygonum species [132]. Interestingly, other species of the genus have demonstrated photoprotective effects. For instance, P. multiflorum extracellular vesicle-like nanovesicles have been explored as a novel natural therapeutic agent against photoaging due to the presence of several antioxidant compounds [133].
5. Conclusions
In the present study, 104 extracts from 41 genera and 49 different species of plants endemic in Greece were evaluated regarding a broad spectrum of biological activities related to skin aging. Many of them were found to possess interesting properties in terms of antioxidant potential, photoprotective capacity, antimelanogenic activity, and inhibition of enzymes such as collagenase, elastase, and proteasomal enzymes. In general, extracts obtained through ASE exhibited higher activities than those of extracts obtained through SFE. Seven extracts showed multiple activities, being highly effective in at least four different assays. Their chemical composition and literature data supported our present findings. Due to the broad spectrum of assays used in the current study, a selection strategy had to be employed in some of the assays, a fact that can be considered as a limitation of the study. Moreover, the multifarious nature of the extracts may lead to masking of certain activities, e.g., during the in vivo zebrafish embryo melanogenesis assay, the presence of a toxic compound in the whole extract could mask the identification of a compound of interest due to using the maximum tolerated concentration. Future studies will be designed employing a bioactivity-driven subfractionation in order to enrich the extracts in the compound of interest or even isolate the single bioactive compound.
In conclusion, novel properties of various plants of the Greek flora identified in the present study, along with the existing knowledge, will prove valuable for their potential use in interventions against skin aging and in cosmetic applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14070824/s1, Table S1: Sequences of the primers used, Table S2: Dereplication tables.
Author Contributions
Conceptualization, H.P., G.S., D.B., M.H., I.P.T. and D.K.; methodology, D.D.G., G.B.L.R., A.A. (Adamantia Agalou), A.F., X.P.L., C.N., A.A. (Aikaterini Argyropoulou), D.M., A.T., I.E., A.P., S.G. and K.K.; validation, H.P. and E.M.; formal analysis, G.B.L.R. and C.N.; Investigation, E.K.; Resources, E.K.; writing—original draft preparation, H.P., D.D.G., G.B.L.R. and D.B.; writing—review and editing, H.P., X.P.L., S.B., E.M., G.S., D.B., M.H., I.P.T. and D.K.; supervision, D.B., M.H., I.P.T. and D.K.; project administration, H.P. and D.K.; funding acquisition, G.S., D.B., M.H., I.P.T. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T2EDK-02583; project acronym: CosmAGE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We would like to acknowledge the use of the equipment and facilities of OPENSCREEN-GR.
Conflicts of Interest
G.S. belongs to the scientific staff of Korres S.A.—Natural Products and C.N., D.M. and S.B. belong to the scientific staff of Pharmagnose. The remaining authors declare that the research was conducted in the absence of any commercial relationships that could be construed as potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pederson, K.; et al. Contoversies in Experimental Dermatology. Exp. Dermatol. 2002, 11, 159–187. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Robert, L.; Labat-Robert, J.; Robert, A.-M. Physiology of Skin Aging. Pathol. Biol. 2009, 57, 336–341. