Identification of Peroxiredoxin (PRX) Genes from Pepper Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Identification of PRX Genes Members
2.3. Analysis of Gene Structure and Regulatory Elements
2.4. Analysis of Localization and Protein Characteristics
2.5. Phylogenetic Analysis and Conserved Motifs
2.6. In Silico Analysis of Possible Protein–Protein Interactions
2.7. Treatment of Sweet Pepper Fruits in an Environment Enriched in Nitric Oxide (NO)
2.8. Protein Extraction from Pepper Fruits
2.9. Immunoprecipitation of Pepper S-Nitrosated Protein with Magnetic Particles
2.10. Electrophoresis in Polyacrylamide Gels Under Denaturing Conditions and Immunoblotting Analysis
2.11. Mass Spectrometry Analysis of S-Nitrosated Protein Obtained by Immunoprecipitation
3. Results
3.1. Identification of Genes Encoding Prxs and Their Chromosomal Localization
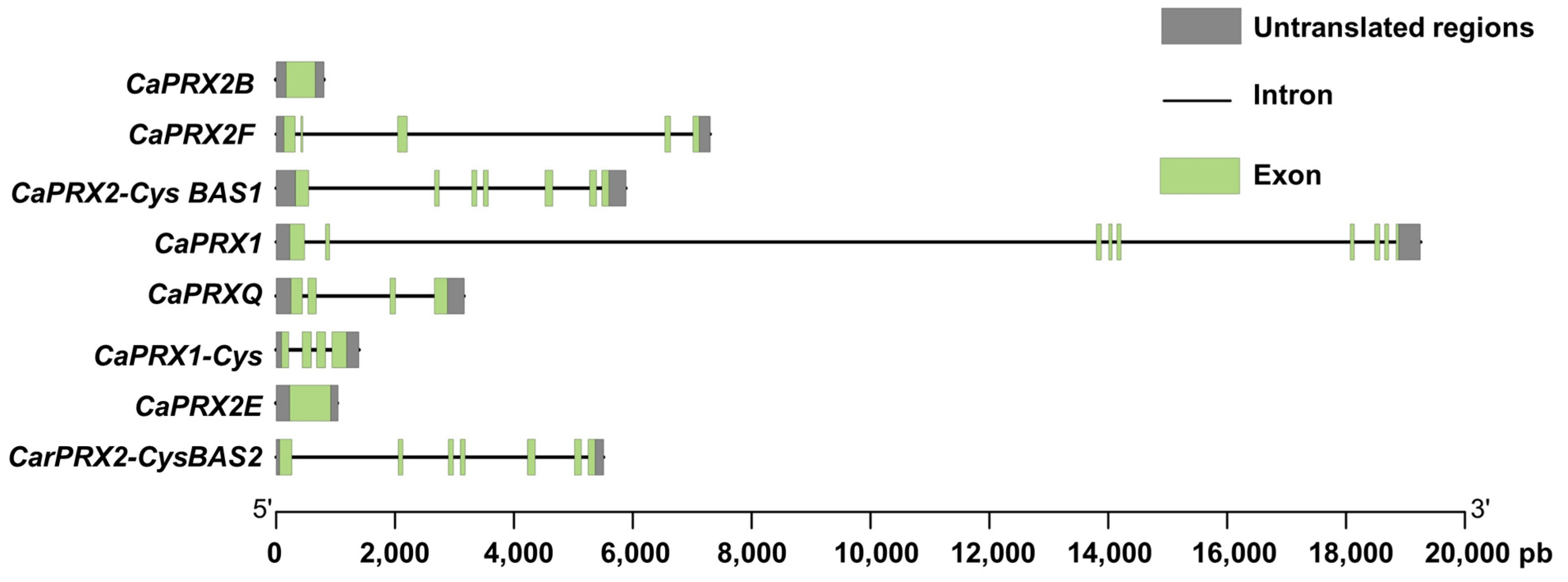
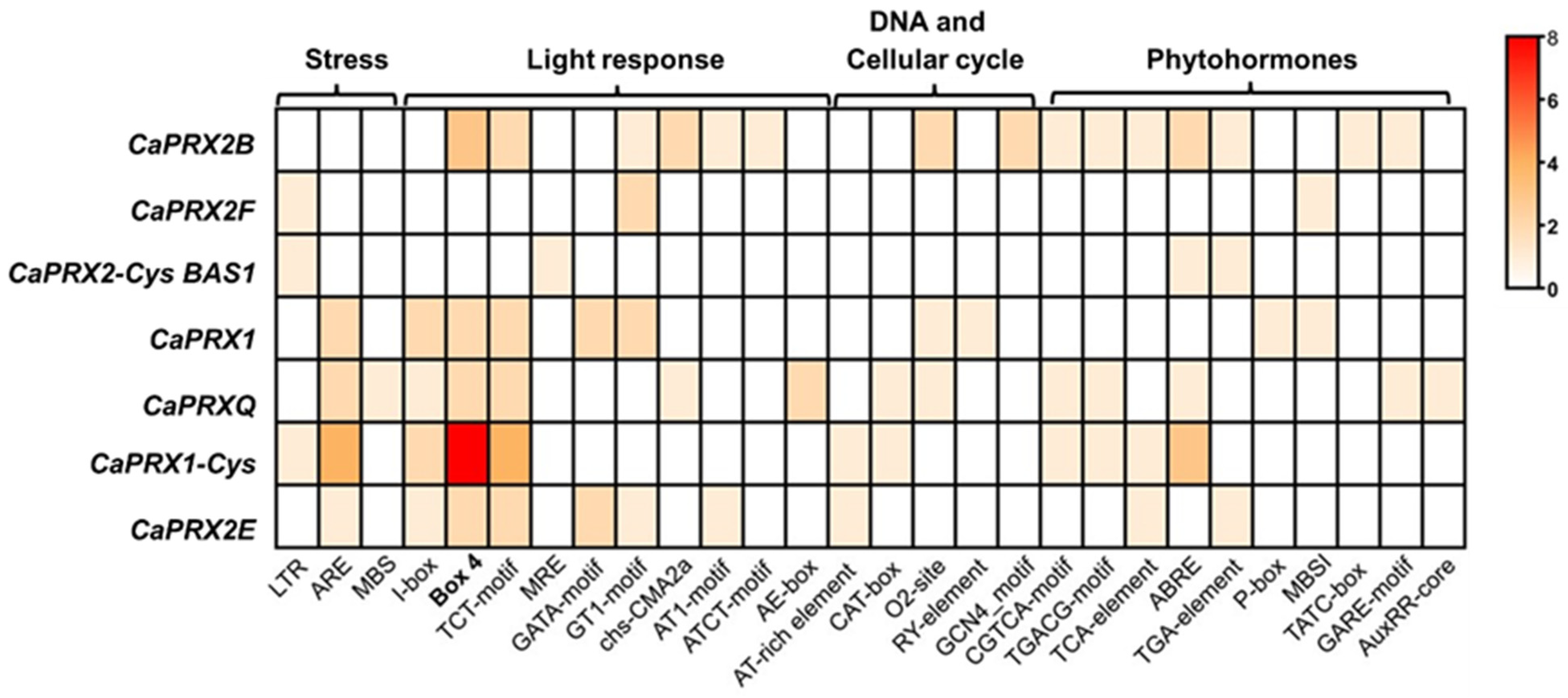
3.2. Analysis of CaPRX Gene Structure and Regulatory Elements
3.3. Analysis of Preserved Prx Protein Motifs
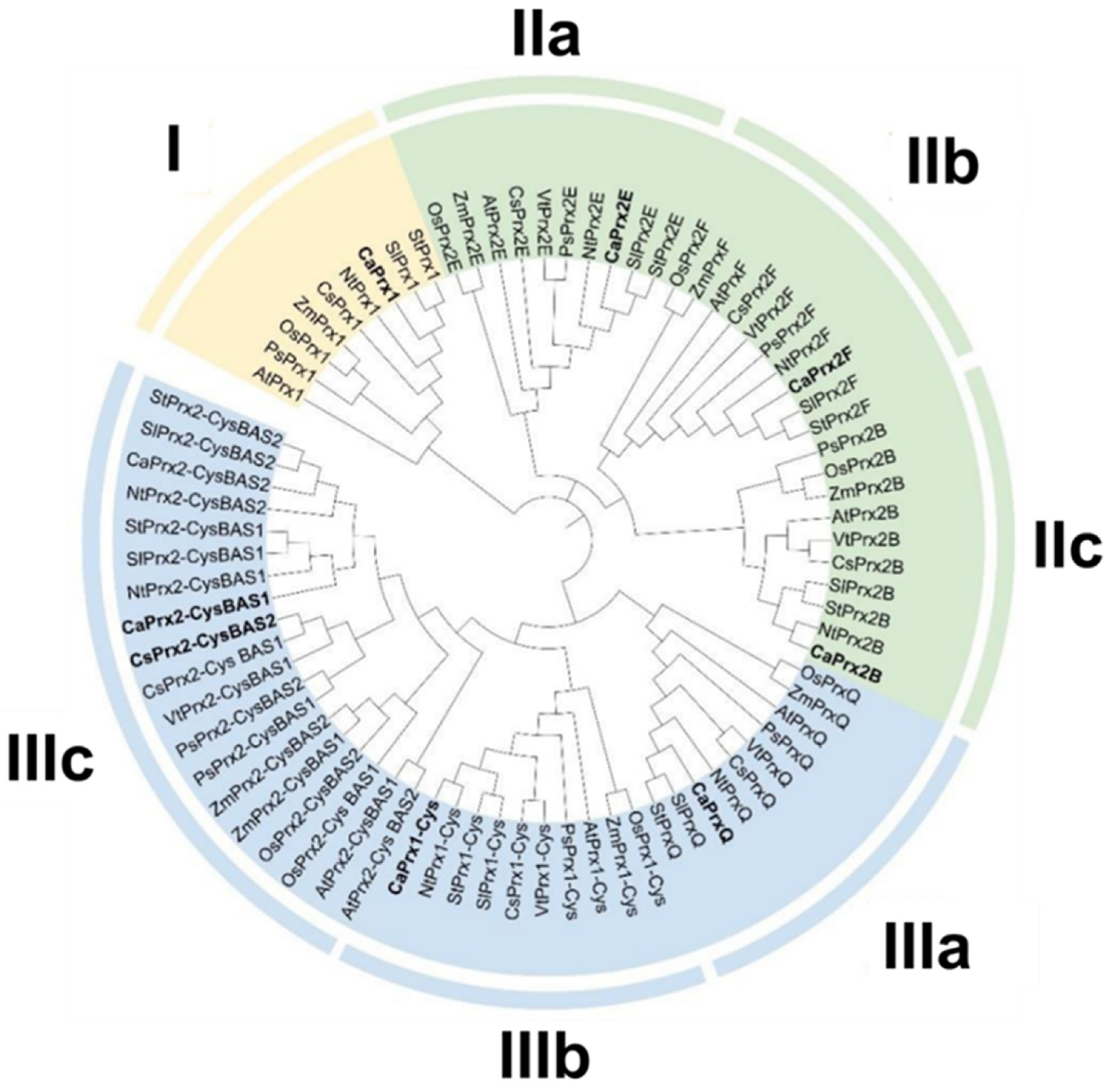
3.4. Phylogenetic Tree
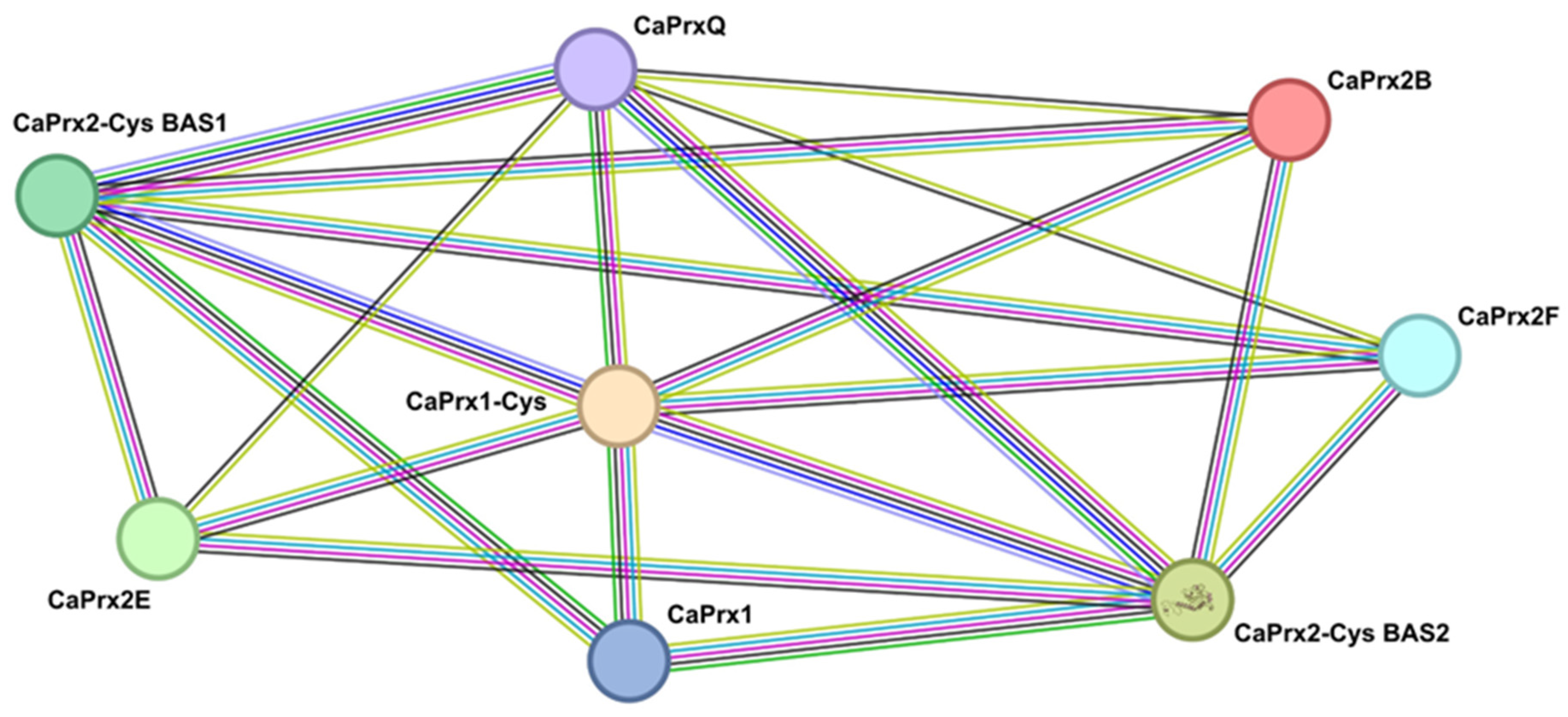
3.5. Possible Protein–Protein Interactions
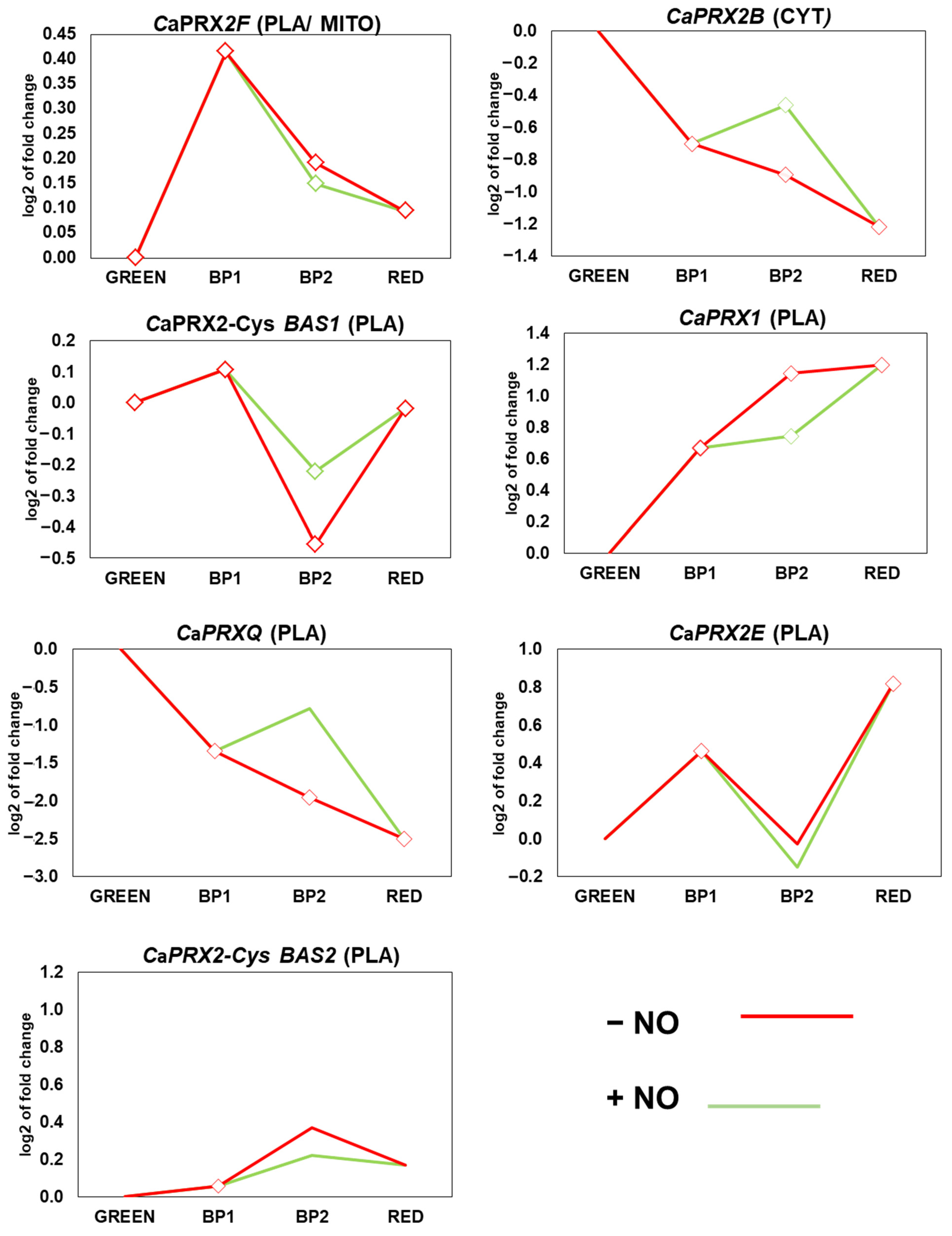
3.6. Expression of CaPRXs Genes in Fruits at Ripening and After Treatment with NO
3.7. Comparison of the Expression of CaPRX Genes in Sweet and Hot Pepper Fruits
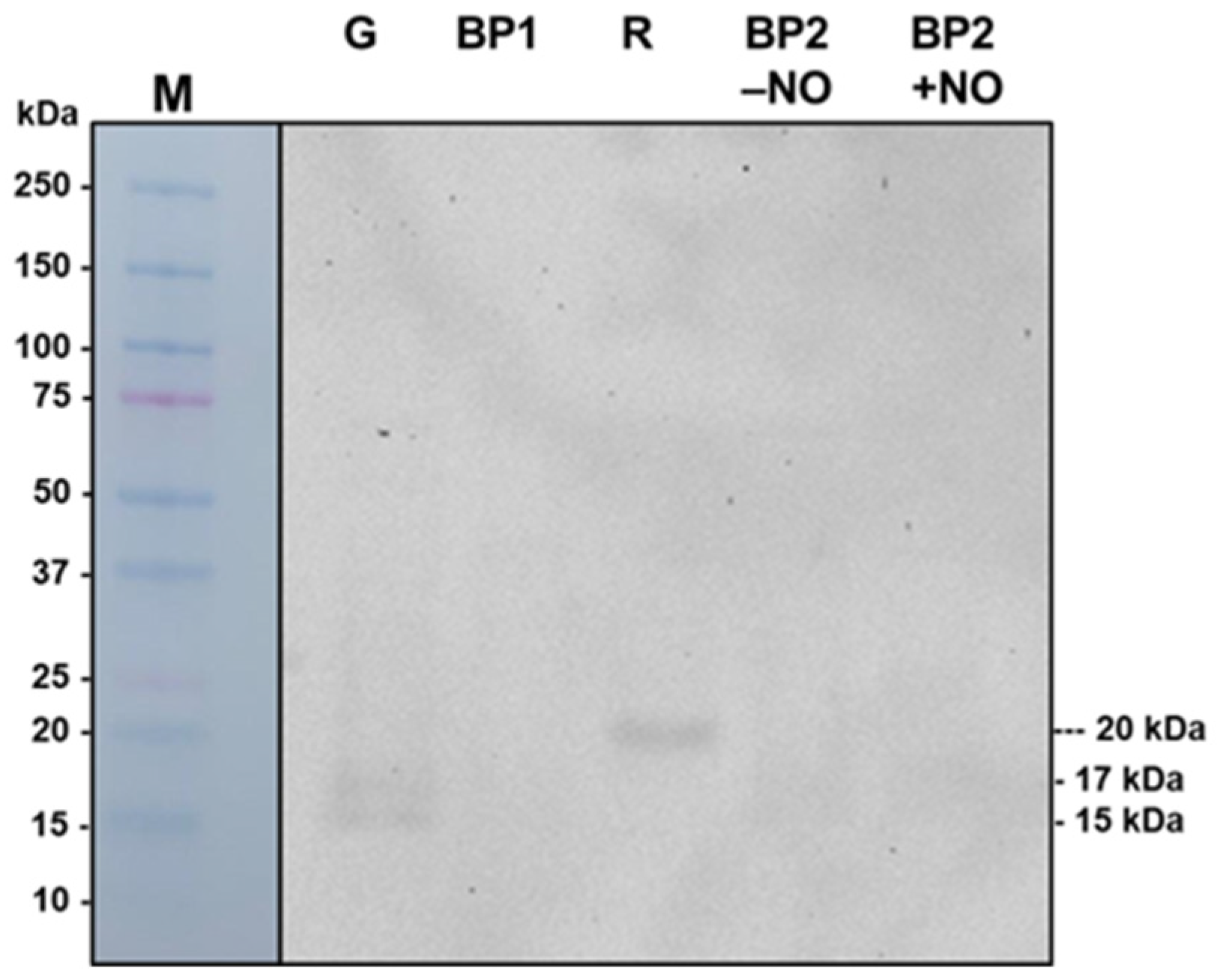
3.8. Immunodetection of Prxs in Pepper Fruits
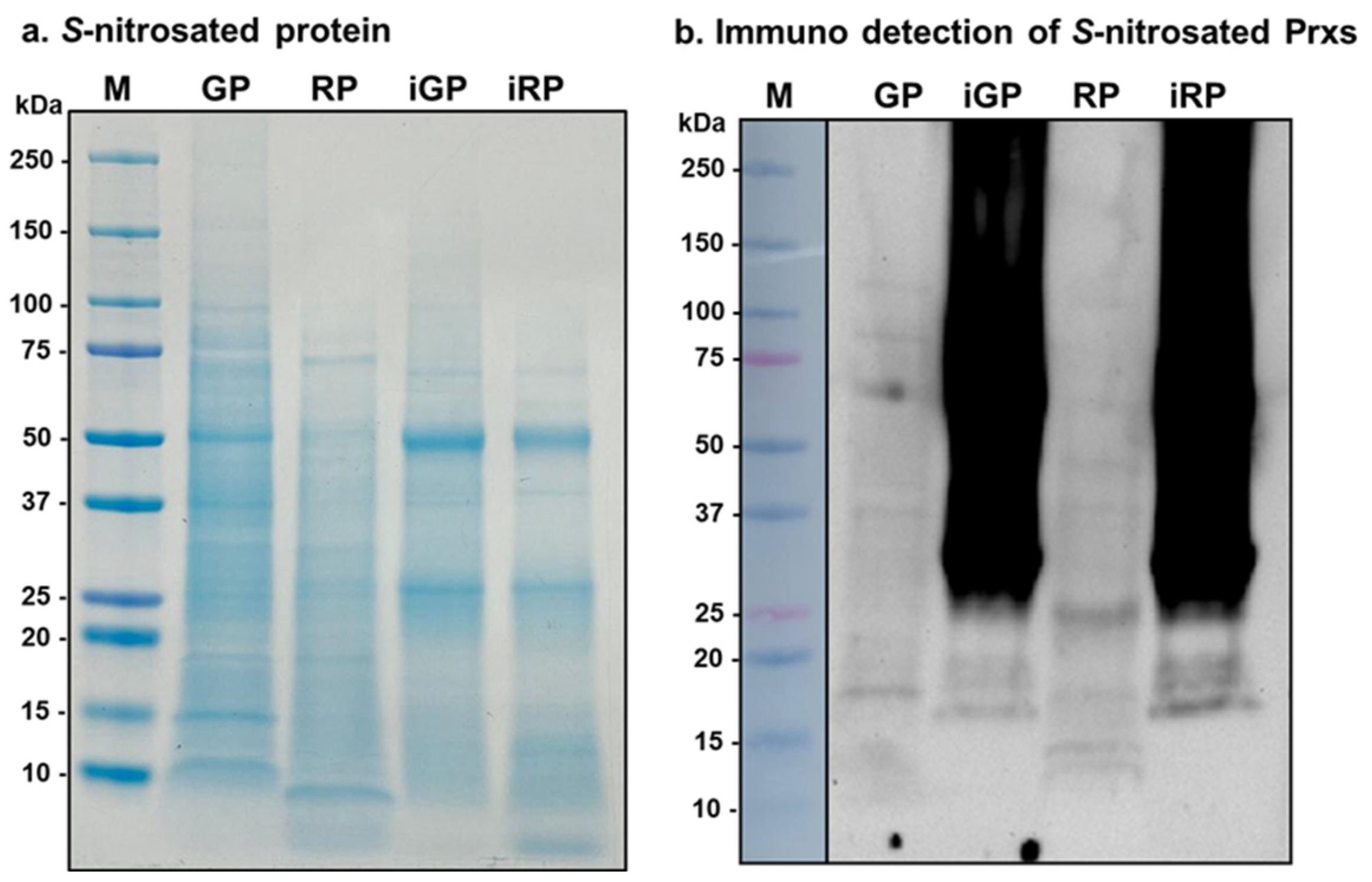
3.9. Immunoprecipitation with Magnetic Beads of S-Nitrosated Proteins and Detection of S-Nitrosated CaPrxs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Cardozo, G.; Mastrogiovanni, M.; Zeida, A.; Viera, N.; Radi, R.; Reyes, A.M.; Trujillo, M. Mitochondrial Peroxiredoxin 3 Is Rapidly Oxidized and Hyperoxidized by Fatty Acid Hydroperoxides. Antioxidants 2023, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, L.; Dietz, K.J. Plant thiol peroxidases as redox sensors and signal transducers in abiotic stress acclimation. Free Radic. Biol. Med. 2022, 193, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Bryk, R.; Griffin, P.; Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 2000, 407, 211–215. [Google Scholar] [CrossRef]
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015, 80, 183–190. [Google Scholar] [CrossRef]
- Dietz, K.J. Plant peroxiredoxins. Annu. Rev. Plant Biol. 2003, 54, 93–107. [Google Scholar] [CrossRef]
