The RAGE Inhibitor TTP488 (Azeliragon) Improves Diabetic Bladder Dysfunction in Leptin-Deficient Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design with TTP488 in ob/ob Mice
2.3. Glycemic Control
2.4. Blood Collection, Serum Processing, and Bladder Extraction
2.5. Measurements of MGO-AGE-RAGE Axis
2.6. Measurements of Glo1 Activity and Protein Expression in Bladder Tissue
2.7. Measurement of Antioxidant Activity in the Bladder Tissue
2.8. Bladder Histology for Collagen Analyses and Second Harmonic Generation (SHG) to Analyze Collagen
2.9. Void Spot Assay on Filter Paper
2.10. Ex Vivo Functional Assays on Isolated Bladders
2.11. Electrical-Field Stimulation (EFS) in Isolated Bladders
2.12. Statistical Analysis
3. Results
3.1. Effect of TTP488 Treatment on Body Weight and Glycemic Parameters
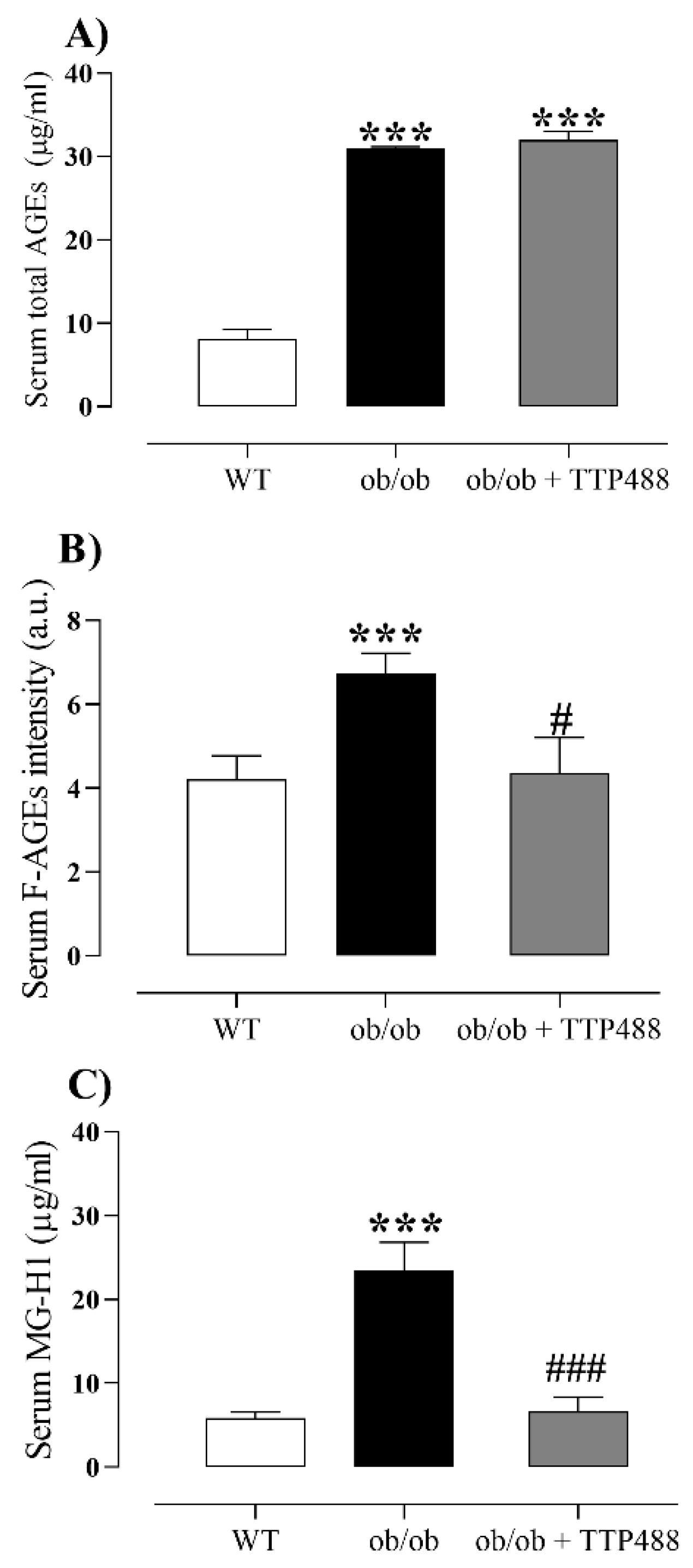
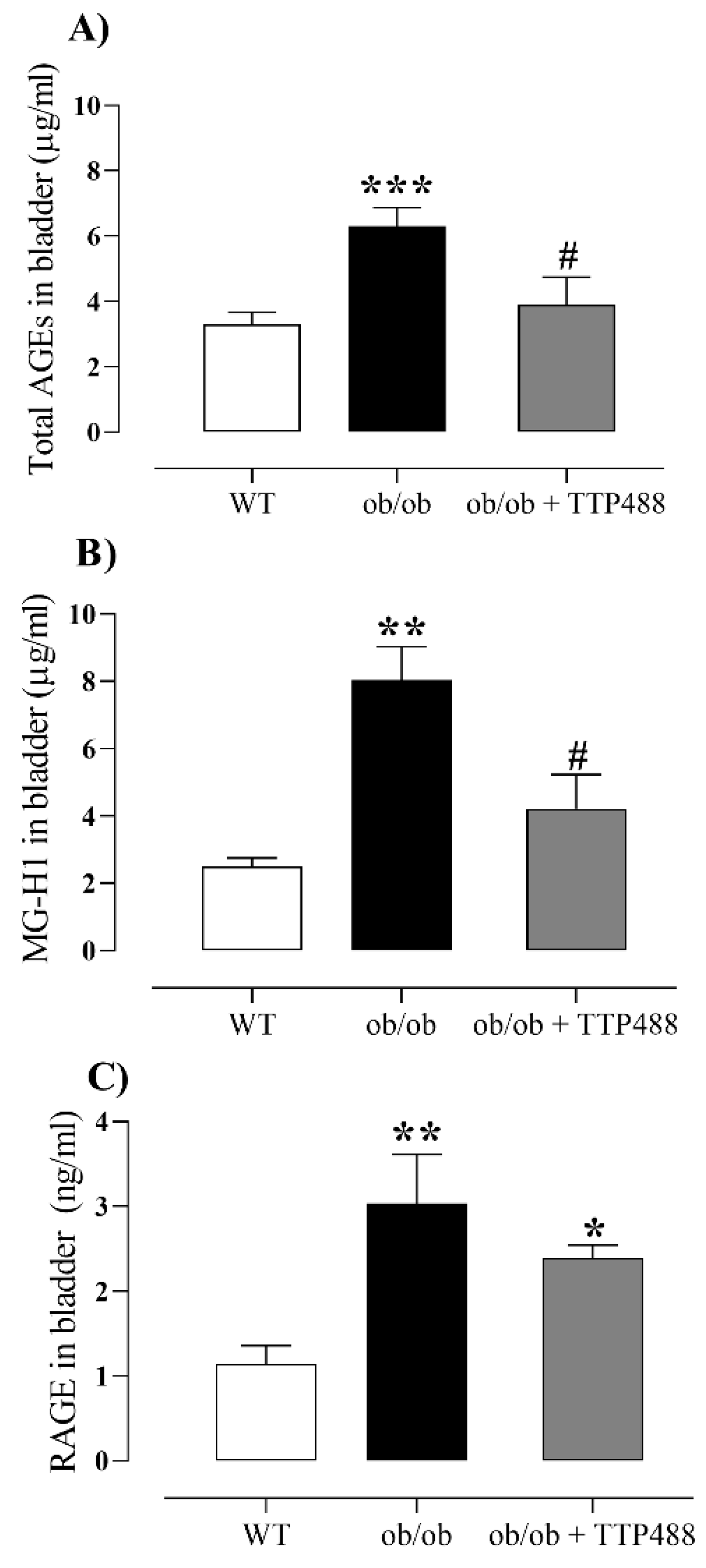
3.2. Effect of TTP488 Treatment on the MGO-AGE-RAGE Axis in Serum and Bladder Tissues
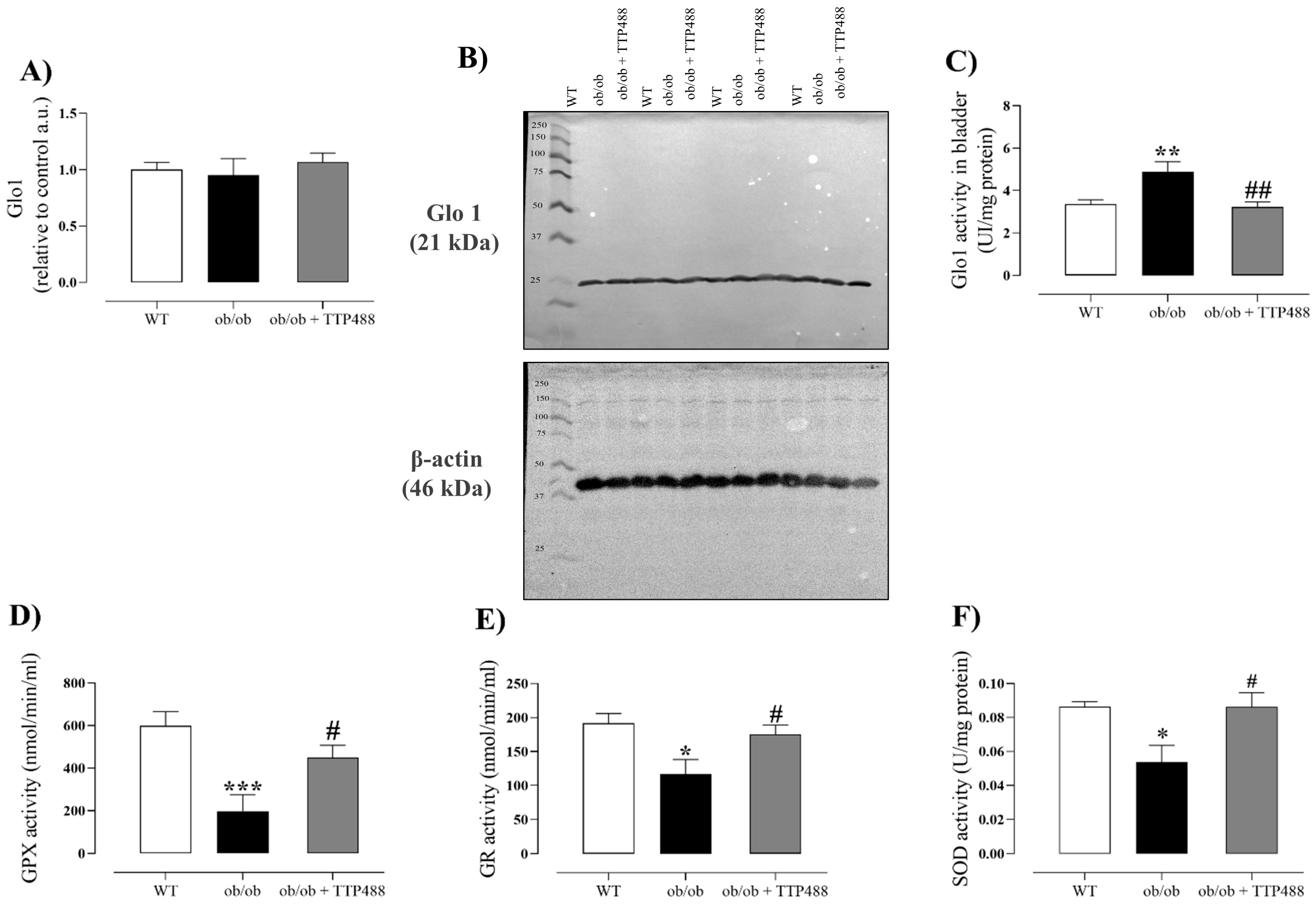
3.3. Effect of TTP488 Treatment on Glo1 Protein Expression and Activity and on Antioxidant System in Bladder
