Abstract
Neurodegenerative, neurodevelopmental, and psychiatric disorders, as well as epilepsy, affect millions of people. Due to their impact on patients’ quality of life, they represent a major health issue. Natural compounds are arising as new treatments for these diseases. Particularly, glucosinolates (GLS) are secondary metabolites found in Cruciferae family plants. Their basic structure consists of a glucose unit linked to a thiohydroximate-O-sulfonate group and an aliphatic, aralkyl, or indolyl side chain, depending on their precursor amino acid. Specifically, aliphatic GLS derive from methionine, aromatic ones from phenylalanine, and indolic ones from tryptophan. Myrosinase (thioglucoside glucohydrolase) is the crucial enzyme for GLS degradation, leading to the production of isothiocyanates (ITCs). ITCs attracted considerable scientific interest for their protective effects against various diseases, thanks to their antioxidant, anti-inflammatory, and neuroprotective properties. Here, we collected the latest evidence regarding ITC effects in neurodegenerative, neurodevelopmental, and psychiatric disorders, including preclinical and clinical studies published in the last decade. These studies evidenced ITCs’ neuroprotective effects, exerted mainly through antioxidant and anti-inflammatory mechanisms. Thus, ITCs’ integration, also through the diet, may represent a safe and efficacious strategy to improve health and limit the risk of neurological and psychiatric disorders. However, new large-scale trials are needed to determine their therapeutic potential, particularly for diseases with no clinical evidence.
1. Introduction
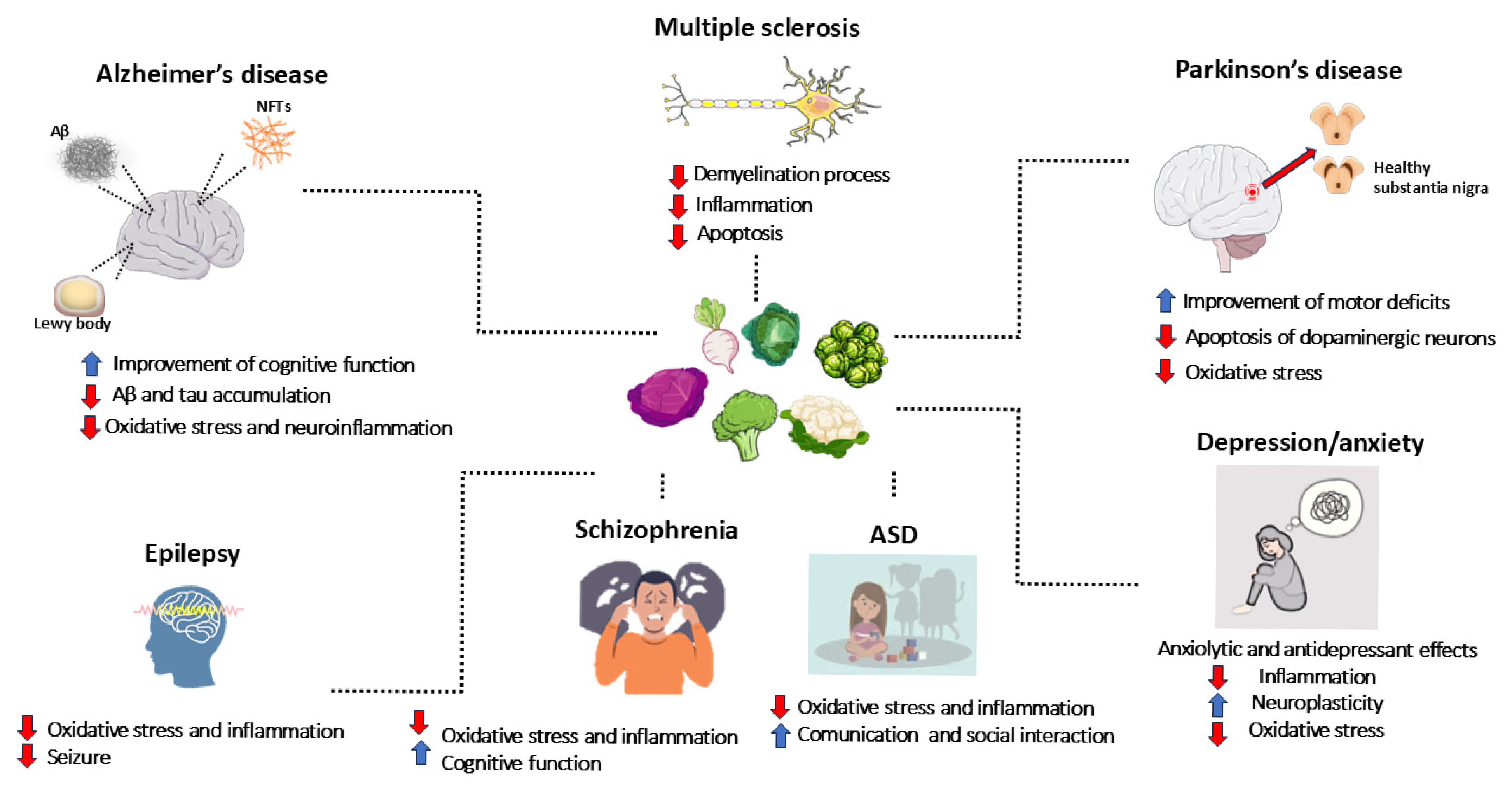
Neurodegenerative diseases, neurodevelopmental disorders, psychiatric disorders, and epilepsy represent a major global health issue, affecting millions of people and significantly impacting patients’ quality of life, while also generating substantial social and economic costs for families and healthcare systems. Neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and other neurodegenerative conditions, are characterized by the progressive loss of neurons in specific brain areas. This irreversible process leads to brain damage, resulting in severe disabilities and premature death [1]. Similarly, neurodevelopmental disorders, such as autism spectrum disorder (ASD) and schizophrenia, affect brain development and severely impair individuals’ ability to socially interact, communicate, and develop cognitive skills. Psychiatric disorders, such as depression and anxiety disorders, are among the most prevalent mental health conditions and constitute one of the leading causes of disability worldwide [2]. Mental disorders diagnosis and classification are based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria, which includes a description of symptoms and their duration [3]. Neurodegenerative diseases, neurodevelopmental disorders, and psychiatric disorders share some common features. Indeed, inflammation and oxidative stress play a key role in the pathogenesis of all these diseases [1,4]. Moreover, given the progressive aging of the global population, the increased life expectancy, and the growing incidence of these disorders, the number of individuals affected is expected to rise significantly, highlighting the urgent need to find effective therapeutic solutions [5]. In this context, the search for new therapeutic agents is increasingly crucial, as current treatments are not always able to effectively address the underlying biological mechanisms of these diseases.
Given the main role played by oxidative stress and inflammation, molecules capable of counteracting these mechanisms could be particularly promising in treating such pathological conditions. Among natural compounds, glucosinolate (GLS) derivatives, namely isothiocyanates (ITCs), are emerging as potential beneficial agents to counteract neurodegenerative diseases, neurodevelopmental disorders, psychiatric disorders, and epilepsy. GLS are secondary metabolites found abundantly in plants of the Cruciferae family (Brassicaceae), including broccoli, cauliflower, cabbage, and other cruciferous vegetables. GLS derivatives, obtained through hydrolysis, have attracted considerable scientific interest for their potential protective effects against a wide range of diseases, thanks to their significant antioxidant, anti-inflammatory, and neuroprotective properties [6].
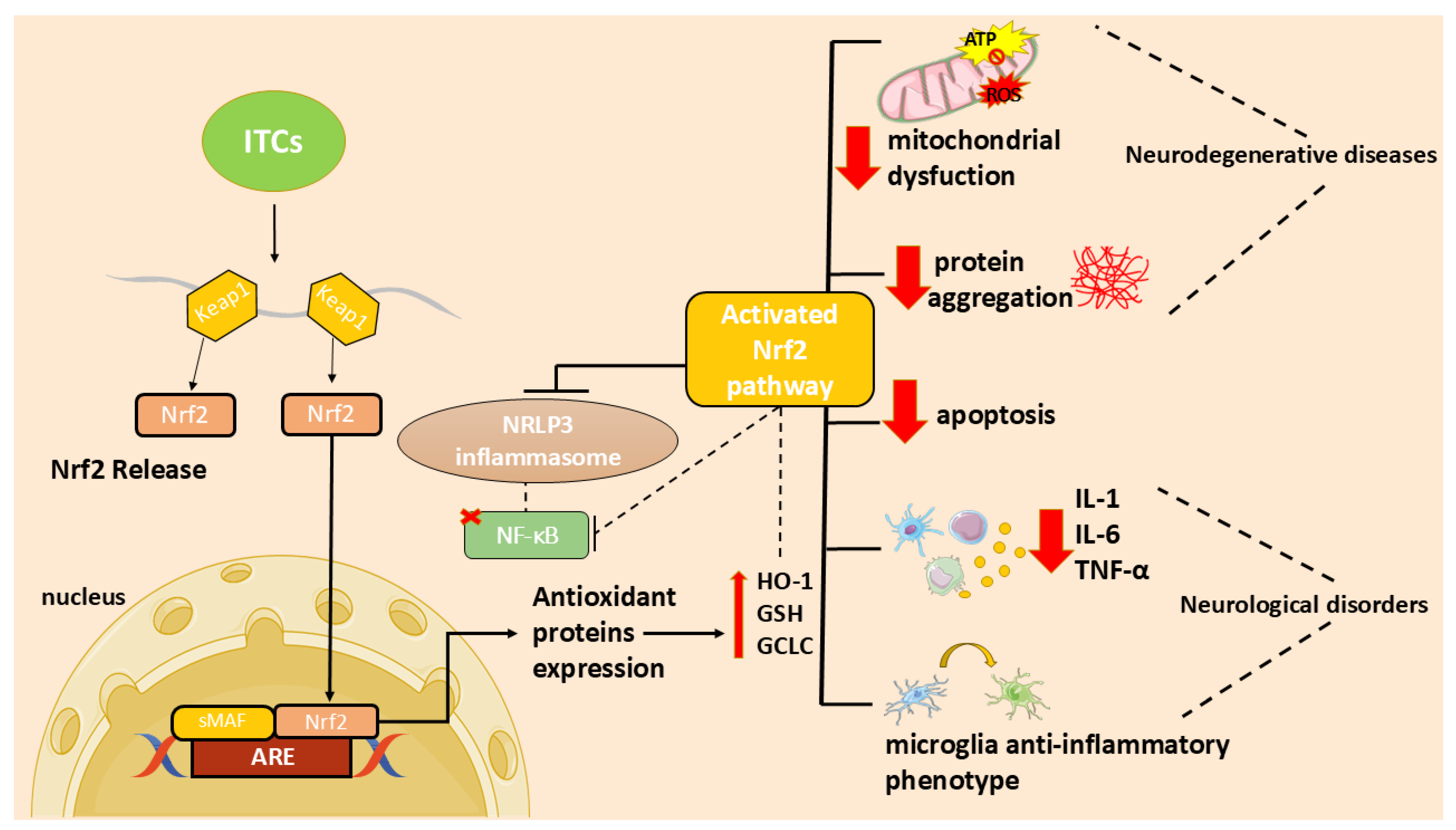
In particular, the ITC ability to activate the nuclear factor 2-related, erythroid-derived factor (Nrf2)/via antioxidant response element (ARE) and to inhibit the nuclear factor (NF)-κB activation [7] may contribute to their antioxidant and anti-inflammatory functions and, consequently, to their neuroprotective effect.
The ITC sulforaphane (SFN) demonstrated powerful neuroprotective effects by modulating oxidative stress and inflammation, as well as promoting mitochondrial function and synaptic protection [8]. Moreover, SFN exhibited neuroprotective effects against oxidative stress by inhibiting the activity of NADPH oxidase Nox4 in endothelial cells and astrocytes [9].
Thus, the benefits of these compounds suggest that they may represent a new strategy to treating neurodegenerative diseases, neurodevelopmental disorders, psychiatric disorders, and epilepsy, offering complementary or alternative therapies to traditional pharmacological options. In vitro and animal model studies play a crucial role in understanding the mechanisms underlying the beneficial effects of GLS derivatives, providing the basis for future clinical studies in humans. However, the number of randomized clinical trials in this field is still limited, highlighting the importance of leveraging preclinical evidence to guide clinical research. Therefore, this review aims to provide an in-depth update on the latest evidence regarding the effects of GLS derivatives in neurodegenerative diseases, neurodevelopmental disorders, and psychiatric disorders, including all preclinical and clinical studies published between 2014 and 2024.
2. Origin, Structure, and Biosynthesis of Glucosinolates and Their Derivatives
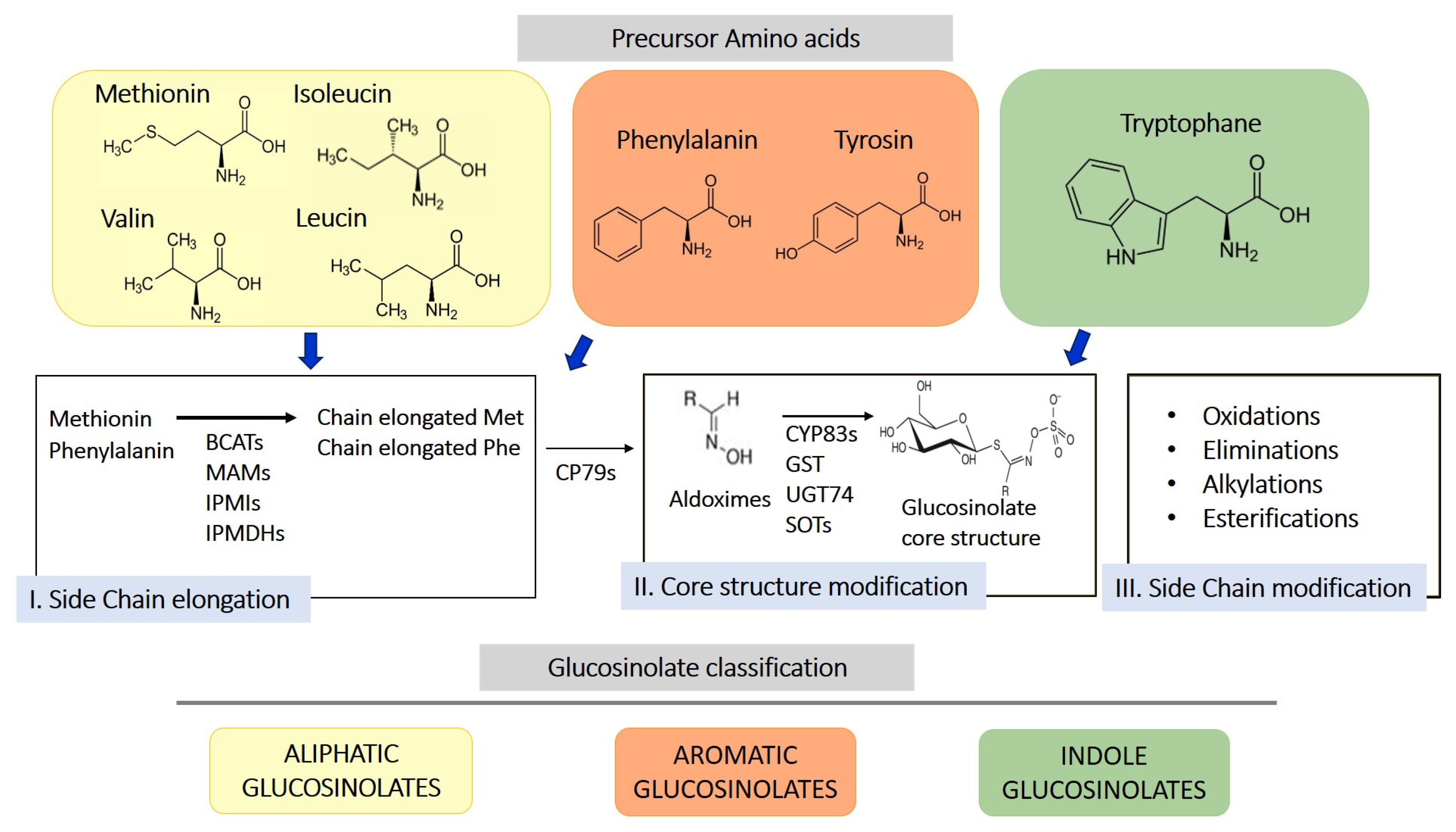
GLS are sulfur-containing secondary metabolites primarily found in plants of the Brassicaceae family, such as broccoli, mustard, cabbage, and cauliflower [10]. Their basic structure consists of a glucose unit linked to a thiohydroximate-O-sulfonate group and an aliphatic, aralkyl, or indolyl side chain (R) [11]. The side chain varies depending on the originating amino acid, classifying GLS into three main groups: aliphatic, aromatic, and indolic. Specifically, aliphatic GLS derive from methionine, aromatic ones from phenylalanine, and indolic ones from tryptophan. The structure and variability of these side chains are key determinants of the chemical and biological properties of the GLS derivates produced through their hydrolysis. More than 200 side groups have been identified [11]. The biosynthesis of GLS is a complex process involving multiple stages (Figure 1). GLS originate from precursor amino acids such as methionine, phenylalanine, tryptophan, and others, undergoing a series of enzymatic reactions, including N-hydroxylation, oxidative decarboxylation, and oxidation, leading to the formation of a sulfur-containing structure [10]. This biosynthetic process can be regulated by environmental factors and genetic variables, determining the concentration of GLS in plants. One of the key genes involved in GLS synthesis is CYP79, which determines which amino acid is used for GLS formation. However, other enzymes, including CYP83s, GSTF, GGP1, SUR1, UGT, SOTs, participate in their formation. The genetic architecture is complex, and the creation of new genes/enzymes plays a role in expanding GLS’s chemical diversity. Thanks to the study of new genomes and to the mechanistic assessment of GLS diversity in the Brassicales, how chemical novelty may arise is beginning to become clearer. Interestingly, small-scale duplications like tandem or distal events play a role in the formation of metabolic novelty. Also, gene loss is important in metabolic diversity across the entire genera [12].
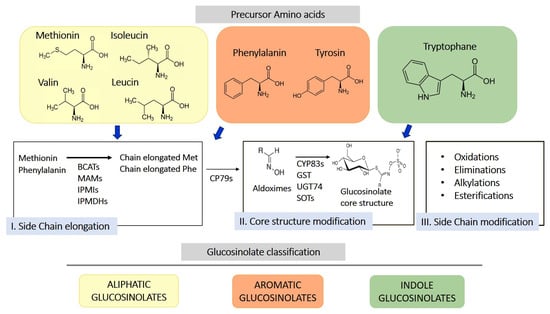
Figure 1.
Schematic representation of biosynthesis of glucosinolates from 3 different amino acid precursors. Depending on the amino acid precursor, glucosinolate can be divided in three different chemical classes: aliphatic, aromatic, and indole glucosinolates. The biosynthesis process is divided into three distinct steps, side chain elongation (for methionine and phenylalanine-derived glucosinolates), core structure formation, and secondary modification. BCAT, branched-chain amino acid aminotransferase; MAM, methylthioalkylmalate synthase; IPMI, isopropylmalate isomerase; IPMDHs, isopropylmalate dehydrogenases; CP79s, cytochromes 79; GST, glutathione-S-transferase; UGT74, glucosyltransferase 74; SOTs, sulfotransferases.
It is important to note that studies demonstrated a wide variability in GLS profile in both cultivated Brassica species and crop wild relatives (CWRs) [13,14] in regards to GLS types and content, with differences in total GLS concentrations of approximately 10-fold between different accessions.