- Soito, L.; Williamson, C.; Knutson, S.T.; Fetrow, J.S.; Poole, L.B.; Nelson, K.J. PREX: PeroxiRedoxin classification indEX, a database of subfamily assignments across the diverse peroxiredoxin family. Nucleic Acids Res. 2011, 39, D332–D337. [Google Scholar] [CrossRef]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; de Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Dreyer, A.; Treffon, P.; Basiry, D.; Jozefowicz, A.M.; Matros, A.; Mock, H.P.; Dietz, K.J. Function and Regulation of Chloroplast Peroxiredoxin IIE. Antioxidants 2021, 10, 152. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Dietz, K.J.; Małecka, A.; Ratajczak, E. Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging. Antioxidants 2022, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Cerveau, D.; Kraut, A.; Stotz, H.U.; Mueller, M.J.; Couté, Y.; Rey, P. Characterization of the Arabidopsis thaliana 2-Cys peroxiredoxin interactome. Plant Sci. 2016, 252, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.; Hecht, H.-J.; Flohé, L. Peroxiredoxins. Biol. Chem. 2002, 383, 347–364. [Google Scholar] [CrossRef]
- Ojeda, V.; Pérez-Ruiz, J.M.; Cejudo, F.J. 2-Cys Peroxiredoxins Participate in the Oxidation of Chloroplast Enzymes in the Dark. Mol. Plant. 2018, 11, 1377–1388. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jung, Y.J.; Shin, M.R.; Park, J.H.; Nawkar, G.M.; Maibam, P.; Lee, E.S.; Kim, K.S.; Paeng, S.K.; Kim, W.Y.; et al. Molecular and functional properties of three different peroxiredoxin isotypes in Chinese cabbage. Mol. Cells 2012, 33, 27–33. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Qi, H.; Zhang, H.; Wang, L.; Zhang, H.; Cui, C.; Ji, G.; Muhammad, S.; Sun, G.; et al. Overexpression of 2-Cys Peroxiredoxin alleviates the NaHCO3 stress-induced photoinhibition and reactive oxygen species damage of tobacco. Plant Physiol. Biochem. 2023, 201, 107876. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.N.; Rani, R.; Kokane, A.D.; Ghosh, D.K.; Tomar, S.; Sharma, A.K. Characterization of a cytoplasmic 2-Cys peroxiredoxin from Citrus sinensis and its potential role in protection from oxidative damage and wound healing. Int. J. Biol. Macromol. 2022, 209 Pt A, 1088–1099. [Google Scholar] [CrossRef]
- Kim, S.Y.; Paeng, S.K.; Nawkar, G.M.; Maibam, P.; Lee, E.S.; Kim, K.S.; Lee, D.H.; Park, D.J.; Kang, S.B.; Kim, M.R.; et al. The 1-Cys peroxiredoxin, a regulator of seed dormancy, functions as a molecular chaperone under oxidative stress conditions. Plant Sci. 2011, 181, 119–124. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.H. Role of Cytosolic 2-Cys Prx1 and Prx2 in Redox Signaling. Antioxidants 2019, 8, 169. [Google Scholar] [CrossRef]
