The Role of Antioxidant Compounds from Citrus Waste in Modulating Neuroinflammation: A Sustainable Solution
Abstract
1. Introduction
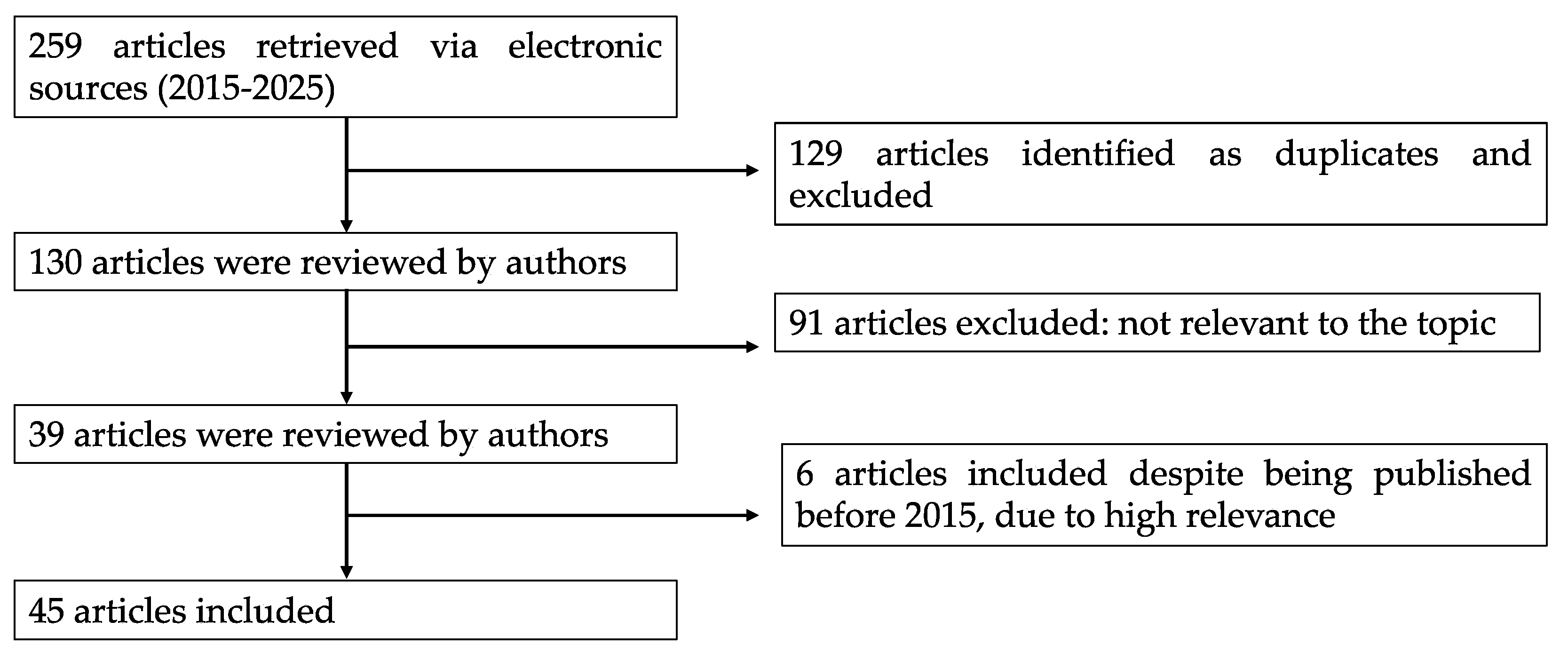
2. Literature Search Strategy
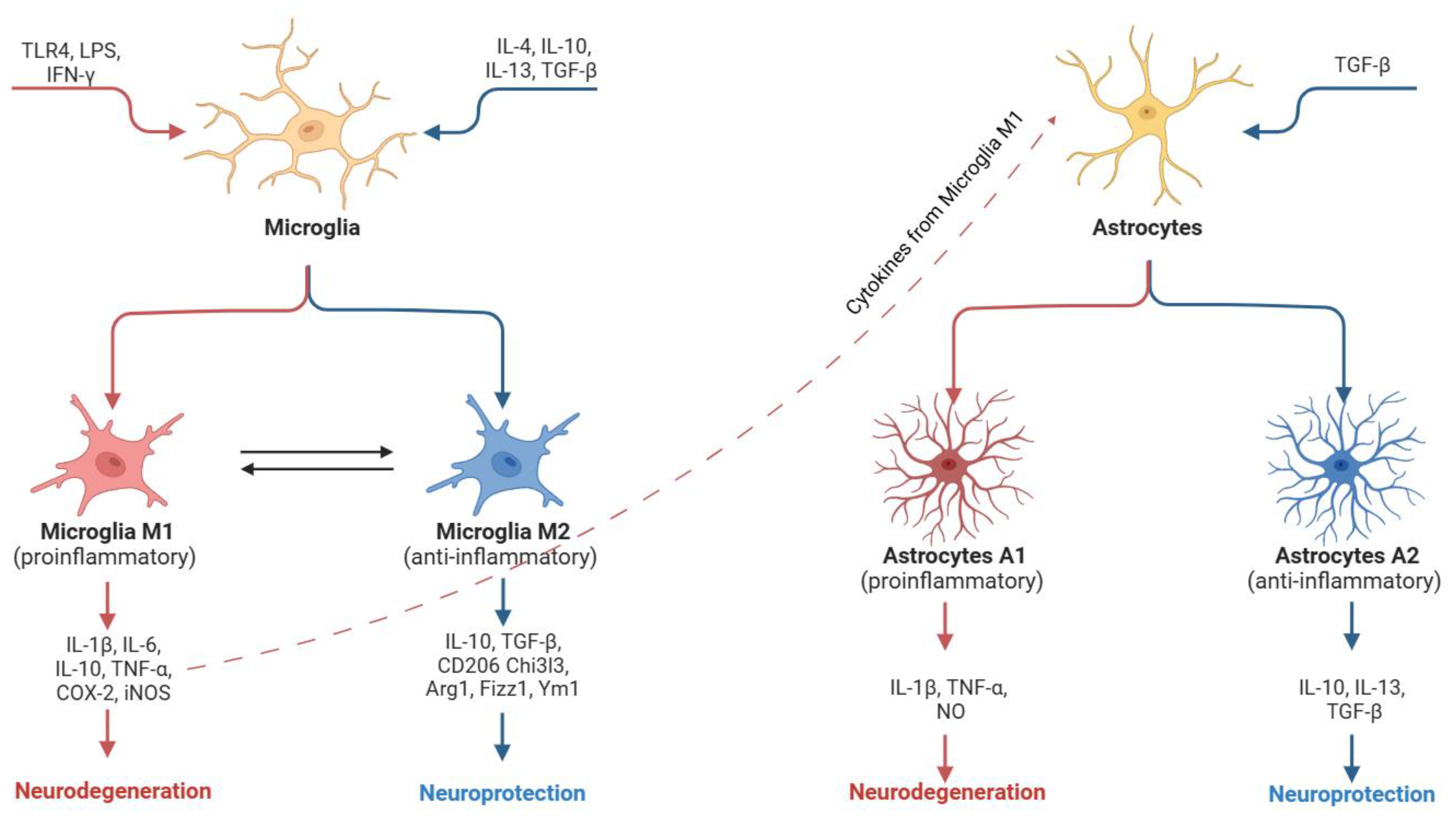
3. Molecular Mechanisms in Neuroinflammation
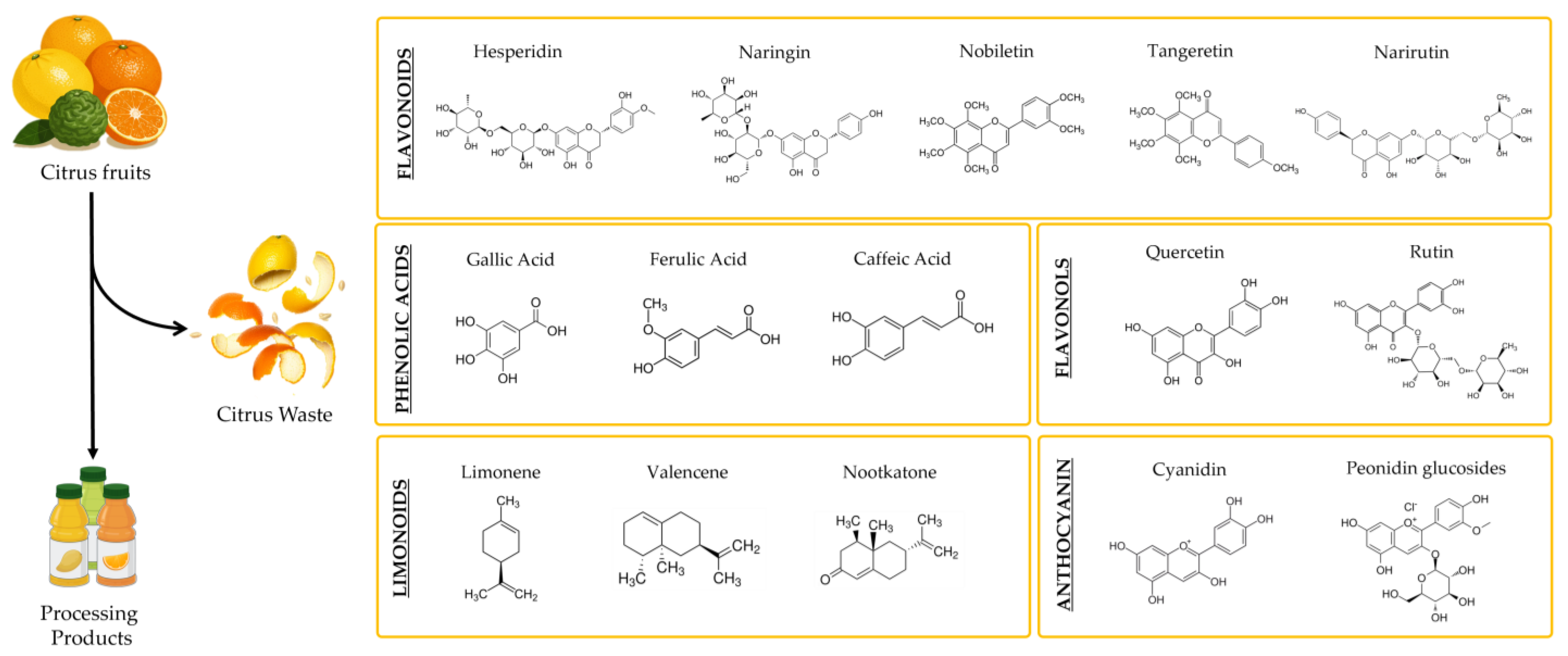
4. Citrus Waste as a Source of Antioxidant Bioactive Compounds
Bioavailability of Bioactive Antioxidant Compounds in Citrus Waste
5. Citrus By-Products and Neuroinflammation: Mechanisms and Potential Applications
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Angeloni, C.; Malaguti, M.; Prata, C.; Freschi, M.; Barbalace, M.C.; Hrelia, S. Mechanisms Underlying Neurodegenerative Disorders and Potential Neuroprotective Activity of Agrifood By-Products. Antioxidants 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.; Punzo, A.; Bonvicini, F.; Perillo, M.; Malaguti, M.; Lorenzini, A.; Foltran, I.; Mercatante, D.; Mandrioli, M.; Rodriguez-Estrada, M.T.; et al. Anti-Inflammatory, Antioxidant and Antibacterial Properties of Tomato Skin and Pomegranate Peel Extracts: A Sustainable Approach for Oral Health Care. Antioxidants 2025, 14, 54. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2022, 11, 20. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Goulas, V.; Manganaris, G.A. Exploring the Phytochemical Content and the Antioxidant Potential of Citrus Fruits Grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar] [CrossRef]
- Medzhitov, R. The Spectrum of Inflammatory Responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Leszek, J.; Barreto, G.E.; Gąsiorowski, K.; Koutsouraki, E.; Ávila-Rodrigues, M.; Aliev, G. Inflammatory Mechanisms and Oxidative Stress as Key Factors Responsible for Progression of Neurodegeneration: Role of Brain Innate Immune System. CNS Neurol. Disord. Drug Targets 2016, 15, 329–336. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of Glial Cells with Neuronal Synapses, from Astrocytes to Microglia and Oligodendrocyte Lineage Cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking Neuroinflammatory Biomarkers in Alzheimer’s Disease: A Strategy for Individualized Therapeutic Approaches? J. Neuroinflamm. 2024, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress. 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in Human Central Nervous System Diseases: A Frontier for New Therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Multiple Roles for Astrocytes as Effectors of Cytokines and Inflammatory Mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef]
- Cohen, J.; Mathew, A.; Dourvetakis, K.D.; Sanchez-Guerrero, E.; Pangeni, R.P.; Gurusamy, N.; Aenlle, K.K.; Ravindran, G.; Twahir, A.; Isler, D.; et al. Recent Research Trends in Neuroinflammatory and Neurodegenerative Disorders. Cells 2024, 13, 511. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of Neuroinflammation in Neurodegenerative Diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Xing, Z.; Peng, C.; Li, D. Autophagy in Neuroinflammation: A Focus on Epigenetic Regulation. Aging Dis. 2024, 15, 739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Pan, J.; Cao, J.; Sun, X.; Li, X.; Zhang, L.; Qin, C. Degradation of NLRP3 by P62-dependent-autophagy Improves Cognitive Function in Alzheimer’s Disease by Maintaining the Phagocytic Function of Microglia. CNS Neurosci. Ther. 2023, 29, 2826–2842. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, V.A.; Mahmood, T.; Ahsan, F.; Wasim, R. Neurodegeneration: Microglia: Nf-Kappab Signaling Pathways. Drug Res. 2022, 72, 496–499. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, P.; Wang, X. Berberine Exerts Neuroprotective Effects in Alzheimer’s Disease by Switching Microglia M1/M2 Polarization Through PI3K-AKT Signaling. Physiol. Res. 2025, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Balak, C.D.; Han, C.Z.; Glass, C.K. Deciphering Microglia Phenotypes in Health and Disease. Curr. Opin. Genet. Dev. 2024, 84, 102146. [Google Scholar] [CrossRef] [PubMed]
- Holtman, I.R.; Skola, D.; Glass, C.K. Transcriptional Control of Microglia Phenotypes in Health and Disease. J. Clin. Investig. 2017, 127, 3220–3229. [Google Scholar] [CrossRef]
- Yu, H.; Chang, Q.; Sun, T.; He, X.; Wen, L.; An, J.; Feng, J.; Zhao, Y. Metabolic Reprogramming and Polarization of Microglia in Parkinson’s Disease: Role of Inflammasome and Iron. Ageing Res. Rev. 2023, 90, 102032. [Google Scholar] [CrossRef]
- Li, J.; Shui, X.; Sun, R.; Wan, L.; Zhang, B.; Xiao, B.; Luo, Z. Microglial Phenotypic Transition: Signaling Pathways and Influencing Modulators Involved in Regulation in Central Nervous System Diseases. Front. Cell Neurosci. 2021, 15, 736310. [Google Scholar] [CrossRef]
- Li, J.; Wang, H. MiR-15b Reduces Amyloid-β Accumulation in SH-SY5Y Cell Line through Targetting NF-ΚB Signaling and BACE1. Biosci. Rep. 2018, 38, BSR20180051. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. NF-Kappa B as a Therapeutic Target in Neurodegenerative Diseases. Expert. Opin. Ther. Targets 2007, 11, 123–132. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Ruan, S.; Wang, J.; Guan, Q.; Zha, L. NADPH Oxidase 4 Regulate the Glycolytic Metabolic Reprogramming of Microglial Cells to Promote M1 Polarization. J. Biochem. Mol. Toxicol. 2023, 37, e23318. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Wu, T.; Zhao, Q.; Zhao, Q.; Cao, Y. LncGBP9/MiR-34a Axis Drives Macrophages toward a Phenotype Conducive for Spinal Cord Injury Repair via STAT1/STAT6 and SOCS3. J. Neuroinflamm. 2020, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xie, W.; Xiao, Q.; Beers, D.R.; Appel, S.H. Protective Effects of an Anti-Inflammatory Cytokine, Interleukin-4, on Motoneuron Toxicity Induced by Activated Microglia. J. Neurochem. 2006, 99, 1176–1187. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Li, B.; Chang, X.; Liang, X.; Liu, T.; Shen, Y.; Zhang, Q.; Yang, X.; Lyu, Y.; Liu, L.; Guo, J.; et al. The Role of Reactive Astrocytes in Neurotoxicity Induced by Ultrafine Particulate Matter. Sci. Total Environ. 2023, 867, 161416. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Pang, Q.-M.; Zhang, Q.; Wu, X.-C.; Yang, R.-L.; Fu, S.-P.; Fan, Z.-H.; Liu, J.; Yu, L.-M.; Peng, J.-C.; Zhang, T. Mechanism of M2 Macrophages Modulating Astrocyte Polarization through the TGF-β/PI3K/Akt Pathway. Immunol. Lett. 2023, 259, 1–8. [Google Scholar] [CrossRef]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte Alterations in Neurodegenerative Pathologies and Their Modeling in Human Induced Pluripotent Stem Cell Platforms. Cell Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef]
- Lana, D.; Ugolini, F.; Nosi, D.; Wenk, G.L.; Giovannini, M.G. The Emerging Role of the Interplay Among Astrocytes, Microglia, and Neurons in the Hippocampus in Health and Disease. Front. Aging Neurosci. 2021, 13, 651973. [Google Scholar] [CrossRef]
