Dual Upcycling of Olive Leaves for the Biocatalytic Synthesis of Antioxidant Cortisone Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of Upcycled Olive Leaves Medium
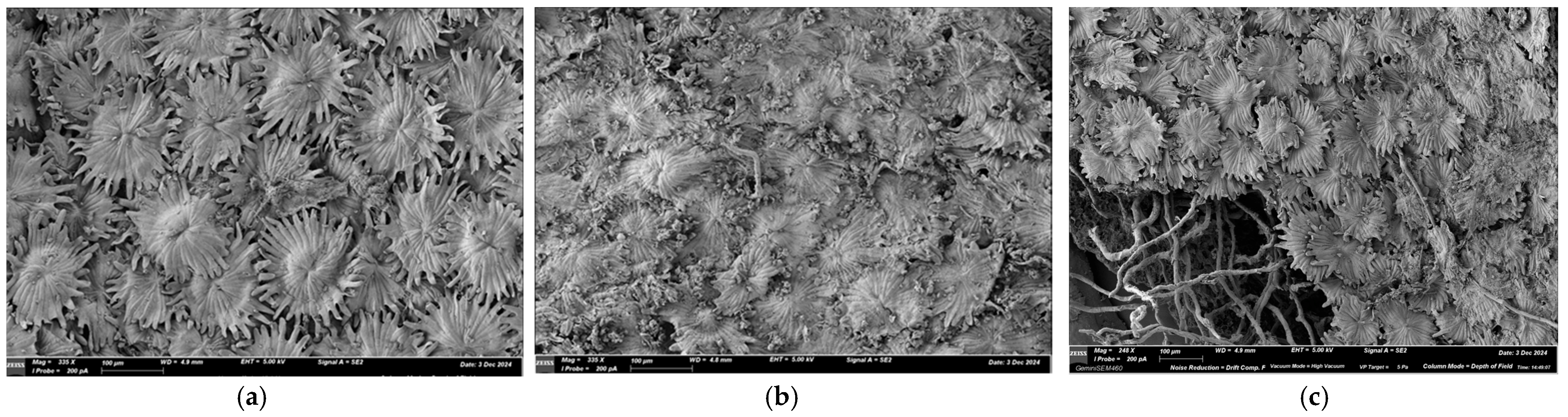
2.2. Determination of Olive Leaves Morphology and Physico-Chemical Properties of Olive Leaf Bioconversion Medium
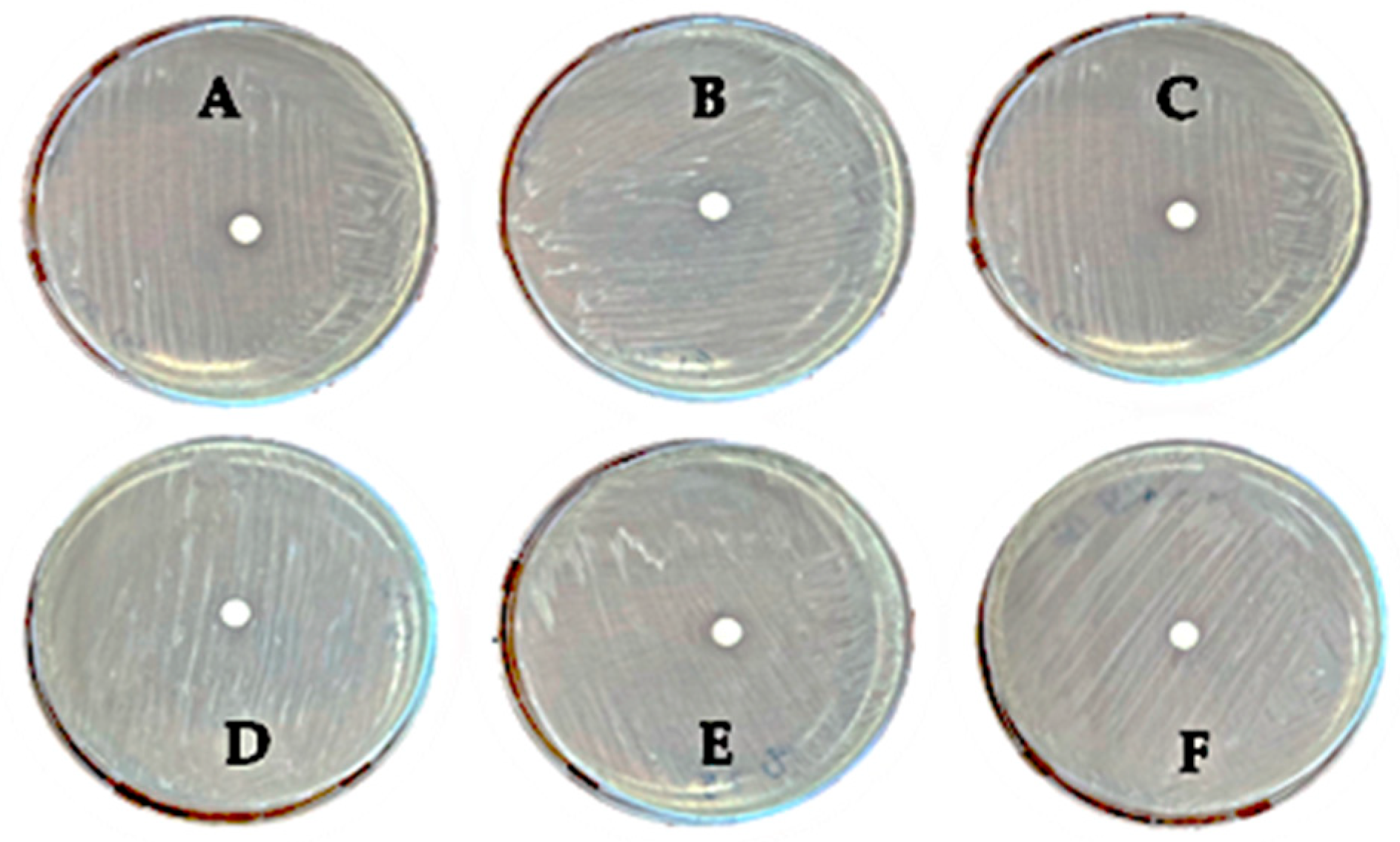
2.3. Susceptibility Test to Upcycled Olive Leaf Medium
2.4. Bioconversion of Cortisone
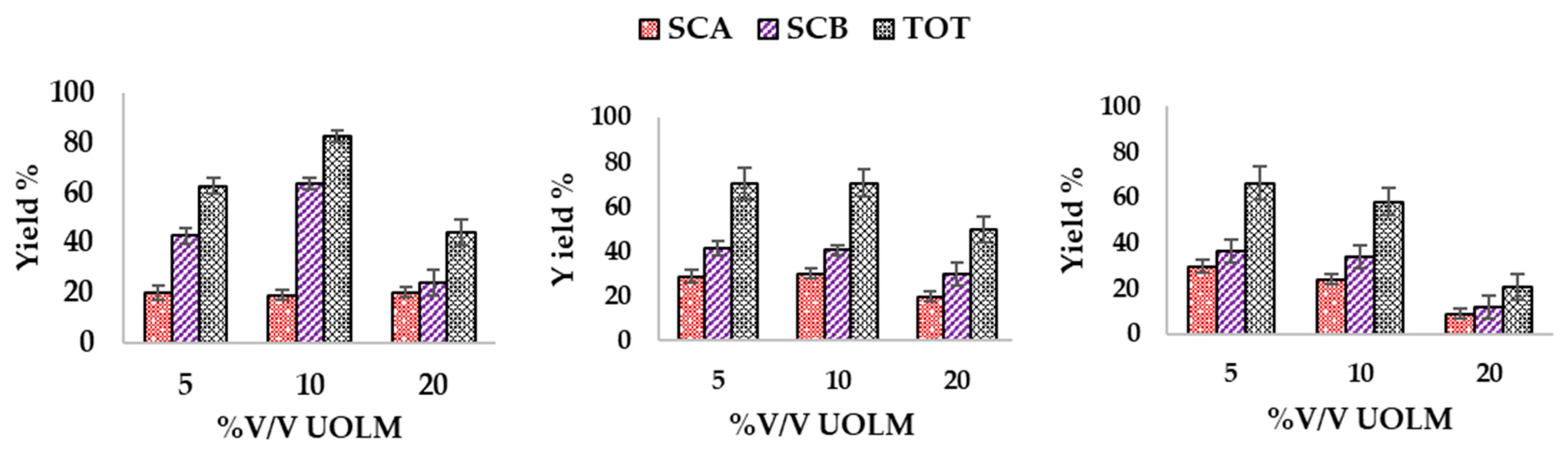
2.5. Effect of Upcycled Olive Leaf Medium and Cortisone Concentration on Biotransformation of Cortisone
2.6. Effect of Initial pH on Biotransformation Performance
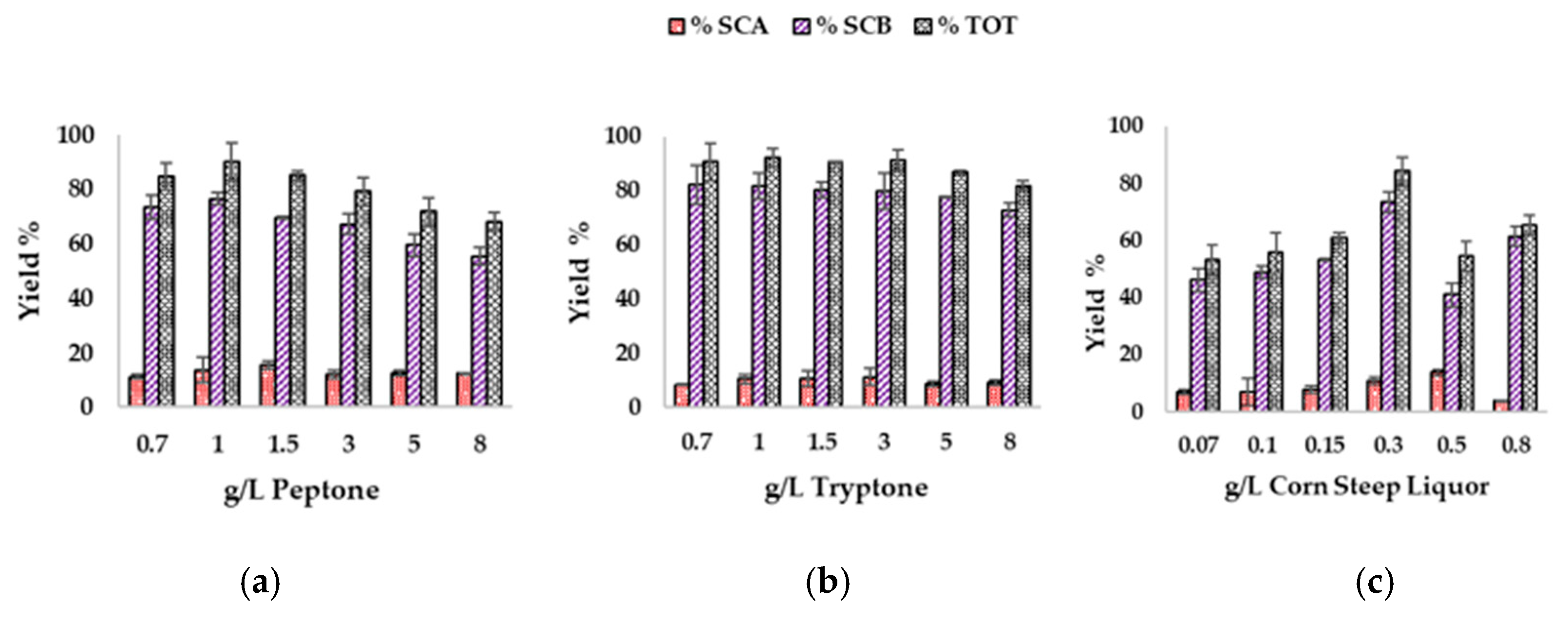
2.7. Effect of Supplement Addition on Biotransformation Performance
2.8. Scale-Up, Isolation and Purification of Biosteroids
2.9. Analytical Determinations
3. Results and Discussion
3.1. Determination of Olive Leaves Morphology and Physico-Chemical Properties of Olive Leaf Bioconversion Medium
3.2. Disk Diffusion Test
3.3. Effect of Upcycled Olive Leaf Medium and Cortisone Concentration on Biotransformation of Cortisone
3.4. Effect of Initial pH of Olive Leaf Upcycled Medium on Biotransformation of Cortisone
3.5. Effect of Supplementation of Olive Leaves Upcycled Medium on Biotransfomation Performances
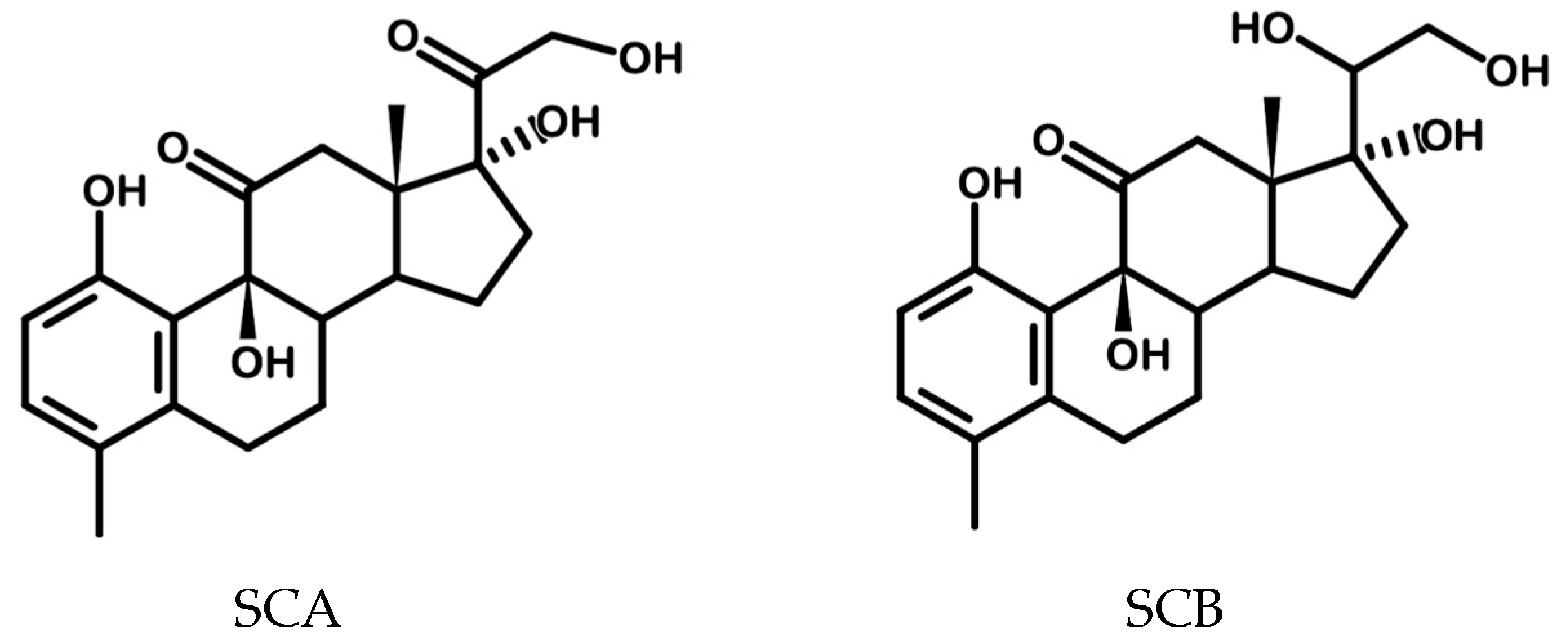
3.6. Scale-Up of Cortisone Bioconversion, Isolation, Purification and Characterization of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived Biomass as a Source of Energy and Chemicals. Biofuels Bioprod. Biorefining 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Masucci, F.; Serrapica, F.; De Luca, L.; Romano, R.; Garofalo, F.; Di Francia, A. Circular Economy on a Small Scale: The Sustainable Use of Olive Tree Biomass Residues as Feed for Lactating Cows in the Sorrento Peninsula. Sustainability 2025, 17, 845. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular Chemistry to Enable a Circular Economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Tunç, Y.; Yaman, M.; Keçe, Y.M.; Yilmaz, K.U.; Yildiz, E.; Güneş, A. Characterization of Olive (Olea europaea L.) Cultivars; Colour Properties, Biochemical Contents, Antioxidant Activity and Nutrient Contents. Genet. Resour. Crop Evol. 2025, 72, 529–541. [Google Scholar] [CrossRef]
- Aydınoğlu, T.; Sargın, S. Production of Laccase from Trametes versicolor by Solid-State Fermentation Using Olive Leaves as a Phenolic Substrate. Bioprocess Biosyst. Eng. 2013, 36, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.A.; Sharma, M.; Sharma, M.; Sharma, G.D.; Passari, A.K.; Bhasin, S. Valorization of Agro-Industrial Residues for Production of Commercial Biorefinery Products. Fuel 2022, 322, 124284. [Google Scholar] [CrossRef]
- Romero-García, J.; Solarte-Toro, J.; Galán-Martín, A.; Ruiz, E.; Castro, E.; Ortiz-Sánchez, M.; Alzate, C.C. Olive Leaves Upgrading Applying a Novel Two-Stage Organosolv Pretreatment: Techno-Economic and Environmental Assessment. Biochem. Eng. J. 2024, 207, 109317. [Google Scholar] [CrossRef]
- Gugel, I.; Marchetti, F.; Costa, S.; Gugel, I.; Baldini, E.; Vertuani, S.; Manfredini, S. 2G-Lactic Acid from Olive Oil Supply Chain Waste: Olive Leaves Upcycling via Lactobacillus casei Fermentation. Appl. Microbiol. Biotechnol. 2024, 108, 379. [Google Scholar] [CrossRef]
