Abstract
Down syndrome (DS), the most common human aneuploidy, is associated with oxidative stress, which contributes to morphological abnormalities, immune dysfunction, cognitive impairment and accelerated ageing. This article aims to provide an overview of the studies on oxidative stress in DS, in particular the investigation of endogenous and exogenous antioxidants, with a focus on endogenous systems. A literature search in MEDLINE and Scopus based on the PRISMA 2020 criteria revealed 41 relevant studies that mainly analysed blood samples (plasma or serum) and occasionally saliva or urine. The findings suggest that oxidative stress in DS is multifactorial and results from an imbalance of superoxide dismutase activity, overexpression of genes on chromosome 21, mitochondrial dysfunction and inflammation. Despite extensive studies over the decades, new sources and mechanisms for oxidative stress in DS continue to emerge, further highlighting the complexity of DS. The recognition that oxidative stress is a hallmark of DS emphasises the need to develop more sensitive and specific methods to detect it and to investigate the associated metabolic pathways in DS in more detail. The expansion of in vivo studies could facilitate the development of targeted interventions aimed at mitigating oxidative damage and ultimately improving outcomes for individuals with DS.
1. Introduction
Down syndrome (DS) or trisomy 21 (Ts21) is the most common aneuploidy in the human population and is characterised by a complex and distinct clinical phenotype with intellectual disability, hypotonia and craniofacial dysmorphia. Overexpression of a gene in a region on chromosome 21 leads to an imbalance in the neurological, immunological, endocrine and biochemical processes of the cell. To varying degrees, individuals with DS also exhibit different phenotypes, including disorders of the immune system, respiratory system, endocrine system, gastrointestinal tract, urinary tract and musculoskeletal system. Individuals with DS are prone to premature ageing and earlier development of Alzheimer’s disease [1,2,3,4]. In addition to these diseases, individuals with DS can also suffer from congenital heart defects (CHDs) and have an increased susceptibility to autoimmune diseases, epilepsy, thyroid disease and leukaemia [2,5,6].
The oxidative stress hypothesis offers a possible explanation for several diseases associated with DS. Oxidative stress is a metabolic state of the body caused by an imbalance in the production of free radicals and their reactive metabolites [7,8]. Free radicals are reactive oxygen and nitrogen species that constantly circulate in the body and occur as a side effect of many reactions in the body [9,10]. Studies suggest that free radicals play an important role in the body’s immune response against infectious agents. For instance, the immune system utilises free radicals to fight pathogens. Free radicals have been recognised as important signalling molecules, first for nitric oxide (NO) and then for other reactive species [11]. Furthermore, studies have shown that hormones such as insulin regulate free radical levels and that they can act as important regulators of metabolic processes in the body. It is therefore now clear that free radicals can play a crucial role in various biological processes, including cell signalling, defence against pathogens and the regulation of metabolic pathways [12,13]. However, due to their high reactivity, free radicals can participate in chain reactions in which a single triggering event damages many molecules [14,15]. Under normal conditions, they are removed from the body by antioxidant processes. When these natural mechanisms are disrupted, the radicals accumulate excessively and lead to disease. Mitochondria play an important role in protecting the body from oxidative stress, and their integrity is key to various cellular processes [16,17,18,19]. Reactive oxygen species (ROS) are formed as a by-product of normal cell metabolism during the oxidative reaction of the mitochondrial respiratory chain [20]. It has been shown that this group of molecules could prove an important role in human cellular signalling and defence mechanisms. In moderate amounts, reactive oxygen species play a key role in biological processes such as the destruction of pathogenic organisms, the promotion of wound healing and the repair of damaged tissue [21,22]. Mitochondrial ROS are a significant source of reactive oxygen species trapped in the double membranes of mitochondria. However, both intracellular disorders and external (environmental) factors can lead to excessive production of ROS (superoxide anion (O2•−), hydroxyl radical (·OH), hydrogen peroxide (H2O2)). For example, increased oxygen availability, a more intensive metabolism and prolonged stress conditions stimulate ROS production, while increased permeability of the mitochondrial membrane enables the direct release of ROS into the cytosol [22,23]. Mitochondria are particularly susceptible to oxidative damage, as the electrons separated from the electron transport chain in the inner membrane react with oxygen to form the superoxide anion. This anion is unstable and cannot diffuse through the membrane, but it quickly transforms into membrane-permeable H2O2 [24]. It is also important to emphasise the interaction between ROS and NO, a reactive nitrogen species (RNS). NO can react with O2•− to form peroxynitrite (ONOO−), a powerful oxidising agent that plays an important role in both cellular signalling and oxidative damage. This interaction between RNS and ROS is crucial for the regulation of numerous cellular processes, including neuronal function and cellular homeostasis. Any disruption of their balance can lead to increased oxidative stress and cellular damage [25,26].
Excessive production of ROS leads to modifications of important biomolecules such as lipids (peroxidation), proteins (aggregation, denaturation), carbohydrates and nucleic acids (changes in the structure of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)) [22,27,28]. These changes put the body in a state of oxidative stress, which leads to damage and/or changes in cell function and ultimately puts the body in a state of morphological abnormalities, immune dysfunction, cognitive impairment, premature ageing and an increased risk of cancer [1,7,22,29,30]. To prevent damage caused by the excessive production of ROS, cells use an antioxidant defence system, i.e., they use substances that can delay or prevent the oxidation of the oxidisable substrate [31,32]. Antioxidant systems regulate gene expression and signalling pathway connectivity to maintain redox balance and the integrity of cellular components (including lipids, proteins and nucleic acids) [22]. The human body produces several enzymatic and non-enzymatic antioxidants (endogenous antioxidants) that serve to balance the effects of excessive amounts of oxidants [33], and the mechanisms of their action consist of capturing and reduce reactive oxygen species and to repair or replace damaged target molecules [34]. If there is a deficiency of endogenous antioxidants, it is possible to ingest substances that act as a defence mechanism against oxidative stress (exogenous antioxidants), e.g., vitamin C, vitamin E, natural flavonoids, carotenoids and various other compounds, via food and dietary supplements [7,32,35]. Endogenous antioxidants have several key roles in protecting the body: they participate in the neutralisation of excessive amounts of ROS, maintain redox potential homeostasis, participate in the elimination of harmful substances, support the cells of the immune system and contribute to increasing the cells’ resistance to external oxidative factors [32,36,37]. The general endogenous system includes enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPxs) and thioredoxin (Trx), hydrophilic antioxidants such as urate, ascorbate, glutathione and flavonoids, and lipophilic free-radical antioxidants such as tocopherols, carotenoids and ubiquinol [33,38,39]. To assess the relationship between oxidative stress and various diseases and health conditions, including cardiovascular diseases, neurodegenerative disorders and cancer, stable biomarkers, i.e., measurable substances that indicate the presence of oxidative stress in the body, are used [40]. Examples of oxidative stress biomarkers include protein carbonyls (PCs) and advanced glycation end products (AGEs), oxidised low-density lipoproteins, oxidised metabolites, antioxidant enzymes and genes or proteins that are activated in response to oxidative stress [40,41]. In clinical practise, biomarkers are most commonly measured from venous blood and urine samples [41], and somewhat less frequently studies are conducted on saliva, tissue or spinal cord samples [41,42,43]. When measuring biomarkers, different methods are used depending on the type of biomarker and the sample being analysed.