These compounds play a defensive role in plants, protecting them from environmental stress, pathogens, and parasites [15]. GLSs are stable molecules within plant cells. However, when plant tissue is damaged (e.g., through chewing or crushing), GLSs come into contact with the myrosinase (MYR) enzyme, which catalyzes their hydrolysis. This enzyme is typically stored separately from GLS in plant species [16]. The hydrolysis process leads to the formation of a β-D-glucose molecule and an unstable aglycone (thiohydroximate-O-sulfonate). Spontaneous rearrangements of these intermediates generate sulfate ions and metabolites with structures that vary depending on the GLS side chain and the physicochemical conditions. Under acidic pH and a Fe2+-rich environment, nitrile formation is favored [17]. At neutral pH, ITC formation is preferred; these compounds are unstable and easily break down into thiocyanate ion and indole-3-carbinol [18]. If the side chain has a β-hydroxy function, ITCs spontaneously cyclize into oxazolidine-2-thione [10,19,20]. Although GLS are biochemically inactive, their hydrolysis by MYR transforms them into biologically active compounds, such as ITCs, which are known for their multiple health benefits. ITCs, deriving from GLS hydrolysis, are a class of molecules characterized by the R-N=C=S general formula, where R represents an alkyl or aryl group. Structural variations in GLS influence the formation of different types of ITCs through enzymatic hydrolysis. Specifically, glucoraphanin (GRA) gives rise to SFN, sinigrin (SIN) generates allyl isothiocyanate (AITC), gluconasturtiin (GST) produces phenethyl isothiocyanate (PEITC), glucoerucin (GER) converts into erucin (ER), glucotropaeolin (GTL) leads to benzyl isothiocyanate (BITC), and glucomoringin (GMG) is converted into moringin (MOR) [21]. The functional group responsible for the biological activity of ITCs is the central electrophilic carbon of the R-N=C=S group, often referred to as the reactive site, which is particularly susceptible to nucleophilic attack [22]. Many studies have highlighted the antioxidant potential of ITCs, particularly their ability to stimulate phase II enzymes, although research demonstrating their direct antioxidant activity remains limited [23]. Additionally, ITCs exhibit other beneficial properties, including anti-inflammatory, antimicrobial, neuroprotective, and cardioprotective activities [24]. These compounds are generally considered safe, with no significant adverse effects observed in humans [25,26].
3. Metabolism and Bioavailability of Isothiocyanates
In humans, ITC metabolism begins with their conjugation to glutathione (GSH) through the action of glutathione S-transferases (GSTs) [27]. Subsequently, the enzyme gamma-glutamyl transpeptidase (GTP) catalyzes the isothiocyanate-Cys-Gly production, which is then converted into Cys-isothiocyanate by the cysteinylglycinase (CG) enzyme. Finally, N-acetyl transferase (NAT) facilitates the N-acetylcysteine isothiocyanate (NAC-ITC) formation [28,29]. ITCs’ bioavailability is influenced by several factors, including food matrix composition, the type of source material (food or supplement), preparation method (raw or cooked), storage, and intestinal metabolism [8]. After ingestion, some studies showed that intact GLS can be partially absorbed in the stomach, while those not absorbed continue through the gastrointestinal tract to the small intestine, where they may be hydrolyzed by plant-derived MYR, and the resulting products are absorbed [8]. When MYR is inactivated, unmetabolized GLS reach the colon, where they are processed by the gut microbiota due to their hydrophilic nature (thioglucosidic and sulfate groups). Heat treatment of plant matrices tends to denature MYR, with an intensity depending on temperature and cooking time, regardless of the method used, such as boiling [30], steaming [31,32], or microwaving [33]. Despite the thermal destruction of MYR enzymatic activity, consuming cooked vegetables still results in the intake of GLS hydrolysis derivatives. Data showed that raw cruciferous consumption improved ITCs’ bioavailability. Boiling significantly reduced GLS levels, while steam cooking, microwaving, and stir-frying did not cause significant changes in their content. Storage reduced GLS content compared to fresh harvest broccoli [8]. Clinical studies showed higher bioavailability of ITCs compared to GLS, with significantly increased urinary excretion in subjects consuming ITCs directly versus those ingesting GLS, reflecting the necessity of enzymatic conversion before absorption [34,35]. Moreover, the presence of MYR in vegetable preparations directly affects ITCs’ bioavailability, with fresh broccoli producing three times higher levels than cooked broccoli [31]. MYR activity is therefore crucial for ITC absorption, as demonstrated by comparisons between fresh broccoli sprouts and GLS-rich broccoli powders lacking MYR, which showed lower conversion and delayed urinary excretion of ITCs [36]. These findings highlight the importance of preparation methods and dietary composition in the pharmacology of ITCs, emphasizing the need for further research to optimize their bioavailability and clinical applications [37].
4. Neuroinflammation and Oxidative Stress
Neuroinflammation is a complex mechanism that occurs in the central nervous system (CNS) as a result of brain damage that leads to the activation of resident cells, including astrocytes, impairing their neuroprotective role and leading to the release of inflammatory mediators [38]. Oxidative stress is a consequence of the unregulated release of reactive oxygen species (ROS) that, in turn, triggers oxidative deterioration of molecules leading to several disorders. Neuroinflammation and oxidative stress are major players in the onset and progression of neurodegenerative disorders and are deeply linked. Indeed, inflammatory cells produce ROS increasing oxidative stress that, in turn, increase pro-inflammatory mediators [1].
In recent years, researchers focused on the use of natural compounds that modulate these processes. In particular, ITCs, due to their ability to activate the Nrf2 pathway, may act as both antioxidants and modulators of inflammation [7]. In addition, ITCs reduce inflammation and apoptosis through several mechanisms [7]. These molecules can reduce neuroinflammation inhibiting NF-κB translocation, pro-inflammatory cytokines production, as well as oxidative species generation and apoptotic neuronal death [7].
Preclinical Studies on Glucosinolate or Isothiocyanates in Neuroinflammation and Oxidative Stress
The use of ITCs could be an important approach to contrast neuroinflammation through the modulation of different pathways. An in vitro study of lipopolysaccharide (LPS)-induced inflammation showed that SFN boosted the release of an-ti-inflammatory cytokines (interleukin (IL)-10, IL-4) and inhibited inflammatory me-diators, such as inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokines by attenuating the pJNK/JNK pathway [39]. Similarly, Qin and colleagues observed that SFN inhibited necroptosis by blocking the release of inflammatory mediators and preventing NF-κB activation in LPS-induced BV-2 microglia cells [40]. SFN exerted a protective action in LPS- and ATP-induced N9 microglial cells by inhibiting inflammasome activation and pyroptotic death [41]. SFN exerted a neuroprotective effect in a model of methylglyox-al (MGO)-induced neuroinflammation, a toxic byproduct of glucose degradation, in-hibiting pro-inflammatory cytokine production, blocking NF-κB translocation, and de-creasing ROS [42]. Additionally, SFN had a neuroprotective effect on both adult and aged mouse microglial cells, reducing the release of pro-inflammatory cytokines by ac-tivating Nrf2. These findings suggested that SFN was able to control inflammation and may help to reduce age-related cognitive decline [43]. SFN was also able to prevent the mitochondrial dysfunctions promoted by chlorpyrifos (CPF), an inhibitor of acetylcho-linesterase, or LPS in SH-SY5Y and microglia BV-2 cells, respectively, increasing heme oxygenase-1 (HO-1) enzyme [44]. Moreover, SFN stimulates the elongation of micro-glia cells, both in vitro and in vivo, counteracting LPS-induced abnormalities and promoting a shift from an amoeboid (pro-inflammatory) to a more branched (an-ti-inflammatory) form, reprogramming the microglia towards a less inflammatory state [45]. In another study, Wang and colleagues demonstrated that SFN reduced neuroinflammation both in vitro and in vivo. In vitro, using an LPS-induced BV-2 cell model, SFN inhibited the NF-κB pathway by upregulating Cezanne, a deubiquitinating enzyme. In vivo, SFN improved neurocognitive function by modulating the ubiquiti-nation of TRAF6 and RIP1 through Cezanne, leading to the inhibition of the NF-κB pathway [46]. Accordingly, the treatment with SFN improved learning and memory deficits in an LPS in vivo model through the modulation of the mTOR pathway, as well as increasing the expression of brain-derived neurotrophic factor (BDNF), a protein involved in the process of synaptic formation [47]. The neuroprotective effects of SFN were demonstrated in an in vitro and in vivo study using an Okadaic Acid (OKA)-induced model. OKA is a potent selective inhibitor of serine/threonine phos-phatase 1 (PP1) and 2 (PP2A), and in vivo studies showed that the administration of OKA causes mitochondrial dysfunction, oxidative stress, and cell death, in addition to neuroinflammatory processes that compromise memory. SFN reduced levels of pro-inflammatory mediators, such as NF-kB and tumor necrosis factor (TNF)-α, in-creased Nrf2 expression, normalized ROS and GSH levels, and improved memory def-icits in the OKA-induced model [48]. SFN was able to exert anti-inflammatory effects by reducing astrogliosis in a tauopathy mouse model [49].
A study examined the effects of SFN combined with NAC in vitro using co-cultures of neurons and BV2 cells, and in an in vivo model of traumatic brain injury (TBI). In vitro results showed a more pronounced neuroprotective and antioxidant ef-fect in cells treated with SFN alone, compared to the combination with NAC. Howev-er, in the in vivo model, the combination of SFN and NAC reduced the levels of in-flammatory biomarkers, with a modest improvement in deficits in rats with TBI [50].
A study showed that SFN reduced inflammation not only in the brain but also pe-ripherally. In LPS-treated mice, SFN decreased pro-inflammatory cytokine production in the hippocampus and liver, but did not improve the response to LPS-induced dis-ease [51]. Neuroinflammation can also affect the retina, leading to degeneration that is found in various pathologies, such as retinitis pigmentosa. An in vivo experiment showed that SFN exerted a protective effect on the retina, reducing inflammation, in-flammatory markers, and glial cell activation, thus preventing retinal degeneration associated with these processes [52]. In a hyperammonemia model linked to cognitive impairment in liver cirrhosis and minimal hepatic encephalopathy (MHE), SFN treat-ment shifted macrophages from M1 to M2, reduced hippocampal inflammation, and normalized GAT-3 expression. This decreased GABA levels, restored the glutamate–nitric oxide–cGMP pathway, and improved learning and motor coordination [53]. The activation of NLRP3-mediated inflammasome plays a key role in the neuroinflamma-tion process during acute ischemic stroke. In an in vivo study, treatment with SFN re-duced stroke volume by about 40%, significantly improving functional recovery com-pared to control animals treated with a vehicle, thanks to the ability of SFN to inhibit the activation of NLRP3 [54].
The consumption of SFN-enriched broccoli sprouts improved the neuroinflam-matory process, reducing the activation of NF-κB pathway, cytokine release, apoptosis, and improving memory deficits [55]. It was shown that a diet including 10% broccoli could be useful in reducing this aging-related neuroinflammation [56].
BITC is an ITC found in several cruciferous vegetables, known for its neuroprotec-tive properties. Treatment with BITC reduced the levels of IL-1β and inhibited the ac-tivation of the NLRP3 inflammasome in LPS-induced microglial cells. In addition, it also decreased the production of ROS and the activation of the transcription factor NF-κB [57]. An extract of Eruca sativa seed was also able to inhibit NLRP3 in motor neurons stimulated with LPS by counteracting apoptosis and pro-inflammatory cyto-kine production [58]. Jafaaru et al. showed that pre-treatment with glu-comoringin-isothiocyanate (GMG-ITC), an ITC derived from moringa oleifera, reduced ROS production by activating Nrf2, influencing the MAPK pathway, and lowering NF-κB levels in H2O2-treated cell line [59]. Another ITC that had a neuroprotective ac-tivity is AITC, an aliphatic ITC from Wasabia japonica (wasabi). AITC reduced the re-lease of TNF-α, IL-6, PGE2, and NO by inhibiting COX-2 and iNOS production in LPS-induced BV2 cells [60]. The study by Latronico and coworkers evaluated the ef-fects of three ITCs (AITC, PEITC, and SFN) in LPS-induced astrocytes, showing that all three compounds inhibited the release of metalloproteinase (MMP)-2 and MMP-9 [61]. Based on the beneficial properties of ITCs, the compound ITH12674 was synthesized combining melatonin and SFN. ITH12674 provided anti-inflammatory and antioxi-dant effects, activating Nrf2 and increasing GSH levels [62]. Secondarily, ITH12674 improved locomotion and social interactions in animals treated with LPS, showing benefits at both cellular and behavioral levels [63]. Based on the data provided, ITCs may be an effective approach to reducing neuroinflammation and oxidative stress. An overview of these studies is presented in Table 1.

Table 1.
Preclinical studies: results on the effects of glucosinolate or isothiocyanate treatments in neuroinflammation and oxidative stress models.
5. Neurodegenerative Disorders
5.1. Alzheimer’s Disease (AD)
AD is a common neurodegenerative disorder, affecting about 10% of individuals over 80 years old. Despite extensive research, its exact pathogenesis remains unclear [64]. However, it is known that neuronal loss is linked to the formation of senile plaques due to the accumulation of amyloid β (Aβ). Under normal conditions, Aβ has regulatory functions, aids in axonal growth, and influences synaptic plasticity. Another key feature of AD is the hyperphosphorylation of the tau protein, leading to neurofibrillary tangles that disrupt intraneuronal communication [65]. Studies show that the accumulation of Aβ and tau in AD is caused by factors like protein folding dysfunction in the endoplasmic reticulum, increased oxidative stress, and impaired protein elimination through the proteasome and autophagy [66,67]. Currently, there is no treatment able to cure AD or delay the disease progression. The discovery of new natural compounds with novel therapeutic effects is a fast-growing field of interest for the treatment of AD. The intake of foods or plant extracts with antioxidant properties could have beneficial effects on human health and improve brain functions [68].
Preclinical Studies on Glucosinolates or Isothiocyanates in AD
SFN is one of the main ITCs studied for the treatment of AD. In an in silico study, it was found that SFN was able to regulate 45 targets involved in different processes implicated in AD pathophysiology, such as inflammation, insulin resistance, and apoptosis [69]. A docking study on two different types of broccoli, Romanesco broccoli (RB) and purple broccoli (PB), revealed that extracts from both varieties exhibit antioxidant and anticholinergic properties [70]. One study revealed, using electrospray ionization mass spectrometry (ESI-MS), that the SFN reduced the aggregation of Aβ1–40 [71]. A computational study showed that SFN could be considered as a possible inhibitor of the β-site amyloid precursor protein-cleaving enzyme 1 (BACE1), which is involved in the production and deposition of Aβ. The inhibition of BACE1 could play an important role in AD prevention [72].
In the pathogenesis of AD, oxidative stress is a crucial event leading to neuronal damage; the modulation of Nrf2 expression could be one of the key strategies for treating AD [73]. In Villavicencio-Tejo and colleagues’ study, SFN increased the expression of antioxidant proteins such as catalase, NAD(P)H quinone oxidoreductase (NQO1), and Nrf2, as well as preventing mitochondrial dysfunctions in immortalized cortical neurons transfected with tau protein [74]. There are several molecular mechanisms regulating Nrf2 expression. SFN was able to act at the epigenetic level, increasing the expression of Nrf2 and then enhancing antioxidant and anti-inflammatory capacity in an in vitro model of AD (N2a/APP cells) [75]. Furthermore, SFN showed a neuroprotective effect in a model of nitrosative stress induced by sodium nitroprusside (SNP) [76]. Impairment of the autophagic process leads to the accumulation of Aβ aggregates. SFN was able to enhance the phagocytosis in microglial cells exposed to Aβ, helping to remove Aβ aggregates via the reduction in inflammation [77]. Moreover, SFN reduced Aβ and tau accumulation by increasing the levels of heat shock protein (HSP) 70 and the chaperone protein CHIP. This decreased plaque formation, inhibited apoptosis, and alleviated memory deficits [78,79]. SFN increased the expression of a specific water channel protein, aquaporin-4 (AQP4), via the p38 MAPK pathway [80]. SFN can also restore the levels of MerTK, a protein involved in the process of Aβ phagocytosis. Downregulation of MerTK contributes to chronic inflammation, a key process in AD. Restoring its function helps improve amyloid removal and reduce inflammation, thus counteracting the disease [81]. These results may suggest how SFN modulates the clearance of Aβ, tau, and other soluble proteins.
The activation of inflammasome is involved in the Aβ-induced inflammation process. One study showed that SFN inhibited NLRP3 in Aβ-activated microglial cells, reducing pro-inflammatory cytokine release, ROS production, and cytostatic autophagy [82]. In addition, SFN could reverse the M2 phenotype to an M1-like phenotype in activated BV-2 cells. This process reduced inflammation by downregulating the MAPK/NF-κB signaling pathway. Typically, M2 microglia are involved in anti-inflammatory and tissue repair functions, whereas M1 microglia are activated in response to stimuli like Aβ, leading to a pro-inflammatory response [83].
The in vitro results were also replicated in in vivo models. SFN exerted neuroprotection via the increase in BDNF expression and key proteins involved in neuronal plasticity and memory, such as TrkB and p-CREB [84]. In addition, SFN was able to inhibit apoptosis and oxidative stress by reducing MAPK activation (ERK1/2, JNK, and p38) and increasing GSH levels in a model induced by MGO, a glycation product that is present at high levels in the cerebrospinal fluid of AD patients [85]. SFN in different AD models ameliorated spatial cognitive impairment, reduced Aβ deposition [86], and regulated specific histone deacetylases (HDACs), resulting in diminished plaque accumulation [87]. In addition, SFN was able to reduce the deposition of Aβ and improve behavioral deficits in an AD model, where disease-like lesions were induced by the combined administration of D-galactose and aluminum [88]. SFN inhibited tau protein phosphorylation in an in vivo model of AD via the PI3K/Akt/GSK-3β pathway and suppressed anti-inflammatory markers such as NF-κB, NO, TNF-α, and IL-6 [89]. In addition, it inhibited cathepsin B and caspase-1 production, decreasing IL-1β release by blocking Aβ-induced inflammasome [90]. It also reduced oxidative stress, enhanced mitochondrial function in the brain, and improved cognitive impairments seen in both AD and type 2 Diabetes Mellitus, where oxidative stress plays a key role in disease progression and raises the risk of developing AD [91]. In addition, thanks to the activation of Nrf2, SFN mitigated cognitive decline and hippocampal AD-like lesions in diabetic mice [92]. SFN was also able to ameliorate cognitive vascular impartment (CVI) through the reduction in Aβ and p-tau accumulation [93]. Furthermore, SFN was able to inhibit apoptosis and Aβ-induced DNA damage. This is possible through its action on the PI3K-Akt signaling pathway and the BRCA1 gene, which plays an important role in DNA protection [94]. SFN also reduced the loss of cholinergic neurons [95], improved neurobehavioral disorders, and exerted anxiolytic effects [96].