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and Lipofuscin as Hallmarks of Skin Aging. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2017, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Eller, M.S.; Yaar, M. Telomere-Mediated Effects on Melanogenesis and Skin Aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Mavrogonatou, E.; Zervou, S.-K.; Triantis, T.; Hiskia, A.; Kletsas, D. Natural Product-Derived Senotherapeutics: Extraction and Biological Evaluation Techniques. Methods Mol. Biol. 2025, 2906, 315–359. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.V. Biodiversity Hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and in Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef]
- Agalou, A.; Thrapsianiotis, M.; Angelis, A.; Papakyriakou, A.; Skaltsounis, A.-L.; Aligiannis, N.; Beis, D. Identification of Novel Melanin Synthesis Inhibitors from Crataegus Pycnoloba Using an in Vivo Zebrafish Phenotypic Assay. Front. Pharmacol. 2018, 9, 265. [Google Scholar] [CrossRef]
- Beis, D. Zebrafish Research in Greece: Swimming against the Current. Int. J. Dev. Biol. 2022, 66, 155–161. [Google Scholar] [CrossRef]
- Giardoglou, P.; Beis, D. On Zebrafish Disease Models and Matters of the Heart. Biomedicines 2019, 7, 15. [Google Scholar] [CrossRef]
- Fotopoulou, A.; Angelopoulou, M.T.; Pratsinis, H.; Mavrogonatou, E.; Kletsas, D. A Subset of Human Dermal Fibroblasts Overexpressing Cockayne Syndrome Group B Protein Resist UVB Radiation-Mediated Premature Senescence. Aging Cell 2025, 24, e14422. [Google Scholar] [CrossRef]
- Kavvoura, D.-A.; Stefanakis, M.K.; Kletsas, D.; Katerinopoulos, H.E.; Pratsinis, H. Biological Activities of Ceratonia Siliqua Pod and Seed Extracts: A Comparative Analysis of Two Cretan Cultivars. Int. J. Mol. Sci. 2023, 24, 12104. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Angelopoulou, M.; Rizou, S.V.; Pratsinis, H.; Gorgoulis, V.G.; Kletsas, D. Activation of the JNKs/ATM-P53 Axis Is Indispensable for the Cytoprotection of Dermal Fibroblasts Exposed to UVB Radiation. Cell Death Dis. 2022, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Beekman, B.; Drijfhout, J.W.; Bloemhoff, W.; Ronday, H.K.; Tak, P.P.; te Koppele, J.M. Convenient Fluorometric Assay for Matrix Metalloproteinase Activity and Its Application in Biological Media. FEBS Lett. 1996, 390, 221–225. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H.; Kobayashi, S. Inhibition Effects of (+)-Catechin–Aldehyde Polycondensates on Proteinases Causing Proteolytic Degradation of Extracellular Matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. A Guide for the Laboratory Use of Zebrafish (Danio Rerio). In THE ZEBRAFISH BOOK, 4th ed.; Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 5 May 2025).
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Mahgoub Awadelkareem, A.; Ashraf, S.A. Recent Insights into Green Extraction Techniques as Efficient Methods for the Extraction of Bioactive Components and Essential Oils from Foods. CyTA-J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Colanesi, S.; Taylor, K.L.; Temperley, N.D.; Lundegaard, P.R.; Liu, D.; North, T.E.; Ishizaki, H.; Kelsh, R.N.; Patton, E.E. Small Molecule Screening Identifies Targetable Zebrafish Pigmentation Pathways. Pigment. Cell Melanoma Res. 2012, 25, 131–143. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Li, K.; Landriault, M.; Fingas, M.; Llompart, M. Accelerated Solvent Extraction (ASE) of Environmental Organic Compounds in Soils Using a Modified Supercritical Fluid Extractor. J. Hazard. Mater. 2003, 102, 93–104. [Google Scholar] [CrossRef]