In DS, the presence of an extra copy of chromosome 21 leads to overexpression of several genes that regulate oxidative stress, in particular the enzyme Cu/Zn superoxide dismutase (SOD1; OMIM: 147450) and the amyloid precursor protein (APP) [44,45,46,47]. This overexpression of SOD1 increases the conversion of O2•− to H2O2 without a corresponding upregulation of downstream antioxidant enzymes, including CAT, which directly scavenges H2O2. This enzymatic imbalance disrupts redox homeostasis and leads to a chronic state of oxidative stress in cells, especially in neurons. Such persistent oxidative stress is a major contributor to the neuropathological and systemic phenotypes that occur in DS. In the central nervous system, oxidative damage to lipids, proteins and nucleic acids impedes neurodevelopment, disrupts synaptic plasticity and accelerates neuronal ageing, leading to cognitive deficits and intellectual disability [48,49,50]. Furthermore, oxidative stress is a key factor in the development of Alzheimer’s disease-like neuropathology by promoting the aggregation of amyloid-beta and hyperphosphorylation of tau, both of which are characteristic of early-onset neurodegeneration in DS. Outside the central nervous system, oxidative stress impairs the functionality of immune cells and can disrupt normal embryonic development, leading to immune dysregulation. In addition, ROS are associated with damage to vascular endothelial cells, possibly contributing to CHDs and metabolic disorders associated with DS [19,51,52]. Studies have shown that individuals with Ts21 have altered mitochondrial structures and reduced mitochondrial connectivity within the mitochondrial network and that mitochondrial dysfunction or damage due to oxidative stress may contribute to the pathogenesis of DS [45,53,54,55].
The aim of this systematic review is to provide an overview of studies conducted in individuals with DS, analysing endogenous and exogenous antioxidants from different types of samples that can serve as biomarkers for the presence of oxidative stress in the body, with a focus on the analysis of endogenous antioxidants in the body of individuals with DS.
2. Materials and Methods
This systematic review is based on the updated guidelines for reporting in systematic reviews published in the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 (PRISMA 2020) Guidelines [56]. To identify all relevant articles published up to 2024, two leading databases were searched: Medline and Scopus. The articles were found with the following keywords and their combinations: Down syndrome, trisomy 21, oxidative stress, reactive oxygen species and endogenous antioxidants. Once duplicates had been removed, the titles of all remaining articles were thoroughly checked. It was determined what specific information was sought in all included studies. This included the type of study, confirmation that the study was directly related to DS, the inclusion of live human subjects, the presence of a control group, indicators of oxidative stress and specific biomarkers. To extract relevant data from the selected studies, a customised data extraction form was used, which was developed independently and in accordance with the recommendations of the PRISMA 2020 guidelines. This form contained essential information that served as the core criteria for the evaluation of all studies. After a thorough review of available studies, articles analysing endogenous antioxidants as indicators of oxidative stress in individuals with DS, exogenous antioxidants and relevant biomarkers were included in this systematic review. For demonstration in this systematic review, English-language articles were selected that present studies in living subjects (foetuses, children and adults), including study (DS) and control (non-DS) groups with comparable characteristics (age) and with results that can be appropriately read and compared with other relevant articles. Non-English language articles, articles with studies conducted in DS mouse models, articles with post-mortem studies, articles focusing on patient treatment, author manuscripts, randomised controlled trials, case reports, clinical trials, congress abstracts, letters, replies, commentaries, editorials, other systematic reviews and reports with incomplete or unavailable data were removed from the systematic review. The article extraction was carried out by two authors independently on the basis of the inclusion criteria, and any differences were resolved after detailed discussion and comparison, either in dialogue or with the involvement of a third author [57]. To effectively detect and mitigate reporting bias, this systematic review was conducted according to a prospectively developed and publicly available protocol that was registered with PROSPERO (registration number CRD420251012690) and complied with PRISMA 2020 guidelines prior to the start of screening [56,58]. Two independent reviewers systematically assessed the risk of bias in predefined domains for each eligible study and categorised each domain as low, high or unclear risk of bias, after which an overall judgement was made accordingly. Disagreements were resolved by consensus discussions or, if necessary, by consulting a third reviewer. To ensure adherence to this methodology, only studies categorised as low or certain risk of bias were considered for further screening [59,60]. As a result of this transparent and protocol-driven approach, a total of 41 articles were included in this systematic review.
This systematic review has several limitations. The results of the articles analysed were based on a selected sample of subjects, which may pose a methodological problem in some cases (e.g., small number of subjects, variations in the number, sex and age of subjects, etc.). Due to different approaches in the presentation of the numerical values of the analysed parameters in the literature and sometimes the absence of these values in individual articles, these data were also not included in the final summary table. However, the present article was prepared in accordance with the updated PRISMA 2020 guidelines [56] and provides a systematic review of the studies on biomarkers of oxidative stress in individuals with DS.
3. Results and Discussion
Initially, a total of 3695 documents were identified in this literature search, of which 1408 were from MEDLINE and 2367 from Scopus. After eliminating 1490 duplicate articles and 1748 articles for other reasons (incomparable results, poorly presented results, samples without a control group, incomplete articles with missing sections, and articles with titles and introductions in English while the rest of the text was written in another language), 457 articles were screened. Additionally, five records were categorised as not available (not available for download or not published). The inclusion and exclusion criteria were then reviewed and finally 41 articles were included in this study. The study process and the PRISMA 2020 flowchart are shown in Figure 1.
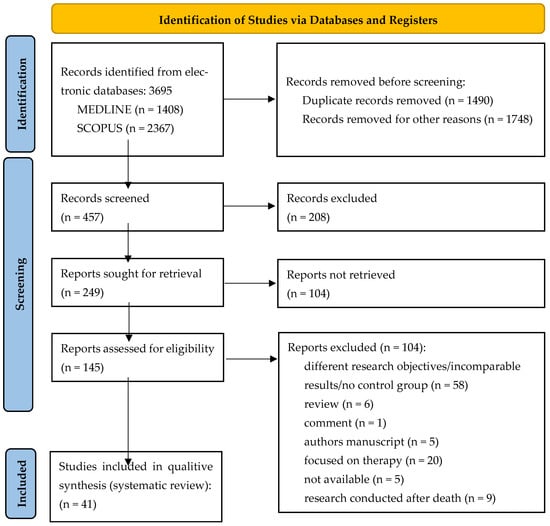
Figure 1.
PRISMA 2020 flow chart for new systematic reviews that included searches of databases and registers only.
The articles were published between 1988 and 2024 (the last search was conducted on 31 December 2024) and analysed various endogenous antioxidants which can indicate the intensity of oxidative stress in the body, as well as exogenous antioxidants and characteristic biomarkers of oxidative stress. The number, sex and age of the test subjects also varied. In 20 articles the exact number of male and female subjects is given, while in the other articles only the number of subjects per group is given. In 30 articles the exact age range of the test subjects is given, while in the other articles the mean value (average) is given. The studies included in the systematic review comprise investigations of various biomarkers of oxidative stress (particularly endogenous antioxidants) in individuals with DS. Of the 41 articles identified, 25 included an analysis based on a blood sample (including serum and plasma), while the remaining articles included an analysis based on a urine sample (5 articles), saliva (4 articles), amniotic fluid (4 articles), deciduous teeth (1 article), abdominal skin (1 article) and cerebral cortex (1 article). All of these biomarkers were included in the overview of data from the final selected studies (Supplementary File S1). Detailed summaries of the included articles can be found in Table 1, which lists the main components of each article (first author’s surname, year of publication, sex and age of subjects, indicator/oxidative stress factor studied, sample processed and the p-value for individual biomarkers/factors due to the significance of differences between subjects). Therefore, this article provides a systematic review of the current literature on the results of studies on biomarkers of oxidative stress in individuals with DS.

Table 1.
Summary of the selected articles.
The results presented in Table 1 show a significant disturbance of the antioxidant balance and indicative oxidative damage in individuals with DS compared to the control group. Key alterations include increased SOD activity, decreased reduced glutathione (GSH) levels, and increased levels of malondialdehyde (MDA) and 8-hydroxy-2’-deoxyguanosine (8-OHdG). These changes therefore indicate a remarkable shift in the balance between oxidants and antioxidants in individuals with DS.