The LPS model was used to investigate the link between neuroinflammation and AD. Studies showed that systemic LPS administration in vivo leads to behavioral changes such as learning and memory deficits, reduced appetite, decreased movement, and weight loss, which are clinically associated with neurodegenerative diseases in humans [97]. In addition, consecutive administration of LPS in mice could potentially increase the production and aggregation of Aβ in the cerebral cortex [98]. Moreover, Alzharani et al. found that SFN pre-treatment in an LPS model improved memory and reduced oxidative stress, Aβ accumulation, and auto cells activation. These effects were mediated through the AMPK signaling pathway, which resulted in decreased caspase-3 expression [99]. Several studies have been conducted on the effects of SFN on cognitive impairment associated with AD. Ho Sub et al. used an in vivo scopolamine (SCOP) model to assess the effect of SFN on memory deficits. SFN treatment improved memory performance, reversing SCOP-induced declines in short-term and long-term memory, and increased BDNF and CREB levels, enhancing hippocampal activity [100]. In addition, SCOP was used in an in vivo model of zebrafish. In this model, SFN was able to enhance the cognitive function in zebrafish inducted by SCOP through the attenuation of cholinergic neural loss [101]. Furthermore, SFN protected the cerebral vascular system during inflammation by activating Nrf2, blocking NF-κB, and downregulating E-cadherin and VCAM. This also helps reduce the recruitment of pathogenic leukocytes to the brain [102]. The antioxidant effect of broccoli ITC extract was tested in Caenorhabditis elegans. Hortal and colleagues showed in C. elegans that the broccoli extract (BRO) was able to reduce the concentration of ROS through induction of the transcriptional factor skn-1/Nrf2 and its downstream genes gst-4 and hsps. In addition, BRO reduced Aβ-induced paralysis [103]. Another ITC tested in in vitro and in vivo models is 6-methylsulfinyl hexyl isothiocyanate (6-MSITC). 6-MSITC improved memory deficits in Aβ1–42-induced mice by activating Nrf2, which increased disintegrin A and metalloproteinase 17 (ADAM17) levels, a key factor in synaptic function [104]. It also reduced cognitive impairment in an AD mouse model by suppressing oxidative stress, apoptosis, and neuroinflammation [105,106]. MOR, extracted from Moringa oleifera, is considered a potential treatment for AD. Moringa oleifera, also known as the “miracle tree,” is a member of the Moringaceae family, which includes 12 other species [107]. MOR reduced the expression of genes involved in different processes such as senescence, autophagy, and mitophagy, and mitigated the cytotoxicity induced by Aβ by decreasing caspase expression [108]. Additionally, pre-treatment with MOR was also able to inhibit the gene involved in mitophagy in stem cells of the human periodontal ligament. In neurodegenerative disease, stem cell-based therapeutic intervention offered an alternative approach meant to repair the damaged tissue [109]. ITCs are considered as H2S donors. Based on this property, a synthetic compound was developed and tested in vitro, obtained by combining the ITCs with memantine, also called Memit. The study showed the neuroprotective effects of Memit in LPS-treated cells, demonstrating a reduction in ROS levels and an inhibition of Aβ aggregation [110]. In addition, other H2S-releasing compounds have been synthesized by combining rivastigmine, an acetylcholinesterase (AChE) inhibitor with two ITCs, SFN and ER, an ITC from Eruca sativa Mill. The results showed that these new compounds had anti-inflammatory and antioxidant action in LPS-induced microglia cells. In addition, the compounds exerted neuroprotective effects against Aβ-induced toxicity in SH-SY5Y cells [111]. From the collection of in vitro and in vivo data, although further studies and clinical approaches are needed, the use of ITCs could represent a new approach for the treatment of AD. An overview of the described studies is presented in Table 2.

Table 2.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments in AD models.
5.2. Parkinson’s Disease (PD)
PD is a neurodegenerative disorder characterized by both motor symptoms—such as bradykinesia, rigidity, postural instability, tremor—and non-motor symptoms, including autonomic dysfunction, cognitive issues, pain, sleep, and mood disorders. One cause of neurodegeneration is the loss of dopaminergic neurons in the substantia nigra and other brain regions. The disease is also linked to the accumulation of Lewy bodies (LBs), cytoplasmic inclusions primarily composed by the protein α-synuclein (α-syn), which leads to neuronal death [64]. The most common treatment for PD is L-DOPA, a dopamine precursor that compensates for dopamine deficiency but does not arrest disease progression. Additionally, many patients do not respond to dopaminergic therapy, and L-DOPA may increase ROS, further contributing to neurodegeneration [112]. Phytochemicals present in plant-based foods, including ITCs, may provide an improvement in symptomology and disease progression [113].
5.2.1. Preclinical Studies on Glucosinolate or Isothiocyanates in PD
A study on red cabbage extracts showed neuroprotective effects in an in vitro model of α-Syn-induced PD [114]. One study identified SFN as a potent H2S donor among 15 compounds derived from cruciferous vegetables. Due to its ability to release H2S, SFN has been tested in an in vivo model of PD, showing improvements in motor skills, a reduction in dopaminergic neuron apoptosis, as well as anti-inflammatory and antioxidant effects [115]. In accordance with a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model, SFN was able to reduce the production of ROS through the modulation of the Nrf2 pathway [116,117]. Yaoyu Pu and colleagues also demonstrated that the dietary intake of GRA, the precursor of SFN, increased Nrf2 levels and antioxidant enzymes such as HO-1 and NQO1 in MPTP-induced mice. GRA also demonstrated a neuroprotective effect against MPTP-induced dopaminergic toxicity in the brain. These findings suggested that consuming cruciferous vegetables may help prevent PD [118]. Also, chronic oral rotenone administration induces key PD features, such as selective dopaminergic neurodegeneration, abnormal behaviors, and Lewy body formation. Zhou’s study showed that rotenone decreased the expression of Nrf2, HO-1, and NQO1 in the cortex and striatum, while SFN treatment increased these genes and reduced oxidative stress [119]. SFN also provided neuroprotection against rotenone-induced toxicity by modulating autophagy [119]. An in vitro and in vivo study showed that SFN and GRA through Nrf2 activation inhibited an age-dependent transcription factor CCAAT/enhancer-binding protein β (C/EBPβ). This factor is a key factor involved in the regulation of α-Syn expression in PD and aging brains and is also implicated in the neuroinflammatory process [120].
Treatment with cypermethrin, a synthetic pesticide, can damage mitochondrial function and cause the overexpression and aggregation of α-Syn, a typical PD feature. In this model, pretreatment with SFN, both in vitro and in vivo, reduced α-Syn aggregation, oxidative stress, and death of dopaminergic neurons [121].
Methyl-binding protein CpG2 (MeCP2) is a transcriptional repressor that blocks the transcription of BDNF and mutations in its gene cause Rett syndrome (RTT). RTT is classified into typical and atypical forms. RTT patients normally show normal development in the first 6 to 18 months of life, followed by a rapid regression with severe intellectual disability, motor issues, and autism-like symptoms. RTT symptoms include deceleration in head growth, loss of purposeful hand movements, abnormal gait, repetitive hand-wringing motions, loss of language function, breathing irregularities, and cognitive impairments. Some atypical cases of RTT present mutations in cyclin-dependent kinase-like 5 (CDKL5). The diagnostic criteria for atypical RTT include the presence of regression associated with 2 or more of the main criteria for classic RTT (loss of acquired purposeful hand skills, loss of acquired spoken language, gait abnormalities, stereotypic hand movements), a period of regression followed by recovery or stabilization, and 5 out of 11 supportive criteria (breathing difficulties, bruxism, impaired sleep pattern, abnormal muscle tone, peripheral vasomotor disturbances, scoliosis/kyphosis, delayed growth, small cold hands and feet, inappropriate laughter or screaming spells, decreased pain sensation, and intense eye communication) [122]. The preserved speech variant is a milder RTT form, in which patients show the same stages of this condition and make slow progress in manual and verbal abilities [123]. RTT patients with mutations in the MeCP2 show motor deficits in association with intellectual disability. Thus, it was supposed that MeCP2 may also be involved in PD pathogenesis. An in vitro and in vivo study showed that SFN was able to exert a neuroprotective effect by increasing the expression of BDNF and suppressing the expression of MeCP2. This result showed that MeCP2 could be a new therapeutic target [124].
Additionally, SFN was able to decrease apoptosis in in vitro and in vivo PD models by the modulation of the mTOR pathway [119]. A study compared the effects of ER and SFN. The biotransformation mechanisms of both compounds were similar [125]. Both ER and SFN enhanced Nrf2 and GSH expression, reduced ROS, and prevented apoptosis in a 6-hydroxydopamine (6-OHDA)-induced SH-SY5Y model. In addition, in vivo, both compounds enhanced motility, reduced damage to dopaminergic neurons, and increased levels of tyrosine hydroxylase (TH), a marker of dopaminergic function. They also reduced DNA fragmentation and neuronal death, confirming the beneficial effects observed in in vitro studies [126]. The neuroprotection ability of ER was also tested in the C. elegans model. The results showed that ER reduced α-Syn aggregation and restored motor deficits [127]. Besides ER, the neuroprotective effects of GMG were also evaluated in PD models. In a study by Giacoppo et al., the effects of GMG and its bioactivated form, MOR, were compared. The results revealed that MOR was more effective than GMG in reducing the production of pro-inflammatory cytokines, such as TNF-α and IL-1β, and in lowering the expression of the TLR4 receptor, which plays a role in the release of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. In vivo results showed that MOR had a stronger neuroprotective effect than GMG, reducing MPTP-induced toxicity at the dopaminergic level. Behavioral tests revealed improved motor coordination and reduced bradykinesia in mice treated with MOR compared to those treated with GMG. Additionally, MOR pre-treatment preserved neuronal cell integrity and enhanced synaptic communication. MPTP mice pre-treated with MOR showed reduced expression of cleaved caspase-9, p53, and STAT1 proteins, which are involved in apoptosis, compared to those treated with GMG. Both in vitro and in vivo data suggested that MOR may be a promising candidate for PD treatment due to its ability to modulate various molecular pathways [128].
Synthetic ITCs were evaluated in in vitro and in vivo models. ITC-3 reduced cytotoxicity induced by MPTP and BH4 by increasing Nrf2 levels and boosting oxidative stress-related proteins such as GCLC, GCLM, and HO-1. In vivo results showed that ITC-3 can suppress microglial activation, protect dopaminergic neurons from degeneration, and reduce motor deficits associated with PD [129]. Moreover, another synthetic ITC, ITC-57, produced similar results in vitro and in vivo [130]. Therefore, it is evident from the data that the application of synthetic ITCs could be a novel approach for PD treatment.
Oxidative stress plays a crucial role in the pathogenesis of PD. Among the main defense mechanisms, there is GST, a natural antioxidant that protects the brain from oxidative stress and neurodegeneration. In a model of zinc-induced neurodegeneration, it was observed that it inhibited the expression of GST-π, increasing oxidative stress and contributing to the degeneration of dopaminergic neurons. However, one study showed that pre-treatment with BITC can reverse the negative effects of zinc by increasing levels of GST-π, improving behavioral disorders and reducing both oxidative stress and apoptosis markers [131]. A study by Morroni et al. showed the neuroprotective effects of 6-MSITC. Morroni et al. demonstrated that in an in vivo PD model induced by 6-OHDA, preventive treatment with 6-MSITC reduced 6-OHDA-induced neurotoxicity in mice. Additionally, 6-MSITC decreased oxidative stress and apoptosis, while improving behavioral disorders, particularly motor deficits [132]. An overview of the described studies is presented in Table 3.

Table 3.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments in PD models.
5.2.2. Clinical Studies on Glucosinolates or Isothiocyanates in PD
A clinical trial was conducted on PD patients to evaluate the efficacy of broccoli seed tea (BST), enriched with SFN, in inducing Nrf2 gene activation. The case study involved 17 patients, who were given a special BST for four weeks, containing a daily dose of 25–40 μmol of SFN (once per week during the first week with a possibility to increase to twice per week from the second week). A new Redox Stress Test technique was used to analyze the effects of the treatment, identifying symptoms related to oxidative stress, a major cause of disorders in PD, and observing the impact of Nrf2 pathway activation on these symptoms. Although the results did not show precise statistical values (p-value), thus making statistical significance uncertain, it was found that the BST treatment improved non-motor symptoms such as fatigue, constipation, and urinary urgency, but without affecting motor symptoms. These preliminary results suggested that oxidative stress plays a crucial role in the onset of non-motor symptoms, both in the central nervous system and peripheral organs. A redox imbalance, in fact, is responsible for progressive neurological damage in the brain, which initially is not easily detectable. Specific neurological symptoms do not manifest themselves until many years later, when dopamine deficiency causes impairment of motor control, leading to the onset of a movement disorder typical of PD [133]. Actually, another randomized placebo-controlled phase 2 trial (NCT05084365) is currently underway to test the effects of SFN on 100 PD patients.
5.3. Multiple Sclerosis (MS)
MS is an immune-mediated inflammatory disorder of the CNS, affecting over 2 million people globally [134]. The disease onset is driven by the loss of blood–brain barrier (BBB) integrity, providing T lymphocytes to cross. Once activated, T cells initiate demyelination, reactive gliosis, and neuronal degeneration [134]. Neurodegeneration, oxidative stress, and neuroinflammation play a main role in the progression of MS. Indeed, ROS production, due to an impaired antioxidative system, triggers the release of pro-inflammatory cytokines, which worsen the pathology [135]. Oligodendrocytes appear to be particularly susceptible to oxidative stress and to cytokines, which can induce their death, inhibiting differentiation and altering demyelination processes [136]. New neuroprotective therapies are needed to reduce long-term neurological disability, and the use of natural compounds with antioxidant properties could represent a potential protective approach.
Preclinical Studies on Glucosinolates or Isothiocyanates in MS
An in vitro study on oligodendrocytes showed that SFN exerted a neuroprotective action reducing the production of ROS and promoting differentiation [137]. In accordance with the main role of inflammation in MS, recent studies showed that the macrophage migration inhibitory factor (MIF) represents a key factor in several autoimmune diseases, including MS, where it is found to be overregulated. One study showed that some synthetic ITCs, particularly those with an aromatic structure, were able to inhibit the activity of MIF. This mechanism could open up new therapeutic prospects for the treatment of inflammatory diseases [138]. Given the complexity of the disease, to date, the majority of the studies investigating the protective effects of phytocompounds in MS were carried out in in vivo models. One of the most commonly used in vivo models for studying MS is the experimental autoimmune encephalitis (EAE) model. It is characterized by the perivascular infiltration of inflammatory cells, leading to demyelination in the brain and spinal cord through an autoimmune cascade mediated by CD4+ T cells [139]. SFN is one of the phytocompound tested in MS in in vivo models. SFN can cross the BBB, a property that has paved the way for its potential use in the treatment of MS. Yoo and colleagues, in their study, showed that SFN treatment reduced iNOS levels, inhibited inflammatory cell infiltration, and prevented demyelination in the spinal cord of EAE mice [140]. In addition, bioactive GRA was observed to exert neuroprotective effects in an experimental in vivo model of EAE. The study showed that bioactive GRA was able to prevent cell death by reducing caspase-3 levels, counteracting the BBB dysfunction through the increase in the expression of tight junction proteins and modulating genes involved in inflammatory pathways [141]. Galea et al. evaluated the effects of synthetic SFN SFX-01 in a PLP 139–151-induced EAE model. The research assessed the use of SFX-01 for both therapeutic and preventive purposes. Histological analysis of the spinal cord showed improved demyelination, reduced apoptotic cells, and decreased symptom severity [142]. These results suggested that SFN could be used as an integrative therapy in the treatment of MS due to its anti-inflammatory properties. In addition to SFN, GMG-ITC was also tested in vivo for the treatment of MS. Galuppo and collaborators showed that GMG-ITC treatment reduced lymphocyte infiltration, demyelination, and axonal loss in MOG mice. It also modulated neuroinflammation by affecting the MAPK pathway, lowering TNF-α levels, and decreasing ROS production [143]. In addition, GMG-ITC exerted anti-inflammatory effects by normalizing the Wnt-beta catenin pathway and inhibiting GSK3B, which blocked the release of inflammation mediators like IL-1β, IL-6, and COX-2 [144]. Additionally, Giacoppo et al. showed that GMG-ITC provided neuroprotection through the modulation of apoptosis-related genes and increased Nrf2 expression, demonstrating antioxidant properties [144]. In another study, Giacoppo and coworkers demonstrated that the topical application of a MOR-based cream helped relieve neuropathic pain associated with MS. While the exact causes of neuropathic pain are not fully understood, it is believed to involve inflammatory processes and ion channel dysfunction. Treatment with MOR-based cream reduced pro-inflammatory cytokines (TNF-α, IFN-γ) and increased levels of the anti-inflammatory cytokine IL-10. Additionally, it provided neuroprotective effects by blocking ion channels [145]. The data collected suggested that the use of ITCs could represent a new approach to the treatment of the disease. An overview of the described studies is presented in Table 4.

Table 4.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments in MS models.
6. Neurodevelopmental Disorders, Psychiatric Conditions, and Epilepsy
Recent clinical and preclinical studies explored the use of GLS and their derivates as potential treatments for neurodevelopmental conditions, psychiatric disorders, and epilepsy. These studies represent the first attempts to address disorders of heterogeneous origin and pathophysiology by targeting a limited number of common pathways through the use of natural compounds such as ITCs and GLS. Preclinical evidence suggests that GLS and their metabolites, particularly SFN, possess various biological properties relevant to neurological and psychiatric conditions [146,147]. However, it remains unclear whether there are key mechanisms shared across multiple disorders or if these mechanisms are specific to each condition. Supported by mechanistic data, recent preclinical and clinical studies were developed to explore the use of SFN in treating autism, psychiatric conditions such as depression and schizophrenia, and epilepsy.
6.1. Autism Spectrum Disorder (ASD)
ASD is a neurodevelopmental disorder characterized by deficits in communication and social interaction, as well as repetitive behaviors or activities [148]. To date, the etiopathogenesis of ASD remains unknown. According to estimates from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network, ASD affects approximately 1 in 36 children and is four times more common in boys than in girls [149]. Currently, no pharmacological therapy has been approved to reduce the core symptoms or address the pathophysiological processes associated with ASD [150]. GLS and their derivates have gained increasing interest for their potential therapeutic effects in ASD, thanks to their ability to modulate key pathophysiological pathways, including oxidative stress, inflammation, and gut microbiota regulation.
6.1.1. Preclinical Studies on Glucosinolate or Isothiocyanates in ASD
Preclinical studies were conducted to elucidate the molecular mechanisms by which SFN alleviates autism-like symptoms. A key mechanism of SFN is the activation of Nrf2, a transcription factor regulating the expression of genes involved in antioxidant defense and inflammatory response. At the cellular level, SFN restored alterations in the thioredoxin 1/thioredoxin reductase 1 (Trx1/TrxR1) system in neutrophils of individuals with ASD, a crucial process for redox balance. Notably, exposure to methylmercury, an environmental contaminant associated with increased ASD risk, inhibited TrxR1 activity and increased ROS production in neutrophils of ASD subjects. SFN reversed these effects via Nrf2 activation [151]. Encapsulation of SFN in extracellular vesicles (EVs) from amniotic fluid (SFN@EVs) enhanced its stability and targeted delivery, improving its bioavailability. SFN@EVs protected hPC-12 cells from oxidative stress by activating the Nrf2 pathway, which in turn reduces the expression of IL-6, a key pro-inflammatory cytokine linked to ASD [152]. Additionally, studies in chick embryos exposed to sodium valproate (an ASD model) confirmed the protective role of SFN@EVs, improving embryonic survival and preserving normal gray and white matter structure in the brain [152]. SFN also modulated immune responses. In peripheral blood mononuclear cells (PBMCs) from healthy donors, SFN treatment increased the expression of cytoprotective enzymes such as NQO1, HO-1, and AKR1C1 while suppressing LPS-induced inflammation by lowering IL-6, IL-1β, COX-2, and TNF-α levels. Similar effects were confirmed in PBMCs from ASD children treated with SFN for 14 days (NCT02561481) [153].
