From Chemistry to Pharmacology: Exploring the Anti-Inflammatory and Antioxidant Potential of Novel Dexketoprofen Amide Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthetic Procedures
2.3. In Vivo Studies
2.3.1. Assessment of Acute Toxicity
2.3.2. Assessment of In Vivo Anti-Inflammatory Potential in Wistar Albino Rats
- Solvent—negative control group of animals treated with distilled water with a few drops of Tween 80;
- Dexketoprofen—positive control group of animals treated with dexketoprofen;
- Group 1—animals treated with derivative 1;
- Group 2—animals treated with derivative 2;
- Group 3—animals treated with derivative 3;
- Group 4—animals treated with derivative 4;
- Group 5—animals treated with derivative 5.
2.3.3. Evaluation of Synthesized Compounds’ Effect on Systemic and Local Redox State
Determination of Pro-Oxidative Parameters
Determination of Antioxidative Parameters
2.4. In Vitro Investigation of COX-1 and COX-2 Inhibitory Activity
2.5. In Silico Molecular Docking Studies
3. Results and Discussion
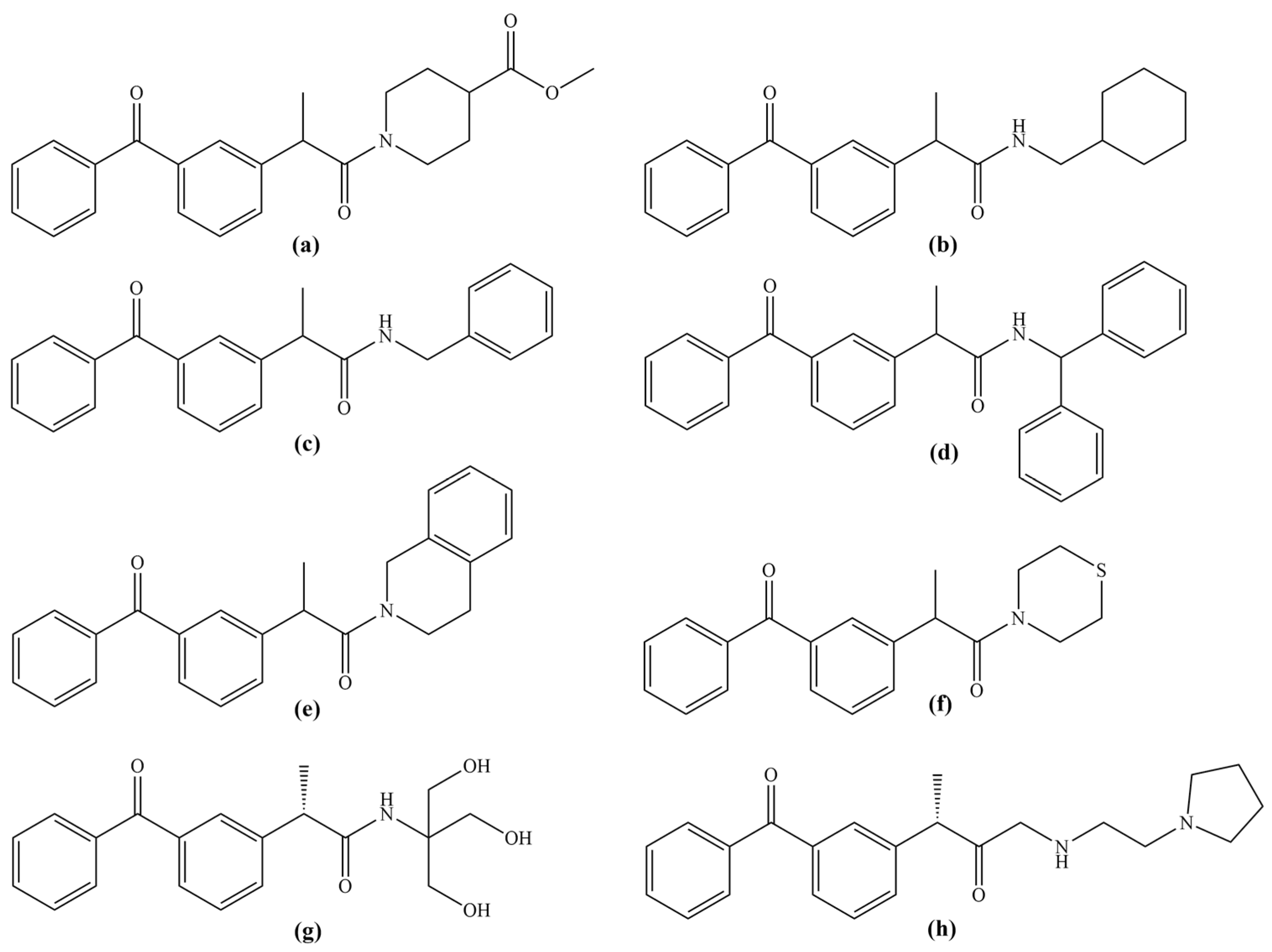
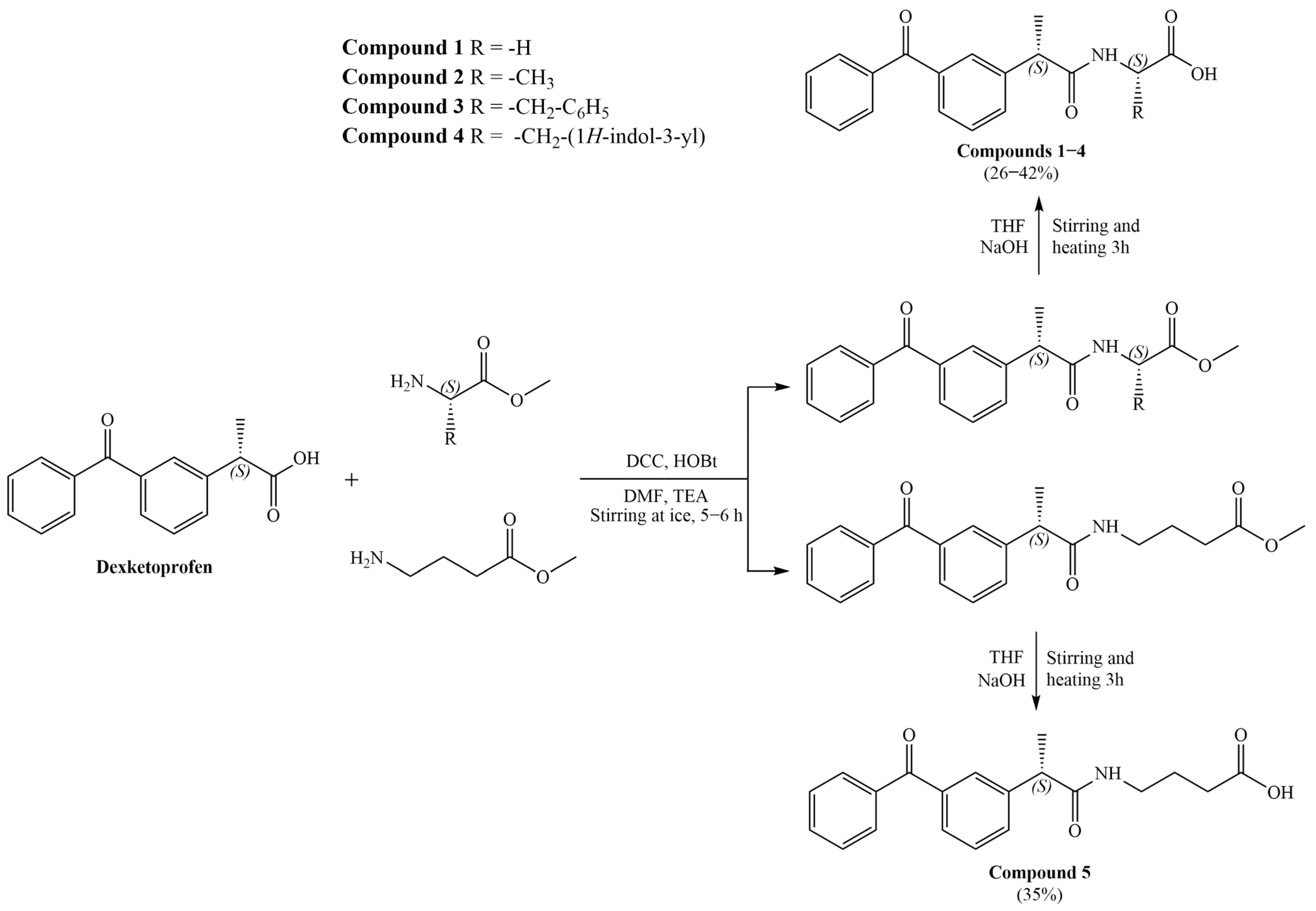
3.1. General Procedure for the Synthesis of Amide Derivatives of Dexketoprofen
3.2. Evaluation of Acute Toxicity
3.3. In Vivo Evaluation of the Anti-Inflammatory Potential
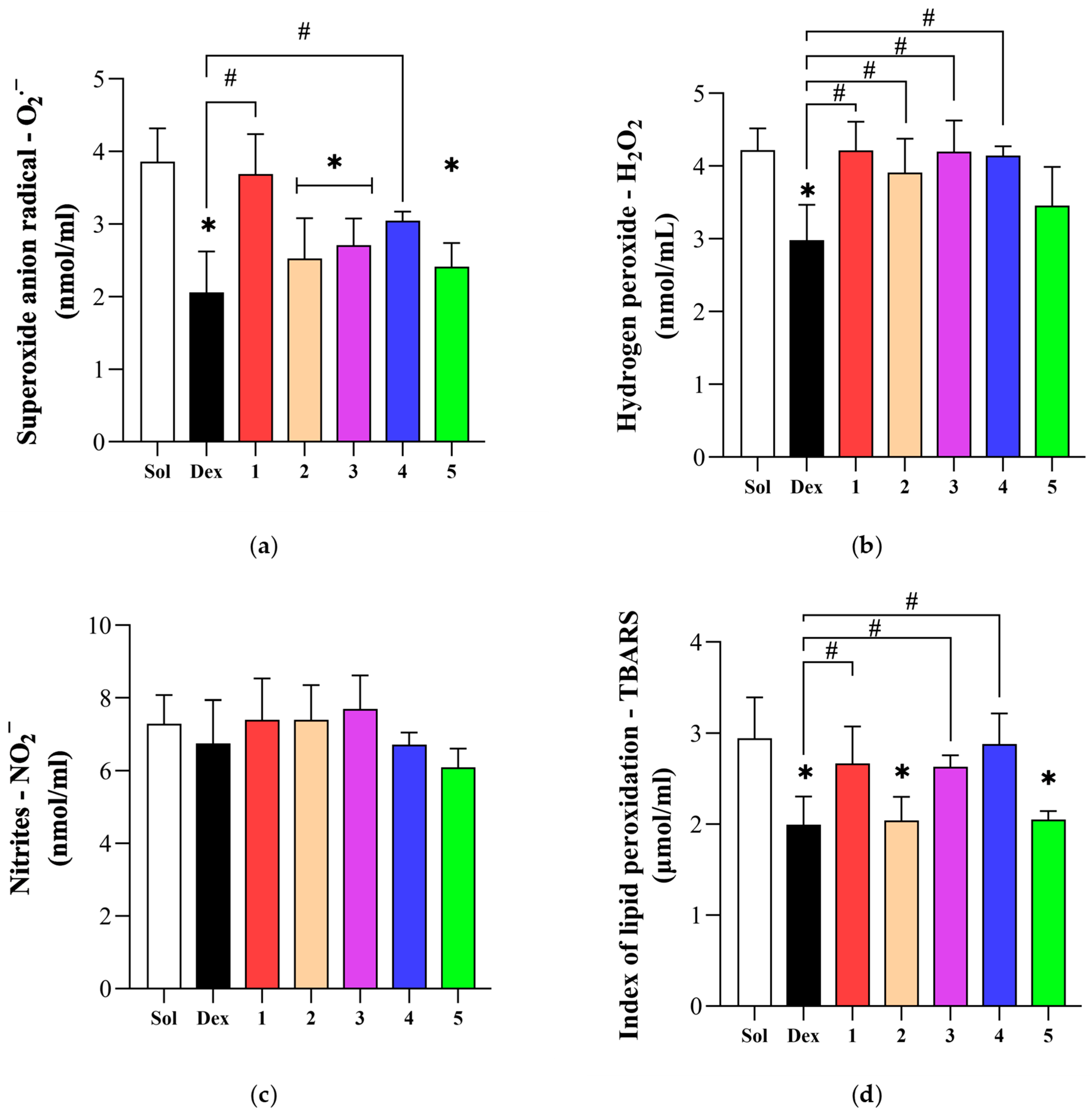
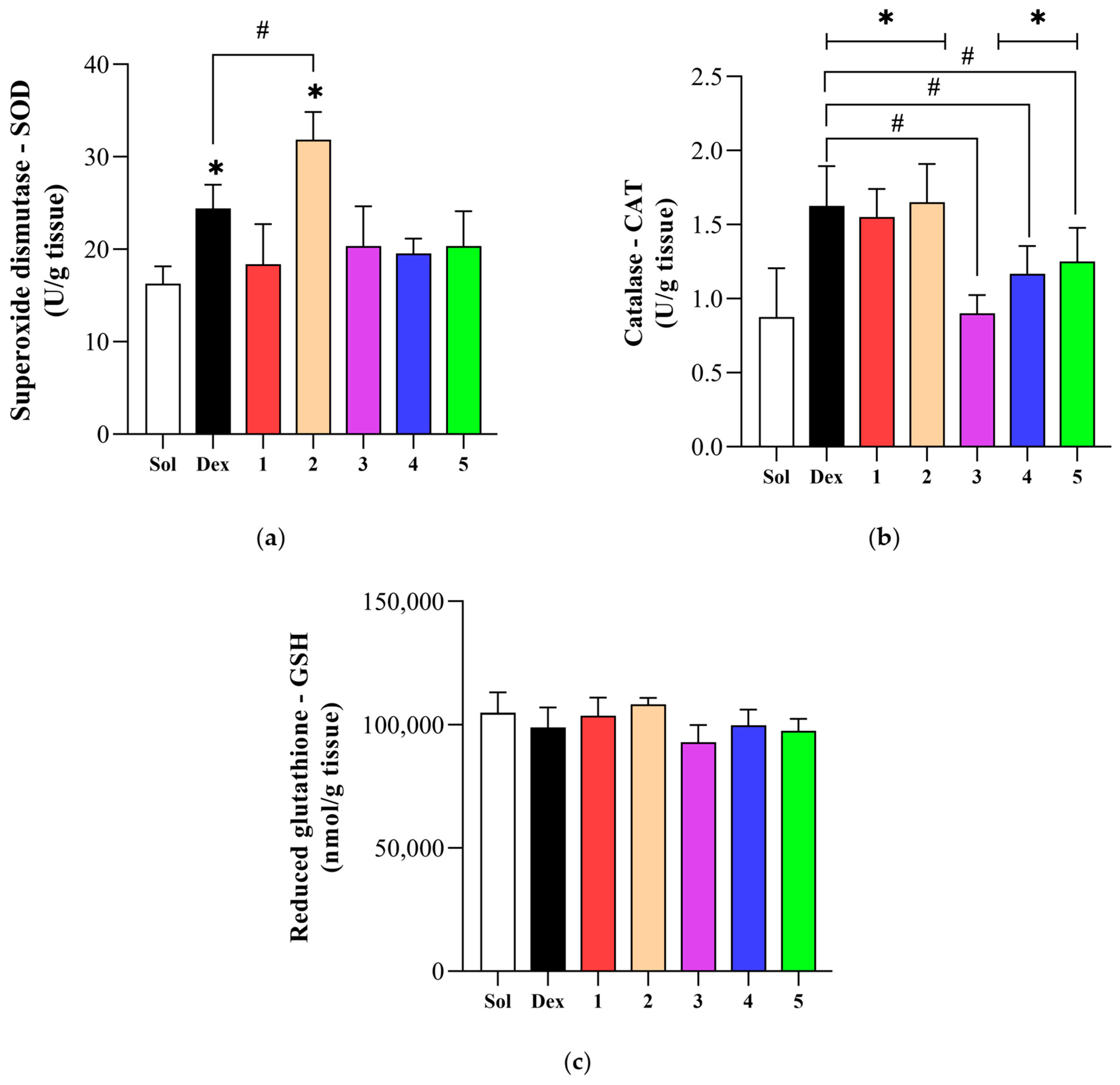
3.4. Effect of Novel Compounds on Systemic Oxidative Stress Parameters
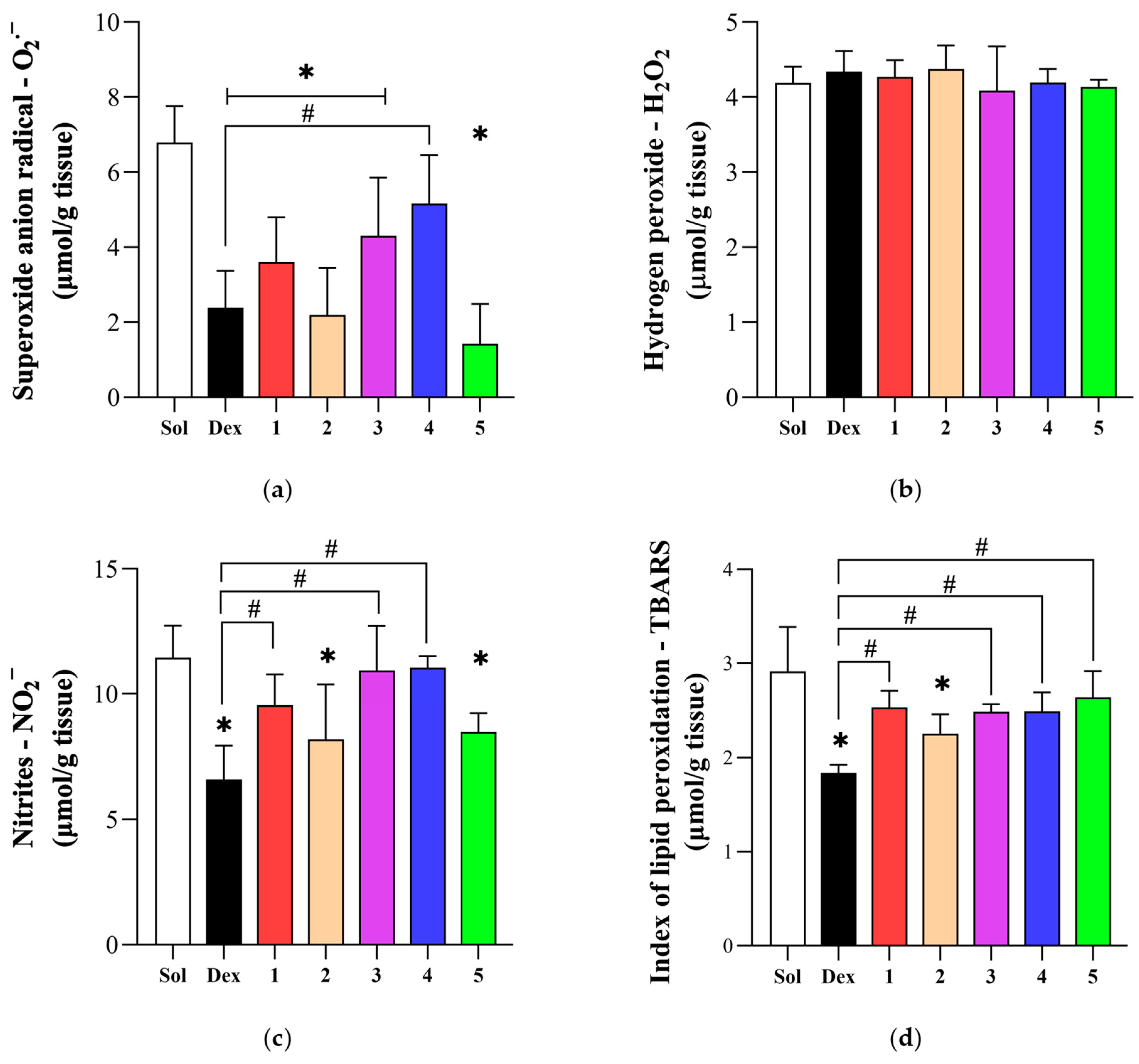
3.5. Effect of Novel Compounds on Local Oxidative Stress Parameters
3.6. Investigation of COX-1 and COX-2 Inhibitory Properties
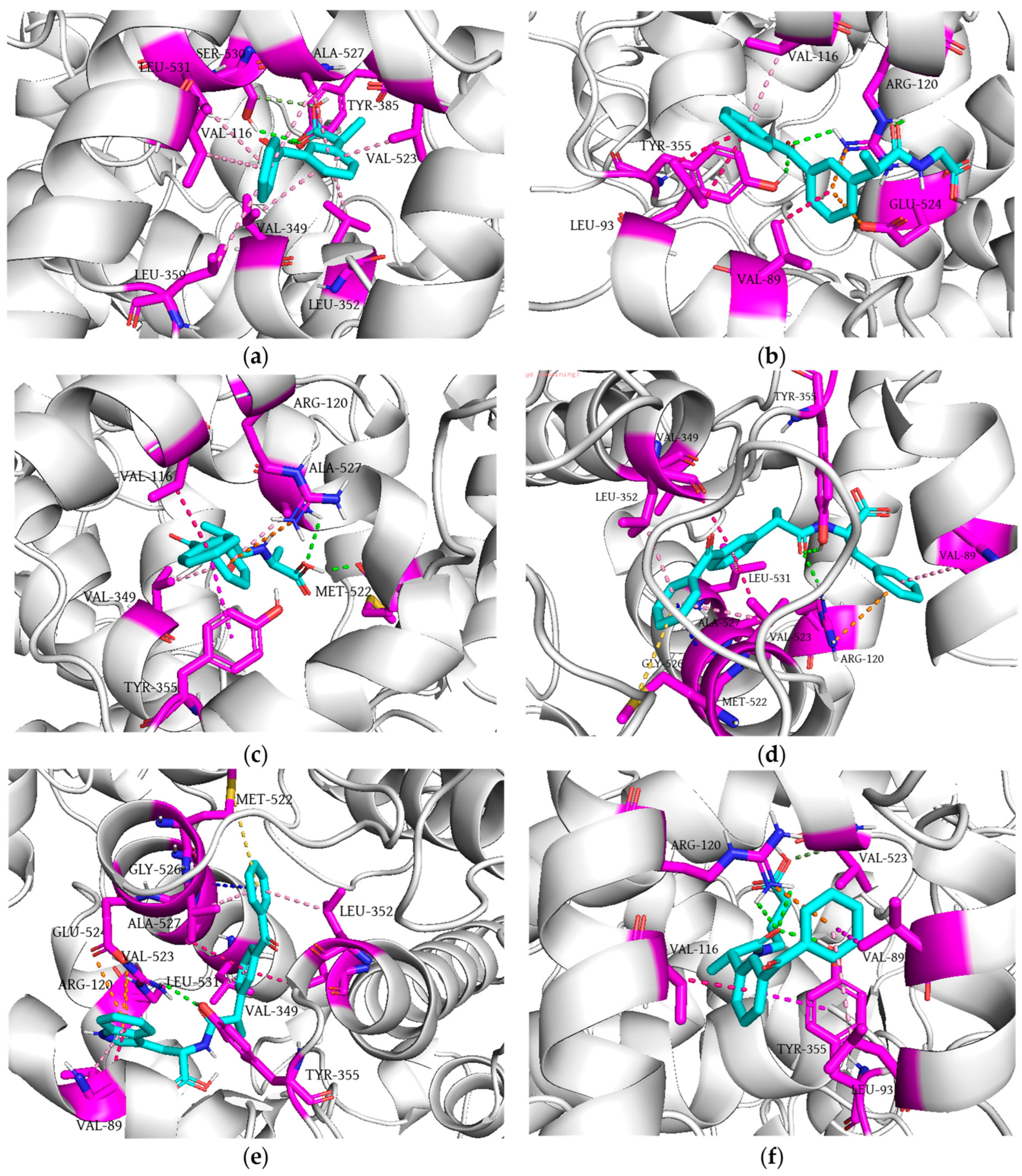
3.7. Molecular Docking Simulation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Aramouni, K.; Assaf, R.; Shaito, A.; Fardoun, M.; Al-Asmakh, M.; Sahebkar, A.; Eid, A.H. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J. Cell. Physiol. 2023, 238, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurologia 2024, 39, 292–301. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Ionuț, I.; Nastasă, C.; Fizeșan, I.; Pîrnău, A.; Vlase, L.; Palage, M.; Oniga, S.; Oniga, O. Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation. Pharmaceutics 2022, 15, 136. [Google Scholar] [CrossRef]
- Bano, B.; Kanwal Hameed, S.; Lateef, M.; Wadood, A.; Shams, S.; Hussain, S.; Ain, N.U.; Perveen, S.; Taha, M.; Khan, K.M. Unsymmetrical thiourea derivatives: Synthesis and evaluation as promising antioxidant and enzyme inhibitors. Future Med. Chem. 2024, 16, 497–511. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, antioxidant and cytoprotective activity evaluation of C-3 substituted indole derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef]