- Jarret, R.L.; Barboza, G.E.; da Costa Batista, F.R.; Berke, T.; Chou, Y.Y.; Hulse-Kemp, A.; Ochoa-Alejo, N.; Tripodi, P.; Veres, A.; Garcia, C.C.; et al. Capsicum-an abbreviated compendium. J. Am. Soc. Hortic. Sci. 2019, 144, 3–22. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.H.; Mustafa, Y.F. Sweet Bell Pepper: A Focus on Its Nutritional Qualities and Illness-Alleviated Properties. Indian. J. Clin. Biochem. 2024, 39, 459–469. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive Compounds and Fruit Quality of Green Sweet Pepper Grown under Different Colored Shade Netting during Postharvest Storage. J. Food Sci. 2015, 80, H2612–H2618. [Google Scholar] [CrossRef]
- Kasampalis, D.S.; Tsouvaltzis, P.; Ntouros, K.; Gertsis, A.; Gitas, I.; Moshou, D.; Siomos, A.S. Nutritional composition changes in bell pepper as affected by the ripening stage of fruits at harvest or postharvest storage and assessed non-destructively. J. Sci. Food Agric. 2022, 102, 445–454. [Google Scholar] [CrossRef]
- Marra, F.; Maffia, A.; Canino, F.; Petrovicova, B.; Mallamaci, C.; Russo, M.; Iftikhar Hussain, M.; Muscolo, A. Enhancing the nutritional value of sweet bell pepper through moderate NaCl salinity. Heliyon 2023, 9, e22439. [Google Scholar] [CrossRef] [PubMed]
- Rödiger, A.; Agne, B.; Dobritzsch, D.; Helm, S.; Müller, F.; Pötzsch, N.; Baginsky, S. Chromoplast differentiation in bell pepper (Capsicum annuum) fruits. Plant J. 2021, 105, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Momo, J.; Kumar, A.; Islam, K.; Ahmad, I.; Rawoof, A.; Ramchiary, N. A comprehensive update on Capsicum proteomics: Advances and future prospects. J. Proteom. 2022, 261, 104578. [Google Scholar] [CrossRef]
- Seong, G.U.; Yun, D.Y.; Cho, J.S.; Park, S.K.; Lee, G.S.; Choi, J.H.; Park, K.J.; Lim, J.H. Ripening-related metabolic changes in different chili pepper cultivars revealed by nuclear magnetic resonance spectroscopy. J. Sci. Food Agric. 2025. [Google Scholar] [CrossRef]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Ortega-Albero, N.; Barchi, L.; Fita, A.; Díaz, M.; Martínez, F.; Luna-Prohens, J.M.; Rodríguez-Burruezo, A. Genetic diversity, population structure, and phylogeny of insular Spanish pepper landraces (Capsicum annuum L.) through phenotyping and genotyping-by-sequencing. Front. Plant Sci. 2024, 15, 1435427. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.; Jaiswal, V.; Rawoof, A.; Kumar, A.; Nitin, M.; Chhapekar, S.S.; Kumar, N.; Ahmad, I.; Islam, K.; Brahma, V.; et al. Identification of genes involved in fruit development/ripening in Capsicum and development of functional markers. Genomics 2019, 111, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.D.; Sun, H.T.; Yang, L.; Zheng, M.L.; Zhang, J.; Guo, D.L. Hydrogen peroxide priming triggers splicing memory in grape berries. Plant Mol. Biol. 2024, 114, 129. [Google Scholar] [CrossRef]