Monocytes from ASD subjects showed a reduced expression and activity of Nrf2, leading to increased NF-κB activation and overexpression of IL-6, IL-1β, iNOS, and nitrotyrosine, markers of inflammation and nitrative stress. SFN treatment restored Nrf2 activity, blocking NF-κB activation and reducing inflammation and oxidative stress in monocytes from ASD subjects. It also upregulated antioxidant enzymes, such as SOD1, GPx1, and GR, enhancing cellular defenses [154]. In BTBR T + Itpr3tf/J (BTBR) mice, a widely used ASD model, SFN improved social interaction and reduced repetitive behaviors such as self-grooming and marble burying. These beneficial effects correlated with reduced Th17 immune responses, as indicated by lower expression of STAT3, RORC, IL-17A, and IL-23R in CD4+ T cells. SFN also reduced oxidative stress markers (NF-κB, iNOS, and lipid peroxidation) in the cerebellum and neutrophils [155]. In BTBR and C57BL/6 (C57) mice, SFN treatment enhanced enzymatic antioxidant defenses in neutrophils and the cerebellum by increasing SOD, GPx, and GR activity, suggesting a neuroprotective effect of SFN through redox balance modulation [155]. In a maternal immune activation (MIA) ASD model, SFN not only improved social deficits but also altered gut microbiota composition, particularly affecting Bacillales, Staphylococcaceae, and Hamophilus families, suggesting a possible microbiota–brain interaction [156]. Furthermore, GRA-derived SFN demonstrated protective effects against MIA-induced ASD. Maternal dietary supplementation with 0.1% GRA during pregnancy and lactation prevents cognitive and social impairments in juvenile offspring. In adult MIA-exposed mice, a GRA-enriched diet restored parvalbumin immunoreactivity in the medial prefrontal cortex (mPFC), a biomarker of neurodevelopmental disorders [157]. These findings highlight SFN as a promising nutritional and therapeutic strategy for mitigating oxidative stress, neuroinflammation, and behavioral abnormalities in ASD, offering potential benefits for both children and at-risk populations. An overview of the described studies is presented in Table 5.

Table 5.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments in ASD models.
6.1.2. Clinical Studies on Glucosinolate or Isothiocyanates and ASD
The first evidence of the beneficial effects of SFN in individuals with ASD emerged from a double-blind, randomized, placebo-controlled trial conducted at Massachusetts General Hospital (NCT01474993) between 2011 and 2013 [158]. The study aimed to improve core ASD symptoms and underlying biochemical abnormalities, such as oxidative stress, antioxidant deficiency, and inflammation, through SFN administration. Male participants aged 13 to 27 years with moderate-to-severe ASD received daily capsules containing SFN derived from broccoli sprouts (50–150 µmoL; approximately 1.4 µmoL/kg/day) for 18 weeks, followed by a 4-week washout period. SFN treatment led to significant and reversible improvements in social interaction, abnormal behavior, and verbal communication in treated subjects (n = 29) compared to the placebo group (n = 15). However, these effects diminished after the washout period, indicating the treatment’s transient nature. SFN exhibited a favorable safety profile, with only minor adverse effects such as gastrointestinal discomfort, fever, seasonal allergies, irritability, and cough. Following this trial, a subset of responders (n = 10) continued SFN-based dietary supplements under caregiver supervision to maintain the observed behavioral improvements. These individuals were periodically monitored over three years, with many continuing to report sustained benefits [159]. This clinical success prompted the development of five additional trials investigating SFN’s effects in ASD (NCT02561481, NCT02677051, NCT02909959, NCT02654743, and NCT02879110), some of which have since been completed. The first of these studies (NCT02654743), led by Robert Hendren, was an open-label study designed to explore associations between urinary metabolites and clinical improvements. Metabolomic analysis of 15 school-aged children with ASD identified 77 urinary metabolites correlated with symptom reduction following daily SFN supplementation with GRA and the enzyme MYR. These urinary metabolites were linked to oxidative stress, amino acid metabolism, gut microbiome-related amino acid metabolism, neurotransmitter metabolism, stress response hormones, and sphingomyelin metabolism [160]. A subsequent randomized, double-blind, placebo-controlled clinical trial involving 60 children with ASD (aged 4 to 12 years) evaluated SFN in combination with risperidone, a standard treatment for irritability in ASD. Participants were assessed using the Aberrant Behavior Checklist (ABC)—Community Edition at weeks 5 and 10. Significant improvements were observed in the Irritability and Hyperactivity/Noncompliance subscales in the SFN-treated group compared to the placebo group, with a significant effect over time for both symptoms. However, no significant changes were observed in other behavioral domains, including lethargy/social withdrawal, stereotypic behavior, or inappropriate speech. SFN treatment was well tolerated, with no increase in adverse effects, suggesting its potential as an adjunctive treatment for managing irritability and hyperactivity in ASD [161]. Building on these findings, a randomized controlled clinical trial (NCT02561481) was conducted to further investigate SFN’s therapeutic potential in ASD [162]. This study enrolled 45 children with ASD (aged 3 to 12 years), who were randomized to receive either GRA + MYR or placebo for 15 weeks, followed by a 15-week open-label phase and a 6-week no-treatment period. Although no statistically significant differences were found in the primary outcome measure evaluating symptom severity, caregivers reported a significant improvement in aberrant behavior (secondary outcome) in the GRA + MYR group compared to placebo. Additionally, significant biomarker changes were detected, including reduced inflammatory cytokines (IL-6, TNF-α) and improved oxidative stress markers. Treatment was well tolerated, with minor side effects such as insomnia, irritability, and abdominal discomfort in some children. While these results highlight SFN’s potential benefits, further research is needed to confirm its long-term efficacy. More recently, a prospective, double-blind, randomized, placebo-controlled study examined the potential benefits of SFN on behavioral and cognitive symptoms in children with ASD (aged 3 to 7 years) [163]. Despite a 36-week treatment period (50 µmol of SFN daily), no statistically significant differences were observed between the SFN and placebo groups across multiple assessment scales, including the Autism Diagnostic Observation Schedule-2 (ADOS-2), the Social Responsiveness Scale-2 (SRS-2), and the ABC. These findings raise questions about SFN’s efficacy in younger children with ASD and highlight the need for larger, longer-term studies to account for individual response variability and placebo effects. Magner et al. [163] concluded that while SFN possesses antioxidant and neuroprotective properties, current evidence does not support its use in preschool-aged children with ASD.
A longitudinal clinical study explored SFN’s impact on gut microbiota, an emerging factor in ASD pathophysiology. Children treated with GRA + MYR for 12 weeks showed significant improvements in verbal and non-verbal communication, as assessed by the OSU Autism Rating Scale-DSM-IV (OARS-4). However, microbiota alterations in treated subjects were less pronounced than those reported in animal models [156], although correlations were found between specific bacterial taxa and behavioral improvements [156]. In a multicenter, randomized, double-blind, placebo-controlled trial, SFN’s efficacy in children with ASD was further evaluated [164]. The study enrolled 108 children (aged 3–15 years), revealing significant improvements in clinician-rated assessment scales, with 30% of SFN-treated participants exhibiting a ≥30% symptom reduction after 12 weeks. However, no significant differences emerged in caregiver-reported evaluations. The benefits of SFN appeared more pronounced in children over the age of 10, suggesting age-related differences in treatment responsiveness. These discrepancies between clinician and caregiver assessments underscore the need for further studies to refine dosing strategies and establish SFN’s clinical utility. Despite promising evidence suggesting SFN’s role in redox regulation, inflammation, and microbiota modulation in ASD, clinical findings remain inconsistent. While some trials report significant behavioral and communication improvements, others do not find statistically meaningful effects. This variability may stem from differences in study design, treatment duration, dosage, participant age, and SFN bioavailability. Additionally, SFN’s precise mechanisms of action in gut microbiota modulation and neuroinflammation remain unclear. Thus, further large-scale clinical studies are essential to confirm SFN’s efficacy, optimize dosing regimens, and develop personalized therapeutic strategies for individuals with ASD. An overview of the described studies is presented in Table 6. Recent studies explored the effect of SFN on specific genetic polymorphisms, such as those of the COMT and CBS genes, which are involved in the metabolism of sulfur-containing compounds. In particular, it has been found that some autistic patients with homozygous genotypes (rs4633-TT and rs4680-AA) in COMT variants exhibit a more pronounced response to SFN intake, whereas others with heterozygous genotypes (rs4633-CT and rs4680-AG) may metabolize the compound differently. These findings suggest that the effectiveness of GLS metabolites may vary depending on individual genetic variants, highlighting the importance of personalized nutritional treatment based on the patient’s genetic profile [165].

Table 6.
Clinical studies: results on the effects of glucosinolates or isothiocyanates treatments on behavior and health in individuals with ASD.
6.2. Schizophrenia
Schizophrenia is a progressive neurodevelopmental disorder marked by positive symptoms (hallucinations, delusions) and negative symptoms (e.g., apathy, reduced ability to experience pleasure) that severely impairs cognitive functions, perception, decision-making ability, and emotional regulation, profoundly affecting daily life and interpersonal relationships [166,167]. The etiology of schizophrenia is complex, with a strong genetic component. Unlike disorders such as ASD, schizophrenia is generally treated with high doses of antipsychotic medications that, although effective in controlling symptoms, can cause significant long-term side effects. In recent years, research has focused on the early identification of the prodromal phase of schizophrenia and the adoption of preventive intervention strategies. Since cognitive impairment is often a prodromal symptom, early intervention at this stage could significantly reduce the risk of psychopathological progression into adulthood [168,169]. In this context, increasing attention has been directed toward the integration of ITCs and GLS, powerful natural antioxidants, into the diet. These compounds, due to their neuroprotective effects and ability to modulate oxidative stress—a key factor in the pathogenesis of schizophrenia—could represent a promising therapeutic strategy for the treatment and prevention of cognitive deficits in this condition.
6.2.1. Preclinical Studies on Glucosinolates or Isothiocyanates in Schizophrenia
Several studies highlighted the involvement of neuroinflammatory processes in schizophrenia, with a particular focus on the aberrant activation of microglia, the brain’s resident immune cells. SFN exhibited neuroprotective properties by modulating microglial activity and reducing oxidative stress in hiPSC-derived microglia-like cells (iMGLC). It enhanced microglial phagocytosis after 24 h of treatment and exerted anti-inflammatory and antioxidant effects by activating the Nrf2 pathway.
Specifically, SFN increased GCLM and HMOX1 expression in control iMGLC and healthy twin iMGLC, but not in iMGLC derived from twins affected by schizophrenia. It also downregulated FOS, a marker of the pro-inflammatory NF-κB pathway, only in control cells. These findings suggested that SFN can enhance microglial function but has a variable response in schizophrenia, indicating potential differences in microglial reactivity [170]. Preclinical studies conducted on animal models highlighted the neuroprotective potential of GRA and its derivative SFN, in cognitive deficits associated with schizophrenia. In a study on mice, prophylactic treatment with SFN, a potent activator of the transcription factor Nrf2, showed beneficial effects against phencyclidine-induced cognitive deficits. This was achieved by reducing the increase in 8-oxo-dG-positive cells (a marker of oxidative DNA damage) and preventing the decrease in parvalbumin-positive cells (GABAergic inhibitory neurons that express parvalbumin and play a role in cognitive impairment in schizophrenia) in mPFC and hippocampus. Moreover, phencyclidine-induced cognitive deficits were improved by subsequent therapeutic treatment with SFN [171]. Interestingly, dietary intake of SFN’s precursor, GRA, during childhood and adolescence prevented the onset of cognitive deficits, the increase in 8-oxo-dG-positive cells, and the reduction in parvalbumin-positive cells in the brain (mPFC and CA1) induced by phencyclidine in adult mice. Genetic studies also revealed an epistatic interaction between the NRF2 and KEAP1 genes, influencing cognitive abilities in schizophrenic patients. These findings suggest that dietary intake of SFN or its precursors during childhood and adolescence may prevent the onset of psychosis in adulthood [171].
MIA during pregnancy has been associated with cognitive deficits and reduced parvalbumin immunoreactivity in the mPFC of adult offspring—alterations characteristic of schizophrenia. However, preclinical studies in mice demonstrated that dietary supplementation with GRA during childhood and adolescence can prevent these deficits by improving gene expression in key brain regions, such as the prefrontal cortex and hippocampus. Specifically, GRA regulated the expression of the suppressor of fermentation-induced loss of stress resistance protein 1 (Sf1) gene, whose altered function has been observed in post-mortem brain tissue and hair follicles of schizophrenia patients. Gene expression analysis revealed that MIA disrupts the activity of genes related to the centrosome (which is involved in various cellular processes, including migration, division, and differentiation). Notably, GRA supplementation was able to normalize these genetic patterns, suggesting a possible role of these genes in the pathogenesis of psychosis.
These findings indicate that consuming GRA-rich foods, such as broccoli sprouts, may have a preventive and therapeutic role in cognitive disorders associated with schizophrenia [172]. ITCs showed promising potential in mitigating the adverse effects of antipsychotic drugs. SFN demonstrated significant hepatoprotective and metabolic benefits against olanzapine-induced liver injury, particularly in the presence of a high-fat diet (HFD). In female C57BL/6J mice, SFN reduced hepatic fat accumulation, improved insulin resistance, and lowered plasma transaminases (ALT and AST), key markers of liver damage. Additionally, it alleviated oxidative stress by reducing 4-hydroxynonenal (4-HNE) adducts, suggesting that Nrf2 pathway activation could be a valuable strategy to counteract olanzapine-induced metabolic dysfunction, especially in obesity [173]. Similarly, AITC exhibited protective effects in female BALB/c mice, preventing olanzapine-induced weight gain and adiposity after six weeks of treatment. AITC improved glucose and lipid metabolism by lowering fasting blood glucose, enhancing glucose tolerance, and reducing insulin resistance—effects comparable to metformin. Furthermore, it mitigated inflammation by downregulating NF-κB, TNF-α, and IL-6 and reduced hepatic lipid accumulation by modulating lipogenic and lipolytic gene expression. At the hypothalamic level, AITC restored appetite regulation by counteracting the upregulation of orexigenic neuropeptides (AgRP and NPY), reinforcing its potential as a metabolic protective agent in antipsychotic treatment [174]. In male Wistar rats, SFN further demonstrated its efficacy by reducing weight gain, BMI, and food intake while improving blood pressure and lipid profile (increasing HDL and reducing LDL, VLDL, triglycerides, and total cholesterol). Additionally, SFN alleviated oxidative stress by enhancing antioxidant enzyme activity (SOD, catalase) and reducing lipid peroxidation (MDA) and nitrite levels. At the hepatic level, it suppressed pro-inflammatory cytokines (TNF-α, IL-6, NF-κB) and improved liver function markers (AST, ALT, TBIL), while modulating key metabolic and inflammatory pathways [175]. Together, these findings highlight the therapeutic potential of ITCs, particularly SFN and AITC, in alleviating the metabolic and hepatotoxic side effects of olanzapine, supporting their role as promising adjunct therapies in antipsychotic-induced metabolic disorders. An overview of the described studies is presented in Table 7.

Table 7.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments on schizophrenia models.
6.2.2. Clinical Studies on Glucosinolate or Isothiocyanates and Schizophrenia
Several clinical studies examined the efficacy of GLS and ITCs, particularly GRA and SFN, in treating cognitive deficits and symptoms of schizophrenia.
A pilot study evaluated the effect of daily supplementation with 30 mg of GRA for eight weeks in schizophrenic patients treated with antipsychotics. The study found a significant improvement in the Accuracy component of the One Card Learning Task (OCLT), a test assessing visual recognition, attention, and short-term memory. Although no significant changes were observed in other cognitive parameters measured using the CogState battery or in scores on the Positive and Negative Syndrome Scale (PANSS), and no variations were noted in BDNF levels, the results suggest a potential beneficial effect on cognitive function, with no relevant adverse effects [176]. In a subsequent study, a randomized, double-blind, placebo-controlled trial (NCT02810964) involved 64 participants, 58 of whom completed the protocol. They were treated for 16 weeks with broccoli extracts containing GRA + MYR. The GRA + MYR-based extracts did not result in significant differences between the treated and placebo groups in either psychotic symptoms or cognitive functions, as measured by the PANSS and the MATRICS Consensus Cognitive Battery (MCCB). However, the authors suggested that future studies should explore higher doses of SFN and include patients at an earlier stage of the disease to better determine treatment efficacy [177]. Another 22-week randomized, double-blind study found that administration of GRA and MYR led to significant improvements in three secondary outcome cognitive domains: spatial working memory, verbal learning, and reasoning/problem-solving, although it did not show significant effects on the MCCB global composite score (primary outcome). These improvements ranged from small to moderate in magnitude and varied depending on the dosage, suggesting a direct effect of SFN on cognitive function, rather than an indirect improvement linked to psychotic symptom control. Additionally, in the higher-dose GRA group, a slight reduction in Calgary Depression Scale scores was observed, with a more pronounced effect in female patients [178]. Finally, another study (NCT03451734) examined the efficacy of SFN in patients with predominant negative symptoms, using a dose of 90 mg/day for 24 weeks in addition to standard antipsychotic therapy. The results showed a significant reduction in the PANSS negative subscale total score, as well as improvements in SOD activity and high-sensitivity C-reactive protein (HsCRP) levels, suggesting that SFN may exert an anti-inflammatory effect, which could be beneficial in treating negative symptoms. However, no significant effects were found on positive symptoms of PANSS [179]. Overall, these studies suggest that while GLS and ITCs have not shown significant direct effects on global psychotic symptoms, they may have a positive impact on certain cognitive functions and negative symptoms of schizophrenia. Therefore, while the results are promising, further studies are needed to confirm their potential as adjunctive treatments for cognitive deficits and negative symptoms, as well as to optimize dosages and administration methods. An overview of the described studies is presented in Table 8.

Table 8.
Clinical studies: results on the effects of glucosinolates or isothiocyanates treatments on behavior and health in individuals with schizophrenia.
6.3. Depression and Anxiety
Depression and anxiety are among the most prevalent psychiatric disorders worldwide and represent a leading cause of disability and reduced quality of life [180,181]. Depression is characterized by persistent sadness, loss of interest in daily activities, changes in appetite and sleep patterns, and suicidal thoughts. Unfortunately, available antidepressant treatments are not always effective, and many patients experience relapses or undesirable side effects [182]. According to the World Health Organization, more than 350 million people suffer from depression, with a significant impact on public health [183,184]. Although the precise pathophysiological mechanisms of depression are not yet fully understood, numerous findings suggest a key role of inflammation in its onset and progression [185]. At the same time, chronic stress is known to contribute to the development of depressive and anxiety disorders through the hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis, leading to an increased release of corticosteroids and dysregulation of the immune and inflammatory response [186]. Currently, pharmacological treatments for depression primarily include selective serotonin reuptake inhibitors (SSRIs), which are considered first-line treatment for depressive symptoms due to their good tolerability [187]. However, despite their widespread use, several challenges remain in their application, including non-response in a significant proportion of patients and the presence of intolerable side effects, such as sexual dysfunction, sleep disturbances, and, in some cases, undesirable cardiovascular effects [188,189]. Furthermore, SSRI treatment requires a 4–6 weeks period before clinical improvements become apparent [190]. These limitations have driven the exploration of new pharmacological strategies to achieve faster symptom relief. In recent years, research has focused on the role of nutrition in the prevention and treatment of depression and anxiety. High intake of fruits, vegetables, fish, and whole grains has been associated with a reduced risk of developing depressive disorders [191]. In this context, bioactive natural compounds, such as GCLs and ITCs, have gained increasing attention for their potential neuroprotective and anti-inflammatory effects. Specifically, ITCs, particularly SFN and GRA, showed promising effects in the treatment of depression and anxiety through the modulation of various neurobiological mechanisms.