- Qian, K.; Jiang, X.; Liu, Z.-Q.; Zhang, J.; Fu, P.; Su, Y.; Brazhe, N.A.; Liu, D.; Zhu, L.-Q. Revisiting the Critical Roles of Reactive Astrocytes in Neurodegeneration. Mol. Psychiatry 2023, 28, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, B.; Liu, W.; Tan, C.; Chen, H.; Wang, X. Emerging Role of Non-Coding RNAs in Neuroinflammation Mediated by Microglia and Astrocytes. J. Neuroinflamm. 2023, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Gill, K.; Kumar, P.; Kumar, A.; Kapoor, B.; Sharma, R.; Joshi, A.K. Comprehensive Mechanistic Insights into the Citrus Genetics, Breeding Challenges, Biotechnological Implications, and Omics-Based Interventions. Tree Genet. Genomes 2022, 18, 9. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, 12895. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-Refinery of Orange Peels Waste: A New Concept Based on Integrated Green and Solvent Free Extraction Processes Using Ultrasound and Microwave Techniques to Obtain Essential Oil, Polyphenols and Pectin. Ultrason. Sonochem 2015, 24, 72–79. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Marocco, A.; Bernardi, J.; Caaruso, P.; Licciardello, C. Molecular Characterization of Citrus Cultivars: Insight from Recent Sudies. In Citrus—Molecular Phylogeny, Antioxidant Properties and Medicinal Uses; Hayat, K., Ed.; Nova Science Pub Inc.: Abbottabad, Pakistan, 2014; pp. 13–30. ISBN 978-1-63117-985-3. [Google Scholar]
- Peng, Z.; Zhang, H.; Li, W.; Yuan, Z.; Xie, Z.; Zhang, H.; Cheng, Y.; Chen, J.; Xu, J. Comparative Profiling and Natural Variation of Polymethoxylated Flavones in Various Citrus Germplasms. Food Chem. 2021, 354, 129499. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Liang, Z.; Li, S.; Cai, J.; Zhu, Z.; Liu, G. HPLC–DAD–ESI–MS2 Analysis of Phytochemicals from Sichuan Red Orange Peel Using Ultrasound-Assisted Extraction. Food Biosci. 2018, 25, 15–20. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical Characteristics of Citrus Peel and Effect of Conventional and Nonconventional Processing on Phenolic Compounds: A Review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Elkomy, M.H.; Fahim, H.I.; Ashour, M.B.; Naguib, I.A.; Alghamdi, B.S.; Mahmoud, H.U.R.; Ahmed, N.A. Rutin and Quercetin Counter Doxorubicin-Induced Liver Toxicity in Wistar Rats via Their Modulatory Effects on Inflammation, Oxidative Stress, Apoptosis, and Nrf2. Oxid. Med. Cell Longev. 2022, 2022, 2710607. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Alvarez-Rivera, G.; Gallego, R.; Fagundes, M.B.; Valdés, A.; Mendiola, J.A.; Ibañez, E.; Cifuentes, A. Neuroprotective Potential of Terpenoid-Rich Extracts from Orange Juice by-Products Obtained by Pressurized Liquid Extraction. Food Chem. X 2022, 13, 100242. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice By-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and Ripening Impact on Phenolic Composition and Antioxidant Activity of Mandarin (Citrus reticulate Blanco) and Bitter Orange (Citrus aurantium L.) Seeds Extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Xu, G.H.; Chen, J.C.; Liu, D.H.; Zhang, Y.H.; Jiang, P.; Ye, X.Q. Minerals, Phenolic Compounds, and Antioxidant Capacity of Citrus Peel Extract by Hot Water. J. Food Sci. 2008, 73, C11–C18. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Wang, M.; Gafner, S.; Manthey, J.; Khan, I. Liquid Chromatography-Electrospray Ionization Mass Spectrometry Analysis of Limonoids and Flavonoids in Seeds of Grapefruits, Other Citrus Species, and Dietary Supplements. Planta Med. 2016, 82, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, A.; Zannini, M.; De Angeli, M.; D’Arca, D.; Minischetti, V.; Conte, A.; Tagliazucchi, D. Bioaccessibility of Flavones, Flavanones, and Flavonols from Vegetable Foods and Beverages. Biology 2024, 13, 1081. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial Effects of Naringenin in Liver Diseases: Molecular Mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Chee, W.S.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.-N.; Williamson, G. Bioavailability Is Improved by Enzymatic Modification of the Citrus Flavonoid Hesperidin in Humans: A Randomized, Double-Blind, Crossover Trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in Humans of the Flavanones Hesperidin and Narirutin after the Ingestion of Two Doses of Orange Juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the Citrus Flavanone Aglycones Hesperetin and Naringenin after Single Oral Administration in Human Subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef]
- Manners, G.D.; Jacob, R.A.; Breksa; Schoch, T.K.; Hasegawa, S. Bioavailability of Citrus Limonoids in Humans. J. Agric. Food Chem. 2003, 51, 4156–4161. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Yang, W.; Ma, J.; Liu, Z.; Lu, Y.; Hu, B.; Yu, H. Effect of Naringenin on Brain Insulin Signaling and Cognitive Functions in ICV-STZ Induced Dementia Model of Rats. Neurol. Sci. 2014, 35, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kuboyama, T.; Tohda, C. A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer’s Disease and Its Target Molecule. Front. Pharmacol. 2017, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wang, D.; Tian, Y.; Wang, M.; Liu, R.; Xia, Z.; Huang, Y. Nanoemulsion for Improving the Oral Bioavailability of Hesperetin: Formulation Optimization and Absorption Mechanism. J. Pharm. Sci. 2021, 110, 2555–2561. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, W.; Shi, L.; Yang, P.; Yang, L.; Zhao, M.; Luo, L. Narirutin Reduces Microglia-Mediated Neuroinflammation by Inhibiting the JAK2/STAT3 Pathway in MPP+/MPTP-Induced Parkinson’s Disease Models. Exp. Neurol. 2025, 389, 115232. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Cifuentes, A.; Valdés, A. Omics Approaches to Investigate the Neuroprotective Capacity of a Citrus Sinensis (Sweet Orange) Extract in a Caenorhabditis Elegans Alzheimer’s Model. Food Res. Int. 2023, 172, 113128. [Google Scholar] [CrossRef]
- Arcone, R.; D’Errico, A.; Nasso, R.; Rullo, R.; Poli, A.; Di Donato, P.; Masullo, M. Inhibition of Enzymes Involved in Neurodegenerative Disorders and Aβ1–40 Aggregation by Citrus Limon Peel Polyphenol Extract. Molecules 2023, 28, 6332. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bosco, F.; Guarnieri, L.; Nucera, S.; Ruga, S.; Oppedisano, F.; Tucci, L.; Muscoli, C.; Palma, E.; Giuffrè, A.M.; et al. Protective Role of an Extract Waste Product from Citrus Bergamia in an In Vitro Model of Neurodegeneration. Plants 2023, 12, 2126. [Google Scholar] [CrossRef] [PubMed]
- Adhikari-Devkota, A.; Kurauchi, Y.; Yamada, T.; Katsuki, H.; Watanabe, T.; Devkota, H.P. Anti-neuroinflammatory Activities of Extract and Polymethoxyflavonoids from Immature Fruit Peels of Citrus ‘Hebesu’. J. Food Biochem. 2019, 43, e12813. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, K.; Zhou, Y.; Chen, L.; Wang, F.; Yi, X.; Huang, Y.; Ge, Y.; Chen, X.; Liao, D.; et al. Rutin Hydrate Relieves Neuroinflammation in Zebrafish Models: Involvement of NF-ΚB Pathway as a Central Network. Fish Shellfish Immunol. 2023, 141, 109062. [Google Scholar] [CrossRef]