- Fioroni, N.; Mouquet-Rivier, C.; Meudec, E.; Cheynier, V.; Boudard, F.; Hemery, Y.; Laurent-Babot, C. Antioxidant Capacity of Polar and Non-Polar Extracts of Four African Green Leafy Vegetables and Correlation with Polyphenol and Carotenoid Contents. Antioxidants 2023, 12, 1726. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.-P.; Huang, D.; Ong, C.N. Antioxidant Activity and Profiles of Common Vegetables in Singapore. Food Chem. 2010, 120, 993–1003. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.V.; Wagner, E.; Wiseman, S.; Put, F.V.D.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects Their Phenolic and Vitamin C Composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (Cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants 2021, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Parić, A.; Muratović, E.; Murtić, S.; Subašić, M.; Pustahija, F. Biochemical-Physiological Responses of Reseda Lutea, Epilobium Dodonaei, and Gentianella Ciliata to Stress in an Open Pit of an Abandoned Iron Mine. Int. J. Phytoremediation 2025, 27, 1110–1118. [Google Scholar] [CrossRef]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of Some Greek Aromatic Plants for Antioxidant Activity. Phytother. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef]
- Ertaş, A.; Boğa, M.; Yılmaz, M.A.; Yeşil, Y.; Haşimi, N.; Kaya, M.Ş.; Temel, H.; Kolak, U. Chemical Compositions by Using LC-MS/MS and GC-MS and Biological Activities of Sedum sediforme (Jacq.) Pau. J. Agric. Food Chem. 2014, 62, 4601–4609. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Tedeschi, P.; Marchetti, N.; Gessi, S. Antioxidant and Antiinflammatory Effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum Plant Extracts in Macrophage and Microglial Cells. Cells 2021, 10, 2691. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Živković, J.; Ristić, M.; Kschonsek, J.; Westphal, A.; Mihailović, M.; Filipović, V.; Böhm, V. Comparison of Chemical Profile and Antioxidant Capacity of Seeds and Oils from Salvia sclarea and Salvia officinalis. Chem. Biodivers. 2017, 14, e1700344. [Google Scholar] [CrossRef]
- Makarova, K.; Sajkowska-Kozielewicz, J.J.; Zawada, K.; Olchowik-Grabarek, E.; Ciach, M.A.; Gogolewski, K.; Dobros, N.; Ciechowicz, P.; Freichels, H.; Gambin, A. Harvest Time Affects Antioxidant Capacity, Total Polyphenol and Flavonoid Content of Polish St John’s Wort’s (Hypericum perforatum L.) Flowers. Sci. Rep. 2021, 11, 3989. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical Composition, Antioxidant, Anti-Tyrosinase, Anti-Cholinesterase and Cytotoxic Activities of Essential Oils of Six Algerian Plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ornano, L.; Ballero, M.; Sanna, C.; Bruno, M.; Rosselli, S.; et al. Bioactive Constituents of Juniperus turbinata Guss. from La Maddalena Archipelago. Chem. Biodivers. 2018, 15, e1800148. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Jaradat, N.; Hattab, S.; Al-hihi, S.; Juma’a, R. Traditional Palestinian Medicinal Plant Cercis Siliquastrum (Judas Tree) Inhibits the DNA Cell Cycle of Breast Cancer – Antimicrobial and Antioxidant Characteristics. Eur. J. Integr. Med. 2019, 27, 90–96. [Google Scholar] [CrossRef]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant Activity of Selected Plant Species; Potential New Sources of Natural Antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Branković, S.; Kovačević, N.; Kitić, D.; Veljković, S. Comparative Study of Spasmolytic Properties, Antioxidant Activity and Phenolic Content of Arbutus unedo from Montenegro and Greece. Phytother. Res. 2011, 25, 749–754. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A New Flavone from Antioxidant Extracts of Pistacia Terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxid. Med. Cell Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Alilou, M. Naringenin Attenuates CCl4 -Induced Hepatic Inflammation by the Activation of an Nrf2-Mediated Pathway in Rats. Clin. Exp. Pharmacol. Physiol. 2014, 41, 416–422. [Google Scholar] [CrossRef]
- Johnson, J.; Maher, P.; Hanneken, A. The Flavonoid, Eriodictyol, Induces Long-Term Protection in ARPE-19 Cells through Its Effects on Nrf2 Activation and Phase 2 Gene Expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2398–2406. [Google Scholar] [CrossRef]
- Ke, Z.; Fan, C.; Li, J.; Wang, L.; Li, H.; Tian, W.; Yu, Q. Nobiletin Intake Attenuates Hepatic Lipid Profiling and Oxidative Stress in HFD-Induced Nonalcoholic-Fatty-Liver-Disease Mice. Molecules 2023, 28, 2570. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-S.; Zhang, Y.; Liu, B.; Li, C.-B.; Wu, J.; Li, Y. Nomilin Protects against Cerebral Ischemia-Reperfusion Induced Neurological Deficits and Blood-Brain Barrier Disruption via the Nrf2 Pathway. Food Funct. 2019, 10, 5323–5332. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.-Y.; Chien, J.-C.; Tung, Y.-C.; Wu, T.-Y.; Liao, J.-A.; Wei, G.-J. Tangeretin and 4’-Demethyltangeretin Prevent Damage to Mouse Hepatocytes from Oxidative Stress by Activating the Nrf2-Related Antioxidant Pathway via an Epigenetic Mechanism. Chem. Biol. Interact. 2023, 382, 110650. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Song, C.; Chen, J.; Zhou, L.; Jiang, X.; Cao, X.; Sun, Y.; Zhang, Q. Limonin Ameliorates Acetaminophen-Induced Hepatotoxicity by Activating Nrf2 Antioxidative Pathway and Inhibiting NF-κB Inflammatory Response via Upregulating Sirt1. Phytomedicine 2020, 69, 153211. [Google Scholar] [CrossRef]
- Morquio, A.; Rivera-Megret, F.; Dajas, F. Photoprotection by Topical Application of Achyrocline satureioides (‘Marcela’). Phytother. Res. 2005, 19, 486–490. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Žunec, S.; Katić, A.; Gašić, U.; Milojković Opsenica, D.; Kopjar, N. Protective Role of Strawberry Tree (Arbutus unedo L.) Honey against Cyto/Genotoxic Effects Induced by Ultraviolet B Radiation in Vitro. J. Apic. Res. 2024, 63, 513–522. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Henao-Zuluaga, K.; Gallardo, C.; Atehortúa, L.; Puertas-Mejía, M.A. Novel In Vitro Antioxidant and Photoprotection Capacity of Plants from High Altitude Ecosystems of Colombia. Photochem. Photobiol. 2016, 92, 150–157. [Google Scholar] [CrossRef]
- Gherboudj, O.; Boutaghane, N.; Kabouche, Z.; Djeblia, L.; Zerrougui, L.; Bekrar, M. Phytochemical Profiles and Evaluation of the Biological Potential of Ethyl Acetate and N-Butanol Fractions of the Aerial Parts of Cistus albidus L. Nat. Prod. Res. 2025, 39, 2642–2648. [Google Scholar] [CrossRef]
- Martorana, M.; Arcoraci, T.; Rizza, L.; Cristani, M.; Bonina, F.P.; Saija, A.; Trombetta, D.; Tomaino, A. In Vitro Antioxidant and in Vivo Photoprotective Effect of Pistachio (Pistacia vera L., Variety Bronte) Seed and Skin Extracts. Fitoterapia 2013, 85, 41–48. [Google Scholar] [CrossRef]
- Otto, A.I.; Riou, L.; Marionnet, C.; Mori, T.; Sarasin, A.; Magnaldo, T. Differential Behaviors toward Ultraviolet A and B Radiation of Fibroblasts and Keratinocytes from Normal and DNA-Repair-Deficient Patients1. Cancer Res. 1999, 59, 1212–1218. [Google Scholar]
- Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibañez, E. Use of Compressed Fluids for Sample Preparation: Food Applications. J. Chromatogr. A 2007, 1152, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In Vivo Bioactivity Assessment on Epilobium Species: A Particular Focus on Epilobium angustifolium and Its Components on Enzymes Connected with the Healing Process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef] [PubMed]
- Stamou, P.; Mikropoulou, E.V.; Chalkiadaki, M.; Basdeki, A.; Antoniadi, L.; Poigny, S.; Halabalaki, M. Revealing the Potential of Chios Mastic Gum and Its Constituents for Cosmetic Applications through Chemical Profiling and Biological Evaluation. Cosmetics 2024, 11, 155. [Google Scholar] [CrossRef]
- Kamli, H.; Ali, A.-A.M.; Salem, Y.H.; Shaikh, A.; El-Nashar, H.A.S. Chemical Profiling and Enzyme Inhibitory Activities of Essential Oil Isolated from Pistacia khinjuk Leaves: Insights On GC-MS Analysis and Skin Aging-Relevant Enzymes. Chem. Biodivers. 2024, 21, e202302096. [Google Scholar] [CrossRef] [PubMed]
- Senol Deniz, F.S.; Orhan, I.E.; Duman, H. Profiling Cosmeceutical Effects of Various Herbal Extracts through Elastase, Collagenase, Tyrosinase Inhibitory and Antioxidant Assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, N.; Yan, C.; Zheng, Z.; Xi, J.; Cao, P. The Potential of Paeonia lactiflora Pall Seeds Oil as a Pure Natural Cosmetics Raw Material: In Vitro Findings. J. Cosmet. Dermatol. 2024, 23, 1875–1883. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and Evaluation of Dermo-Cosmetic Activities of the Invasive Plant Species Polygonum cuspidatum. Plants 2023, 12, 83. [Google Scholar] [CrossRef]
- Eruygur, N.; Buyukyildirim, T.; Tetik Rama, S.; Ayaz, F.; Tekin, M.; Tuzcu, M.; Akcakavak, G.; Abdullah Yilmaz, M. Phytochemical Profiling and Biological Activity of Achillea sintenisii Hub.-Mor. Chem. Biodivers. 2023, 20, e202201258. [Google Scholar] [CrossRef]
- Amer, R.I.; Ezzat, S.M.; Aborehab, N.M.; Ragab, M.F.; Mohamed, D.; Hashad, A.; Attia, D.; Salama, M.M.; El Bishbishy, M.H. Downregulation of MMP1 Expression Mediates the Anti-Aging Activity of Citrus Sinensis Peel Extract Nanoformulation in UV Induced Photoaging in Mice. Biomed. Pharmacother. 2021, 138, 111537. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Jeong, Y.-U.; Kim, J.-H.; Park, Y.-J. 3,5,6,7,8,3’,4’-Heptamethoxyflavone, a Citrus Flavonoid, Inhibits Collagenase Activity and Induces Type I Procollagen Synthesis in HDFn Cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, F.; Zhang, L.; Yu, H.; Yu, F.; Chen, J. The Effect of Active Components from Citrus Fruits on Dentin MMPs. Arch. Oral. Biol. 2017, 83, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a Hundred Plant Extracts as Tyrosinase and Elastase Inhibitors, Two Enzymatic Targets of Cosmetic Interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules 2022, 27, 855. [Google Scholar] [CrossRef]
- Tilkat, E.A.; Batibay, H.; Yener, I.; Yilmaz, P.K.; Akdeniz, M.; Kaplan, A.; Ercisli, S.; Ertas, A.; Holubec, V. Determination of Enzyme Inhibition Potential and Anticancer Effects of Pistacia khinjuk Stocks Raised in In Vitro and In Vivo Conditions. Agronomy 2021, 11, 154. [Google Scholar] [CrossRef]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Schmidt, C.; Fronza, M.; Goettert, M.; Geller, F.; Luik, S.; Flores, E.M.M.; Bittencourt, C.F.; Zanetti, G.D.; Heinzmann, B.M.; Laufer, S.; et al. Biological Studies on Brazilian Plants Used in Wound Healing. J. Ethnopharmacol. 2009, 122, 523–532. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and Anti-Inflammatory Flavonol Glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical Compositions and Anti-Skin-Ageing Activities of Origanum vulgare L. Essential Oil from Tropical and Mediterranean Region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef]