- Mahmood, K.A.S.; Ahmed, J.H.; Jawad, A.M. Non-steroidal anti-inflammatory drugs (NSAIDs), free radicals and reactive oxygen species (ros): A review of literature. Med. J. Basrah Univ. 2009, 27, 46–53. [Google Scholar] [CrossRef][Green Version]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Zhong, M.; Lu, Y.; Li, S.; Li, X.; Liu, Z.; He, X.; Zhang, Y. Synthesis, cytotoxicity, antioxidant activity and molecular modeling of new NSAIDs-EBS derivatives. Eur. J. Med. Chem. 2023, 259, 115662. [Google Scholar] [CrossRef]
- Ivanov, I.; Manolov, S.; Bojilov, D.; Marc, G.; Dimitrova, D.; Oniga, S.; Oniga, O.; Nedialkov, P.; Stoyanova, M. Novel Flurbiprofen Derivatives as Antioxidant and Anti-Inflammatory Agents: Synthesis, In Silico, and In Vitro Biological Evaluation. Molecules 2024, 29, 385. [Google Scholar] [CrossRef]
- Raza, A.; Abbas Khan, M.; Ahmad, I.; Ur Rehman, S.; Khaliq, S.; Ahmed, J.; Awan, B.; Ullah, F.; Masood, A.; Ahmed, N. Design, Synthesis, and Biological Evaluation of Dexibuprofen Derivatives as Novel Anti-Inflammatory, Antioxidant and Molecular Docking Studies. Chem. Biodivers. 2023, 20, e202300482. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Marc, G.; Rekka, E.A. Design, Synthesis and Evaluation of Antioxidant and NSAID Derivatives with Antioxidant, Anti-Inflammatory and Plasma Lipid Lowering Effects. Molecules 2024, 29, 1016. [Google Scholar] [CrossRef]
- Novelli, R.; Aramini, A.; Boccella, S.; Bagnasco, M.; Cattani, F.; Ferrari, M.P.; Goisis, G.; Minnella, E.M.; Allegretti, M.; Pace, V. Ketoprofen lysine salt has a better gastrointestinal and renal tolerability than ketoprofen acid: A comparative tolerability study in the Beagle dog. Biomed. Pharmacother. 2022, 153, 113336. [Google Scholar] [CrossRef]
- Graziosi, A.; Senatore, M.; Gazzaniga, G.; Agliardi, S.; Pani, A.; Scaglione, F. Ketoprofen Lysine Salt vs. Ketoprofen Acid: Assessing the Evidence for Enhanced Safety and Efficacy. Life 2025, 15, 659. [Google Scholar] [CrossRef]
- Cimini, A.; Brandolini, L.; Gentile, R.; Cristiano, L.; Menghini, P.; Fidoamore, A.; Antonosante, A.; Benedetti, E.; Giordano, A.; Allegretti, M. Gastroprotective effects of L-lysine salification of ketoprofen in ethanol-injured gastric mucosa. J. Cell. Physiol. 2015, 230, 813–820. [Google Scholar] [CrossRef]