3.1. Enzymatic Antioxidants
It is known that excessive activity of the SOD1 in individuals with DS primarily contributes to increased oxidative stress. An imbalance in the activity of SOD and other essential antioxidant enzymes such as CAT and GPx can lead to an accumulation of H2O2, potentially resulting in damage to cellular structures. A well-known hypothesis is that the increased expression and activity of SOD1 due to DS occurs without a corresponding increase in the activities of CAT and GPx, which are critical for the effective degradation of H2O2 [31,46]. This oxidative imbalance is thought to be a primary pathophysiological mechanism contributing to the development of the disorders associated with DS. These include increased neurological dysfunction, cardiovascular diseases, the occurrence of haematological abnormalities, accelerated cellular ageing processes and an increased susceptibility to bacterial infections [7,46,94,96,97]. Many studies have shown that the activity of SOD in the blood of individuals with DS varies greatly [47,48,52,64]. This observation is consistent with the fact that the SOD1 gene is located on chromosome 21, which is upregulated by DS [98,99], and later studies have confirmed these findings [48,74]. In a study conducted by Garlet et al., an increased activity of SOD was found in serum samples from individuals with DS. Furthermore, CAT activity was significantly increased in these individuals compared to the control group. However, even the increased CAT activity does not appear to be sufficient to effectively neutralise the excess H2O2 resulting from the increased SOD1 activity, which further increases oxidative stress in individuals with DS [46]. In contrast to the other enzymes analysed, the activity of glutathione S-transferase (GST) was significantly lower in individuals with DS compared to the control group, indicating the presence of oxidative stress in these individuals [46,62]. The increased activity of SOD1 can lead to an accumulation of ·OH, which subsequently reduces the effectiveness of GST as part of the secondary antioxidant defence system [31,62]. Moreover, lower GST activity is associated with lower levels of GSH, an important non-enzymatic antioxidant that plays a crucial role in the neutralisation of H2O2 [46,63,66,100].
Besides blood and serum, saliva has also been recognised as a valuable biological sample for the assessment of oxidative stress in individuals with DS. Several studies have shown statistically significant differences in total protein (TP) content, total antioxidant status (TAS) and SOD activity in saliva of individuals with DS compared to the control group [82,83,84,85]. In recent years, a growing number of studies have investigated the biomarkers of oxidative stress in the amniotic fluid (AF) of pregnant women carrying a foetus with Ts21. The results of these studies indicate a significant increase in the activity of SOD, an increased content of oxidised proteins and an elevated content of products of lipid peroxidation. At the same time, the activity of important antioxidant enzymes such as CAT and GPx decreases significantly. This imbalance contributes to an increased overall level of oxidative damage [47,79,91,92]. These changes in biomarkers indicate the potential for early detection of oxidative stress in utero caused by different biological pathways [101]. The collected data indicate that oxidative stress during pregnancy in a foetus with Ts21 is a complex pathophysiological process that can significantly affect foetal development [79].
3.2. Non-Enzymatic Antioxidants
Non-enzymatic antioxidants such as GSH play an important role in defence against oxidative stress by rapidly protecting cellular integrity through various antioxidant mechanisms, but their levels can often be reduced in individuals with DS [33,81]. GSH is a potent non-enzymatic antioxidant for the detoxification of H2O2 and can influence elevated ROS levels both through direct interaction and indirectly by modulating the corresponding signalling pathways [63,66,100,102]. Studies indicate a significant correlation between decreased GSH levels and increased oxidative stress in individuals with DS [70,71,103]. Low GSH levels can impair the effectiveness of enzymatic antioxidants such as GST and GPx and thus contribute to elevated oxidative stress [46].
Analyses of blood and plasma samples have shown that individuals with DS have significantly lower GSH levels compared to control groups, indicating an impaired antioxidant defence system in this group [1,46,63]. It has also been shown that a progressive decline in GSH levels with increasing age, suggesting that oxidative stress may increase over time. In particular, older individuals with DS show a marked decline in GSH levels, which increases their susceptibility to oxidative damage. It was also found that TAS levels were significantly lower in individuals with DS compared to the control group, indicating a reduced capacity of the antioxidant system in this group. These findings suggest possible oxidative damage that may contribute to the pathophysiological processes associated with DS, including accelerated ageing and tissue damage [46,63,72,77]. In a study conducted by Campos et al., urine samples from individuals with DS aged between one and twelve years showed elevated uric acid (UA) levels compared to a control group of the same age [89]. This finding is consistent with the results of Garlet et al. who reported a significant increase in UA levels in the blood of individuals with DS aged up to 14 years compared to a control group [46]. The results also align with the study by Žitňanová et al. which showed that individuals with DS have significantly increased UA and allantoin (Alla) levels. Moreover, there is a positive correlation between these levels and the age of individuals with DS compared to controls, further supporting the notion of progressive ROS in individuals with DS [69]. The relatively high urate levels may indicate a compensatory antioxidant response to the prolonged oxidative stress associated with DS. In contrast, no statistically significant differences in UA concentrations were found between the DS group and the corresponding control group in adults aged 43 to 61 years [1,46,88].
3.3. Biomarkers of Oxidative Damage
Individuals with DS have an increased susceptibility to reactive ROS, which can cause damage that leads to degenerative changes in various tissues, including the brain, heart, eyes and thyroid [72,73,75,78]. As a result, there is a significant increase in lipid and protein peroxidation, characterised by increased MDA levels in the erythrocytes, and this increase can impair the function of various enzymes [68,76]. The increase in lipid peroxidation is also associated with higher levels of ROS and RNS, indicating a disturbed homeostatic balance in oxidative metabolism in individuals with DS. Due to its high chemical reactivity, MDA can cause additional structural and functional changes in the cells [65]. Elevated levels of MDA and protein carbonyls (PCs) have been found in the plasma of individuals with DS, which has been confirmed by several previous studies [67,103,104]. These biomarkers can have a negative effect on enzyme activities and disrupt the structure and function of cell membranes, ultimately leading to a change in cellular homeostasis. Oxidative damage to proteins is of particular concern as it can lead to changes in their structure, enzyme activity and signalling pathways, ultimately contributing to accelerated ageing and the development of various diseases [72,81,90]. Interestingly, there were also no significant differences in the plasma levels of 4-hydroxynonenal (4-HNE) between individuals with DS and those without DS [80].
Saliva has also proven to be an effective sample for the assessment of oxidative stress. Studies have shown significantly elevated levels of MDA, PCO and 8-OHdG in the saliva of individuals with DS [82,83,85]. In particular, 8-OHdG concentrations were higher in adults with DS (over 30 years old) than in age-matched controls and younger individuals with DS (1–12 years old). The results suggest increased oxidative stress activity in the saliva of individuals with DS, possibly indicating pathological processes associated with increased oxidative stress in this aneuploidy, such as accelerated ageing. This observation is consistent with the finding that progressive oxidative stress can occur in individuals with DS [85]. In studies conducted by Campos et al. in 2010 and 2011, they analysed the concentrations of specific biomarkers for oxidative stress in the urine of younger and older individuals with and without DS [88,89]. This is in line with previous studies that have shown that the urine of younger individuals often contains elevated levels of biomarkers associated with oxidative stress [88,105,106]. In their 2011 study, Campos et al. observed elevated levels of 8-OHdG and dityrosine (diTyr) in younger individuals with DS (under 10 years of age) compared to a control group. Biomarkers such as F2-isoprostane (F2-isoPs), thiobarbituric acid-reacting substances (TBARS) and advanced glycation end products were also significantly elevated in this younger group, with some biomarkers showing a negative correlation with age. In contrast, no significant differences between the levels of F2-isoPs and TBARS were found between the group with DS and the control group in the older individuals. It is worth noting that significantly higher diTyr levels were consistently found in the urine of individuals with DS in all age groups compared to controls [87]. In contrast, a study by Toluna et al. analysing Alla and F2-isoPs as biomarkers of oxidative stress found no significant increase in oxidative stress in individuals with DS compared to controls [86]. These results indicate that, contrary to previous assumptions, individuals with DS do not necessarily have elevated levels of oxidative stress when analysed using these specific biomarkers. These discrepancies are likely due to the heterogeneity associated with certain biomarkers and assays. Biomarkers of DNA and protein oxidation, such as 8-OHdG and diTyr, reflect redox pathways that show only a weak correlation with the urate- and lipid-based biomarkers analysed, including Alla and 2,3-dinor-8-iso-prostaglandin F2α-III (2,3-dinor-iPF2α-III). Studies have shown that the agreement between these different methods is only modest. Furthermore, the age-related compensatory upregulation of antioxidants and repair mechanisms observed in individuals with DS may normalise signs of systemic lipid peroxidation, although certain DNA and protein adducts may remain elevated. Therefore, it is likely that pathway- and age-specific effects contribute to the seemingly contradictory results [91,107,108].