6.3.1. Preclinical Studies on Glucosinolate or Isothiocyanates and Depression/Anxiety
Depression is often associated with reduced neuronal plasticity, particularly in brain regions involved in mood and emotion regulation, such as the hippocampus and prefrontal cortex. An in vitro study showed that SFN enhanced neuronal plasticity by promoting neurite outgrowth in PC12 cells in a concentration-dependent manner. The inhibition of Nrf2 via siRNA significantly attenuated this effect, confirming the crucial role of Nrf2 in this process. SFN also increased Nrf2 protein levels, and this increase was blocked by Nrf2 siRNA, further supporting its involvement in neurite growth [192]. In a mouse model of acute and chronic stress, repeated SFN administration, particularly at a dose of 10 mg/kg/day, exerted antidepressant and anxiolytic effects. In behavioral tests, repeated SFN treatment for 14 days significantly reduced immobility time in the tail suspension test (TST) at 10 mg/kg/day and the forced swim test (FST) at 3 mg/kg/day and 10 mg/kg/day, improving depressive symptoms in mice subjected to chronic mild stress, with antidepressant effects comparable to fluoxetine. Furthermore, SFN at 10 mg/kg/day increased sucrose preference in the sucrose preference test (SPT), indicating an improvement in anhedonia, and reduced anxiety-like behaviors in the open field test (OFT) at 10 mg/kg/day while decreasing latency to feed in the novelty-suppressed feeding test (NSF) at 3 mg/kg/day and 10 mg/kg/day. At the neurobiological level, SFN regulated the HPA axis, lowering serum corticosterone and adrenocorticotropic hormone (ACTH) levels. It also counteracted stress-induced increases in pro-inflammatory cytokines such as IL-6 and TNF-α, demonstrating anti-inflammatory action [146]. In a social defeat stress model, SFN pre-treatment mitigated reductions in social interaction and sucrose preference, indicating protective effects against social avoidance and anhedonia. These improvements were associated with Nrf2 pathway activation, restoration of BDNF levels, and modulation of its receptor TrkB signaling in key brain regions such as CA3, dentate gyrus (DG), and prefrontal cortex (PFC), suggesting enhanced neuronal function [192]. Moreover, GRA treatment in the juvenile and adolescent phases prevented depressive behaviors in adulthood [192]. In a mouse model of LPS-induced depression, preventive treatment with SFN significantly reduced TNF-α and increased IL-10, demonstrating anti-inflammatory effects. Behaviorally, SFN dose-dependently reduced immobility time in the TST and FST on LPS-treated mice. At the molecular level, SFN counteracted alterations in BDNF, PSD-95, and GluA1 levels, key proteins for neuroplasticity, restoring their levels in the PFC and hippocampus, thereby improving neuroplasticity and preventing LPS-induced dendritic spine loss in these regions, a phenomenon associated with depression [193]. Similarly, GRA supplementation (0.1%) during youth prevented the onset of depressive behaviors induced by LPS in adulthood, suggesting preventive LPS-induced depressive behaviors in adulthood by preserving BDNF and synaptic protein levels, while also preventing excessive spine density increases in the nucleus accumbens (NAc). Furthermore, GRA supplementation (0.1%) between the 5th and 8th week of life significantly attenuated the increase in immobility time in the TST and FST in adulthood without affecting control mice behavior [193]. SFN has also shown analgetic and antidepressant effects in chronic neuropathic pain models, reducing nociceptive responses, mechanical and thermal hypersensitivity, and depression-like behaviors. These effects were mediated by Nrf2 activation, increased HO-1 expression, and reduced oxidative stress, along with the modulation of MAPK proteins (JNK, ERK1/2, p-38), which are involved in inflammatory and neuroplasticity processes. Furthermore, SFN enhanced morphine’s analgesic efficacy by counteracting neuropathy-induced reductions in opioid receptors [194].
In an in vivo Alzheimer’s disease-associated depression model, SFN counteracted depression-like effects induced by β-amyloid oligomers (AβOs), improving performance in the FST, OFT, and SPT of rats. This effect was accompanied by reduced oxidative stress (decreased MDA levels), neuroinflammation (reduced IL-1β and TNF-α levels), and preservation of the serotonergic system (increased TPH2 and SERT levels), suggesting a neuroprotective effect [96]. Similarly, GRA exhibited significant antidepressant properties in a depressive phenotype rat model, restoring serotonin and norepinephrine levels while reducing NF-κB activation, kynurenine production, and ROS levels in the PFC [195]. The daily intraperitoneal administration of slow-releasing hydrogen sulfide donors, such as AITC and phenyl isothiocyanate (PITC), has demonstrated positive effects on depression associated with chronic osteoarthritis pain, induced by intra-articular injection of monosodium iodoacetate, in female C57BL/6 mice. Specifically, both treatments significantly reduced the immobility time in behavioral tests used to assess depressive symptoms in osteoarthritis mice, showing a clear antidepressant effect. These effects were also associated with modulation of antioxidant enzyme levels (HO-1, NQO1, GSTM1, GSTA1). However, in tests specifically designed to assess anxiety (EPM and OFT), neither AITC nor PITC were able to normalize the anxiety-like behaviors induced by osteoarthritis [196]. Subsequently, the same research group demonstrated that AITC and PITC were able to significantly reduce depressive-like behaviors also associated with chronic neuropathic pain induced by chronic constriction of the sciatic nerve in male C57BL/6 mice. Specifically, in the TST, mice with neuropathic pain exhibited increased immobility time, indicative of depressive behavior. Interestingly, administration of AITC and PITC reduced this parameter in both the nerve-injured mice and the control group, suggesting an antidepressant effect that is independent of the presence of chronic pain [197]. These findings suggested that slow-releasing hydrogen sulfide donors, despite not being effective in alleviating anxiety related to chronic pain, may represent a promising strategy for the treatment of depression associated with osteoarthritis and neuropathic pain. SFN’s rapid antidepressant effects were further confirmed in murine models, where it attenuated LPS-induced reductions in Nrf2 and BDNF levels, counteracting the increased expression of BDNF transcriptional repressors (HDAC2, mSin3A, and MeCP2) in the mPFC and hippocampus. These effects correlated with improved synaptic transmission and reduced depressive behaviors, but were absent in Nrf2 knockout mice, confirming the essential role of this pathway [198]. In the chronic social defeat stress (CSDS) model, SFN promoted stress resilience by regulating Nrf2 and MeCP2 in the mPFC and microglia, leading to increased BDNF expression and dendritic spine restoration. Additionally, SFN reduced pro-inflammatory microglia (iNOS+) while activating the anti-inflammatory phenotype (arginase1+), leading to behavioral improvements in the social interaction test (SIT), FST, and SPT, reinforcing its therapeutic potential for depression [199]. Further studies suggested that Nrf2 activation via SFN restored TREM2 expression, enhanced the anti-inflammatory microglial phenotype (arginase 1+), and improved dendritic spine density in the mPFC of CSDS-exposed mice, highlighting a possible therapeutic mechanism for depression. The Nrf2-TREM2 pathway appeared crucial in BDNF-TrkB signaling modulation, essential for synaptic plasticity and stress resilience [200]. Beyond SFN, an aqueous extract of Raphanus sativus sprouts (AERSS), rich in ITCs (sulforaphene, SFN, and iberin), demonstrated anxiolytic effects in experimental models. Treatment with 30 mg/kg and 100 mg/kg i.p., or 500 mg/kg orally (p.o.), significantly increased time spent in the open arms of the elevated maze, comparable to diazepam and buspirone. Mechanistic studies suggested that these effects involve GABAA/BDZs and 5-HT1A receptors, implicating both the GABAergic and serotonergic systems [201]. In a mouse model of olfactory bulbectomy (OB)-induced depression, SFN (10 mg/kg for 14 days) significantly improved depressive behaviors, reducing hyperactivity and normalizing self-care and motivation. Biochemical analysis revealed that SFN enhanced total antioxidant capacity (TAC) in the frontal cortex and serum, increasing SOD activity [202]. Collectively, these preclinical studies suggest that GLS and their derivates, like SFN, offer a promising strategy for the prevention and/or treatment of depression and anxiety. Their beneficial effects appear to stem from a combination of anti-inflammatory, antioxidant, and neurotrophic properties, with the ability to modulate the HPA axis, serotonergic and noradrenergic neurotransmission, BDNF signaling, and synaptic plasticity. Additionally, dietary GRA supplementation could also offer a long-term preventive approach, reducing mood disorder vulnerability. An overview of the described studies is presented in Table 9.

Table 9.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments on depression/anxiety models.
6.3.2. Clinical Study on Sulforaphane in Depression/Anxiety
There are multiple physical and mental factors underlying the development of depressive symptoms, and depression is a particular concern among patients undergoing major surgical procedures. In a double-blind, randomized, placebo-controlled trial, the efficacy and safety of SFN in the treatment of depression induced by cardiac surgery, such as percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), was evaluated. After six weeks of treatment, patients (n = 30) treated with a tablet containing 30 mg/day of SFN (appro. 169.2 μmoL/day of SFN) showed a significant improvement in Hamilton Rating Scale for Depression (HAM-D) scores compared to the placebo group (n = 30). The remission rate was also higher in the SFN group, although the difference was not statistically significant. Additionally, SFN treatment was well tolerated, with no significant differences in common side effects between the groups. These findings suggest that SFN could be a safe and effective therapy for alleviating depressive symptoms in patients undergoing cardiac surgery. However, further studies with larger samples and longer follow-up periods are needed to confirm these effects [203].
6.4. Epilepsy
Epilepsy is a chronic neurological disorder characterized by spontaneous and recurrent seizures, often associated with abnormal, hypersynchronous neuronal discharges in the brain [204]. While epilepsy can arise from diverse etiologies, temporal lobe epilepsy (TLE) is among the most common and clinically significant forms, particularly due to its frequent association with cognitive dysfunctions that severely impact patients’ quality of life [205]. Approximately 30% of epilepsy cases are classified as drug-resistant, underscoring the urgent need for more effective and targeted therapies [206]. This need is particularly acute in pediatric populations, where seizures may disrupt brain maturation and result in long-term neurodevelopmental impairments [207]. In recent years, increasing attention has been paid to the role of oxidative stress and mitochondrial dysfunction in the pathophysiology of epilepsy, both in adult and immature brains [208]. Persistent oxidative damage can contribute to neuronal injury, synaptic dysfunction, and cognitive deficits observed in both experimental and clinical epilepsy [209]. Accordingly, antioxidants have emerged as potential neuroprotective agents, especially those capable of activating endogenous defense mechanisms, such as the Nrf2/HO-1 signaling pathway [210]. Natural compounds derived from dietary sources, such as GLS derivatives and ITCs have shown promise in modulating oxidative and inflammatory pathways, making them attractive candidates for complementary therapeutic strategies [210].
Preclinical Studies on Glucosinolate or Isothiocyanates in Epilepsy
Activation of the Nrf2–ARE signaling pathway by SFN has demonstrated notable neuroprotective effects in various epilepsy models. In a chronic amygdala kindling model, SFN treatment suppressed seizure progression, reduced post-discharge duration, and significantly improved seizure-induced cognitive deficits, as assessed by the Morris water maze test. These benefits correlated with decreased oxidative stress markers, lower MDA levels, and higher hippocampal GSH content, as well as the upregulation of Nrf2 nuclear translocation alongside increased expression of antioxidant enzymes HO-1 and NQO1. The absence of such effects in Nrf2-deficient mice underscores the pivotal role of Nrf2 in mediating SFN’s neuroprotection [211]. Further studies confirm SFN’s anticonvulsant and antioxidant properties across multiple seizure models, where pre-treatment raised seizure thresholds and reduced status epilepticus (SE) incidence. These effects were linked to enhanced systemic and hippocampal antioxidant defenses, including elevated SOD activity and improved mitochondrial respiration, specifically boosting ATP-linked oxygen consumption and restoring mitochondrial complexes I and II activity SE-impaired. However, SFN did not prevent hippocampal neuronal loss post-SE, suggesting that early bioenergetic improvements may not fully confer neuroprotection in severe epilepsy models [212]. Despite its generally favorable safety profile, high doses of SFN (200 mg/kg) exhibited proconvulsant effects by lowering seizure thresholds and potentiating carbamazepine’s anticonvulsant efficacy through possible pharmacokinetic interactions. Toxicity signs included sedation, hypothermia, neuromuscular impairment, mortality (LD50 of 212.67 mg/kg), and leukopenia, highlighting the importance of dose considerations and safety evaluations for clinical translation [213]. Combinatorial antioxidant therapy with SFN and NAC during epileptogenesis in a rat SE model delayed seizure onset, prevented disease progression, and rescued cognitive function. This treatment normalized hippocampal redox balance, prevented mitochondrial dysfunction, reduced oxidative and inflammatory damage (including HMGB1 translocation), and attenuated neuronal loss, outperforming either agent alone. These results indicate that early antioxidant intervention may offer lasting disease-modifying effects [214]. In vitro and in vivo temporal lobe epilepsy models further support SFN’s neuroprotective and antioxidant effects via Nrf2 activation, with restored GSH levels, reduced ROS production, increased antioxidant capacity, and diminished hippocampal neuronal loss [215]. Similarly, in immature rats subjected to Li-pilocarpine SE, SFN improved brain metabolism and cerebral blood flow without altering seizure severity or acute neuronal death, suggesting its benefits derive from enhancing metabolic and vascular responses during epileptogenesis [216]. In another lithium–pilocarpine SE model in immature rats, SFN pre-treatment (two doses of 5 mg/kg, 48 h and 24 h before SE) significantly reduced oxidative stress and mitochondrial dysfunction. SFN attenuated superoxide production and levels of 3-nitrotyrosine and 4-hydroxynonenal during both acute and post-SE periods (up to 3 weeks) and partially preserved mitochondrial complex I activity. These effects occurred without modifying seizure severity, indicating that SFN’s protective actions may rely more on Nrf2-mediated mitochondrial support than on direct anticonvulsant activity [217]. Other ITCs, such as SIN, demonstrated anticonvulsant, antioxidant, and anti-inflammatory effects in pentylenetetrazole-treated rats by delaying seizure onset, improving memory, increasing antioxidant enzyme levels (SOD and CAT), and downregulating pro-inflammatory genes (Il1b and Nlrp3) [218]. Moreover, BITC treatment in a lithium–pilocarpine epilepsy model improved cognitive and motor functions, reduced neuronal loss, and enhanced Nrf2 pathway activity and antioxidant defenses, suggesting its therapeutic potential against epilepsy-related neurodegeneration [219]. Overall, preclinical evidence indicates that GLS and ITCs exert multifaceted neuroprotective effects in epilepsy models primarily via Nrf2-mediated antioxidant and metabolic modulation. While these compounds show promise as adjunctive therapies to mitigate oxidative stress and cognitive decline in epilepsy, further research is necessary to clarify optimal dosing and long-term safety. An overview of the described studies is presented in Table 10.

Table 10.
Preclinical studies: results on the effects of glucosinolates or isothiocyanates treatments on epilepsy models.
7. Adverse Effects
GLS and ITCs generally present a favorable safety profile, but their consumption at high doses can lead to adverse effects. Preclinical studies showed that high amounts of GRA can suppress phase I enzymes in the liver and lungs, affecting the metabolism of endogenous substances and increasing the risk of bioactivation of polycyclic aromatic hydrocarbons (PAHs) into carcinogenic forms [220,221]. Moreover, a GLS-rich diet led to reduced food intake and growth in rats, with increased mortality and alterations in thyroid function, including thyroid gland enlargement and decreased plasma levels of thyroid hormones [222]. However, moderate consumption of Brassicaceae vegetables does not appear to pose a significant clinical risk, especially in individuals with adequate iodine intake [223]. Regarding SFN, some clinical studies have reported gastrointestinal disturbances and a burning sensation in the throat following ingestion at high doses, effects that can be mitigated with gradual exposure [224]. The adverse events reported were generally mild, such as nausea or vomiting associated with the odor or ingestion of SFN preparations [35]. Moreover, ITCs showed a dose-dependent effect on the generation of ROS and the breakage of double-stranded DNA, contributing to cytotoxic effects in cancer cells, but being well tolerated in normal cells due to more efficient DNA repair mechanisms [225,226,227,228,229]. Even in studies with patients suffering from neuropsychiatric disorders, treatment with SFN-rich extracts was well tolerated, with no significant differences in adverse events between treated and placebo groups, despite some serious adverse events being reported [177]. Additionally, a clinical trial showed a high completion rate (73.77%), as well as no evidence of significant adverse events related to the administration of SFN, as confirmed by routine tests on liver, kidney, and metabolic function [179]. In conclusion, GLS and ITCs are generally well tolerated and offer numerous health benefits, thanks to their antioxidant, anti-inflammatory, and potentially chemopreventive properties. Although excessive consumption may require caution, particularly in individuals predisposed to thyroid dysfunction or those exposed to environmental mutagens, clinical studies suggest that moderate doses are safe and free from significant adverse effects. With balanced use, these compounds could represent valuable nutritional support for the prevention and management of various pathological conditions, with a generally favorable safety profile.
8. Clinical Challenges and Future Perspectives
Despite the growing body of evidence supported by numerous preclinical studies and emerging clinical data, the clinical translation of ITCs remains limited and requires further investigation. One of the main challenges is the definition of effective and safe dosage for humans. In clinical studies, widely variable doses of ITC have been used; for example, SFN has been administered in doses ranging from below 25 to above 500 μmol/day, commonly delivered through broccoli sprout extracts or standardized supplements. However, inter-individual differences in metabolism influenced by the activity of gut microbiota and glutathione-S-transferase enzyme activity can significantly affect ITCs’ bioavailability and therapeutic response. Moreover, administration methods play a crucial role in therapeutic outcomes. Oral administration is currently the most studied and practical route, but it is susceptible to variability due to food matrix effects, individual absorption, as well as dietary and metabolic factors. Promising strategies include the use of standardized extracts with known GLS-to-ITC conversion rates, or encapsulated formulations to improve stability and bioavailability. Patient selection also plays a critical role. Individuals with elevated levels of oxidative stress or systemic inflammation, common features in many neurodegenerative and psychiatric disorders, are likely to benefit the most from ITC-based interventions. However, this remains a hypothesis that requires validation through clinical trials designed with stratified patient cohorts and guided by specific biomarkers in order to identify those subpopulations that respond most effectively to ITC supplementation. To enable successful clinical translation, future trials should focus on defining therapeutic windows, optimizing formulations, and identifying predictive biomarkers of response to ensure effective clinical use. Moreover, long-term safety studies are essential to evaluate potential adverse effects and drug–nutrient interactions, especially in combination with conventional pharmacological treatments. Mechanistic research should also be extended to lesser-known ITCs to better characterize their molecular targets, particularly in relation to brain-specific signaling pathways and epigenetic modulation. Integrating multi-omics approaches and personalized medicine frameworks may further improve our understanding of individual variability in ITC response and support more tailored neuroprotective strategies.
9. Methods
All articles included in this review were selected through the PubMed databases, considering publications from 2014 to 2024. The research was conducted using the following keywords: “isothiocyanate” or “glucosinolate” or “sulforaphane” or “glucoraphanin” or “moringin” in combination with “neuroinflammation” or “alzheimer” or “parkinson” or “multiple sclerosis” or “autism” or “schizophrenia” or “depression” or “anxiety” or “epilepsy” (and other related terms). From these search criteria, a total of 412 articles were found, 84 of which were excluded as they were reviews, resulting in a total of 328 studies that were examined by two reviewers. The inclusion criteria focused exclusively on original studies, written in the English language, investigating naturally occurring plant-derived ITCs and GLS and their effects on neuropsychiatric disorders, neurodegenerative diseases, neurodevelopment diseases, and other brain health-related conditions. All articles related to the use of ITCs in experimental assays, such as fluorescein isothiocyanate (FITC) or Rhodamine B isothiocyanate (RITC), as well as those centered on other non-relevant phytochemicals, were excluded. At the end of the selection process, 142 articles were selected, of which 128 preclinical studies and 15 clinical studies were collected.