- Olivera, E.R.; Luengo, J.M. Steroids as Environmental Compounds Recalcitrant to Degradation: Genetic Mechanisms of Bacterial Biodegradation Pathways. Genes 2019, 10, 512. [Google Scholar] [CrossRef]
- Costa, S.; Tedeschi, P.; Ferraro, L.; Beggiato, S.; Grandini, A.; Manfredini, S.; Buzzi, R.; Sacchetti, G.; Valacchi, G. Biological Activity of New Bioactive Steroids Deriving from Biotransformation of Cortisone. Microb. Cell Factories 2022, 21, 250. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, T.H.; Dogru, M.; Tsubota, K. Tearful Relations: Oxidative Stress, Inflammation and Eye Diseases. Arq. Bras. Oftalmol. 2008, 71, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Di Marco, B.; Marchetti, F.; Costa, S.; Baldini, E.; Baldisserotto, A.; Gugel, I.; Vertuani, S.; Strettoi, E.; Manfredini, S. Dual-Action Steroid Derivatives with Anti-Inflammatory and Antioxidant Potency: An in Vitro Study. Biomed. Pharmacother. 2025, 186, 117940. [Google Scholar] [CrossRef]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the Production of Biobased Chemicals in a Circular Economy: A Perspective for the Period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- de Castro, A.; Brenes, M. Fermentation of Washing Waters of Spanish-Style Green Olive Processing. Process Biochem. 2001, 36, 797–802. [Google Scholar] [CrossRef]
- Nasrabadi, T.; Ruegner, H.; Sirdari, Z.Z.; Schwientek, M.; Grathwohl, P. Using Total Suspended Solids (TSS) and Turbidity as Proxies for Evaluation of Metal Transport in River Water. Appl. Geochem. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Barbari, R.; Tupini, C.; Buzzi, R.; Durini, E.; Lampronti, I.; Manfredini, S.; Baldini, E.; Vertuani, S. Multifunctional Profiling of Moringa oleifera Leaf Extracts for Topical Application: A Comparative Study of Different Collection Time. Antioxidants 2023, 12, 411. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Gugel, I.; Costa, S.; Baldisserotto, A.; Foletto, A.; Gugel, I.; Baldini, E.; Manfredini, S.; Vertuani, S. A Sustainable Approach to Valuable Polyphenol and Iridoid Antioxidants from Medicinal Plant By-Products. Antioxidants 2024, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Gugel, I.; Costa, S.; Baldini, E.; Bellonzi, G.; Vertuani, S.; Manfredini, S. Cloud Point Extraction Coupled HPLC for a Green Downstream Process and Quantification of 1, 9β, 17, 21-Tetrahydoxy-4-Methyl-19-nor-9β-Pregna-1, 3, 5 (10)-Trien-11, 20-Dione and 1, 9β, 17, 20β, 21-Pentahydoxy-4-Methyl-19-nor-9β-Pregna-1, 3, 5 (10)-Trien-11-One Obtained by Microbial Biotransformation of Cortisone. Sustain. Chem. Pharm. 2024, 37, 101345. [Google Scholar]
- Zappaterra, F.; Costa, S.; Summa, D.; Bertolasi, V.; Semeraro, B.; Pedrini, P.; Buzzi, R.; Vertuani, S. Biotransformation of Cortisone with Rhodococcus rhodnii: Synthesis of New Steroids. Molecules 2021, 26, 1352. [Google Scholar] [CrossRef]
- Fernández, V.; Almonte, L.; Bahamonde, H.A.; Galindo-Bernabeu, A.; Sáenz-Arce, G.; Colchero, J. Chemical and Structural Heterogeneity of Olive Leaves and Their Trichomes. Commun. Biol. 2024, 7, 352. [Google Scholar] [CrossRef]
- Magyari-Pavel, I.Z.; Moacă, E.-A.; Avram, Ș.; Diaconeasa, Z.; Haidu, D.; Ștefănuț, M.N.; Rostas, A.M.; Muntean, D.; Bora, L.; Badescu, B.; et al. Antioxidant Extracts from Greek and Spanish Olive Leaves: Antimicrobial, Anticancer and Antiangiogenic Effects. Antioxidants 2024, 13, 774. [Google Scholar] [CrossRef]