- Atzeni, F.; Masala, I.F.; Bagnasco, M.; Lanata, L.; Mantelli, F.; Sarzi-Puttini, P. Comparison of Efficacy of Ketoprofen and Ibuprofen in Treating Pain in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Pain Ther. 2021, 10, 577–588. [Google Scholar] [CrossRef]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future prospects of ketoprofen in improving the safety of the gastric mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef]
- Siódmiak, J.; Siódmiak, T.; Tarczykowska, A.; Czirson, K.; Dulęba, J.; Marszałł, M.P. Metabolic chiral inversion of 2-arylpropionic acid derivatives (profens). Med. Res. J. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Hanna, M.; Moon, J.Y. A review of dexketoprofen trometamol in acute pain. Curr. Med. Res. Opin. 2019, 35, 189–202. [Google Scholar] [CrossRef]
- Rudy, A.C.; Liu, Y.; Brater, C.; Hall, S.D. Stereoselective pharmacokinetics and inversion of (R)- ketoprofen in healthy volunteers. J. Clin. Pharmacol. 1998, 38, 3S–10S. [Google Scholar] [CrossRef]
- Ghezzi, P.; Melillo, G.; Meazza, C.; Sacco, S.; Pellegrini, L.; Asti, C.; Porzio, S.; Marullo, A.; Sabbatini, V.; Caselli, G.; et al. Differential contribution of R and S isomers in ketoprofen anti-inflammatory activity: Role of cytokine modulation. J. Pharmacol. Exp. Ther. 1998, 287, 969–974. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Marnett, A.B.; Kozak, K.R.; Remmel, R.P.; Marnett, L.J. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: Facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc. Natl. Acad. Sci. USA 2000, 97, 925–930. [Google Scholar] [CrossRef]
- Deplano, A.; Karlsson, J.; Fowler, C.J.; Onnis, V. The fatty acid amide hydrolase and cyclooxygenase-inhibitory properties of novel amide derivatives of carprofen. Bioorg. Chem. 2020, 101, 104034. [Google Scholar] [CrossRef]
- Deplano, A.; Karlsson, J.; Moraca, F.; Svensson, M.; Cristiano, C.; Morgillo, C.M.; Fowler, C.J.; Russo, R.; Catalanotti, B.; Onnis, V. Design, synthesis and in vitro and in vivo biological evaluation of flurbiprofen amides as new fatty acid amide hydrolase/cyclooxygenase-2 dual inhibitory potential analgesic agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 940–953. [Google Scholar] [CrossRef]
- Alam, A.; Ali, M.; Rehman, N.U.; Latif, A.; Shah, A.J.; Wazir, N.U.; Lodhi, M.A.; Kamal, M.; Ayaz, M.; Al-Harrasi, A.; et al. Synthesis and characterization of biologically active flurbiprofen amide derivatives as selective prostaglandin-endoperoxide synthase II inhibitors: In vivo anti-inflammatory activity and molecular docking. Int. J. Biol. Macromol. 2023, 228, 659–670. [Google Scholar] [CrossRef]