10. Conclusions
This review provides an in-depth description of preclinical and clinical studies conducted over the past ten years on the effects of GLS and ITCs in neurological, neurodevelopmental, and psychiatric disorders as well as epilepsy (Figure 2). The molecular mechanisms underlying their beneficial effects and their potential positive impact on patients’ quality of life were highlighted. The collected evidence suggests that ITCs are bioactive molecules of great interest for the prevention and treatment of numerous neurodegenerative, neurodevelopmental, and psychiatric disorders. Preclinical research and early clinical studies indicate that these compounds may exert neuroprotective effects through antioxidant mechanisms (primarily via activation of the Nrf2 pathway) and anti-inflammatory actions, helping to mitigate conditions such as depression, schizophrenia, ASD, AD, PD, and MS (Figure 3). For these reasons they may represent an alternative pharmacological strategy also in association with standard therapies. Although each disease discussed in this review has distinct clinical and pathological features, it is evident that ITCs exert beneficial effects primarily through two core mechanisms: modulation of oxidative stress and immune regulation. In neurodegenerative diseases, such as AD, PD, MS, and epilepsy, the dominant mechanism of ITCs involves the activation of the Nrf2 pathway. This pathway enhances cellular antioxidant defenses, promotes mitochondrial integrity, and supports proteostasis, all of which are crucial in counteracting protein aggregation and neuronal degeneration. In contrast, in psychiatric and neurodevelopmental disorders, such as schizophrenia, depression, and ASD, ITCs appear to act more prominently on immune regulation. These effects include the suppression of pro-inflammatory cytokines, inhibition of NF-κB activation, and promotion of anti-inflammatory phenotypes in microglia. Interestingly, some mechanisms overlap. For example, Nrf2 activation also indirectly regulates inflammation, while modulation of immune responses can reduce oxidative stress. This dual action suggests that ITCs may target shared pathophysiological mechanisms across these disorders, although with different predominant effects depending on the type of disease. A comparative analysis thus reveals that while antioxidant pathways (e.g., Nrf2, HO-1 induction) are central in neurodegenerative disorders and epilepsy, immune-modulatory effects (e.g., inhibition of NF-κB, cytokine regulation) are more pronounced under psychiatric and neurodevelopmental conditions. Understanding these nuanced differences will be essential for tailoring therapeutic strategies based on disease-specific pathological hallmarks.
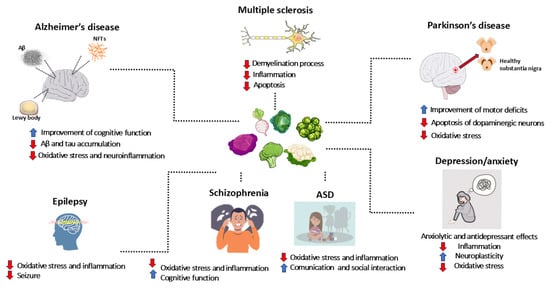
Figure 2.
Potential benefits of glucosinolate derivatives in neurodegenerative diseases, neurodevelopmental disorders, and psychiatric conditions: insights from preclinical and clinical studies. The figure was created using the vector image bank of Servier Medical Art by Servier (http://smart.servier.com/ (accessed on 1 April 2025)). Servier Medical Art is licensed under CC BY 4.0.
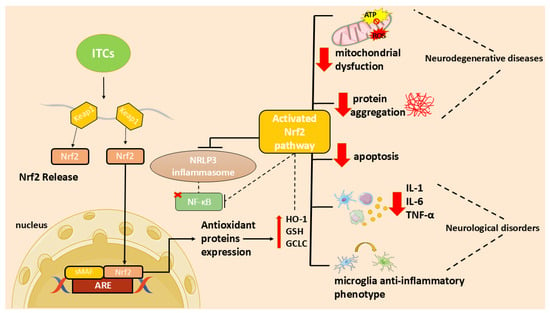
Figure 3.
Overview of ITC effects on the Nrf2 pathway and their anti-inflammatory actions. Both antioxidant and anti-inflammatory properties mediate their neuroprotective actions. However, in neurodegenerative diseases, the dominant mechanism of ITCs involves antioxidant actions, while in neurological disorders the immunoregulatory effect is prevalent.
While preclinical data from animal and in vitro studies are promising, further clinical research with large-scale trials is needed to determine the true therapeutic potential of these compounds and establish their optimal administration in humans, particularly for diseases such as AD, PD, MS, and epilepsy, where clinical evidence remains limited. Of note, SFN is the most studied ITC, and then the molecular mechanisms underlying its neuroprotective actions were discovered. Instead, more studies are needed for less-indagated ITCs to deepen the knowledge on their protective actions. In the meantime, integrating cruciferous vegetables into the diet may represent a safe and potentially effective nutritional strategy to enhance brain health and reduce the risk of neurological and psychiatric disorders. Moreover, the consumption of cruciferous vegetables provides GLS and other beneficial phytocompounds, including flavonoids and phenols, which may synergistically enhance neuroprotective effects.
Author Contributions
Conceptualization, E.M.; writing—original draft preparation, C.M. and G.C.; writing—review and editing, A.G. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
Funded by the European Union—Next Generation EU—PNRR M6C2—Investment 2.1 Enhancement and strengthening of biomedical research in the NHS” (project title: A composition comprising glucoraphanin, myrosinase, and a buffered solution for use in the treatment of neurodegenerative diseases; Project code: PNRR-POC-2022-12376049).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MS | Multiple sclerosis |
| ASD | Autism spectrum disorder |
| GLS | Glucosinolate |
| ITCs | Isothiocyanates |
| Nrf2 | Nuclear factor 2-related erythroid-derived factor |
| ARE | Antioxidant response element |
| NF-κB | Nuclear factor-κB |
| SFN | Sulforaphane |
| MYR | Myrosinase |
| GRA | Glucoraphanin |
| SIN | Sinigrin |
| AITC | Allyl isothiocyanate |
| GST | Gluconasturtiin |
| PEITC | Phenethyl isothiocyanate |
| GER | Glucoerucin |
| ER | Erucin |
| GTL | Glucotropaeolin |
| BITC | Benzyl isothiocyanate |
| GMG | Glucomoringin |
| MOR | Moringin |
| GSH | Glutathione |
| GST | Glutathione S-transferases |
| GTP | Gamma-glutamyl transpeptidase |
| CG | Cysteinylglycinase |
| NAT | N-acetyl transferase |
| NAC-ITC | N-acetylcysteine isothiocyanate |
| CNS | Central nervous system |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| COX | Cyclooxygenase |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| MGO | Methylglyoxal |
| CPF | Chlorpyrifos |
| HO-1 | Heme oxygenase-1 |
| BDNF | Brain-derived neurotrophic factor |
| OKA | Okadaic Acid |
| TNF | Tumor necrosis factor |
| TBI | Traumatic brain injury |
| MHE | Minimal hepatic encephalopathy |
| GMG-ITC | Glucomoringin-isothiocyanate |
| MMP | Metalloproteinase |
| Aβ | Amyloid β |
| RB | Romanesco broccoli |
| PB | Purple broccoli |
| ESI-MS | Electrospray ionization mass spectrometry |
| BACE1 | β-site amyloid precursor protein cleaving enzyme 1 |
| NQO1 | NAD(P)H quinone oxidoreductase |
| SNP | Sodium nitroprusside |
| HSP | Heat shock protein |
| AQP4 | Aquaporin-4 |
| HDACs | Histone deacetylases |
| CVI | Cognitive vascular impartment |
| SCOP | Scopolamine |
| BRO | Broccoli extract |
| 6-MSITC | 6-methylsulfinyl hexyl isothiocyanate |
| ADAM17 | Metalloproteinase 17 |
| AChE | Acetylcholinesterase |
| LB | Lewy bodies |
| α-syn | α-synuclein |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| C/EBPβ | CCAAT/enhancer-binding protein β |
| MeCP2 | Methyl-binding protein CpG2 |
| RTT | Rett syndrome |
| 6-OHDA | 6-hydroxydopamine |
| TH | Tyrosine hydroxylase |
| BST | Broccoli seed tea |
| BBB | Blood–brain barrier |
| MIF | Migration inhibitory factor |
| EAE | Experimental autoimmune encephalitis |
| Trx1/TrxR1 | Thioredoxin 1/thioredoxin reductase 1 |
| EVs | Extracellular vesicles |
| PBMCs | Peripheral blood mononuclear cells |
| MIA | Maternal immune activation |
| mPFC | Medial prefrontal cortex |
| ABC | Aberrant Behavior Checklist |
| ADOS-2 | Autism Diagnostic Observation Schedule-2 |
| SRS-2 | Social Responsiveness Scale-2 |
| OARS-4 | OSU Autism Rating Scale-DSM-IV |
| iMGLC | hiPSC-derived microglia-like cells |
| 4-HNE | 4-hydroxynonenal |
| OCLT | One Card Learning Task |
| MCCB | MATRICS Consensus Cognitive Battery |
| HPA | Hypothalamic–pituitary–adrenal |
| SSRIs | Selective serotonin reuptake inhibitors |
| TST | Tail suspension test |
| FST | Forced swim test |
| SPT | Sucrose preference test |
| OFT | Open field test |
| ACTH | Adrenocorticotropic hormone |
| DG | Dentate gyrus |
| PFC | Prefrontal cortex |
| NAc | Nucleus accumbens |
| AβOs | β-amyloid oligomers |
| PITC | Phenyl isothiocyanate |
| CSDS | Chronic social defeat stress |
| SIT | Social interaction test |
| TAC | Total antioxidant capacity |
| PCI | Percutaneous coronary intervention |
| TLE | Temporal lobe epilepsy |
| SE | Status epilepticus |
| CABG | Coronary artery bypass grafting |
| HAM-D | Hamilton Rating Scale for Depression |
| PAHs | Polycyclic aromatic hydrocarbons |
References
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- GBD Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Doernberg, E.; Hollander, E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.S.; Luca, A.C.; Ciobica, A. Potential Novel Therapies for Neurodevelopmental Diseases Targeting Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 6640206. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.Y.; Chen, X.; Liu, G.H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel Sources and Biological Potential. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 1–58. [Google Scholar]
- Giacoppo, S.; Galuppo, M.; Montaut, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia 2015, 106, 12–21. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Liu, J.; Chandaka, G.K.; Zhang, R.; Parfenova, H. Acute antioxidant and cytoprotective effects of sulforaphane in brain endothelial cells and astrocytes during inflammation and excitotoxicity. Pharmacol. Res. Perspect. 2020, 8, e00630. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Bird, K.A.; Ramos, A.A.; Kliebenstein, D.J. Phylogenetic and genomic mechanisms shaping glucosinolate innovation. Curr. Opin. Plant Biol. 2025, 85, 102705. [Google Scholar] [CrossRef]
- Garcia, G.; Treccarichi, S.; Arena, D.; Ben Ammar, H.; Maggioni, L.; Branca, F. Capturing the L. wild relatives diversity for improving nutraceutical traits of cole crops. Genet. Resour. Crop Evol. 2025. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Bianchi, G.; Picchi, V.; Campanelli, G.; Ficcadenti, N.; Treccarichi, S.; Arena, D.; Sestili, S.; Branca, F. Compositional traits of hybrid populations of Brassica oleracea L. var. italica (broccoli) and Brassica oleracea L. var. botrytis (cauliflower) during four organic breeding cycles. J. Food Compos. Anal. 2024, 131, 106209. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Williams, D.J.; Critchley, C.; Pun, S.; Chaliha, M.; O’Hare, T.J. Differing mechanisms of simple nitrile formation on glucosinolate degradation in Lepidium sativum and Nasturtium officinale seeds. Phytochemistry 2009, 70, 1401–1409. [Google Scholar] [CrossRef]
- Kim, S.H.; Ochar, K.; Iwar, K.; Lee, Y.J.; Kang, H.J.; Na, Y.W. Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS. Int. J. Mol. Sci. 2024, 25, 4829. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Burcul, F.; Generalic Mekinic, I.; Radan, M.; Rollin, P.; Blazevic, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Prabu, S.L.; Umamaheswari, A.; Puratchikody, A. Phytopharmacological potential of the natural gift Moringa oleifera Lam and its therapeutic application: An overview. Asian Pac. J. Trop. Med. 2019, 12, 485–498. [Google Scholar] [CrossRef]
- Yadav, K.; Dhankhar, J.; Preeti, K. Isothiocyanates—A Review of their Health Benefits and Potential Food Applications. Curr. Res. Nutr. Food Sci. 2022, 10, 476–502. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Kolm, R.H.; Mannervik, B.; Talalay, P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem. Biophys. Res. Commun. 1995, 206, 748–755. [Google Scholar] [CrossRef]
- Egner, P.A.; Kensler, T.W.; Chen, J.G.; Gange, S.J.; Groopman, J.D.; Friesen, M.D. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem. Res. Toxicol. 2008, 21, 1991–1996. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Stephenson, K.K.; Wade, K.L.; Ye, L.; Talalay, P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr. Cancer 2006, 55, 53–62. [Google Scholar] [CrossRef]
- Getahun, S.M.; Chung, F.L. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol. Biomark. Prev. 1999, 8, 447–451. [Google Scholar]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea Var. capitata) cooked for different durations. J. Agric. Food Chem. 2006, 54, 7628–7634. [Google Scholar] [CrossRef]
- Rouzaud, G.; Young, S.A.; Duncan, A.J. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol. Biomark. Prev. 2004, 13, 125–131. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508. [Google Scholar]
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: Results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395. [Google Scholar] [CrossRef]
- Cramer, J.M.; Jeffery, E.H. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr. Cancer 2011, 63, 196–201. [Google Scholar] [CrossRef]
- Tang, L.; Zirpoli, G.R.; Guru, K.; Moysich, K.B.; Zhang, Y.; Ambrosone, C.B.; McCann, S.E. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1806–1811. [Google Scholar] [CrossRef]
- Rivest, S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-kappaB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Qin, S.; Yang, C.; Huang, W.; Du, S.; Mai, H.; Xiao, J.; Lu, T. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-kappaB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res. 2018, 133, 218–235. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunol. Lett. 2021, 233, 20–30. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-kappaB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, 792. [Google Scholar] [CrossRef]
- Townsend, B.E.; Johnson, R.W. Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp. Gerontol. 2016, 73, 42–48. [Google Scholar] [CrossRef]
- Brasil, F.B.; de Almeida, F.J.S.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. The isothiocyanate sulforaphane prevents mitochondrial impairment and neuroinflammation in the human dopaminergic SH-SY5Y and in the mouse microglial BV2 cells: Role for heme oxygenase-1. Metab. Brain Dis. 2023, 38, 419–435. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Wu, J.; Hu, P.; Xu, X.; Zhang, Y.; Wang, D.; Chen, Z.; Huang, C. Sulforaphane triggers a functional elongation of microglial process via the Akt signal. J. Nutr. Biochem. 2019, 67, 51–62. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, Q.; Wang, J.; Yu, L.S.; Chen, L.W. Sulforaphane mitigates LPS-induced neuroinflammation through modulation of Cezanne/NF-kappaB signalling. Life Sci. 2020, 262, 118519. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, B.; Zhang, B.; Li, S.; Huang, N.; Zhan, G.; Jiang, R.; Yang, L.; Wu, Y.; Miao, L.; et al. Sulforaphane Alleviates Lipopolysaccharide-induced Spatial Learning and Memory Dysfunction in Mice: The Role of BDNF-mTOR Signaling Pathway. Neuroscience 2018, 388, 357–366. [Google Scholar] [CrossRef]
- Dwivedi, S.; Rajasekar, N.; Hanif, K.; Nath, C.; Shukla, R. Sulforaphane Ameliorates Okadaic Acid-Induced Memory Impairment in Rats by Activating the Nrf2/HO-1 Antioxidant Pathway. Mol. Neurobiol. 2016, 53, 5310–5323. [Google Scholar] [CrossRef]
- Castro-Sanchez, S.; Garcia-Yague, A.J.; Kugler, S.; Lastres-Becker, I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019, 22, 101118. [Google Scholar] [CrossRef]
- Kyyriainen, J.; Kajevu, N.; Banuelos, I.; Lara, L.; Lipponen, A.; Balosso, S.; Hamalainen, E.; Das Gupta, S.; Puhakka, N.; Natunen, T.; et al. Targeting Oxidative Stress with Antioxidant Duotherapy after Experimental Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 10555. [Google Scholar] [CrossRef]
- Townsend, B.E.; Johnson, R.W. Sulforaphane reduces lipopolysaccharide-induced proinflammatory markers in hippocampus and liver but does not improve sickness behavior. Nutr. Neurosci. 2017, 20, 195–202. [Google Scholar] [CrossRef]
- Canto, A.; Martinez-Gonzalez, J.; Miranda, M.; Olivar, T.; Almansa, I.; Hernandez-Rabaza, V. Sulforaphane Modulates the Inflammation and Delays Neurodegeneration on a Retinitis Pigmentosa Mice Model. Front. Pharmacol. 2022, 13, 811257. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Gonzalez-Usano, A.; Agusti, A.; Balzano, T.; Llansola, M.; Felipo, V. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflamm. 2016, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.; Bieber, M.; Kraft, P.; Weber, A.N.R.; Stoll, G.; Schuhmann, M.K. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav. Immun. 2021, 92, 223–233. [Google Scholar] [CrossRef]
- Subedi, L.; Cho, K.; Park, Y.U.; Choi, H.J.; Kim, S.Y. Sulforaphane-Enriched Broccoli Sprouts Pretreated by Pulsed Electric Fields Reduces Neuroinflammation and Ameliorates Scopolamine-Induced Amnesia in Mouse Brain through Its Antioxidant Ability via Nrf2-HO-1 Activation. Oxidative Med. Cell. Longev. 2019, 2019, 3549274. [Google Scholar] [CrossRef]
- Townsend, B.E.; Chen, Y.J.; Jeffery, E.H.; Johnson, R.W. Dietary broccoli mildly improves neuroinflammation in aged mice but does not reduce lipopolysaccharide-induced sickness behavior. Nutr. Res. 2014, 34, 990–999. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, D.S.; Jung, W.K.; Yoo, J.S.; Yim, M.J.; Choi, Y.H.; Park, S.; Seo, S.K.; Choi, J.S.; Lee, Y.M.; et al. Benzyl isothiocyanate inhibits inflammasome activation in E. coli LPS-stimulated BV2 cells. Int. J. Mol. Med. 2016, 38, 912–918. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquo, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Nordin, N.; Rosli, R.; Shaari, K.; Bako, H.Y.; Saad, N.; Noor, N.M.; Abdull Razis, A.F. Neuroprotective effects of glucomoringin-isothiocyanate against H(2)O(2)-Induced cytotoxicity in neuroblastoma (SH-SY5Y) cells. Neurotoxicology 2019, 75, 89–104. [Google Scholar] [CrossRef]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and Anti-Inflammatory Activities of Allyl Isothiocyanate through Attenuation of JNK/NF-kappaB/TNF-alpha Signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacology 2021, 29, 561–571. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Rada, P.; Cuadrado, A.; Lopez, M.G.; Garcia, A.G.; Leon, R. Melatonin-sulforaphane hybrid ITH12674 induces neuroprotection in oxidative stress conditions by a ‘drug-prodrug’ mechanism of action. Br. J. Pharmacol. 2015, 172, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; Buendia, I.; Duarte, P.; FernandezMendivil, C.; Negredo, P.; Cuadrado, A.; Lopez, M.G.; Leon, R. Melatonin-sulforaphane hybrid ITH12674 attenuates glial response in vivo by blocking LPS binding to MD2 and receptor oligomerization. Pharmacol. Res. 2020, 152, 104597. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Johnson, G.V. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 449–463. [Google Scholar] [CrossRef]