- Kamal El-Sawah, T.; Mohamed El-Shahawy, R.; Ibrahim Nageeb, A.; Mohamed Atalla, K. Antimicrobial Activity of Olive Leaves Extracts and Application of Leaves Powder in Meat Preservation. Fayoum J. Agric. Res. Dev. 2024, 38, 45–55. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; Cruz, P.A.; da Fonseca, M.M.R.; Xavier-Filho, L. Antibacterial Properties of the Extract of Abelmoschus esculentus. Biotechnol. Bioprocess Eng. 2011, 16, 971–977. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Venkateshwaran, K.; Vanitha, J.; Ganesh, M.; Vasudevan, M.; Sivakumar, T. Evaluation of Antibacterial Activity, Phenol and Flavonoid Contents of Thespesia populnea Flower Extracts. Pak. J. Pharm. Sci. 2009, 22, 282–286. [Google Scholar]
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature Review on Furfural Production from Lignocellulosic Biomass. Nat. Resour. 2016, 7, 115. [Google Scholar] [CrossRef]
- Mateo, S.; Mateo, P.; Barbanera, M.; Buratti, C.; Moya, A.J. Acid Hydrolysis of Olive Tree Leaves: Preliminary Study towards Biochemical Conversion. Processes 2020, 8, 886. [Google Scholar] [CrossRef]
- Maniyam, M.N.; Sjahrir, F.; Ibrahim, A.L. Bioremediation of Cyanide by Optimized Resting Cells of Rhodococcus Strains Isolated from Peninsular Malaysia. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 98. [Google Scholar] [CrossRef]
- Hughes, K.; Sulaiman, I. The Ecology of Rhodococcus equi and Physicochemical Influences on Growth. Vet. Microbiol. 1987, 14, 241–250. [Google Scholar] [CrossRef]