- Marjanović, M.; Zorc, B.; Pejnović, L.; Zovko, M.; Kralj, M. Fenoprofen and ketoprofen amides as potential antitumor agents. Chem. Biol. Drug Des. 2007, 69, 222–226. [Google Scholar] [CrossRef]
- Dhokchawle, B.V.; Tauro, S.J.; Bhandari, A.B. Ester Prodrugs of Ketoprofen: Synthesis, Hydrolysis Kinetics and Pharmacological Evaluation. Drug Res. 2016, 66, 46–50. [Google Scholar] [CrossRef]
- Gaitan, G.; Ahuir, F.J.; Del Soldato, P.; Herrero, J.F. Comparison of the antinociceptive activity of two new NO-releasing derivatives of the NSAID S-ketoprofen in rats. Br. J. Pharmacol. 2004, 143, 533–540. [Google Scholar] [CrossRef]
- Al Mekhlafi, S.; Alkadi, H.; El-Sayed, M.I.K. Synthesis and anti-inflammatory activity of novel Ketoprofen and Ibuprofen derivatives. J. Chem. Pharm. Res. 2015, 7, 503–510. [Google Scholar]
- Rajić, Z.; Hadjipavlou-Litina, D.; Pontiki, E.; Balzarini, J.; Zorc, B. The novel amidocarbamate derivatives of ketoprofen: Synthesis and biological activity. Med. Chem. Res. 2011, 20, 210–219. [Google Scholar] [CrossRef]
- Uludağ, M.O.; Ergün, B.Ç.; Alkan, D.A.; Ercan, N.; Özkan, G.Y.; Banoğlu, E. Stable ester and amide conjugates of some NSAIDs as analgesic and antiinflammatory compounds with improved biological activity. Turk. J. Chem. 2011, 35, 427–439. [Google Scholar] [CrossRef]
- Rajić, Z.; Hadjipavlou-Litina, D.; Pontiki, E.; Kralj, M.; Suman, L.; Zorc, B. The novel ketoprofen amides--synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem. Biol. Drug Des. 2010, 75, 641–652. [Google Scholar] [CrossRef]
- Manolov, S.; Bojilov, D.; Ivanov, I.; Marc, G.; Bataklieva, N.; Oniga, S.; Oniga, O.; Nedialkov, P. Synthesis, Molecular Docking, Molecular Dynamics Studies, and In Vitro Biological Evaluation of New Biofunctional Ketoprofen Derivatives with Different N-Containing Heterocycles. Processes 2023, 11, 1837. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Gavalas, A.; Rekka, E.A. Amides of non-steroidal anti-inflammatory drugs with thiomorpholine can yield hypolipidemic agents with improved anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2016, 26, 910–913. [Google Scholar] [CrossRef]