Significant differences in oxidative status were also observed during the prenatal period when it comes to DS. The analysis performed by Odetti et al. revealed elevated levels of lipid and protein oxidation in foetuses with Ts21 compared to a control group. The concentration of glycation products, especially AGEs, was increased. These are substances that can lead to tissue and organ damage and are associated with the development of various diseases. These results indicate that significant oxidative stress occurs in the brains of foetuses with Ts21, suggesting that this stress will be felt throughout their lives. Oxidative stress has the potential to damage cell structure and function, which may contribute to the neurological complications typically associated with DS [95].
3.4. Inflammatory and Neurological Biomarkers Associated with Oxidative Stress
Previous studies have highlighted that the incidence of infectious and autoimmune diseases is significantly elevated in individuals with DS, regardless of factors such as sex, age, family history or other risk variables [109]. A study by Tarani et al. from 2020 [5] specifically investigated neuroinflammatory biomarkers in the serum of prepubertal individuals with DS (aged between one year and nine years and six months), focusing on nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), oxidative free radical defence (FORD) indicators, oxygen free radical (FORT) levels and cytokines, all of which play a crucial role in neuroinflammation and oxidative stress processes. The results showed no significant differences in NGF, FORD and FORT levels when comparing individuals with DS to the control group. However, the analysis revealed a significant increase in BDNF levels and a remarkable decrease in all analysed cytokines in individuals with DS compared to the control group. Furthermore, statistically significant sex-specific differences were observed in the serum cytokine levels of individuals with DS. This study emphasises the importance of investigating neuroinflammatory processes in DS in order to develop targeted strategies for the early detection and possible treatment of inflammatory conditions in these individuals [5].
Sun et al. explored the relationship between dopamine-related oxidative stress and mitochondrial dysfunction in dopaminergic neurons of individuals with DS (age six to ten years). Their analysis of dopaminergic neurons differentiated from deciduous teeth-derived stem cells of these individuals suggests that dysregulation of dopamine homeostasis may contribute to oxidative stress and mitochondrial dysfunction in the dopaminergic system of individuals with DS. This study emphasises the importance of understanding the specific biological changes that occur in the dopamine neurons of individuals with DS. The identification of increased oxidative stress and mitochondrial dysfunction provides the basis for further studies focussing on the development of targeted therapies or interventions to improve the neurological prognosis and overall quality of life of individuals with DS [93].
3.5. Metabolic Mediators of Oxidative Stress
Studies from 2016 were among the first to reveal that citrate metabolism contributes significantly to the oxidative stress observed in individuals with DS [110,111]. Accordingly, Convertini et al. found that individuals with DS aged three to five years had higher citrate levels compared to a control group of healthy individuals of the same age. The increased concentrations of citrate entering the cytosol from the mitochondria have been shown to promote the production of reactive species, including ROS and RNS, and thus increase the oxidative load on the cells [61]. Namely, citrate serves as a source of acetyl units for lipid biosynthesis, and its breakdown produces oxaloacetate, which is a precursor for the synthesis of nicotinamide adenine dinucleotide phosphate (NADPH) [111,112]. Since NADPH is a crucial reducing agent for the enzyme-mediated generation of ROS and RNS by NADPH oxidase and inducible nitric oxide synthase (iNOS), its role is particularly important. Nevertheless, the exact molecular mechanisms that lead to elevated citrate levels in individuals with DS are not yet fully understood [61].
Recent studies have also found elevated levels of asprosin, a relatively new antioxidant protein, in the plasma and AF of pregnant women carrying foetuses diagnosed with Ts21 [79,113]. Asprosin plays several vital roles in the body, particularly in the regulation of glucose metabolism, cell apoptosis, appetite and the modulation of central and peripheral nervous system functions [79,114]. The increased presence of asprosin in pregnancies with a foetus affected by Ts21 could represent an adaptive response to the oxidative stress that presumably occurs during intrauterine development [79,113,115]. Over time, a growing number of studies have investigated the biomarkers of oxidative stress in the AF of pregnant women carrying a foetus with Ts21. The elevated levels of asprosin observed in these pregnancies support the hypothesis that foetuses with Ts21 experience oxidative disturbances in the early stages of development. This emphasises the hypothesis of early onset and accumulation of oxidative damage that may have clinical implications for life after birth [47,79,91,92].
4. Conclusions
This article provides a systematic review of studies on biomarkers of oxidative stress in individuals with DS, with a particular focus on endogenous antioxidants. The analysis of the selected studies shows that the development of oxidative stress in individuals with DS is a complex process caused not only by an imbalance of SOD activity or overexpression of certain genes on chromosome 21, but also by other, less explored sources (e.g., high citrate levels, mitochondrial dysfunction, inflammatory processes, etc.). Indicators of oxidative stress, including antioxidants and biomarkers, are analysed using a variety of methods and samples. Despite extensive studies and a considerable amount of data on oxidative stress in DS, there are still notable limitations on this topic. The heterogeneity of study populations, particularly in terms of age, sex and methodological approaches, poses a challenge to the interpretation of these results. The standardisation of biomarkers of oxidative stress in DS and the uniform application of experimental protocols across studies are essential prerequisites for drawing definitive and valid conclusions. Longitudinal studies that track changes in oxidative stress biomarkers over time, especially as individuals with DS age, are essential for a better understanding of the progression of oxidative damage and its relationship to DS-related pathologies as well as other oxidative stress-related diseases common in this aneuploidy. Subgroup analyses targeting specific comorbidities or age groups may help to identify tailored therapeutic approaches. Future studies should emphasise the clinical potential of antioxidant therapies and other strategies to reduce oxidative stress in DS to improve quality of life and prolong life expectancy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14070816/s1, Supplementary File S1: Analysed Values from Body Fluids (Blood Except Serum and Plasma, Serum, Plasma, Saliva, Urine and Amniotic Fluid).