- Nijholt, D.A.; van Haastert, E.S.; Rozemuller, A.J.; Scheper, W.; Hoozemans, J.J. The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies. J. Pathol. 2012, 226, 693–702. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Inflammation in Alzheimer’s disease: Amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 2009, 87, 181–194. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Vu, G.H.; Nguyen, H.D. Molecular mechanisms of sulforaphane in Alzheimer’s disease: Insights from an in-silico study. Silico Pharmacol. 2024, 12, 96. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; El-Shiekh, R.A.; Mohamed, O.G.; Al-Karmalawy, A.A.; Tripathi, A.; Abdel-Baki, P.M. LC/MS-Based Metabolomics Reveals Chemical Variations of Two Broccoli Varieties in Relation to Their Anticholinesterase Activity: In vitro and In silico Studies. Plant Foods Hum. Nutr. 2024, 79, 359–366. [Google Scholar] [CrossRef]
- Nagaveni, V.; Lakshmi, V.V.; Prabhakar, S. Sulforaphane interaction with amyloid beta 1-40 peptide studied by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2171–2180. [Google Scholar] [CrossRef]
- Youn, K.; Yoon, J.H.; Lee, N.; Lim, G.; Lee, J.; Sang, S.; Ho, C.T.; Jun, M. Discovery of Sulforaphane as a Potent BACE1 Inhibitor Based on Kinetics and Computational Studies. Nutrients 2020, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Jimenez-Moreno, N.; Garcia-Yague, A.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rabano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio-Tejo, F.; Olesen, M.A.; Aranguiz, A.; Quintanilla, R.A. Activation of the Nrf2 Pathway Prevents Mitochondrial Dysfunction Induced by Caspase-3 Cleaved Tau: Implications for Alzheimer’s Disease. Antioxidants 2022, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Denzer, I.; Munch, G.; Pischetsrieder, M.; Friedland, K. S-allyl-L-cysteine and isoliquiritigenin improve mitochondrial function in cellular models of oxidative and nitrosative stress. Food Chem. 2016, 194, 843–848. [Google Scholar] [CrossRef]
- Chilakala, R.R.; Manchikalapudi, A.L.; Kumar, A.; Sunkaria, A. Sulforaphane Attenuates Abeta Oligomers Mediated Decrease in Phagocytic Activity of Microglial Cells. Neuroscience 2020, 429, 225–234. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears Amyloid-beta and Tau in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800240. [Google Scholar] [CrossRef]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; d’Erme, M.; et al. Neuroprotective Effect of Brassica oleracea Sprouts Crude Juice in a Cellular Model of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef]
- Yang, C.; Qin, S.; Zhang, J.; Wang, Y.; Li, H.; Lu, T. Sulforaphane Upregulates Cultured Mouse Astrocytic Aquaporin-4 Expression through p38 MAPK Pathway. J. Healthc. Eng. 2022, 2022, 1144124. [Google Scholar] [CrossRef]
- Jhang, K.A.; Park, J.S.; Kim, H.S.; Chong, Y.H. Sulforaphane rescues amyloid-beta peptide-mediated decrease in MerTK expression through its anti-inflammatory effect in human THP-1 macrophages. J. Neuroinflamm. 2018, 15, 75. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Yang, C.; Dong, B.; Fu, Y.; Wang, Y.; Gong, M.; Liu, T.; Qiu, P.; Xie, W.; et al. Sulforaphane attenuates microglia-mediated neuronal damage by down-regulating the ROS/autophagy/NLRP3 signal axis in fibrillar Abeta-activated microglia. Brain Res. 2023, 1801, 148206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, C.; Zhang, S.; Cao, T.; Peng, R.; Guo, W.; Yan, Y.; Xie, S.; Peng, X.; Lu, T.; et al. Sulforaphane reverses Abeta fiber-mediated M1 type microglia polarization and neuroinflammation-mediated necroptosis of neural stem cells by downregulating the MAPK/NF-kappaB signaling pathways. Nan Fang Yi Ke Da Xue Xue Bao 2023, 43, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Choi, B.R.; Yang, H.; Hwang, Y.; Park, J.H.; LaFerla, F.M.; Han, J.S.; Lee, K.W.; Kim, J. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol. Nutr. Food Res. 2017, 61, 1600194. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.T.; Yang, H.Y.; Wang, W.; Wu, Q.Q.; Tian, Y.R.; Jia, J.P. Sulforaphane Inhibits the Generation of Amyloid-beta Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease (PS1V97L) Transgenic Mice. J. Alzheimers Dis. 2018, 62, 1803–1813. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Zhan, Z.; Li, X.; Zhou, F.; Xing, A.; Jiang, C.; Chen, Y.; An, L. Beneficial Effects of Sulforaphane Treatment in Alzheimer’s Disease May Be Mediated through Reduced HDAC1/3 and Increased P75NTR Expression. Front. Aging Neurosci. 2017, 9, 121. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid beta deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Xu, Q.Q.; Xian, Y.F.; Lin, Z.X. Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3beta Pathway in Experimental Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef]
- An, Y.W.; Jhang, K.A.; Woo, S.Y.; Kang, J.L.; Chong, Y.H. Sulforaphane exerts its anti-inflammatory effect against amyloid-beta peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol. Aging 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Khan, W.U.; Salman, M.; Ali, M.; Majid, H.; Yar, M.S.; Akhtar, M.; Parvez, S.; Najmi, A.K. Neuroprotective Effects of Sulforaphane in a rat model of Alzheimer’s Disease induced by Abeta (1-42) peptides. Neurochem. Int. 2024, 179, 105839. [Google Scholar] [CrossRef]
- Pu, D.; Zhao, Y.; Chen, J.; Sun, Y.; Lv, A.; Zhu, S.; Luo, C.; Zhao, K.; Xiao, Q. Protective Effects of Sulforaphane on Cognitive Impairments and AD-like Lesions in Diabetic Mice are Associated with the Upregulation of Nrf2 Transcription Activity. Neuroscience 2018, 381, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Li, X.; Hu, Q.; Mao, L.; Shao, Y.; Han, M.; Zhang, S.; Ejaz, I.; Mesbah, L.; et al. Sulforaphane suppresses Abeta accumulation and tau hyperphosphorylation in vascular cognitive impairment(VCI). J. Nutr. Biochem. 2025, 136, 109803. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Mazzon, E.; Iori, R.; Chiricosta, L.; Artimagnella, O. Bioactivated Glucoraphanin Improves Cell Survival, Upregulating Phospho-AKT, and Modulates Genes Involved in DNA Repair in an In Vitro Alzheimer’s Disease Model: A Network-Transcriptomic Analysis. Nutrients 2024, 16, 4202. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Fang, L.; Li, X.; Zhao, Y.; Shi, W.; An, L. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. Int. J. Mol. Sci. 2014, 15, 14396–14410. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C.; Quan, M.; Li, T.; Jia, J. Sulforaphane Reverses the Amyloid-beta Oligomers Induced Depressive-like Behavior. J. Alzheimers Dis. 2020, 78, 127–137. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Alzahrani, N.A.; Bahaidrah, K.A.; Mansouri, R.A.; Aldhahri, R.S.; Abd El-Aziz, G.S.; Alghamdi, B.S. Possible Prophylactic Effects of Sulforaphane on LPS-Induced Recognition Memory Impairment Mediated by Regulating Oxidative Stress and Neuroinflammatory Proteins in the Prefrontal Cortex Region of the Brain. Biomedicines 2024, 12, 1107. [Google Scholar] [CrossRef]
- Park, H.S.; Hwang, E.S.; Choi, G.Y.; Kim, H.B.; Park, K.S.; Sul, J.Y.; Hwang, Y.; Choi, G.W.; Kim, B.I.; Park, H.; et al. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021, 238, 113467. [Google Scholar] [CrossRef]
- Rajesh, V.; Ilanthalir, S. Cognition Enhancing Activity of Sulforaphane Against Scopolamine Induced Cognitive Impairment in Zebra Fish (Danio rerio). Neurochem. Res. 2016, 41, 2538–2548. [Google Scholar] [CrossRef]
- Holloway, P.M.; Gillespie, S.; Becker, F.; Vital, S.A.; Nguyen, V.; Alexander, J.S.; Evans, P.C.; Gavins, F.N.E. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vasc. Pharmacol. 2016, 85, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hortal, M.D.; Romero-Marquez, J.M.; Lopez-Bascon, M.A.; Sanchez-Gonzalez, C.; Xiao, J.; Sumalla-Cano, S.; Battino, M.; Forbes-Hernandez, T.Y.; Quiles, J.L. In Vitro and In Vivo Insights into a Broccoli Byproduct as a Healthy Ingredient for the Management of Alzheimer’s Disease and Aging through Redox Biology. J. Agric. Food Chem. 2024, 72, 5197–5211. [Google Scholar] [CrossRef] [PubMed]
- Carnicero-Senabre, D.; Jimenez-Villegas, J.; Alvarez-Garrote, S.; Escoll, M.; Cuadrado, A.; Rojo, A.I. NRF2 activation by 6-MSITC increases the generation of neuroprotective, soluble alpha amyloid precursor protein by inducing the metalloprotease gene ADAM17. Free Radic. Biol. Med. 2025, 227, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Uruno, A.; Matsumaru, D.; Ryoke, R.; Saito, R.; Kadoguchi, S.; Saigusa, D.; Saito, T.; Saido, T.C.; Kawashima, R.; Yamamoto, M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020, 40, e00467-19. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Protective Effects of 6-(Methylsulfinyl)hexyl Isothiocyanate on Abeta(1-42)-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and Apoptosis in Mice. Int. J. Mol. Sci. 2018, 19, 2083. [Google Scholar] [CrossRef]
- Azlan, U.K.; Khairul Annuar, N.A.; Mediani, A.; Aizat, W.M.; Damanhuri, H.A.; Tong, X.; Yanagisawa, D.; Tooyama, I.; Wan Ngah, W.Z.; Jantan, I.; et al. An insight into the neuroprotective and anti-neuroinflammatory effects and mechanisms of Moringa oleifera. Front. Pharmacol. 2022, 13, 1035220. [Google Scholar] [CrossRef]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/alpha-CD Pretreatment Induces Neuroprotection in an In Vitro Model of Alzheimer’s Disease: A Transcriptomic Study. Curr. Issues Mol. Biol. 2021, 43, 197–214. [Google Scholar] [CrossRef]
- Chiricosta, L.; Gugliandolo, A.; Diomede, F.; Pizzicannella, J.; Trubiani, O.; Iori, R.; Tardiolo, G.; Guarnieri, S.; Bramanti, P.; Mazzon, E. Moringin Pretreatment Inhibits the Expression of Genes Involved in Mitophagy in the Stem Cell of the Human Periodontal Ligament. Molecules 2019, 24, 3217. [Google Scholar] [CrossRef]
- Sestito, S.; Daniele, S.; Pietrobono, D.; Citi, V.; Bellusci, L.; Chiellini, G.; Calderone, V.; Martini, C.; Rapposelli, S. Memantine prodrug as a new agent for Alzheimer’s Disease. Sci. Rep. 2019, 9, 4612. [Google Scholar] [CrossRef]
- Sestito, S.; Pruccoli, L.; Runfola, M.; Citi, V.; Martelli, A.; Saccomanni, G.; Calderone, V.; Tarozzi, A.; Rapposelli, S. Design and synthesis of H(2)S-donor hybrids: A new treatment for Alzheimer’s disease? Eur. J. Med. Chem. 2019, 184, 111745. [Google Scholar] [CrossRef]
- Francardo, V.; Recchia, A.; Popovic, N.; Andersson, D.; Nissbrandt, H.; Cenci, M.A. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 42, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Duda, J.E. Dietary modifications in Parkinson’s disease: A neuroprotective intervention? Med. Hypotheses 2015, 85, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Gomez-Perez, L.S.; Zepeda, F.; Vidal, R.L.; Grunenwald, F.; Mejias, N.; Pasten, A.; Araya, M.; Ah-Hen, K.S. Assessment of Bio-Compounds Content, Antioxidant Activity, and Neuroprotective Effect of Red Cabbage (Brassica oleracea var. Capitata rubra) Processed by Convective Drying at Different Temperatures. Antioxidants 2023, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, X.; Gong, L.; Zhai, X.; Wang, K.; Qiu, X.; Zhang, H.; Tang, Z.; Jiang, H.; Wang, X. Screening of H(2)S donors with a red emission mitochondria-targetable fluorescent probe: Toward discovering a new therapeutic strategy for Parkinson’s disease. Biosens. Bioelectron. 2023, 237, 115521. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, M.Q.; Chen, Z.Y.; Wu, X.B.; Xia, Z.B.; Chai, J.Y.; Yin, X.P. Sulforaphane prevents PC12 cells from oxidative damage via the Nrf2 pathway. Mol. Med. Rep. 2019, 19, 4890–4896. [Google Scholar] [CrossRef]
- Izumi, Y.; Kataoka, H.; Inose, Y.; Akaike, A.; Koyama, Y.; Kume, T. Neuroprotective effect of an Nrf2-ARE activator identified from a chemical library on dopaminergic neurons. Eur. J. Pharmacol. 2018, 818, 470–479. [Google Scholar] [CrossRef]
- Pu, Y.; Qu, Y.; Chang, L.; Wang, S.M.; Zhang, K.; Ushida, Y.; Suganuma, H.; Hashimoto, K. Dietary intake of glucoraphanin prevents the reduction of dopamine transporter in the mouse striatum after repeated administration of MPTP. Neuropsychopharmacol. Rep. 2019, 39, 247–251. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Hu, Z.; Cai, X.; Yang, J.; Sun, X.; et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci. Rep. 2016, 6, 32206. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, L.; Cao, Q.; Luo, H.; Yao, W.; Zhang, J.C. Inhibition of abnormal C/EBPbeta/alpha-Syn signaling pathway through activation of Nrf2 ameliorates Parkinson’s disease-like pathology. Aging Cell 2023, 22, e13958. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, M.P. A Complex Interplay of DJ-1, LRRK2, and Nrf2 in the Regulation of Mitochondrial Function in Cypermethrin-Induced Parkinsonism. Mol. Neurobiol. 2024, 61, 953–970. [Google Scholar] [CrossRef]
- Asuncion, R.M.D.; Ramani, P.K. Rett Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Renieri, A.; Mari, F.; Mencarelli, M.A.; Scala, E.; Ariani, F.; Longo, I.; Meloni, I.; Cevenini, G.; Pini, G.; Hayek, G.; et al. Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant). Brain Dev. 2009, 31, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zou, Q.; Zhao, X.; Zhang, Y.; Qu, Y.; Wang, N.; Murayama, S.; Qi, Q.; Hashimoto, K.; Lin, S.; et al. Regulation of BDNF transcription by Nrf2 and MeCP2 ameliorates MPTP-induced neurotoxicity. Cell Death Discov. 2022, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Piberger, A.L.; Budnowski, J.; Herz, C.; Schreiner, M.; Blaut, M.; Hartwig, A.; Lamy, E.; Hanske, L.; Rohn, S. Bioavailability and biotransformation of sulforaphane and erucin metabolites in different biological matrices determined by LC-MS-MS. Anal. Bioanal. Chem. 2015, 407, 1819–1829. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Djemil, A.; D’Amico, M.; Pruccoli, L.; Cantelli-Forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865. [Google Scholar] [CrossRef]
- Balducci, M.; Perez, J.T.; Del Rio, C.T.; Perez, M.C.; Carranza, A.D.V.; Gomez Escribano, A.P.; Vazquez-Manrique, R.P.; Tarozzi, A. Erucin, a Natural Isothiocyanate, Prevents Polyglutamine-Induced Toxicity in Caenorhabditis elegans via aak-2/AMPK and daf-16/FOXO Signaling. Int. J. Mol. Sci. 2024, 25, 12220. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The Isothiocyanate Isolated from Moringa oleifera Shows Potent Anti-Inflammatory Activity in the Treatment of Murine Subacute Parkinson’s Disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef]
- Lee, J.A.; Son, H.J.; Park, K.D.; Han, S.H.; Shin, N.; Kim, J.H.; Kim, H.R.; Kim, D.J.; Hwang, O. A Novel Compound ITC-3 Activates the Nrf2 Signaling and Provides Neuroprotection in Parkinson’s Disease Models. Neurotox. Res. 2015, 28, 332–345. [Google Scholar] [CrossRef]
- Lee, J.A.; Son, H.J.; Kim, J.H.; Park, K.D.; Shin, N.; Kim, H.R.; Kim, E.M.; Kim, D.J.; Hwang, O. A novel synthetic isothiocyanate ITC-57 displays antioxidant, anti-inflammatory, and neuroprotective properties in a mouse Parkinson’s disease model. Free Radic. Res. 2016, 50, 1188–1199. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Mittra, N.; Singh, B.K.; Singh, C. Inhibition of glutathione S-transferase-pi triggers c-jun N-terminal kinase-dependent neuronal death in Zn-induced Parkinsonism. Mol. Cell. Biochem. 2019, 452, 95–104. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Tarozzi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotection by 6-(methylsulfinyl)hexyl isothiocyanate in a 6-hydroxydopamine mouse model of Parkinson’s disease. Brain Res. 2014, 1589, 93–104. [Google Scholar] [CrossRef]
- Wright, A.F. The Redox Stress Test: A novel technique reveals oxidative stress in Parkinson’s disease. Med. Res. Arch. 2024, 12, 4955. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Chartier, G.; Quandt, J. Modulating inflammation and neuroprotection in multiple sclerosis. J. Neurosci. Res. 2018, 96, 927–950. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, B.; Ignarro, L.J.; Montestruque, S.; Smoll, A.; Merrill, J.E. Nitric oxide as a potential pathological mechanism in demyelination: Its differential effects on primary glial cells in vitro. Neuroscience 1994, 61, 575–585. [Google Scholar] [CrossRef]
- Lim, J.L.; van der Pol, S.M.; Baron, W.; McCord, J.M.; de Vries, H.E.; van Horssen, J. Protandim Protects Oligodendrocytes against an Oxidative Insult. Antioxidants 2016, 5, 30. [Google Scholar] [CrossRef]
- Spencer, E.S.; Dale, E.J.; Gommans, A.L.; Rutledge, M.T.; Vo, C.T.; Nakatani, Y.; Gamble, A.B.; Smith, R.A.; Wilbanks, S.M.; Hampton, M.B.; et al. Multiple binding modes of isothiocyanates that inhibit macrophage migration inhibitory factor. Eur. J. Med. Chem. 2015, 93, 501–510. [Google Scholar] [CrossRef]
- Ellwardt, E.; Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp. Neurol. 2014, 262 Pt A, 8–17. [Google Scholar] [CrossRef]
- Yoo, I.H.; Kim, M.J.; Kim, J.; Sung, J.J.; Park, S.T.; Ahn, S.W. The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis. J. Korean Med. Sci 2019, 34, e197. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. The protective effects of bioactive (RS)-glucoraphanin on the permeability of the mice blood-brain barrier following experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol Sci 2014, 18, 194–204. [Google Scholar]
- Galea, I.; Copple, I.M.; Howat, D.W.; Franklin, S. SFX-01 reduces residual disability after experimental autoimmune encephalomyelitis. Mult. Scler. Relat. Disord. 2019, 30, 257–261. [Google Scholar] [CrossRef]
- Galuppo, M.; Giacoppo, S.; De Nicola, G.R.; Iori, R.; Navarra, M.; Lombardo, G.E.; Bramanti, P.; Mazzon, E. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2014, 95, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Soundara Rajan, T.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. Moringin activates Wnt canonical pathway by inhibiting GSK3beta in a mouse model of experimental autoimmune encephalomyelitis. Drug Des. Devel. Ther. 2016, 10, 3291–3304. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Iori, R.; Bramanti, P.; Mazzon, E. Topical moringin-cream relieves neuropathic pain by suppression of inflammatory pathway and voltage-gated ion channels in murine model of multiple sclerosis. Mol. Pain 2017, 13, 1744806917724318. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, Q.; Zhao, P.; Gao, Y.; Xi, Y.; Wang, X.; Liang, Y.; Shi, H.; Ma, Y. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behav. Brain Res. 2016, 301, 55–62. [Google Scholar] [CrossRef]