- Kulabaş, N.; Set, İ.; Aktay, G.; Gürsoy, Ş.; Danış, Ö.; Ogan, A.; Erdem, S.S.; Erzincan, P.; Helvacıoğlu, S.; Hamitoğlu, M.; et al. Identification of some novel amide conjugates as potent and gastric sparing anti-inflammatory agents: In vitro, in vivo, in silico studies and drug safety evaluation. J. Mol. Struct. 2023, 1285, 135521. [Google Scholar] [CrossRef]
- Berk, B.; Bender, C.; Akkol, E.K.; Yeşilada, E. Design and synthesis of stable N-[2-(Aryl/heteroaryl substituted) ethyl] propanamide derivatives of (S)-ketoprofen and (S)-ibuprofen as non-ulcerogenic anti-inflammatory and analgesic agents. Acta Pharm. Sci. 2016, 54, 65. [Google Scholar] [CrossRef]
- Nedeljković, N.; Dobričić, V.; Bošković, J.; Vesović, M.; Bradić, J.; Anđić, M.; Kočović, A.; Jeremić, N.; Novaković, J.; Jakovljević, V.; et al. Synthesis and investigation of anti-inflammatory activity of new thiourea derivatives of naproxen. Pharmaceuticals 2023, 16, 666. [Google Scholar] [CrossRef]
- Boarescu, I.; Boarescu, P.M.; Pop, R.M.; Bocșan, I.C.; Gheban, D.; Râjnoveanu, R.M.; Râjnoveanu, A.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Curcumin Nanoparticles Enhance Antioxidant Efficacy of Diclofenac Sodium in Experimental Acute Inflammation. Biomedicines 2021, 10, 61. [Google Scholar] [CrossRef]
- Tomic, Z.; Milijasevic, B.; Sabo, A.; Dusan, L.; Jakovljevic, V.; Mikov, M.; Majda, S.; Vasovic, V. Diclofenac and ketoprofen liver toxicity in rat. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 253–260. [Google Scholar] [CrossRef]
- Tomovic, M.; Petrovic, A.; Nikolic, M.; Mitrovic, S.; Jakovljevic, V.; Pecarski, D. Lady’s Bedstraw as a Powerful Antioxidant for Attenuation of Doxorubicin-Induced Cardiotoxicity. Antioxidants 2023, 12, 1277. [Google Scholar] [CrossRef]
- Rankovic, M.; Draginic, N.; Jeremic, J.; Samanovic, A.M.; Stojkov, S.; Mitrovic, S.; Jeremic, N.; Radonjic, T.; Srejovic, I.; Bolevich, S.; et al. Protective Role of Vitamin B1 in Doxorubicin-Induced Cardiotoxicity in Rats: Focus on Hemodynamic, Redox, and Apoptotic Markers in Heart. Front. Physiol. 2021, 12, 690619. [Google Scholar] [CrossRef]
- Draginic, N.; Andjic, M.; Jeremic, J.; Zivkovic, V.; Kocovic, A.; Tomovic, M.; Bozin, B.; Kladar, N.; Bolevich, S.; Jakovljevic, V.; et al. Anti-inflammatory and Antioxidant Effects of Melissa officinalis Extracts: A Comparative Study. Iran. J. Pharm. Res. 2022, 21, e126561. [Google Scholar] [CrossRef]