- Maurya, S.K.; Raj, K.; Srivastava, A.K. Antidyslipidaemic Activity of Glycyrrhiza glabra in High Fructose Diet Induced Dsyslipidaemic Syrian Golden Hamsters. Indian. J. Clin. Biochem. 2009, 24, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Kim, B.J.; Kim, J.H.; Heo, M.Y.; Kim, H.P. Biological Screening of 100 Plant Extracts for Cosmetic Use (I): Inhibitory Activities of Tyrosinase and DOPA Auto-Oxidation. Int. J. Cosmet. Sci. 1997, 19, 291–298. [Google Scholar] [CrossRef]
- Kang, M.H.; Jang, G.Y.; Ji, Y.-J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The Inhibitory Effect of Glabridin from Licorice Extracts on Melanogenesis and Inflammation. Pigment. Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.D.; Yang, J.Y.; Song, E.S.; Par, J. WStimulation of Melanogenesis by Glycyrrhizin in B16 Melanoma Cells. Exp. Mol. Med. 2001, 33, 131–135. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus Alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef]
- Uysal, S.; Sinan, K.I.; Jekő, J.; Cziáky, Z.; Zengin, G. Chemical Characterization, Comprehensive Antioxidant Capacity, and Enzyme Inhibitory Potential of Leaves from Pistacia terebinthus L. (Anacardiaceae). Food Biosci. 2022, 48, 101820. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Ahmed, S.; Zengin, G.; Selvi, S.; Ak, G.; Cziáky, Z.; Jekő, J.; Rodrigues, M.J.; Custodio, L.; Venanzoni, R.; Flores, G.A.; et al. Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques. Pathogens 2024, 13, 795. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Ancuceanu, R.; Anghel, A.I.; Hovaneț, M.V.; Ciobanu, A.-M.; Lascu, B.E.; Dinu, M. Antioxidant Activity of Essential Oils from Pinaceae Species. Antioxidants 2024, 13, 286. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tamura, N. Inhibitory Compound of Tyrosinase Activity from the Sprout of Polygonum hydropiper L. (Benitade). Biol. Pharm. Bull. 2007, 30, 595–597. [Google Scholar] [CrossRef]
- Murray, A.F.; Satooka, H.; Shimizu, K.; Chavasiri, W.; Kubo, I. Polygonum odoratum Essential Oil Inhibits the Activity of Mushroom Derived Tyrosinase. Heliyon 2019, 5, e02817. [Google Scholar] [CrossRef]
- Schmitt, M.; Alabdul Magid, A.; Hubert, J.; Etique, N.; Duca, L.; Voutquenne-Nazabadioko, L. Bio-Guided Isolation of New Phenolic Compounds from Hippocrepis Emerus Flowers and Investigation of Their Antioxidant, Tyrosinase and Elastase Inhibitory Activities. Phytochem. Lett. 2020, 35, 28–36. [Google Scholar] [CrossRef]
- Demir, S.; Koyu, H.; Yilmaz, M.A.; Tarhan, A.; Ozturk, S.B. Antityrosinase Activity and LC-MS/MS Analysis of Optimized Ultrasound-Assisted Condition Extracts and Fractions from Strawberry Tree (Arbutus unedo L.). J. Food Drug Anal. 2024, 32, 194–212. [Google Scholar] [CrossRef]
- Habachi, E.; Rebey, I.B.; Dakhlaoui, S.; Hammami, M.; Sawsen, S.; Msaada, K.; Merah, O.; Bourgou, S. Arbutus unedo: Innovative Source of Antioxidant, Anti-Inflammatory and Anti-Tyrosinase Phenolics for Novel Cosmeceuticals. Cosmetics 2022, 9, 143. [Google Scholar] [CrossRef]