Author Contributions
Conceptualisation, J.V., G.S., K.V., M.Č., D.V. and K.L.; methodology, G.S., J.V., K.V., J.K., D.V. and A.B.B.; validation, M.M., M.Č., A.B.B., J.K., K.L. and D.V.; investigation, G.S., S.M., S.P., M.F. and R.M.; formal analysis, G.S., S.M., R.M., S.P. and M.F.; data curation, I.D.; S.M., K.V., M.F. and K.L.; writing—original draft preparation, G.S., S.M., I.D., M.Č. and J.V.; writing—review and editing, G.S., S.M., I.D., R.M. and J.V.; visualisation, M.M., S.M., I.D., R.M. and A.B.B.; supervision, J.V., K.L., M.M., M.F., D.V. and A.B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations, Acronyms and Initialisms
The following abbreviations, acronyms and initialisms are used in this manuscript:
| % | percentage |
| / | slash |
| + | plus |
| < | less than |
| = | equal to |
| > | greater than |
| ± | plus-minus |
| × | multiplication |
| ·OH | hydroxyl radical |
| 15-F2t-IsoP | isoprostane |
| 2,3-dinor-iPF2α-III | 2,3-dinor-8-iso-prostaglandin F2α-III |
| 25(OH)D | 25-hydroxyvitamin D |
| 4-HNE | 4-hydroxynonenal |
| 8-OHdG | 8-hydroxy-2’-deoxyguanosine |
| α | alpha |
| β | beta |
| A1AT | alpha-1-antitrypsin |
| ACLY | ATP-citrate lyase |
| ACP1 | acid phosphatase |
| ADA | adenosine deaminase |
| ADP | adenosine monophosphate |
| ADSP | aged Down syndrome patients |
| AF | amniotic fluid |
| AGEs | advanced glycation end products |
| Ala | alanine |
| Alla | allantoin |
| AMP | adenosine monophosphate |
| AnonDSP | aged non-Down syndrome patients |
| APP | amyloid precursor protein |
| Arg | arginine |
| Asn | asparagine |
| Asp | aspartate |
| AT | Republic of Austria |
| ATP | adenosine triphosphate |
| BDNF | brain-derived neurotrophic factor |
| CAT | catalase |
| CBS | cystathionine beta-synthase |
| CC | creative commons |
| CHDs | congenital heart defects |
| CIC | citrate carrier |
| Cit | citrulline |
| Cr | creatinine |
| Cu | copper (lat. cuprum) |
| Cys | cysteine |
| diTyr | dityrosine |
| DNA | deoxyribonucleic acid |
| DNs | dopaminergic neurons |
| DOI | digital object identifier |
| DS | Down syndrome |
| e.g., | for example (lat. exempli gratia) |
| et al. | and others (lat. et alia) |
| etc. | and other similar things (lat. et cetera) |
| F | female |
| F2-dihomo-IsoPs | F2-dihomo-isoprostanes |
| F2-isoPs | F2-isoprostane |
| F4-NeuroPs | F4-neuroprostanes |
| FORD | free oxygen radical defence test |
| FORT | free oxygen radicals |
| FRAP | ferric reducing ability of plasma |
| FRM | free radical metabolism |
| fT4 | free thyroxine |
| G6PDH | glucose-6-phosphate dehydrogenase |
| Gln | glutamine |
| Glu | glutamate |
| Glx | glyoxal |
| Gly | glycine |
| GPx | glutathione peroxidase |
| GPxs | glutathione peroxidases |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GS-Hb | glutathionyl-haemoglobin |
| GSHf | free glutathione |
| GSHt | total glutathione |
| GSSG | glutathione disulfide |
| GST | glutathione S-transferase |
| H2O2 | hydrogen peroxide |
| HDL | high-density lipoprotein |
| His | histidine |
| HX | hypoxanthine |
| i.e., | that is (lat. id est) |
| IE-NPBI | intraerythrocyte non-protein bound iron |
| IL-10 | interleukin-10 |
| IL-12 | interleukin-12 |
| IL-1α | interleukin-1 alpha |
| IL-2 | interleukin-2 |
| IL-6 | interleukin-6 |
| Ile | isoleucine |
| IMA | ischemia-modified albumin |
| iNOS | inducible nitric oxide synthase |
| IP | isoprostane |
| lat. | Latin |
| LDL | low-density lipoprotein |
| Leu | leucine |
| LMWA | enzymatic low molecular weight antioxidants |
| Lys | lysine |
| M | male |
| MCP1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| Met | methionine |
| MGlx | methylglyoxal |
| MHR | methemoglobin reductase |
| mRNA | messenger ribonucleic acid |
| n | sample size |
| N | total sample size |
| N.S. | not significant |
| N/A | not applicable |
| NADPH | reduced nicotinamide adenine dinucleotide phosphate |
| NGF | tumour necrosis factor |
| NO | nitric oxide |
| non-DS | non-Down syndrome |
| NOS | nitric oxide synthase |
| O2•− | superoxide anion |
| OMIM | Online Mendelian Inheritance in Man |
| ONOO− | peroxynitrite |
| Orn | ornithine |
| OSDP | DNA/RNA oxidative stress damage products |
| PCO | protein carbonylation |
| PCs | protein carbonyls |
| Phe | phenylalanine |
| P-NPBI | plasma nonprotein-bound iron |
| PRISMA 2020 | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| PROSPERO | International Systematic Review Registry |
| p-value | probability value |
| R | ratio |
| RNA | ribonucleic acid |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA | sialic acid |
| SAH | S-adenosyl homocysteine |
| SAM | S-adenosylhomocysteine |
| Ser | serine |
| SOD | superoxide dismutase |
| SOD1 | copper-zinc superoxide dismutase |
| SOD2 | superoxide dismutase-2 |
| STI | serum total iron |
| T3 | triiodothyronine |
| TAOC | total antioxidant capacity of saliva |
| TAS | total antioxidant status |
| Tau | taurine |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid-reacting substances |
| TBG | thyroxine-binding globulin |
| TGF-β | transforming growth factor beta |
| TGs | triglycerides |
| th | ordinal indicator |
| tHcy | total homocysteine |
| Thr | threonine |
| TIBC | total iron binding capacity |
| TMR | transmembrane reductase |
| TNF-α | tumour necrosis factor alpha |
| tNOx | total nitrite and nitrate |
| TP | total protein |
| Trp | tryptophan |
| Trx | thioredoxin |
| Ts21 | trisomy 21 |
| TSH | thyroid stimulating hormone |
| tT4 | total thyroxine |
| Tyr | tyrosine |
| UA | uric acid |
| UK | United Kingdom of Great Britain and Northern Ireland |
| Val | valine |
| X | xanthine |
| XO | xanthine oxidase |
| YDSP | younger Down syndrome patients |
| YnonDSP | younger non-Down syndrome |
| Zn | zinc (lat. zincum) |
References
- Pallardó, F.V.; Degan, P.; D’Ischia, M.; Kelly, F.J.; Zatterale, A.; Calzone, R.; Castello, G.; Fernandez-Delgado, R.; Dunster, C.; Lloret, A.; et al. Multiple Evidence for An Early Age Pro-Oxidant State in Down Syndrome Patients. Biogerontology 2006, 7, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down Syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Romano, A.; Zaccaria, G.; Villani, E.R.; Manes Gravina, E.; Vetrano, D.L.; Bernabei, R.; Onder, G. The Burden of Chronic Disease, Multimorbidity, and Polypharmacy in Adults with Down Syndrome. Am. J. Med. Genet. Part A 2020, 182, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Rueda Revilla, N.; Martínez-Cué, C. Antioxidants in Down Syndrome: From Preclinical Studies to Clinical Trials. Antioxidants 2020, 9, 692. [Google Scholar] [CrossRef]
- Tarani, L.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Messina, M.P.; Rasio, D.; De Luca, E.; Putotto, C.; et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with Down Syndrome. J. Immunol. Res. 2020, 2020, 6937154. [Google Scholar] [CrossRef]