- Corpas, F.J.; Taboada, J.; Sánchez-Romera, B.; López-Jaramillo, J.; Palma, J.M. Peroxisomal Sulfite Oxidase (SOX), an alternative source of NO in higher plants which is upregulated by H2S. Plant Physiol. Biochem. 2025, 225, 110000. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Z.; Pan, X.; Yang, T.; An, C.; Wang, Y.; Li, L.; Liao, W.; Wang, C. Effects of reactive oxygen species on fruit ripening and postharvest fruit quality. Plant Sci. 2025, 352, 112391. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Yao, K.; Ding, Z.; Zhang, W.; Zhu, Y.; Su, W.; Liao, W. Nitric oxide delayed tomato fruit coloring by regulating chlorophyll- and nitric oxide delayed tomato fruit coloring by regulating chlorophyll- and carotenoid-related genes in a SlSPL6c-dependent manner. Plant Cell Physiol. 2025, pcaf051. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; Taboada, J.; Palma, J.M.; Corpas, F.J. Pepper catalase: A broad analysis of its modulation during fruit ripening and by nitric oxide. Biochem. J. 2024, 481, 883–901. [Google Scholar] [CrossRef]
- González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Class III Peroxidases (POD) in Pepper (Capsicum annuum L.): Genome-Wide Identification and Regulation during Nitric Oxide (NO)-Influenced Fruit Ripening. Antioxidants 2023, 12, 1013. [Google Scholar] [CrossRef]
- González-Gordo, S.; Rodríguez-Ruiz, M.; López-Jaramillo, J.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Nitric Oxide (NO) Differentially Modulates the Ascorbate Peroxidase (APX) Isozymes of Sweet Pepper (Capsicum annuum L.) Fruits. Antioxidants 2022, 11, 765. [Google Scholar] [CrossRef]
- Hulse-Kemp, A.M.; Maheshwari, S.; Stoffel, K.; Hill, T.A.; Jaffe, D.; Williams, S.R.; Weisenfeld, N.; Ramakrishnan, S.; Kumar, V.; Shah, P.; et al. Reference quality assembly of the 3.5-Gb genome of Capsicum annuum from a single linked-read library. Hortic. Res. 2018, 5, 4. [Google Scholar] [CrossRef]
- Gho, Y.S.; Park, S.A.; Kim, S.R.; Chandran, A.K.N.; An, G.; Jung, K.H. Comparative Expression Analysis of Rice and Arabidopsis Peroxiredoxin Genes Suggests Conserved or Diversified Roles Between the Two Species and Leads to the Identification of Tandemly Duplicated Rice Peroxiredoxin Genes Differentially Expressed in Seeds. Rice 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. String v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar] [CrossRef]
- Palma, J.M.; Ruiz, C.; Corpas, F.J. A Simple and Useful Method to Apply Exogenous NO Gas to Plant Systems: Bell Pepper Fruits as a Model. Methods Mol. Biol. 2018, 1747, 3–11. [Google Scholar]
- Gayte, I.G.; Moreno, R.B.; Zonjic, P.S.; Claro, M.G. DEgenes Hunter—A Flexible R Pipeline for Automated RNA-Seq Studies in Organisms without Reference Genome. Genom. Comput. Biol. 2017, 3, 31. [Google Scholar]