- El Jemli, M.; Ezzat, S.M.; Kharbach, M.; Mostafa, E.S.; Radwan, R.A.; El Jemli, Y.; El-Guourrami, O.; Ahid, S.; Cherrah, Y.; Zayed, A.; et al. Bioassay-Guided Isolation of Anti-Inflammatory and Antinociceptive Metabolites among Three Moroccan Juniperus Leaves Extract Supported with in Vitro Enzyme Inhibitory Assays. J. Ethnopharmacol. 2024, 331, 118285. [Google Scholar] [CrossRef]
- Jegal, J.; Chung, K.W.; Chung, H.Y.; Jeong, E.J.; Yang, M.H. The Standardized Extract of Juniperus communis Alleviates Hyperpigmentation in Vivo HRM-2 Hairless Mice and in Vitro Murine B16 Melanoma Cells. Biol. Pharm. Bull. 2017, 40, 1381–1388. [Google Scholar] [CrossRef]
- Park, J.S.; Ko, K.; Kim, S.-H.; Lee, J.K.; Park, J.-S.; Park, K.; Kim, M.R.; Kang, K.; Oh, D.-C.; Kim, S.Y.; et al. Tropolone-Bearing Sesquiterpenes from Juniperus chinensis: Structures, Photochemistry and Bioactivity. J. Nat. Prod. 2021, 84, 2020–2027. [Google Scholar] [CrossRef]
- Wedel, S.; Manola, M.; Cavinato, M.; Trougakos, I.P.; Jansen-Dürr, P. Targeting Protein Quality Control Mechanisms by Natural Products to Promote Healthy Ageing. Molecules 2018, 23, 1219. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.R.; Sbardella, D.; Santoro, A.M.; Coletta, A.; Oddone, F.; Grasso, G.; Milardi, D.; Lacal, P.M.; Marini, S.; Purrello, R.; et al. The Proteasome as a Druggable Target with Multiple Therapeutic Potentialities: Cutting and Non-Cutting Edges. Pharmacol. Ther. 2020, 213, 107579. [Google Scholar] [CrossRef]
- Elliott, P.J.; Zollner, T.M.; Boehncke, W.-H. Proteasome Inhibition: A New Anti-Inflammatory Strategy. J. Mol. Med. 2003, 81, 235–245. [Google Scholar] [CrossRef]
- Harer, S.L.; Bhatia, M.S.; Bhatia, N.M. Proteasome Inhibitors Mechanism; Source for Design of Newer Therapeutic Agents. J. Antibiot. 2012, 65, 279–288. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome Inhibitors in Cancer Therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, N.; Bustamante, H.; Soza, A.; Burgos, P. Cellular Responses to Proteasome Inhibition: Molecular Mechanisms and Beyond. Int. J. Mol. Sci. 2019, 20, 3379. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy Modulation as a Potential Therapeutic Target for Diverse Diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Ma, J.; Teng, Y.; Huang, Y.; Tao, X.; Fan, Y. Autophagy Plays an Essential Role in Ultraviolet Radiation-Driven Skin Photoaging. Front. Pharmacol. 2022, 13, 864331. [Google Scholar] [CrossRef]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxid. Med. Cell Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Hedtke, T.; Heinz, A.; Andrault, P.-M.; Hoehenwarter, W.; Granville, D.J.; Schmelzer, C.E.H.; Brömme, D. Expression of Elastolytic Cathepsins in Human Skin and Their Involvement in Age-Dependent Elastin Degradation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129544. [Google Scholar] [CrossRef]
- Son, E.D.; Shim, J.H.; Choi, H.; Kim, H.; Lim, K.M.; Chung, J.H.; Byun, S.Y.; Lee, T.R. Cathepsin G Inhibitor Prevents Ultraviolet B-Induced Photoaging in Hairless Mice via Inhibition of Fibronectin Fragmentation. Dermatology 2012, 224, 352–360. [Google Scholar] [CrossRef]
- Moon, D.O. Review of Cathepsin K Inhibitor Development and the Potential Role of Phytochemicals. Molecules 2025, 30, 91. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Albalat, A.; González, F.V. Chapter 6—Natural Products as Cathepsin Inhibitors. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 179–213. ISBN 1572 5995. [Google Scholar]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Diterpenes from the Trunk of Abies holophylla and Their Potential Neuroprotective and Anti-Inflammatory Activities. J. Nat. Prod. 2016, 79, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Feng, L.; Li, S.-M.; Liu, X.-H.; Li, Y.-L.; Wu, L.; Shen, Y.-H.; Tian, J.-M.; Zhang, X.; Liu, X.-R.; et al. Isolation, Structure, and Bioactivities of Abiesadines A–Y, 25 New Diterpenes from Abies Georgei Orr. Bioorganic Med. Chem. 2010, 18, 744–754. [Google Scholar] [CrossRef]