- Vraneković, J.; Slivšek, G.; Majstorović, D. Methyltetrahydrofolate-Homocysteine Methyltransferase Reductase Gene and Congenital Heart Defects in Down Syndrome. Genet. Appl. 2020, 4, 12–17. [Google Scholar] [CrossRef]
- Muchová, J.; Žitňanová, I.; Ďuračková, Z. Oxidative Stress and Down Syndrome. Do Antioxidants Play a Role in Therapy? Physiol. Res. 2014, 63, 535–542. [Google Scholar] [CrossRef]
- Kuhn, E.; Natacci, F.; Corbo, M.; Pisani, L.; Ferrero, S.; Bulfamante, G.; Gambini, D. The Contribution of Oxidative Stress to NF1-Altered Tumors. Antioxidants 2023, 12, 1557. [Google Scholar] [CrossRef]
- Mladenov, M.; Lubomirov, L.; Grisk, O.; Avtanski, D.; Mitrokhin, V.; Sazdova, I.; Keremidarska-Markova, M.; Danailova, Y.; Nikolaev, G.; Konakchieva, R.; et al. Oxidative Stress, Reductive Stress and Antioxidants in Vascular Pathogenesis and Aging. Antioxidants 2023, 12, 1126. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef]
- Du, Y.; Chai, Y.; Zheng, X.; Zheng, Y. Theoretical Study on the Multiple Free Radical Scavenging Reactions of Pyranoanthocyanins. Antioxidants 2023, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Radical-Free Biology of Oxidative Stress. Am. J. Physiol. Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Zana, M.; Janka, Z.; Kálmán, J. Oxidative Stress: A Bridge between Down’s Syndrome and Alzheimer’s Disease. Neurobiol. Aging 2007, 28, 648–676. [Google Scholar] [CrossRef]
- Zamponi, E.; Zamponi, N.; Coskun, P.; Quassollo, G.; Lorenzo, A.; Cannas, S.A.; Pigino, G.; Chialvo, D.R.; Gardiner, K.; Busciglio, J.; et al. Nrf2 Stabilization Prevents Critical Oxidative Damage in Down Syndrome Cells. Aging Cell 2018, 17, e12812. [Google Scholar] [CrossRef]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular Hydrogen: Potential in Mitigating Oxidative-Stress-Induced Radiation Injury. Can. J. Physiol. Pharmacol. 2019, 97, 287–292. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Zbucka-Krętowska, M. The Role of Oxidative Stress in Trisomy 21 Phenotype. Cell. Mol. Neurobiol. 2023, 43, 3943–3963. [Google Scholar] [CrossRef]
- Berdún, R.; Obis, È.; Mota-Martorell, N.; Bassols, A.; Valent, D.; Serrano, J.C.E.; Martín-Garí, M.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Tibau, J.; et al. High-Fat Diet-Induced Obesity Increases Brain Mitochondrial Complex I and Lipoxidation-Derived Protein Damage. Antioxidants 2024, 13, 161. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Hur, J.; Sullivan, K.A.; Schuyler, A.D.; Hong, Y.; Pande, M.; States, D.J.; Jagadish, H.V.; Feldman, E.L. Literature-Based Discovery of Diabetes- and ROS-Related Targets. BMC Med. Genom. 2010, 3, 49. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Vigneron, A.; Vousden, K.H. P53, ROS and Senescence in the Control of Aging. Aging 2010, 2, 471–474. [Google Scholar] [CrossRef]
- Wu, Z.; Du, Y.; Xue, H.; Wu, Y.; Zhou, B. Aluminum Induces Neurodegeneration and Its Toxicity Arises from Increased Iron Accumulation and Reactive Oxygen Species (ROS) Production. Neurobiol. Aging 2012, 33, 199.e1–199.e12. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative Stress: The Lowest Common Denominator of Multiple Diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An Introduction to Free Radical Biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Morales-Gonzalez, J.A.; Morales-Gonzalez, A.; Madrigal-Santillan, E.O. (Eds.) A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; InTech: London, UK, 2016. [Google Scholar]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of Antioxidants in Prophylaxis and Therapy: A Pharmaceutical Perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Juranek, J.K.; Daffu, G.K.; Wojtkiewicz, J.; Lacomis, D.; Kofler, J.; Schmidt, A.M. Receptor for Advanced Glycation End Products and Its Inflammatory Ligands Are Upregulated in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2015, 9, 485. [Google Scholar] [CrossRef]
- Ozbay, I.; Kucur, C.; Koçak, F.E.; Savran, B.; Oghan, F. Advanced Oxidation Protein Product Levels as a Marker of Oxidative Stress in Paediatric Patients with Chronic Tonsillitis. Acta Otorhinolaryngol. Ital. 2016, 36, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Tischfield, J.; Ruddle, F.H. The Linkage of Genes for the Human Interferon-Induced Antiviral Protein and Indophenol Oxidase-B Traits to Chromosome G-21. J. Exp. Med. 1973, 137, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Pallardó, F.V.; Lloret, A.; Lebel, M.; D’Ischia, M.; Cogger, V.C.; Le Couteur, D.G.; Gadaleta, M.N.; Castello, G.; Pagano, G. Mitochondrial Dysfunction in Some Oxidative Stress-Related Genetic Diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 2010, 11, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Garlet, T.R.; Parisotto, E.B.; de Medeiros, G.D.S.; Pereira, L.C.R.; Moreira, E.A.D.M.; Dalmarco, E.M.; Dalmarco, J.B.; Wilhelm Filho, D. Systemic Oxidative Stress in Children and Teenagers with Down Syndrome. Life Sci. 2013, 93, 558–563. [Google Scholar] [CrossRef]
- Bahsi, S.; Bakır, A.; Topçu, V.; Bahsi, T.; Ergüder, B.İ. Evaluation of Oxidant/Antioxidant System, IL-6 and IL-10 Parameters and SOD-Enzyme Activity in Pregnancy with Down Syndrome in Amnion Fluid Analysis. Gazi Med. J. 2022, 33, 53–57. [Google Scholar] [CrossRef]
- Gulesserian, T.; Seidl, R.; Hardmeier, R.; Cairns, N.; Lubec, G. Superoxide Dismutase SOD1, Encoded on Chromosome 21, but Not SOD2 Is Overexpressed in Brains of Patients with down Syndrome. J. Investig. Med. 2001, 49, 41–46. [Google Scholar] [CrossRef]
- Lee, M.; Hyun, D.; Jenner, P.; Halliwell, B. Effect of Overexpression of Wild-type and Mutant Cu/Zn-Superoxide Dismutases on Oxidative Damage and Antioxidant Defences: Relevance to Down’s Syndrome and Familial Amyotrophic Lateral Sclerosis. J. Neurochem. 2001, 76, 957–965. [Google Scholar] [CrossRef]
- Lubec, G. (Ed.) Advances in Down Syndrome Research; Springer: Vienna, Austria, 2003. [Google Scholar]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 Fatty Acids and Antioxidants in Neurological and Psychiatric Diseases: An Overview. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef]
- Ferreira, M.; Rodrigues, R.; Motta, E.; Debom, G.; Soares, F.; de Mattos, B.D.S.; Machado, C.; Stefanello, F.M.; da Silva, T.M.; Martins, C.C.; et al. Evaluation of Extracellular Adenine Nucleotides Hydrolysis in Platelets and Biomarkers of Oxidative Stress in Down Syndrome Individuals. Biomed. Pharmacother. 2015, 74, 200–205. [Google Scholar] [CrossRef]
- Perluigi, M.; Butterfield, D.A. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-like Dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 724904. [Google Scholar] [CrossRef]