- Pátek, M.; Grulich, M.; Nešvera, J. Stress Response in Rhodococcus Strains. Biotechnol. Adv. 2021, 53, 107698. [Google Scholar] [CrossRef]
- Altaf, M.; Naveena, B.; Venkateshwar, M.; Kumar, E.V.; Reddy, G. Single Step Fermentation of Starch to L (+) Lactic Acid by Lactobacillus amylophilus GV6 in SSF Using Inexpensive Nitrogen Sources to Replace Peptone and Yeast Extract–Optimization by RSM. Process Biochem. 2006, 41, 465–472. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Musonge, P. Valorization of Corn Steep Liquor for Improved Value-Added Products: A Review. Recent Innov. Chem. Eng. (Former. Recent Pat. Chem. Eng.) 2024, 17, 26–43. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Kurbanoglu, N.I. Utilization as Peptone for Glycerol Production of Ram Horn Waste with a New Process. Energy Convers. Manag. 2004, 45, 225–234. [Google Scholar] [CrossRef]
- Man, R.; Illias, R.; Shaarani, S.; Arshad, Z.; Mudalip, S.; Sulaiman, S.; Fuzi, S.M.; Abdullah, A. Effect of Tryptone Concentration on Cyclodextrin Glucanotranferase (CGTase) Excretion and Cell Lysis of Immobilized Recombinant Escherichia coli; IOP Publishing: Bristol, UK, 2020; Volume 991, p. 012053. [Google Scholar]
- Yang, T.-W.; Rao, Z.-M.; Zhang, X.; Xu, M.-J.; Xu, Z.-H.; Yang, S.-T. Effects of Corn Steep Liquor on Production of 2, 3-Butanediol and Acetoin by Bacillus subtilis. Process Biochem. 2013, 48, 1610–1617. [Google Scholar] [CrossRef]
- Wahjudi, S.M.W.; Petrzik, T.; Oudenne, F.; Lera Calvo, C.; Büchs, J. Unraveling the Potential and Constraints Associated with Corn Steep Liquor as a Nutrient Source for Industrial Fermentations. Biotechnol. Prog. 2023, 39, e3386. [Google Scholar] [CrossRef]

| Parameter | Content | SD | Units |