- Bošković, J.; Dobričić, V.; Mihajlović, M.; Kotur-Stevuljević, J.; Čudina, O. Synthesis, evaluation of enzyme inhibition and redox properties of potential dual COX-2 and 5-LOX inhibitors. Pharmaceuticals 2023, 16, 549. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Buntrock, R.E. ChemOffice Ultra 7.0. J. Chem. Inf. Comput. Sci. 2002, 42, 1505–1506. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Lee, J.Y.; Yuan, C.; Smith, W.L. Comparison of cyclooxygenase-1 crystal structures: Cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry 2010, 49, 7069–7079. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Biovia, D.S.; Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Richmond, T.J. Dassault Systèmes BIOVIA, Discovery Studio Visualizer, V. 17.2, San Diego: Dassault Systèmes. J. Chem. Phys. 2016, 10, 21–9991. [Google Scholar]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. Available online: http://www.pymol.org/pymol (accessed on 25 May 2025).
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Tziona, P.; Theodosis-Nobelos, P.; Papagiouvannis, G.; Petrou, A.; Drouza, C.; Rekka, E.A. Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol. Molecules 2022, 27, 2104. [Google Scholar] [CrossRef]
- Ghosh, R.; Alajbegovic, A.; Gomes, A.V. NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2015, 2015, 536962. [Google Scholar] [CrossRef]
- Nair, P.; Kanwar, S.S.; Sanyal, S.N. Effects of non steroidal anti-inflammatory drugs on the antioxidant defense system and the membrane functions in the rat intestine. Nutr. Hosp. 2006, 21, 638–649. [Google Scholar]
- Li, Z.; Xu, D.; Li, X.; Deng, Y.; Li, C. Redox Imbalance in Chronic Inflammatory Diseases. BioMed Res. Int. 2022, 2022, 9813486. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Moore, R.A.; Barden, J. Systematic review of dexketoprofen in acute and chronic pain. BMC Clin. Pharmacol. 2008, 8, 11. [Google Scholar] [CrossRef]
- Koksal, E.; Ustun, Y.B.; Bilgin, S.; Aksoy, A.; Das, Y.K.; Yarim, M.; Ozkan, F.; Kaya, C.; Dost, B. The effects of dexketoprofen on renal ischemia-reperfusion injury: An experimental study. Braz. J. Anesthesiol. 2022, 72, 365–371. [Google Scholar] [CrossRef]
- Ustun, Y.B.; Koksal, E.; Kaya, C.; Sener, E.B.; Aksoy, A.; Yarim, G.; Kabak, Y.; Gulbahar, Y. The effects of dexketoprofen on endogenous leptin and lipid peroxidation during liver ischemia reperfusion injury. Int. Surg. 2014, 99, 757–763. [Google Scholar] [CrossRef]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Kiefer, J.R.; Pawlitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Gierse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J.; et al. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef]