- Dai, X.; Jia, Y.; Cao, R.; Zhou, M. Naringin Prevents Cognitive Dysfunction in Aging Rats by Inhibiting Toll-Like Receptor 4 (TLR4)/NF-κ B Pathway and Endoplasmic Reticulum Stress. Evid.-Based Complement. Altern. Med. 2023, 2023, 2919811. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.; Kim, M. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-ΚB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Scordino, M.; Urone, G.; Frinchi, M.; Valenza, C.; Bonura, A.; Cipollina, C.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M.; Mudò, G.; et al. Anti-Apoptotic and Anti-Inflammatory Properties of Grapefruit IntegroPectin on Human Microglial HMC3 Cell Line. Cells 2024, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.-D.; Lee, J.S. Hesperetin Inhibits Neuroinflammation on Microglia by Suppressing Inflammatory Cytokines and MAPK Pathways. Arch. Pharm. Res. 2019, 42, 695–703. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, D.; Yan, B. Eriocitrin Alleviates Oxidative Stress and Inflammatory Response in Cerebral Ischemia Reperfusion Rats by Regulating Phosphorylation Levels of Nrf2/NQO-1/HO-1/NF-ΚB P65 Proteins. Ann. Transl. Med. 2020, 8, 757. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Zhang, L.; Zhuang, Y.; Wang, Y. Efficacy and Molecular Mechanisms of Hesperidin in Mitigating Alzheimer’s Disease: A Systematic Review. Eur. J. Med. Chem. 2025, 283, 117144. [Google Scholar] [CrossRef]
- Ahsan, A.U.; Sharma, V.L.; Wani, A.; Chopra, M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Mol. Neurobiol. 2020, 57, 3589–3602. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Bayav, İ.; Genç, A.; Kandemir, Ö. Neuromodulatory Effects of Hesperidin against Sodium Fluoride-Induced Neurotoxicity in Rats: Involvement of Neuroinflammation, Endoplasmic Reticulum Stress, Apoptosis and Autophagy. Neurotoxicology 2022, 90, 197–204. [Google Scholar] [CrossRef]
- Huang, S.; Tsai, S.; Lin, J.; Wu, C.; Yen, G. Cytoprotective Effects of Hesperetin and Hesperidin against Amyloid β-induced Impairment of Glucose Transport through Downregulation of Neuronal Autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef]
- Khajepour, Z.; Reiszadeh Jahromi, S.; Dabiri, S.; Esmaeili-Mahani, S. Protective Effects of Naringenin against Methamphetamine-Induced Cell Death in Dopaminergic SH-SY5Y Cells. Am. J. Drug Alcohol. Abus. 2024, 50, 807–818. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A.; Shiehzadeh, S.; Hamedi, A. A Review of Citrus Plants as Functional Foods and Dietary Supplements for Human Health, with an Emphasis on Meta-Analyses, Clinical Trials, and Their Chemical Composition. Clin. Nutr. ESPEN 2023, 54, 311–336. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhu, Z.-J.; Ren, S.-P.; Deng, Y.-C.; Xu, J.-Y.; Zhang, S.-M.; Gao, J.-M.; Zhang, Q. Metabolomic Navigated Citrus Waste Repurposing to Restore Amino Acids Disorder in Neural Lesion. Food Chem. 2022, 387, 132933. [Google Scholar] [CrossRef] [PubMed]
- Fatima, J.; Siddique, Y.H. The Neuroprotective Role of Tangeritin. CNS Neurol. Disord. Drug Targets 2025, 24, 144–157. [Google Scholar] [CrossRef]