|---|---|---|---|
| pH | 4.02 | 0.01 | - |
| Dry weight | 1.27 | 0.03 | mg/mL |
| Total suspended solids (TSS) | 6.79 | 0.21 | mg/mL |
| Color(A440-A700) | 1.21 | 0.00 | - |
| Ferric reducing antioxidant power (FRAP) | 3.53 | 0.04 | μmol T/g |
| Total polyphenols (TPC) | 2305.96 | 108.46 | μg GAE/mL |
| Sugars: | |||
| Cellobiose | 0.68 | 0.00 | mg/mL |
| Glucose | 6.00 | 0.00 | mg/mL |
| Arabinose | 0.65 | 0.00 | mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, F.; Gugel, I.; Costa, S.; Gugel, I.; Baldisserotto, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Dual Upcycling of Olive Leaves for the Biocatalytic Synthesis of Antioxidant Cortisone Derivatives. Antioxidants 2025, 14, 821. https://doi.org/10.3390/antiox14070821
Marchetti F, Gugel I, Costa S, Gugel I, Baldisserotto A, Baldini E, Manfredini S, Vertuani S. Dual Upcycling of Olive Leaves for the Biocatalytic Synthesis of Antioxidant Cortisone Derivatives. Antioxidants. 2025; 14(7):821. https://doi.org/10.3390/antiox14070821
Chicago/Turabian StyleMarchetti, Filippo, Irene Gugel, Stefania Costa, Ilenia Gugel, Anna Baldisserotto, Erika Baldini, Stefano Manfredini, and Silvia Vertuani. 2025. "Dual Upcycling of Olive Leaves for the Biocatalytic Synthesis of Antioxidant Cortisone Derivatives" Antioxidants 14, no. 7: 821. https://doi.org/10.3390/antiox14070821
APA StyleMarchetti, F., Gugel, I., Costa, S., Gugel, I., Baldisserotto, A., Baldini, E., Manfredini, S., & Vertuani, S. (2025). Dual Upcycling of Olive Leaves for the Biocatalytic Synthesis of Antioxidant Cortisone Derivatives. Antioxidants, 14(7), 821. https://doi.org/10.3390/antiox14070821