| Compound | 1 | 2 | 3 | 4 | 5 | Control Group |
|---|---|---|---|---|---|---|
| Organ | ||||||
| Kidney | 0.35 ± 0.07 | 0.39 ± 0.05 | 0.36 ± 0.04 | 0.37 ± 0.04 | 0.36 ± 0.06 | 0.38 ± 0.02 |
| Heart | 0.33 ± 0.03 | 0.36 ± 0.04 | 0.34 ± 0.02 | 0.33 ± 0.04 | 0.34 ± 0.02 | 0.35 ± 0.03 |
| Liver | 2.84 ± 0.20 | 3.15 ± 0.50 | 2.98 ± 0.23 | 2.67 ± 0.14 | 3.03 ± 0.41 | 3.05 ± 0.12 |
| Stomach | 0.57 ± 0.07 | 0.61 ± 0.05 | 0.55 ± 0.04 | 0.57 ± 0.06 | 0.58 ± 0.05 | 0.59 ± 0.07 |
| Rat Paw Thickness (mm) (% of Inhibition) | |||||
|---|---|---|---|---|---|
| Experimental Groups | 0 h | 1 h | 2 h | 3 h | 4 h |
| Compound 1 | 3.58 ± 0.13 | 5.68 ± 0.25 (5.263%) | 5.58 ± 0.34 (13.669%) | 5.16 ± 0.34 (16.842%) | 4.50 ± 0.29 (18.824%) |
| Compound 2 | 4.66 ± 0.13 | 6.52 ± 0.28 (16.090%) | 6.36 ± 0.25 (26.619%) * | 5.92 ± 0.19 (33.684%) * | 5.08 ± 0.54 (62.941%) ** |
| Compound 3 | 4.27 ± 0.21 | 6.25 ± 0.25 (10.526%) | 6.10 ± 0.18 (20.863%) * | 5.73 ± 0.23 (22.807%) | 4.93 ± 0.37 (41.176%) |
| Compound 4 | 4.02 ± 0.12 | 5.90 ± 0.37 (15.038%) | 5.77 ± 0.36 (24.460%) * | 5.38 ± 0.34 (28.070%) * | 4.48 ± 0.33 (58.824%) ** |
| Compound 5 | 3.46 ± 0.29 | 5.52 ± 0.25 (7.068%) | 5.38 ± 0.48 (17.122%) | 5.00 ± 0.29 (18.947%) | 4.28 ± 0.37 (27.647%) * |
| Solvent | 3.68 ± 0.41 | 5.90 ± 0.24 | 6.00 ± 0.40 | 5.58 ± 0.37 | 4.82 ± 0.23 |
| Dexketoprofen | 4.10 ± 0.31 | 5.93 ± 0.08 (17.293%) | 5.78 ± 0.15 (27.338%) * | 5.38 ± 0.23 (32.456%) * | 4.53 ± 0.23 (61.765%) ** |
| Compound | COX-1 | COX-2 |
|---|---|---|
| 1 | >100 | >100 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | >100 | 37.55 ± 3.43 |
| 5 | >100 | >100 |
| Dexketoprofen | 0.0015 ± 0.0006 | 0.13 ± 0.02 |
| Compound | COX-1 | COX-2 | ||
|---|---|---|---|---|
| Docking Score | Interacting Residue * | Docking Score | Interacting Residue * | |
| 1 | −8.2 | Arg120 (HBD × 3), Val349 (π-σ), Leu352 (π-σ), Tyr355 (bump), Ile523 (π-alkyl), Ala527 (π-alkyl × 2), Ser530 (HBD) | −9.0 | Val89 (π-σ), Leu93 (π-σ × 2), Val116 (π-alkyl), Arg120 (HBD × 2), Arg120 (π-cation), Tyr355 (HBD), Glu524 (π-anion) |
| 2 | −7.6 | Leu93 (π-alkyl), Leu115 (π-alkyl), Val116 (π-σ × 3), Val119 (π-alkyl), Arg120 (HBD × 2), Tyr355 (π-π), Ile523 (HBA) | −9.4 | Val116 (π-σ), Arg120 (π-cation), Val349 (π-alkyl), Tyr355 (π-π), Met522 (HBA), Ala527 (HBD), Ala527 (π-alkyl) |
| 3 | −8.8 | Arg97 (HBD × 4), Gln192 (CHBD), Tyr355 (HBA) | −9.9 | Val89 (π-alkyl), Arg120 (HBD), Arg120 (π-cation), Val349 (π-σ), Leu352 (π-alkyl), Tyr355 (HBD), Met522 (π-sulfur), Val523 (π-alkyl), Gly526 (amide-π), Ala527 (π-σ), Leu531 (π-alkyl) |