- Haghmorad, D.; Mahmoudi, M.B.; Salehipour, Z.; Jalayer, Z.; Momtazi Brojeni, A.A.; Rastin, M.; Kokhaei, P.; Mahmoudi, M. Hesperidin Ameliorates Immunological Outcome and Reduces Neuroinflammation in the Mouse Model of Multiple Sclerosis. J. Neuroimmunol. 2017, 302, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Vishwas, S.; Kumar, R.; Khursheed, R.; Ramanunny, A.K.; Kumar, R.; Awasthi, A.; Corrie, L.; Porwal, O.; Arshad, M.F.; Alshammari, M.K.; et al. Expanding Arsenal against Neurodegenerative Diseases Using Quercetin Based Nanoformulations: Breakthroughs and Bottlenecks. Curr. Neuropharmacol. 2023, 21, 1558–1574. [Google Scholar] [CrossRef]
- Wani, I.; Koppula, S.; Balda, A.; Thekkekkara, D.; Jamadagni, A.; Walse, P.; Manjula, S.N.; Kopalli, S.R. An Update on the Potential of Tangeretin in the Management of Neuroinflammation-Mediated Neurodegenerative Disorders. Life 2024, 14, 504. [Google Scholar] [CrossRef]
- Shi, L.-B.; Tang, P.-F.; Zhang, W.; Zhao, Y.-P.; Zhang, L.-C.; Zhang, H. Naringenin Inhibits Spinal Cord Injury-Induced Activation of Neutrophils through MiR-223. Gene 2016, 592, 128–133. [Google Scholar] [CrossRef]
- Wang, M.-H.; Yang, C.-C.; Tseng, H.-C.; Fang, C.-H.; Lin, Y.-W.; Soung, H.-S. Naringin Ameliorates Haloperidol-Induced Neurotoxicity and Orofacial Dyskinesia in a Rat Model of Human Tardive Dyskinesia. Neurotox. Res. 2021, 39, 774–786. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Ohizumi, Y. Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders. Nutrients 2021, 13, 145. [Google Scholar] [CrossRef]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4+ T Cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.; Li, S. Citrus Flavonoids and the Intestinal Barrier: Interactions and Effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.; Oh, J. Taming Neuroinflammation in Alzheimer’s Disease: The Protective Role of Phytochemicals through the Gut−brain Axis. Biomed. Pharmacother. 2024, 178, 117277. [Google Scholar] [CrossRef]
- Varesi, A.; Campagnoli, L.I.M.; Carrara, A.; Pola, I.; Floris, E.; Ricevuti, G.; Chirumbolo, S.; Pascale, A. Non-Enzymatic Antioxidants against Alzheimer’s Disease: Prevention, Diagnosis and Therapy. Antioxidants 2023, 12, 180. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Cui, Y.; Ma, X. Dietary Supplementation with Citrus Extract Altered the Intestinal Microbiota and Microbial Metabolite Profiles and Enhanced the Mucosal Immune Homeostasis in Yellow-Feathered Broilers. Front. Microbiol. 2019, 10, 2662. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, N.; Chen, Q.; Dong, L.; Li, Y.; Weng, P.; Wu, Z.; Pan, D.; Liu, L.; Farag, M.A.; et al. Research Advances in Citrus Polyphenols: Green Extraction Technologies, Gut Homeostasis Regulation, and Nano-Targeted Delivery System Application. Crit. Rev. Food Sci. Nutr. 2024, 64, 11493–11509. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, A.Y.; Kim, H.C.; Ryu, D.; Jo, S.A.; Jung, Y.-S. Effects of Natural Polyphenols on Oxidative Stress-Mediated Blood-Brain Barrier Dysfunction. Antioxidants 2022, 11, 197. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Rosales, T.K.O.; Fabi, J.P. Valorization of Polyphenolic Compounds from Food Industry By-Products for Application in Polysaccharide-Based Nanoparticles. Front. Nutr. 2023, 10, 1144677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]

| Compound | Citrus Source | Target Gene/Pathway | Biological Activity | Ref. |
|---|---|---|---|---|