- Panjwani, A.A.; Liu, H.; Fahey, J.W. Crucifers and related vegetables and supplements for neurologic disorders: What is the evidence? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 451–457. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Talalay, P.; Fahey, J.W. Biomarker-Guided Strategy for Treatment of Autism Spectrum Disorder (ASD). CNS Neurol. Disord. Drug Targets 2016, 15, 602–613. [Google Scholar] [CrossRef]
- Alshehri, S.; Ahmad, S.F.; Albekairi, N.A.; Alqarni, S.S.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; Bakheet, S.A.; Nadeem, A. Thioredoxin 1 and Thioredoxin Reductase 1 Redox System Is Dysregulated in Neutrophils of Subjects with Autism: In Vitro Effects of Environmental Toxicant, Methylmercury. Toxics 2023, 11, 739. [Google Scholar] [CrossRef]
- Shahlaei, M.; Saeidifar, M.; Zamanian, A. Sustained release of sulforaphane by bioactive extracellular vesicles for neuroprotective effect on chick model. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2636–2648. [Google Scholar] [CrossRef]
- Liu, H.; Zimmerman, A.W.; Singh, K.; Connors, S.L.; Diggins, E.; Stephenson, K.K.; Dinkova-Kostova, A.T.; Fahey, J.W. Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder. Sci. Rep. 2020, 10, 5822. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Bakheet, S.A.; Ibrahim, K.E.; Alqahtani, F.; Alqinyah, M. Nrf2 activator, sulforaphane ameliorates autism-like symptoms through suppression of Th17 related signaling and rectification of oxidant-antioxidant imbalance in periphery and brain of BTBR T+tf/J mice. Behav. Brain Res. 2019, 364, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, L.; Dai, S.; Zheng, H.; Cui, X.; Ou, J.; Zhang, X. Therapeutic efficacy of sulforaphane in autism spectrum disorders and its association with gut microbiota: Animal model and human longitudinal studies. Front. Nutr. 2023, 10, 1294057. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, A.; Ishima, T.; Hirai, A.; Suzuki, S.; Suganuma, H.; Hashimoto, K. Dietary intake of glucoraphanin during pregnancy and lactation prevents the behavioral abnormalities in the offspring after maternal immune activation. Neuropsychopharmacol. Rep. 2020, 40, 268–274. [Google Scholar] [CrossRef]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Lynch, R.; Diggins, E.L.; Connors, S.L.; Zimmerman, A.W.; Singh, K.; Liu, H.; Talalay, P.; Fahey, J.W. Sulforaphane from Broccoli Reduces Symptoms of Autism: A Follow-up Case Series from a Randomized Double-blind Study. Glob. Adv. Health Med. 2017, 6, 2164957X17735826. [Google Scholar] [CrossRef]
- Bent, S.; Lawton, B.; Warren, T.; Widjaja, F.; Dang, K.; Fahey, J.W.; Cornblatt, B.; Kinchen, J.M.; Delucchi, K.; Hendren, R.L. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol. Autism 2018, 9, 35. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Amirimoghaddam-Yazdi, Z.; Moghaddam, H.S.; Mohammadi, M.R.; Akhondzadeh, S. Sulforaphane as an adjunctive treatment for irritability in children with autism spectrum disorder: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin. Neurosci. 2020, 74, 398–405. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Singh, K.; Connors, S.L.; Liu, H.; Panjwani, A.A.; Lee, L.C.; Diggins, E.; Foley, A.; Melnyk, S.; Singh, I.N.; et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder. Mol. Autism 2021, 12, 38. [Google Scholar] [CrossRef]
- Magner, M.; Thorova, K.; Zupova, V.; Houska, M.; Svandova, I.; Novotna, P.; Triska, J.; Vrchotova, N.; Soural, I.; Jilek, L. Sulforaphane Treatment in Children with Autism: A Prospective Randomized Double-Blind Study. Nutrients 2023, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Smith, R.C.; Tobe, R.H.; Lin, J.; Arriaza, J.; Fahey, J.W.; Liu, R.; Zeng, Y.; Liu, Y.; Huang, L.; et al. Efficacy of Sulforaphane in Treatment of Children with Autism Spectrum Disorder: A Randomized Double-Blind Placebo-Controlled Multi-center Trial. J. Autism Dev. Disord. 2024, 54, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Meral, G.; Aslan, E.S.; Burkay, N.; Alper Acar, E.G.; Karagoz, M.F.; Ozkaya, M.; Sahin, E.; Alp, M.Y. Importance of Using Epigenetic Nutrition and Supplements Based on Nutrigenetic Tests in Personalized Medicine. Cureus 2024, 16, e66959. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.E.; Healey, K.M.; Gagen, E.C.; Roberts, D.L.; Penn, D.L. Social cognition in schizophrenia: Factor structure, clinical and functional correlates. J. Ment. Health 2016, 25, 330–337. [Google Scholar] [CrossRef]
- Kahn, R.S.; Keefe, R.S. Schizophrenia is a cognitive illness: Time for a change in focus. JAMA Psychiatry 2013, 70, 1107–1112. [Google Scholar] [CrossRef]
- Hashimoto, K. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin. Ther. Targets 2014, 18, 1049–1063. [Google Scholar] [CrossRef]
- Ladermann, A.; Edwards, T.B.; Walch, G. Arm lengthening after reverse shoulder arthroplasty: A review. Int. Orthop. 2014, 38, 991–1000. [Google Scholar] [CrossRef]
- Koskuvi, M.; Porsti, E.; Hewitt, T.; Rasanen, N.; Wu, Y.C.; Trontti, K.; McQuade, A.; Kalyanaraman, S.; Ojansuu, I.; Vaurio, O.; et al. Genetic contribution to microglial activation in schizophrenia. Mol. Psychiatry 2024, 29, 2622–2633. [Google Scholar] [CrossRef]
- Shirai, Y.; Fujita, Y.; Hashimoto, R.; Ohi, K.; Yamamori, H.; Yasuda, Y.; Ishima, T.; Suganuma, H.; Ushida, Y.; Takeda, M.; et al. Dietary Intake of Sulforaphane-Rich Broccoli Sprout Extracts during Juvenile and Adolescence Can Prevent Phencyclidine-Induced Cognitive Deficits at Adulthood. PLoS ONE 2015, 10, e0127244. [Google Scholar] [CrossRef]
- Matsuura, A.; Ishima, T.; Fujita, Y.; Iwayama, Y.; Hasegawa, S.; Kawahara-Miki, R.; Maekawa, M.; Toyoshima, M.; Ushida, Y.; Suganuma, H.; et al. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Sci. Rep. 2018, 8, 2158. [Google Scholar] [CrossRef]
- Isaacson, R.H.; Beier, J.I.; Khoo, N.K.; Freeman, B.A.; Freyberg, Z.; Arteel, G.E. Olanzapine-induced liver injury in mice: Aggravation by high-fat diet and protection with sulforaphane. J. Nutr. Biochem. 2020, 81, 108399. [Google Scholar] [CrossRef] [PubMed]
- Kaur Sodhi, R.; Kumar, H.; Singh, R.; Bansal, Y.; Singh, Y.; Kiran Kondepudi, K.; Bishnoi, M.; Kuhad, A. Allyl isothiocyanate, a TRPA1 agonist, protects against olanzapine-induced hypothalamic and hepatic metabolic aberrations in female mice. Biochem. Pharmacol. 2024, 222, 116074. [Google Scholar] [CrossRef] [PubMed]
- El-Shoura, E.A.M.; Abdelzaher, L.A.; Mahmoud, N.I.; Farghaly, O.A.; Sabry, M.; Girgis Shahataa, M.; Salem, E.A.; Saad, H.M.; Elhussieny, O.; Kozman, M.R.; et al. Combined sulforaphane and beta-sitosterol mitigate olanzapine-induced metabolic disorders in rats: Insights on FOXO, PI3K/AKT, JAK/STAT3, and MAPK signaling pathways. Int. Immunopharmacol. 2024, 140, 112904. [Google Scholar] [CrossRef]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62–67. [Google Scholar] [CrossRef]
- Dickerson, F.; Origoni, A.; Katsafanas, E.; Squire, A.; Newman, T.; Fahey, J.; Xiao, J.C.; Stallings, C.; Goga, J.; Khushalani, S.; et al. Randomized controlled trial of an adjunctive sulforaphane nutraceutical in schizophrenia. Schizophr. Res. 2021, 231, 142–144. [Google Scholar] [CrossRef]
- Hei, G.; Smith, R.C.; Li, R.; Ou, J.; Song, X.; Zheng, Y.; He, Y.; Arriaza, J.; Fahey, J.W.; Cornblatt, B.; et al. Sulforaphane Effects on Cognition and Symptoms in First and Early Episode Schizophrenia: A Randomized Double-Blind Trial. Schizophr. Bull. Open 2022, 3, sgac024. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, W.; Lu, X.; Zhou, H.; Huang, J.; Xu, Z.; Liao, H.; Liang, J.; Liang, M.; Ye, C.; et al. The association of SOD and HsCRP with the efficacy of sulforaphane in schizophrenia patients with residual negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1083–1092. [Google Scholar] [CrossRef]
- Maletic, V.; Raison, C.L. Neurobiology of depression, fibromyalgia and neuropathic pain. Front. Biosci. 2009, 14, 5291–5338. [Google Scholar] [CrossRef]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef]
- Stachowicz, K.; Sowa-Kucma, M. The treatment of depression—Searching for new ideas. Front. Pharmacol. 2022, 13, 988648. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Pathogen-Host Defense in the Evolution of Depression: Insights into Epidemiology, Genetics, Bioregional Differences and Female Preponderance. Neuropsychopharmacology 2017, 42, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The Role of Early Life Stress in HPA Axis and Anxiety. Adv. Exp. Med. Biol. 2020, 1191, 141–153. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical Practice Guidelines for the management of Depression. Indian J. Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef]
- Sackeim, H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry 2001, 62 (Suppl. S16), 10–17. [Google Scholar]
- Bigger, J.T.; Glassman, A.H. The American Heart Association science advisory on depression and coronary heart disease: An exploration of the issues raised. Cleve. Clin. J. Med. 2010, 77 (Suppl. S3), S12–S19. [Google Scholar] [CrossRef]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.C.; Ishima, T.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Suganuma, H.; et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016, 6, 30659. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Wu, J.; Ushida, Y.; Suganuma, H.; et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutr. Biochem. 2017, 39, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leanez, S.; Pol, O. Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated With Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Bove, M.; Sikora, V.; Dimonte, S.; Morgese, M.G.; Schiavone, S.; Di Cesare Mannelli, L.; Ghelardini, C.; Trabace, L. Glucoraphanin Triggers Rapid Antidepressant Responses in a Rat Model of Beta Amyloid-Induced Depressive-like Behaviour. Pharmaceuticals 2022, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Batalle, G.; Cabarga, L.; Pol, O. The Inhibitory Effects of Slow-Releasing Hydrogen Sulfide Donors in the Mechanical Allodynia, Grip Strength Deficits, and Depressive-Like Behaviors Associated with Chronic Osteoarthritis Pain. Antioxidants 2019, 9, 31. [Google Scholar] [CrossRef]
- Cabarga, L.; Batalle, G.; Pol, O. Treatment with slow-releasing hydrogen sulfide donors inhibits the nociceptive and depressive-like behaviours accompanying chronic neuropathic pain: Endogenous antioxidant system activation. J. Psychopharmacol. 2020, 34, 737–749. [Google Scholar] [CrossRef]
- Yao, W.; Lin, S.; Su, J.; Cao, Q.; Chen, Y.; Chen, J.; Zhang, Z.; Hashimoto, K.; Qi, Q.; Zhang, J.C. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl. Psychiatry 2021, 11, 140. [Google Scholar] [CrossRef]
- Tang, R.; Cao, Q.Q.; Hu, S.W.; He, L.J.; Du, P.F.; Chen, G.; Fu, R.; Xiao, F.; Sun, Y.R.; Zhang, J.C.; et al. Sulforaphane activates anti-inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol. Sin. 2022, 43, 829–839. [Google Scholar] [CrossRef]
- He, L.; Zheng, Y.; Huang, L.; Ye, J.; Ye, Y.; Luo, H.; Chen, X.; Yao, W.; Chen, J.; Zhang, J.C. Nrf2 regulates the arginase 1(+) microglia phenotype through the initiation of TREM2 transcription, ameliorating depression-like behavior in mice. Transl. Psychiatry 2022, 12, 459. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, L.Y.; Gonzalez-Trujano, M.E.; Moreno, D.A.; Vibrans, H.; Castillo-Juarez, I.; Dorazco-Gonzalez, A.; Soto-Hernandez, M. Pharmacological evaluation of the anxiolytic-like effects of an aqueous extract of the Raphanus sativus L. sprouts in mice. Biomed. Pharmacother. 2023, 162, 114579. [Google Scholar] [CrossRef]
- Panczyszyn-Trzewik, P.; Stachowicz, K.; Misztak, P.; Nowak, G.; Sowa-Kucma, M. Repeated Sulforaphane Treatment Reverses Depressive-like Behavior and Exerts Antioxidant Effects in the Olfactory Bulbectomy Model in Mice. Pharmaceuticals 2024, 17, 762. [Google Scholar] [CrossRef]
- Ghazizadeh-Hashemi, F.; Bagheri, S.; Ashraf-Ganjouei, A.; Moradi, K.; Shahmansouri, N.; Mehrpooya, M.; Noorbala, A.A.; Akhondzadeh, S. Efficacy and safety of sulforaphane for treatment of mild to moderate depression in patients with history of cardiac interventions: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin. Neurosci. 2021, 75, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Bonner, A.M. The Revised Definition and Classification of Epilepsy for Neurodiagnostic Technologists. Neurodiagnostic J. 2018, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Dubey, V.; Dixit, A.B.; Tripathi, M.; Chandra, P.S.; Banerjee, J. Differential Levels of Tryptophan-Kynurenine Pathway Metabolites in the Hippocampus, Anterior Temporal Lobe, and Neocortex in an Animal Model of Temporal Lobe Epilepsy. Cells 2022, 11, 3560. [Google Scholar] [CrossRef]
- Janmohamed, M.; Brodie, M.J.; Kwan, P. Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020, 168, 107790. [Google Scholar] [CrossRef]
- Kubova, H.; Mares, P.; Suchomelova, L.; Brozek, G.; Druga, R.; Pitkanen, A. Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur. J. Neurosci. 2004, 19, 3255–3265. [Google Scholar] [CrossRef]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Jia, W.; Zhang, C.; Boczek, T.; Harding, M.; Liu, Y.; Li, M.; Zhang, S.; Lei, S.; et al. Circulating glutathione peroxidase and superoxide dismutase levels in patients with epilepsy: A meta-analysis. Seizure 2021, 91, 278–286. [Google Scholar] [CrossRef]
- El Badawy, S.A.; Ogaly, H.A.; Abd-Elsalam, R.M.; Azouz, A.A. Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-kappaB signaling pathways on indomethacin-induced gastric injury in rats. Food Funct. 2021, 12, 6001–6013. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Zhang, G.; Fang, H.; Wang, H.; Zang, H.; Xie, T.; Wang, W. Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res. 2014, 1544, 54–61. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Borges, K. Sulforaphane is anticonvulsant and improves mitochondrial function. J. Neurochem. 2015, 135, 932–942. [Google Scholar] [CrossRef]
- Socala, K.; Nieoczym, D.; Kowalczuk-Vasilev, E.; Wyska, E.; Wlaz, P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol. Appl. Pharmacol. 2017, 326, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Pastore, A.; Pascente, R.; Liang, L.P.; Villa, B.R.; et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain A J. Neurol. 2019, 142, e39. [Google Scholar] [CrossRef] [PubMed]
- Sandouka, S.; Shekh-Ahmad, T. Induction of the Nrf2 Pathway by Sulforaphane Is Neuroprotective in a Rat Temporal Lobe Epilepsy Model. Antioxidants 2021, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Danek, J.; Danacikova, S.; Kala, D.; Svoboda, J.; Kapoor, S.; Posusta, A.; Folbergrova, J.; Tauchmannova, K.; Mracek, T.; Otahal, J. Sulforaphane Ameliorates Metabolic Changes Associated With Status Epilepticus in Immature Rats. Front. Cell. Neurosci. 2022, 16, 855161. [Google Scholar] [CrossRef]
- Folbergrova, J.; Jesina, P.; Otahal, J. Protective Effect of Sulforaphane on Oxidative Stress and Mitochondrial Dysfunction Associated with Status Epilepticus in Immature Rats. Mol. Neurobiol. 2023, 60, 2024–2035. [Google Scholar] [CrossRef]
- Aghaie, F.; Rajabi, M.; Hosseini, A.; Moradifar, F.; Koneshlou, S.; Hosseini, A. Preventive Effects of Sinigrin Against the Memory Deterioration in the Pentylenetetrazole-Kindled Male Wistar Rats: Possible Modulation of NLRP3 Pathway. Neuromolecular Med. 2022, 24, 311–319. [Google Scholar] [CrossRef]
- Xiaoyu, C.; Hongzhen, Z.; Nan, P.; Tengwei, G.; Yanan, G.; Yan, G.; Haiyan, L.; Li, M.; Haiya, W.; Yujun, W.; et al. Benzyl isothiocyanate ameliorates cognitive function in mice of chronic temporal lobe epilepsy. Front. Neurol. 2024, 15, 1330102. [Google Scholar] [CrossRef]
- Canistro, D.; Barillari, J.; Melega, S.; Sapone, A.; Iori, R.; Speroni, E.; Paolini, M. Black cabbage seed extract affects rat Cyp-mediated biotransformation: Organ and sex related differences. Food Chem. Toxicol. 2012, 50, 2612–2621. [Google Scholar] [CrossRef]
- Paolini, M.; Perocco, P.; Canistro, D.; Valgimigli, L.; Pedulli, G.F.; Iori, R.; Croce, C.D.; Cantelli-Forti, G.; Legator, M.S.; Abdel-Rahman, S.Z. Induction of cytochrome P450, generation of oxidative stress and in vitro cell-transforming and DNA-damaging activities by glucoraphanin, the bioprecursor of the chemopreventive agent sulforaphane found in broccoli. Carcinogenesis 2004, 25, 61–67. [Google Scholar] [CrossRef]
- Nugon-Baudon, L.; Rabot, S.; Szylit, O.; Raibaud, P. Glucosinolates toxicity in growing rats: Interactions with the hepatic detoxification system. Xenobiotica 1990, 20, 223–230. [Google Scholar] [CrossRef]
- Galanty, A.; Grudzinska, M.; Pazdziora, W.; Sluzaly, P.; Pasko, P. Do Brassica Vegetables Affect Thyroid Function?—A Comprehensive Systematic Review. Int. J. Mol. Sci. 2024, 25, 3988. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, F.; Capasso, S.; Cipollaro, M.; Pagnotta, E.; Carteni, M.; Casale, F.; Iori, R.; Galderisi, U. Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age 2012, 34, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, F.; Capasso, S.; Di Bernardo, G.; Cipollaro, M.; Pagnotta, E.; Carteni, M.; Casale, F.; Iori, R.; Giordano, A.; Galderisi, U. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis 2012, 17, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Paolillo, M.; Lenzi, M.; Colombo, E.; Vallorani, L.; Casadei, L.; Martinelli, C.; Fimognari, C. Sulforaphane induces DNA single strand breaks in cultured human cells. Mutat. Res. 2010, 689, 65–73. [Google Scholar] [CrossRef]
- Xie, J.; Yang, M.R.; Hu, X.; Hong, Z.S.; Bai, Y.Y.; Sheng, J.; Tian, Y.; Shi, C.Y. Moringa oleifera Lam. Isothiocyanate Quinazolinone Derivatives Inhibit U251 Glioma Cell Proliferation through Cell Cycle Regulation and Apoptosis Induction. Int. J. Mol. Sci. 2023, 24, 11376. [Google Scholar] [CrossRef]
- Hac, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Wegrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).