- Izzo, A.; Manco, R.; Cristofaro, T.; de Bonfiglio, F.; Cicatiello, R.; Mollo, N.; Martino, M.D.; Genesio, R.; Zannini, M.; Conti, A.; et al. Overexpression of Chromosome 21 MiRNAs May Affect Mitochondrial Function in the Hearts of Down Syndrome Fetuses. Int. J. Genom. 2017, 2017, 8737649. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, E.; Helguera, P.R. The Shape of Mitochondrial Dysfunction in Down Syndrome. Dev. Neurobiol. 2019, 79, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, 1–9. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpst, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar]
- Pieper, D.; Rombey, T. Where to Prospectively Register a Systematic Review. Syst. Rev. 2022, 11, 8. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef]
- Drucker, A.M.; Fleming, P.; Chan, A.-W. Research Techniques Made Simple: Assessing Risk of Bias in Systematic Reviews. J. Investig. Dermatol. 2016, 136, 109–114. [Google Scholar] [CrossRef]
- Convertini, P.; Menga, A.; Andria, G.; Scala, I.; Santarsiero, A.; Castiglione Morelli, M.A.; Iacobazzi, V.; Infantino, V. The Contribution of the Citrate Pathway to Oxidative Stress in Down Syndrome. Immunology 2016, 149, 423–431. [Google Scholar] [CrossRef]
- Sadiq, M.F.; Hunaiti, A.A.; Alkaraki, A.K. The Association between Anti—Oxidant/Redox Status and Sister Chromatid Exchanges in down Syndrome Individuals. Jordan J. Biol. Sci. 2015, 8, 11–15. [Google Scholar] [CrossRef]
- Sulthana, S.M.; Kumar, S.N.; Sridhar, M.G.; Bhat, B.V.; Rao, K.R. Levels of Non Enzymatic Antioxidants in Down Syndrome. Indian J. Pediatr. 2012, 79, 1473–1476. [Google Scholar] [CrossRef]
- Sulthana, S.M.; Kumar, S.N.; Sridhar, M.G.; Bhat, B.V.; Rao, K.R. Antioxidant Enzyme Activity in Children with Down Syndrome. Curr. Pediatr. Res. 2012, 16, 43–47. [Google Scholar]
- Casado, Á.; López-Fernández, M.E.; Ruíz, R. Lipid Peroxidation in Down Syndrome Caused by Regular Trisomy 21, Trisomy 21 by Robertsonian Translocation and Mosaic Trisomy 21. Clin. Chem. Lab. Med. 2007, 45, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, F.; Rosety-Plaza, M.; Rosety-Rodriguez, M. Glucose-6-Phosphate-Dehydrogenase Is Also Increased in Erythrocytes from Adolescents with Down Syndrome. Down Syndr. Res. Pract. 2006, 11, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.E.; Peres, W.; Salvador, M. Oxidative Stress and Hematologic and Biochemical Parameters in Individuals with Down Syndrome. Mayo Clin. Proc. 2005, 80, 1607–1611. [Google Scholar] [CrossRef]
- Garaiová, I.; Muchová, J.; Šustrová, M.; Blažíček, P.; Sivoňová, M.; Kvasnička, P.; Pueschel, S.; Ďuračková, Z. The Relationship between Antioxidant Systems and Some Markers of Oxidative Stress in Persons with Down Syndrome. Biologia 2004, 59, 787–794. [Google Scholar]
- Žitňanová, I.; Korytár, P.; Aruoma, O.I.; Sustrová, M.; Garaiová, I.; Muchová, J.; Kalnovicová, T.; Pueschel, S.; Duracková, Z. Uric Acid and Allantoin Levels in Down Syndrome: Antioxidant and Oxidative Stress Mechanisms? Clin. Chim. Acta 2004, 341, 139–146. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Giannotti, A.; Bertini, E.; Federici, G.; Digilio, M.C.; Piemonte, F. Glutathione Metabolism and Antioxidant Enzymes in Children with Down Syndrome. J. Pediatr. 2003, 142, 583–585. [Google Scholar] [CrossRef]
- Pogribna, M.; Melnyk, S.; Pogribny, I.; Chango, A.; Yi, P.; James, S.J. Homocysteine Metabolism in Children with Down Syndrome: In Vitro Modulation. Am. J. Hum. Genet. 2001, 69, 88–95. [Google Scholar] [CrossRef]
- Kanavin, Ø.J.; Aaseth, J.; Birketvedt, G.S. Thyroid Hypofunction in Down’s Syndrome: Is It Related to Oxidative Stress? Biol. Trace Elem. Res. 2000, 78, 35–42. [Google Scholar] [CrossRef]
- Brugge, K.; Nichols, S.; Saitoh, T.; Trauner, D. Correlations of Glutathione Peroxidase Activity with Memory Impairment in Adults with Down Syndrome. Biol. Psychiatry 1999, 46, 1682–1689. [Google Scholar] [CrossRef]
- Pastor, M.-C.; Sierra, C.; Doladé, M.; Navarro, E.; Brandi, N.; Cabré, E.; Mira, A.; Serés, A. Antioxidant Enzymes and Fatty Acid Status in Erythrocytes of Down Syndrome Patients. Clin. Chem. 1998, 44, 924–929. [Google Scholar] [CrossRef]
- Gerli, G.; Zenoni, L.; Locatelli, G.F.; Mongiat, R.; Piattoni, F.; Orsini, G.B.; Montagnani, A.; Gueli, M.R.; Gualandri, V. Erythrocyte Antioxidant System in Down Syndrome. Am. J. Med. Genet. Suppl. 1990, 7, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Brás, A.; Monteiro, C.; Rueff, J. Oxidative Stress in Trisomy 21: A Possible Role in Cataractogenesis. Ophthalmic Paediatr. Genet. 1989, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Mangione, R.; Saab, M.W.; Di Stasio, E.; Di Rosa, M.; Tavazzi, B.; Lazzarino, G.; Onder, G.; Carfì, A. Biochemical Discrimination of the Down Syndrome-Related Metabolic and Oxidative/Nitrosative Stress Alterations from the Physiologic Age-Related Changes through the Targeted Metabolomic Analysis of Serum. Antioxidants 2022, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Officioso, A.; Trojsi, F.; Tedeschi, G.; Leoncini, S.; Signorini, C.; Ciccoli, L.; De Felice, C. Increased Non-Protein Bound Iron in Down Syndrome: Contribution to Lipid Peroxidation and Cognitive Decline. Free Radic. Res. 2016, 50, 1422–1431. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Ławicki, S.; Krętowski, A.J.; Zbucka-Krętowska, M. Prenatal Screening of Trisomy 21: Could Oxidative Stress Markers Play a Role? J. Clin. Med. 2021, 10, 2382. [Google Scholar] [CrossRef]
- Žitňanová, I.; Korytár, P.; Sobotová, H.; Horáková, L.; Šustrová, M.; Pueschel, S.; Ďuračková, Z. Markers of Oxidative Stress in Children with Down Syndrome. Clin. Chem. Lab. Med. 2006, 44, 306–310. [Google Scholar] [CrossRef]
- Pinto, M.; Neves, J.; Palha, M.; Bicho, M. Oxidative Stress in Portuguese Children with Down Syndrome. Down Syndr. Res. Pract. 2002, 8, 79–82. [Google Scholar] [CrossRef]
- Domingues, N.B.; Mariusso, M.R.; Tanaka, M.H.; Scarel-Caminaga, R.M.; Mayer, M.P.A.; Brighenti, F.L.; Zuanon, Â.C.C.; Ibuki, F.K.; Nogueira, F.N.; Giro, E.M.A. Reduced Salivary Flow Rate and High Levels of Oxidative Stress in Whole Saliva of Children with Down Syndrome. Spec. Care Dent. 2017, 37, 269–276. [Google Scholar] [CrossRef]
- de Sousa, M.C.; Vieira, R.B.; dos Santos, D.S.; Carvalho, C.A.T.; Camargo, S.E.A.; Mancini, M.N.G.; de Oliveira, L.D. Antioxidants and Biomarkers of Oxidative Damage in the Saliva of Patients with Down’s Syndrome. Arch. Oral Biol. 2015, 60, 600–605. [Google Scholar] [CrossRef]
- Subramaniam, P.; Girish Babu, K.; Mohan Das, L. Assessment of Salivary Total Antioxidant Levels and Oral Health Status in Children with Down Syndrome. Spec. Care Dent. 2014, 34, 193–200. [Google Scholar] [CrossRef]