| 4 | −8.8 | Arg97 (HBD × 2), Gln192 (CHBD), Gln350 (HBD) | −10.8 | Val89 (π-alkyl), Val89 (π-σ), Arg120 (HBD), Arg120 (π-cation × 2), Val349 (π-σ), Leu352 (π-alkyl), Tyr355 (HBD), Met522 (π-sulfur), Val523 (π-alkyl), Glu524 (π-anion), Gly526 (amide-π), Ala527 (π-σ), Leu531 (π-alkyl) |
| 5 | −8.5 | Arg120 (HBD), Val349 (π-σ), Leu352 (π-σ), Ile523 (π-alkyl), Glu524 (HBA), Gly526 (amide-π), Ala527 (π-alkyl), Ser530 (HBD) | −9.2 | Val89 (π-σ), Leu93 (π-alkyl), Val116 (π-σ), Arg120 (HBD × 2), Arg120 (π-cation), Tyr355 (HBD), Tyr355 (π-π), Val523 (CHBD) |
| Dexketoprofen | −8.9 | Arg120 (HBD × 2), Val349 (π-σ), Leu352 (π-alkyl × 2), Ile523 (π-alkyl), Gly526 (amide-π), Ala527 (π-alkyl), Ser530 (HBD) | −8.9 | Tyr385 (HBD), Ser530 (HBD), Ser530 (CHBD), Val349 (π-alkyl), Leu352 (π-alkyl), Val523 (π-alkyl), Ala527 (π-alkyl × 2), Val116 (π-alkyl), Leu359 (π-alkyl), Leu531 (π-alkyl) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karović, M.; Nikolić, M.; Nedeljković, N.; Vesović, M.; Nikolić, M.; Anđić, M.; Lazarević, N.; Jakovljević, V.; Nedeljković, J.; Đaković, S.; et al. From Chemistry to Pharmacology: Exploring the Anti-Inflammatory and Antioxidant Potential of Novel Dexketoprofen Amide Derivatives. Antioxidants 2025, 14, 796. https://doi.org/10.3390/antiox14070796
Karović M, Nikolić M, Nedeljković N, Vesović M, Nikolić M, Anđić M, Lazarević N, Jakovljević V, Nedeljković J, Đaković S, et al. From Chemistry to Pharmacology: Exploring the Anti-Inflammatory and Antioxidant Potential of Novel Dexketoprofen Amide Derivatives. Antioxidants. 2025; 14(7):796. https://doi.org/10.3390/antiox14070796
Chicago/Turabian StyleKarović, Marko, Miloš Nikolić, Nikola Nedeljković, Marina Vesović, Marina Nikolić, Marijana Anđić, Nevena Lazarević, Vladimir Jakovljević, Jelena Nedeljković, Sanja Đaković, and et al. 2025. "From Chemistry to Pharmacology: Exploring the Anti-Inflammatory and Antioxidant Potential of Novel Dexketoprofen Amide Derivatives" Antioxidants 14, no. 7: 796. https://doi.org/10.3390/antiox14070796
APA StyleKarović, M., Nikolić, M., Nedeljković, N., Vesović, M., Nikolić, M., Anđić, M., Lazarević, N., Jakovljević, V., Nedeljković, J., Đaković, S., Bošković, J., & Dobričić, V. (2025). From Chemistry to Pharmacology: Exploring the Anti-Inflammatory and Antioxidant Potential of Novel Dexketoprofen Amide Derivatives. Antioxidants, 14(7), 796. https://doi.org/10.3390/antiox14070796