- Pedrajas, J.R.; McDonagh, B.; Hernández-Torres, F.; Miranda-Vizuete, A.; González-Ojeda, R.; Martínez-Galisteo, E.; Padilla, C.A.; Bárcena, J.A. Glutathione is the resolving thiol for thioredoxin peroxidase activity of 1-Cys peroxiredoxin without being consumed during the catalytic cycle. Antioxid. Redox Signal 2016, 24, 115–128. [Google Scholar] [CrossRef]
- Corpas, F.J.; Pedrajas, J.R.; Palma, J.M.; Valderrama, R.; Rodríguez-Ruiz, M.; Chaki, M.; del Río, L.A.; Barroso, J.B. Immunological evidence for the presence of peroxiredoxin in pea leaf peroxisomes and response to oxidative stress conditions. Acta Physiol. Plant 2017, 39, 57. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Aroca, A.; Romero, L.C.; Gotor, C.; Palma, J.M.; Corpas, F.J. Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S. Antioxidants 2024, 13, 719. [Google Scholar] [CrossRef]
- Liebthal, M.; Maynard, D.; Dietz, K.-J. Peroxiredoxins and Redox Signaling in Plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. 2011, 15, 1129–1159. [Google Scholar] [CrossRef]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric Oxide-Dependent Regulation of Sweet Pepper Fruit Ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef]
- Flohé, L.; Jaeger, T.; Pilawa, S.; Sztajer, H. Thiol-dependent peroxidases care little about homology-based assignments of function. Redox Rep. 2003, 8, 256–264. [Google Scholar] [CrossRef]
- Pedrajas, J.R.; Bárcena, J.A. Peroxiredoxins: Types, Characteristics and Functions in Higher Plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 95–121. [Google Scholar]
- Trujillo, M.; Ferrer-Sueta, G.; Thomson, L.; Flohé, L.; Radi, R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell. Biochem. 2007, 44, 83–113. [Google Scholar]
- Trujillo, M.; Ferrer-Sueta, G.; Radi, R. Peroxynitrite detoxification and its biologic implications. Antioxid. Redox Signal 2008, 10, 1607–1620. [Google Scholar] [CrossRef]
- De Armas, M.I.; Esteves, R.; Viera, N.; Reyes, A.M.; Mastrogiovanni, M.; Alegria, T.G.P.; Netto, L.E.S.; Tórtora, V.; Radi, R.; Trujillo, M. Rapid peroxynitrite reduction by human peroxiredoxin 3: Implications for the fate of oxidants in mitochondria. Free Radic. Biol. Med. 2019, 130, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Villar, S.F.; Ferrer-Sueta, G.; Denicola, A. The multifaceted nature of peroxiredoxins in chemical biology. Curr. Opin. Chem. Biol. 2023, 76, 102355. [Google Scholar] [CrossRef]
- El Msehli, S.; Ben Ayed, R.; Gargouri-Bouzid, R.; Drira, N. Identification and characterization of peroxiredoxins in Medicago truncatula: Differential regulation during salt stress. Plant Mol. Biol. Rep. 2011, 29, 220–232. [Google Scholar]