- Baosong, C.; Sixian, W.; Gaoqiang, L.; Li, B.; Ying, H.; Ruilin, Z.; Hongwei, L. Anti-Inflammatory Diterpenes and Steroids from Peels of the Cultivated Edible Mushroom Wolfiporia Cocos. Phytochem. Lett. 2020, 36, 11–16. [Google Scholar] [CrossRef]
- Lee, T.H.; Subedi, L.; Ha, Y.J.; Moon, G.; Kim, S.Y.; Kim, C.S. Glycosylated Constituents Isolated from the Trunk of Abies Holophylla and Their Anti-Inflammatory and Neurotrophic Activity. Phytochemistry 2021, 192, 112962. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium Spp: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Shawky, E.M.; Elgindi, M.R.; Ibrahim, H.A.; Baky, M.H. The Potential and Outgoing Trends in Traditional, Phytochemical, Economical, and Ethnopharmacological Importance of Family Onagraceae: A Comprehensive Review. J. Ethnopharmacol. 2021, 281, 114450. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, Pharmacology and Traditional Uses of Different Epilobium Species (Onagraceae): A Review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D. Advancing Pistacia terebinthus L. (Anacardiaceae) Research: Food Preservation, Functional Foods, and Nutraceutical Potential. Foods 2025, 14, 1245. [Google Scholar] [CrossRef]
- Winekenstädde, D.; Angelis, A.; Waltenberger, B.; Schwaiger, S.; Tchoumtchoua, J.; König, S.; Werz, O.; Aligiannis, N.; Skaltsounis, A.-L.; Stuppner, H. Phytochemical Profile of the Aerial Parts of Sedum Sediforme and Anti-Inflammatory Activity of Myricitrin. Nat. Prod. Commun. 2015, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.A.; Elwekeel, A.; Moawad, A.; Afifi, N.; Amin, E.; Amir, D.E. Phytochemical Constituents and Biological Activity of Selected Genera of Family Crassulaceae: A Review. South. Afr. J. Bot. 2021, 141, 383–404. [Google Scholar] [CrossRef]
- Trabsa, H.; Krach, I.; Boussoualim, N.; Ouhida, S.; Arrar, L.; Baghiani, A. Evaluation of Anti-Inflammatory and Antioxidant Activities of Sedum Sediforme Extracts. Trop. J. Pharm. Res. 2020, 19, 2109–2114. [Google Scholar] [CrossRef]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Lucarini, D.; Papa, F.; Peron, G.; Dall’Acqua, S. Phytochemical Analysis of the Labdanum-Poor Cistus creticus Subsp. Eriocephalus (Viv.) Greuter et Burdet Growing in Central Italy. Biochem. Syst. Ecol. 2016, 66, 50–57. [Google Scholar] [CrossRef]
- Palaiogiannis, D.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Extraction of Bioactive Compounds from Cistus creticus Leaves and Their Use in the Preparation of Yogurt Desserts. Oxygen 2024, 4, 90–107. [Google Scholar] [CrossRef]
- Idoudi, S.; Tourrette, A.; Bouajila, J.; Romdhane, M.; Elfalleh, W. The Genus Polygonum: An Updated Comprehensive Review of Its Ethnomedicinal, Phytochemical, Pharmacological Activities, Toxicology, and Phytopharmaceutical Formulation. Heliyon 2024, 10, e28947. [Google Scholar] [CrossRef]
- He, J.; Fu, L.; Shen, Y.; Teng, Y.; Huang, Y.; Ding, X.; Xu, D.; Cui, H.; Zhu, M.; Xie, J.; et al. Polygonum multiflorum Extracellular Vesicle-Like Nanovesicle for Skin Photoaging Therapy. Biomater. Res. 2024, 28, 0098. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).