| Hesperidin | C. sinensis C. reticulata C. grandis C. limon | ↑ HO-1, SOD, CAT, GSH ↓ iNOS, COX-2, PGE2, NO ↓ ICAM-1, VCAM-1, IL-1β, IL-6, IL-17 and TNF-α ↑ IL-10, TGF-B ⊣ PDEs ↑ Claudin-5 ↑ AMPK ↓ mTOR ↓ Caspase-3 ↓ APP, Aβ1–42 ↓ AChE activity | Antioxidant activity, ROS scavenging activity, anti-inflammatory activity, vascular permeability activity and BBB protection, anti-apoptotic activity, gut microbiota modulation, reduction in Aβ deposits, autophagy-promoting activity. | [4,7,8,51,52,54,63,64,76,81,91,102,105,117,118] |
| Hesperetin | C. sinensis C. reticulata C. grandis C. limon | ↑ Nrf2/ARE ↑ HO-1, SOD, CAT, GSH ↓ NF-κB ↓ TNF-α, VCAM-1, IL-17, IL-6 and IL-1β. ↑ IL-10, TGF-β ↓ iNOS, COX2 ↓ Caspase-3, Bax, ↑ Bcl-2 ↑ PI3K/Akt, ERK/MAPK ↑ Th2/Treg | Antioxidant activity, ROS scavenging activity, anti-inflammatory activity, anti-apoptotic activity, apoptosis reduction in PD models, reduction in CNS demyelination, immune cell modulation. | [54,76,90,91,93,95,102,105] |
| Naringin | C. sinensis C. limon C. paradisi C. reticulata C. aurantium C. grandis | ↑ SOD-1, CAT, GSH ↓ MDA ↓ LPO ↓ NF-κB, ↓ MAPK (p38) ↓ IL-6, TNF-α, IL-1β, MCP-1, VCAM-1, ICAM-1 ↓ iNOS, NO, ↓ Caspase-3, Bax ↓ GRP78 ↓ CHOP ↓ ATF6 ⊣ PDEs ⊣ TXA2 formation | Antioxidant activity, ROS scavenging activity, anti-inflammatory activity, anti-apoptotic activity, ER stress-reducing activity, vasoprotective activity, cognitive-enhancing activity, cytoprotective (H2O2 damage). | [7,8,51,52,53,54,59,63,76,86,89,102,109] |
| Naringenin | C. sinensis C. limon C. paradisi C. reticulata C. grandis | ↑ Nrf2/ARE ↑ SOD-1, CAT, GSH ↓ MDA ↓ iNOS, COX2 ↓ NF-κB, ↓ MAPK (JNK, p38), ↓ STAT-1, ↑ SOCS3 ⊣ TLR4/NF-κB, ↓ Iba-1, GFAP ↓ IL-1β, IL-17, IL-6, TNF-α, MCP-1, VCAM-1 ↓ BACE1 ↓ Caspase-3, Bax ↑ Bcl-2 ↑ AMPKα ↓ AChE/BChE ↓ GSK-3β activity ↑ ACh ↑ BDNF, NGF ↑ AMPK/ULK1 axis ↓ mTOR ↑ PI3K/Akt, MAPK/ERK ⊣ UV-induced damage | Antioxidant activity, ROS scavenging activity, anti-inflammatory activity, anti-amyloidogenic activity, anti-apoptotic activity, cognitive-enhancing activity, autophagy-promoting activity, cytoprotective activity (UV damage). | [7,52,54,76,77,78,81,85,102,111,118,122] |
| Nobiletin | C. reticulata C. sinensis C. tangerina | ↑ Nrf2/ARE ↑ SOD-1, CAT, GSH ↓ LOP, ↓ MDA, ↓ iNOS, COX-2 ↓ GSSG ↓ MMP-9 ↓ NF-κB, ↓ MAPK (JNK, p38), ↓ TNF-α, IL-1α, IL-17, IL-1β, IL-6, PGE2 ↓ proMMP-1/proMMP-3 ↑ PI3K/Akt, MAPK/ERK ↓ NO ⊣ BACE1 ↓ Caspase-3, Bax, ↑ Bcl-2 ↑ CREB-P ↑ mRNA NR2B, NR2A, NR1, ChAT, mAChR M1 | Antioxidant activity, ROS scavenging activity, anti-inflammatory activity, anti-angiogenic activities, enhancement in glutamatergic and cholinergic neurotransmission, anti-apoptotic activity, improvement in synaptic plasticity, anti-amyloidogenic activity, cognitive-enhancing activity. | [4,51,52,54,56,59,64,81,110,118,119] |
| Eriocitrin | C. limon C. reticulata C. bergamia C. aurantium | ↑ Nrf2 ↑ SOD, HO-1 ↑ NQO1 ↓ MDA ↓ NF-κB p65 ↓ TNF-α, IL-6 ↑ IL-10 ↓Caspase-3, caspase-9 ↑AMPK ↓ mTOR | Antioxidant activity, anti-inflammatory activity, anti-apoptotic activity, autophagy inducer. | [54,65,76,91,94] |
| Rutin | C. aurantium C. reticulata C. grandis C. sinensis | ↑ CAT ⊣ TLR9/NF-κB axis ↓ Alox4a, Alox5 ↓ RelA, ↓ NOS2a ↓ TNF-α, IL-6, IL-1β, CXCL8 ↓ MIF ↓ AChE activity ↓ prnpa, itgb2, ALP | Antioxidant activity, ROS scavenger activity, anti-inflammatory activity, reduction in immune cell infiltration, neuroprotective, and anti-neuroinflammatory activity. | [53,63,85,88] |