- Hu, X.; Kong, X.; Lu, M.; Kong, C.; Liu, Y. Genome-wide identification and expression analysis of peroxiredoxins in maize (Zea mays L.). Acta Physiol. Plant. 2016, 38, 120. [Google Scholar]
- Zhang, Y.; Wang, X.; Tong, L. Genome-wide identification and analysis of peroxiredoxin gene family in poplar (Populus trichocarpa). Plant Omics J. 2015, 8, 246–254. [Google Scholar]
- Vidigal, P.; Carvalho, R.; Amâncio, S.; Carvalho, L. Peroxiredoxins are involved in two independent signalling pathways in the abiotic stress protection in Vitis vinifera. Biol. Plant. 2013, 57, 675–683. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, R.N.; Lopes-da-Silva, J.A.; Mateus, N. Characterization of peroxiredoxins in grape berry skin and their relation to anthocyanin degradation during postharvest storage. Food Chem. 2020, 326, 126999. [Google Scholar]
- Feng, Y.; Wei, R.; Liu, A.; Fan, S.; Che, J.; Zhang, Z.; Tian, B.; Yuan, Y.; Shi, G.; Shang, H. Genome-wide identification, evolution, expression, and alternative splicing profiles of peroxiredoxin genes in cotton. PeerJ 2021, 9, e10685. [Google Scholar] [CrossRef]
- Boubakri, H.; Chihaoui, S.A.; Najjar, E.; Barhoumi, F.; Jebara, M. Comprehensive identification, evolutionary patterns and the divergent response of PRX genes in Phaseolus vulgaris under biotic and abiotic interactions. 3 Biotech 2022, 12, 175. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Rodríguez-Ruiz, M.; Mioto, P.T.; González-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef]
- Terzaghi, W.B.; Cashmore, A.R. Light-Regulated Transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 445–474. [Google Scholar] [CrossRef]
- Lin, M.; Yan, J.; Ali, M.M.; Wang, S.; Tian, S.; Chen, F.; Lin, Z. Isolation and Functional Characterization of a Green-Tissue Promoter in Japonica Rice (Oryza sativa subsp. Japonica). Biology 2022, 11, 1092. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Paul, P.; Agrawal, G.K.; Rakwal, R.; Kim, S.T.; Weckwerth, W.; Gupta, R. Proteomics survey of Solanaceae family: Current status and challenges ahead. J. Proteom. 2017, 169, 41–57. [Google Scholar] [CrossRef]
- Weids, A.J.; Grant, C.M. The yeast peroxiredoxin Tsa1 protects against protein-aggregate-induced oxidative stress. J. Cell Sci. 2014, 127, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kang, S.W.; Yang, C.H.; Rhee, S.G.; Ryu, S.E. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat. Struct. Biol. 1998, 5, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Feinstein, S.I.; Fisher, A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. USA 2004, 101, 3780–3785. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, M.; Mioto, P.; Palma, J.M.; Corpas, F.J. S-Nitrosoglutathione Reductase (GSNOR) Activity is Down-Regulated during Pepper (Capsicum annuum L.) Fruit Ripening. Nitric Oxide 2017, 68, 51–55. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Laxa, M.; Mattè, A.; Zaninotto, F.; Finkemeier, I.; Jones, A.M.; Perazzolli, M.; Vandelle, E.; Dietz, K.J.; Delledonne, M. S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell 2007, 19, 4120–4130. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Albertos, P.; Sanz, C.; Wybouw, B.; De Rybel, B.; Begara-Morales, J.C.; Chaki, M.; Mata-Pérez, C.; Barroso, J.B.; Lorenzo, O. Reversible S-nitrosylation of bZIP67 by peroxiredoxin IIE activity and nitro-fatty acids regulates the plant lipid profile. Cell Rep. 2024, 43, 114091. [Google Scholar] [CrossRef]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A multifaceted oxidizing and nitrating metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Álvarez de Morales, P.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.L.; Wang, Z.G.; Pei, M.S.; Guo, L.L.; Yu, Y.H. Transcriptome analysis reveals mechanism of early ripening in Kyoho grape with hydrogen peroxide treatment. BMC Genom. 2020, 21, 784. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Wang, H.; Lin, M.; Chen, Y.; Fan, Z.; Hung, Y.C.; Lin, Y. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. 2020, 242, 116427. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Pei, M.S.; Wei, T.G.; Yu, Y.H.; Guo, D.L. Molecular cloning and expression analysis of hydrogen peroxide sensors under H2O2 and ROS inhibitor treatment in ‘Kyoho’ grape berry. Postharvest Biol. Technol. 2021, 180, 111617. [Google Scholar] [CrossRef]
- Vall-Llaura, N.; Fernández-Cancelo, P.; Nativitas-Lima, I.; Echeverria, G.; Teixidó, N.; Larrigaudière, C.; Torres, R.; Giné-Bordonaba, J. ROS-scavenging-associated transcriptional and biochemical shifts during nectarine fruit development and ripening. Plant Physiol. Biochem. 2022, 171, 38–48. [Google Scholar] [CrossRef]
- Mansouri, S.; Koushesh Saba, M.; Sarikhani, H. Exogenous melatonin delays strawberry fruit ripening by suppressing endogenous ABA signaling. Sci. Rep. 2023, 13, 14209. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Zhang, S.; Sun, L.; Yan, P.; Feng, Y.; Zhao, Z. Phosphorylation of MdWRKY70L by MdMPK6/02G mediates reactive oxygen accumulation to regulate apple fruit senescence. Plant Biotechnol. J. 2025, 23, 2386–2399. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; Taboada, J.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Characterization of leucine aminopeptidase (LAP) activity in sweet pepper fruits during ripening and its inhibition by nitration and reducing events. Plant Cell Rep. 2024, 43, 92. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vargas, M.A.; Taboada, J.; Palma, J.M.; Corpas, F.J. H2O2-generating polyamine oxidases (PAOs) are modulated during sweet pepper ripening: Spermine oxidase (SpmOX) as a case study of post-translational modification regulation. Plant Sci. 2025, 359, 112606. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vargas, M.A.; López-Jaramillo, J.; González-Gordo, S.; Taboada, J.; Palma, J.M.; Corpas, F.J. Peroxisomal H2O2-generating sulfite oxidase (SOX) from pepper fruits is negatively modulated by NO and H2S. Plant Physiol. Biochem. 2025, 221, 109591. [Google Scholar] [CrossRef] [PubMed]
- Umate, P. Genome-wide analysis of thioredoxin fold superfamily peroxiredoxins in Arabidopsis and rice. Plant Signal Behav. 2010, 5, 1543–1546. [Google Scholar] [CrossRef]
- Poole, L.B.; Nelson, K.J. Distribution and Features of the Six Classes of Peroxiredoxins. Mol. Cells 2016, 39, 53–59. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Chr | Protein ID | Length (aa) | Mw (kDa) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| PRX2B | 107840788 | 1 | XP_016540190.1 | 162 | 17.41 | 5.80 | Cytosol |
| PRX2F | 107841069 | 1 | XP_016540565.1 | 191 | 20.78 | 9.00 | Plastid |
| PRX2-CysBAS1 | 107846522 | 1 | XP_016546376.1 | 270 | 29.61 | 7.57 | Plastid |
| PRX1 | 107867717 | 4 | XP_016569570.1 | 256 | 28.47 | 9.53 | Plastid |
| PRX Q | 107877928 | 7 | XP_016580227.2 | 211 | 23.18 | 9.60 | Plastid |
| PRX1-Cys | 107848474 | 9 | XP_016548730.1 | 219 | 24.31 | 6.45 | Cytosol/Nucleus |
| PRX2E | 107843634 | 10 | XP_016543463.1 | 229 | 24.60 | 8.26 | Plastid |
| PRX2-CysBAS2 | 107843729 | 10 | XP_016543590.1 | 265 | 29.04 | 6.75 | Plastid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Mellado, F.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Identification of Peroxiredoxin (PRX) Genes from Pepper Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO). Antioxidants 2025, 14, 817. https://doi.org/10.3390/antiox14070817
Ramírez-Mellado F, González-Gordo S, Palma JM, Corpas FJ. Identification of Peroxiredoxin (PRX) Genes from Pepper Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO). Antioxidants. 2025; 14(7):817. https://doi.org/10.3390/antiox14070817
Chicago/Turabian StyleRamírez-Mellado, Fátima, Salvador González-Gordo, José M. Palma, and Francisco J. Corpas. 2025. "Identification of Peroxiredoxin (PRX) Genes from Pepper Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO)" Antioxidants 14, no. 7: 817. https://doi.org/10.3390/antiox14070817
APA StyleRamírez-Mellado, F., González-Gordo, S., Palma, J. M., & Corpas, F. J. (2025). Identification of Peroxiredoxin (PRX) Genes from Pepper Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO). Antioxidants, 14(7), 817. https://doi.org/10.3390/antiox14070817