- Komatsu, T.; Duckyoung, Y.; Ito, A.; Kurosawa, K.; Maehata, Y.; Kubodera, T.; Ikeda, M.; Lee, M.-C. Increased Oxidative Stress Biomarkers in the Saliva of Down Syndrome Patients. Arch. Oral Biol. 2013, 58, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Tolun, A.A.; Scarbrough, P.M.; Zhang, H.; McKillop, J.-A.; Wang, F.; Kishnani, P.S.; Millington, D.S.; Young, S.P.; Il’yasova, D. Systemic Oxidative Stress, as Measured by Urinary Allantoin and F(2)-Isoprostanes, Is Not Increased in Down Syndrome. Ann. Epidemiol. 2012, 22, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of Urinary Biomarkers of Oxidative/Nitrosative Stress in Children with Down Syndrome. Life Sci. 2011, 89, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of Urinary Biomarkers of Oxidative/Nitrosative Stress in Adolescents and Adults with Down Syndrome. Biochim. Biophys. Acta 2011, 1812, 760–768. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Urinary Uric Acid and Antioxidant Capacity in Children and Adults with Down Syndrome. Clin. Biochem. 2010, 43, 228–233. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Clements, D.; MacLeod, K. Biomarkers of Oxidative Stress Are Significantly Elevated in Down Syndrome. Free Radic. Biol. Med. 1998, 25, 1044–1048. [Google Scholar] [CrossRef]
- Perluigi, M.; di Domenico, F.; Fiorini, A.; Cocciolo, A.; Giorgi, A.; Foppoli, C.; Butterfield, D.A.; Giorlandino, M.; Giorlandino, C.; Eugenia Schininà, M.; et al. Oxidative Stress Occurs Early in Down Syndrome Pregnancy: A Redox Proteomics Analysis of Amniotic Fluid. Proteom. Clin. Appl. 2011, 5, 167–178. [Google Scholar] [CrossRef]
- Perrone, S.; Longini, M.; Bellieni, C.V.; Centini, G.; Kenanidis, A.; De Marco, L.; Petraglia, F.; Buonocore, G. Early Oxidative Stress in Amniotic Fluid of Pregnancies with Down Syndrome. Clin. Biochem. 2007, 40, 177–180. [Google Scholar] [CrossRef]
- Sun, X.; Kato, H.; Sato, H.; Han, X.; Hirofuji, Y.; Kato, T.A.; Sakai, Y.; Ohga, S.; Fukumoto, S.; Masuda, K. Dopamine-Related Oxidative Stress and Mitochondrial Dysfunction in Dopaminergic Neurons Differentiated from Deciduous Teeth-Derived Stem Cells of Children with Down Syndrome. FASEB BioAdv. 2022, 4, 454–467. [Google Scholar] [CrossRef]
- Rodríguez-Sureda, V.; Vilches, Á.; Sánchez, O.; Audí, L.; Domínguez, C. Intracellular Oxidant Activity, Antioxidant Enzyme Defense System, and Cell Senescence in Fibroblasts with Trisomy 21. Oxid. Med. Cell. Longev. 2015, 2015, 509241. [Google Scholar] [CrossRef]
- Odetti, P.; Angelini, G.; Dapino, D.; Zaccheo, D.; Garibaldi, S.; Dagna-Bricarelli, F.; Piombo, G.; Perry, G.; Smith, M.; Traverso, N.; et al. Early Glycoxidation Damage in Brains from Down’s Syndrome. Biochem. Biophys. Res. Commun. 1998, 243, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Ani, C.; Grantham-McGregor, S.; Muller, D. Nutritional Supplementation in Down Syndrome: Theoretical Considerations and Current Status. Dev. Med. Child Neurol. 2000, 42, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.M.L.; Goulart, M.O.F.; Moura, J.B.D.F.; Manfredini, V.; Benfato, M.D.S.; Kubota, L.T. Espécies Reativas de Oxigênio e de Nitrogênio, Antioxidantes e Marcadores de Dano Oxidativo Em Sangue Humano: Principais Métodos Analíticos Para Sua Determinação. Quim. Nova 2007, 30, 1323–1338. [Google Scholar] [CrossRef]
- Michelson, A.M.; McCord, J.M.; Fridovich, I. (Eds.) Superoxide and Superoxide Dismutases; Academic Press: London, UK, 1977. [Google Scholar]
- Sinet, P.-M.; Lejeune, J.; Jerome, H. Trisomy 21 (Down’s Syndrome) Glutathione Peroxidase, Hexose Monophosphate Shunt and I.Q. Life Sci. 1979, 24, 29–33. [Google Scholar] [CrossRef]
- Muchova, J.; Garaiova, I.; Sustrova, M.; Liptakova, A.; Blazicek, P.; Kvasnicka, P.; Durackova, Z. The Redox State of Glutathione in Erythrocytes of Individuals with Down Syndrome. Bratisl. Lek. Listy 2007, 108, 70–74. [Google Scholar]
- Rejc, B.; Karas-Kuželički, N.; Osredkar, J.; Geršak, K. Correlation between Markers of DNA and Lipid Oxidative Damage in Maternal and Fetoplacental Compartment in the Mid-Trimester of Pregnancy. J. Perinat. Med. 2017, 45, 413–419. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Muchová, J.; Sustrová, M.; Garaiová, I.; Liptáková, A.; Blazícek, P.; Kvasnicka, P.; Pueschel, S.; Duracková, Z. Influence of Age on Activities of Antioxidant Enzymes and Lipid Peroxidation Products in Erythrocytes and Neutrophils of Down Syndrome Patients. Free Radic. Biol. Med. 2001, 31, 499–508. [Google Scholar] [CrossRef]
- Brooksbank, B.W.L.; Balazs, R. Superoxide Dismutase, Glutathione Peroxidase and Lipoperoxidation in Down’s Syndrome Fetal Brain. Brain Res. 1984, 318, 37–44. [Google Scholar] [CrossRef]
- Kauffman, L.D.; Sokol, R.J.; Jones, R.H.; Awad, J.A.; Rewers, M.J.; Norris, J.M. Urinary F2-Isoprostanes in Young Healthy Children at Risk for Type 1 Diabetes Mellitus. Free Radic. Biol. Med. 2003, 35, 551–557. [Google Scholar] [CrossRef]
- Tsukahara, H. Biomarkers for Oxidative Stress: Clinical Application in Pediatric Medicine. Curr. Med. Chem. 2007, 14, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Watters, J.L.; Satia, J.A.; Costa, K.-A.; da Boysen, G.; Collins, L.B.; Morrow, J.D.; Milne, G.L.; Swenberg, J.A. Comparison of Three Oxidative Stress Biomarkers in a Sample of Healthy Adults. Biomarkers 2009, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Rossetto Giaccherino, R.; Lanfranco, F.; Motta, G.; Gori, D.; Arvat, E.; Ghigo, E.; Giordano, R. Endocrine Autoimmunity in Down’s Syndrome. Front. Horm. Res. 2017, 48, 133–146. [Google Scholar] [CrossRef]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial Dysfunction in Down Syndrome: Molecular Mechanisms and Therapeutic Targets. Mol. Med. 2018, 24, 2. [Google Scholar] [CrossRef]
- Mosaoa, R.; Kasprzyk-Pawelec, A.; Fernandez, H.R.; Avantaggiati, M.L. The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules 2021, 11, 141. [Google Scholar] [CrossRef]
- Pecze, L.; Randi, E.B.; Szabo, C. Meta-Analysis of Metabolites Involved in Bioenergetic Pathways Reveals a Pseudohypoxic State in Down Syndrome. Mol. Med. 2020, 26, 102. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.; Zhu, L.; Zhang, B.; Feng, P.; Gao, E.; Xu, C.; Wang, X.; Yi, W.; Sun, Y. Asprosin Improves the Survival of Mesenchymal Stromal Cells in Myocardial Infarction by Inhibiting Apoptosis via the Activated ERK1/2-SOD2 Pathway. Life Sci. 2019, 231, 116554. [Google Scholar] [CrossRef]
- Yuan, M.; Li, W.; Zhu, Y.; Yu, B.; Wu, J. Asprosin: A Novel Player in Metabolic Diseases. Front. Endocrinol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Luís, C.; Fernandes, R.; Soares, R.; von Hafe, P. A State of the Art Review on the Novel Mediator Asprosin in the Metabolic Syndrome. Porto Biomed. J. 2020, 5, e108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).