| Narirutin | C. reticulata C. sinensis | ↑ SOD, CAT, GSH, and HO-1 ↓ TNFα, IL-6, and IL-1β ↓ NF-kB ⊣ AChE ↑ IκBα ⊣ JAK2/STAT3 | Antioxidant, activity, oxidative stress reduction, anti-inflammatory activity, synaptic plasticity improvement, neuronal apoptosis reduction, memory and learning improvement, attenuates systemic and cerebral inflammation, cognitive decline prevention. | [8,54,73,83] |
| Tangeretin | C. reticulata C. sinensis | ↓ TNFα, IL-6, Il-2, and IL-1β ↑ SOD-1, CAT, HO-1 ↓ iNOS, COX-2 ↑ Nrf2/HO-1 ↓ NF-kB ↑ PI3K/Akt ⊣ AChE ↑ IκBα | Anti-inflammatory activity, oxidative stress reduction, neural cell death reduction, neurogenesis increase, cognition and memory improvement, neurodegeneration reduction, mitigated neurological abnormalities and acute brain injury mitigation, synaptic impairment reduction, Aβ aggregation inhibition. | [4,51,52,56,59,64,81,104,107,110] |
| Quercetin | C. reticulata C. sinensis | ↑ Nrf2/ARE ⊣ AChE and BChE ↑ SOD-1, CAT, GSH, and GPx1 ↓ TNFα, IL-6, and IL-1β ↓ NF-kB ↓ BACE1 ⊣ Iba-1, and GFAP ↑ ATP synthesis | Antioxidant activity, oxidative stress reduction, ROS/RNS scavenger activity, anti-inflammatory activity, neuron protection, anti-amyloidogenic properties, Aβ aggregation inhibition, mood, motor, memory deficits, and learning function improvement, tau phosphorylation reduction, inhibition of platelet aggregation, cognitive enhancement, mitochondrial dysfunction modulation. | [54,59,60,63,85,106,118] |
| Limonene | C. limon C. sinensis C. aurantium | ↓ TNF-α, IL-6, and IL-1β ⊣ AChE and BChE ⊣ LOX | Antioxidant activity, ROS/RNS scavenger activity, anti-inflammatory capacity, oxidative stress protection, reduction in Aβ deposits. | [52,61,62,81] |
| Limonin | Citrus spp. | ↑ TPH | Neuroprotective effects, anti-apoptotic activity, amino acid content upregulation. | [103] |
| Phenolic acids (e.g., gallic acid, ferulic acid, and caffeic acid) | Citrus spp. | ⊣ BACE1 activity ↓ TNFα, and IL-1β ↑ SOD-1, CAT, and GPx1 ↓ GFAP ↑ Nrf2/HO-1 | Antioxidant activity, ROS scavenger activity, anti-apoptotic activity, microglial inhibition activation, reduction in Aβ deposits, improvement in spatial cognitive and memory functions, neuroinflammation attenuation, synaptic strength increasing. | [53,63,64,81,84,118] |
| Pectin | Citrus spp. | ↑ MAPK/ERK ↓ PI3K/Akt ↓ NF-kB | Antioxidant activity, ROS scavenger activity, neuroinflammatory response inhibition, microglial inhibition activation, anti-apoptotic activity. | [81,91,92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silla, A.; Punzo, A.; Caliceti, C.; Barbalace, M.C.; Hrelia, S.; Malaguti, M. The Role of Antioxidant Compounds from Citrus Waste in Modulating Neuroinflammation: A Sustainable Solution. Antioxidants 2025, 14, 581. https://doi.org/10.3390/antiox14050581
Silla A, Punzo A, Caliceti C, Barbalace MC, Hrelia S, Malaguti M. The Role of Antioxidant Compounds from Citrus Waste in Modulating Neuroinflammation: A Sustainable Solution. Antioxidants. 2025; 14(5):581. https://doi.org/10.3390/antiox14050581
Chicago/Turabian StyleSilla, Alessia, Angela Punzo, Cristiana Caliceti, Maria Cristina Barbalace, Silvana Hrelia, and Marco Malaguti. 2025. "The Role of Antioxidant Compounds from Citrus Waste in Modulating Neuroinflammation: A Sustainable Solution" Antioxidants 14, no. 5: 581. https://doi.org/10.3390/antiox14050581
APA StyleSilla, A., Punzo, A., Caliceti, C., Barbalace, M. C., Hrelia, S., & Malaguti, M. (2025). The Role of Antioxidant Compounds from Citrus Waste in Modulating Neuroinflammation: A Sustainable Solution. Antioxidants, 14(5), 581. https://doi.org/10.3390/antiox14050581