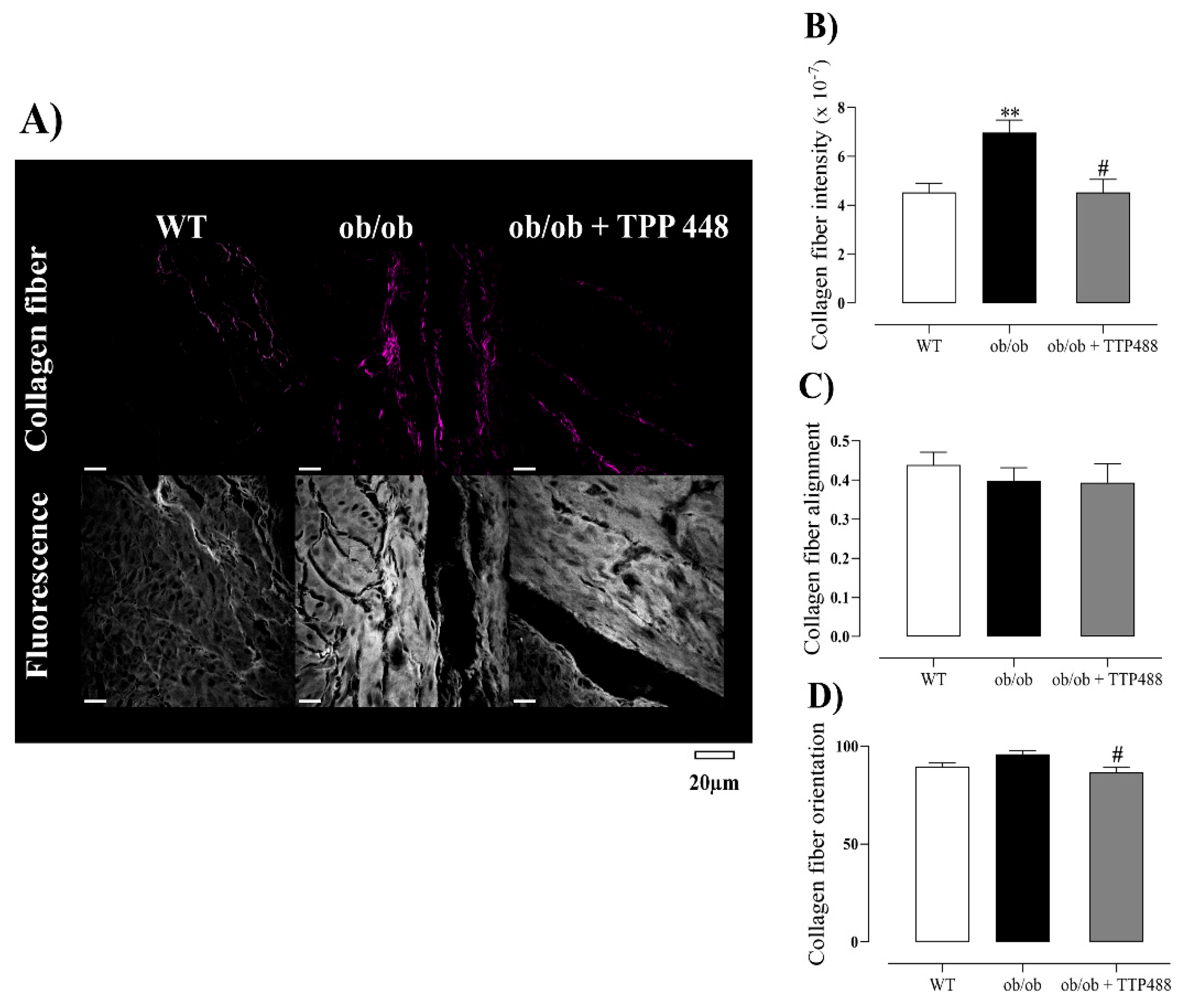
3.4. Effect of TTP488 Treatment on Collagen
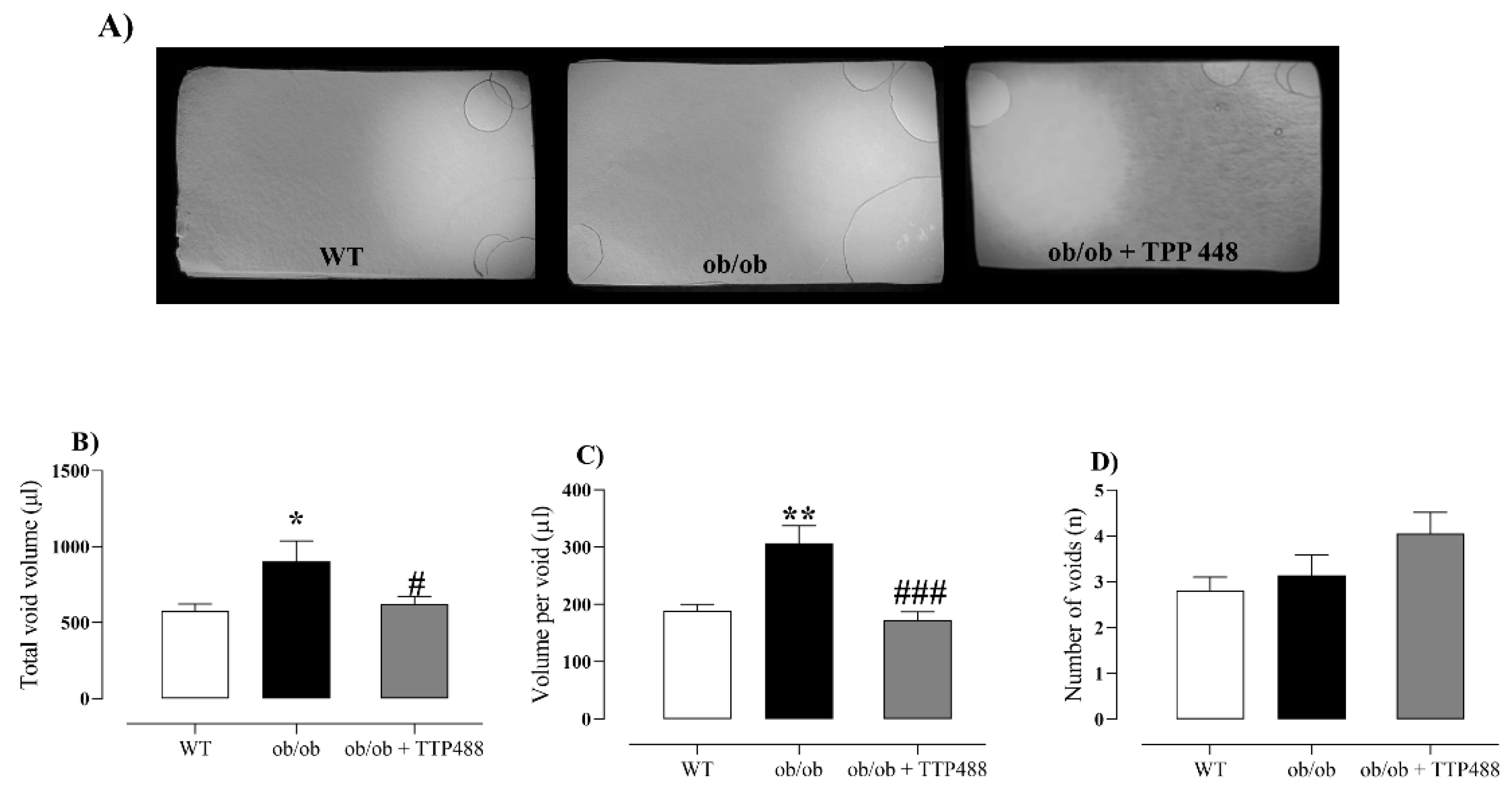
3.5. Effect of TTP488 Treatment on Voiding Behavior Assessed by Filter Paper Assay and Water Consumption
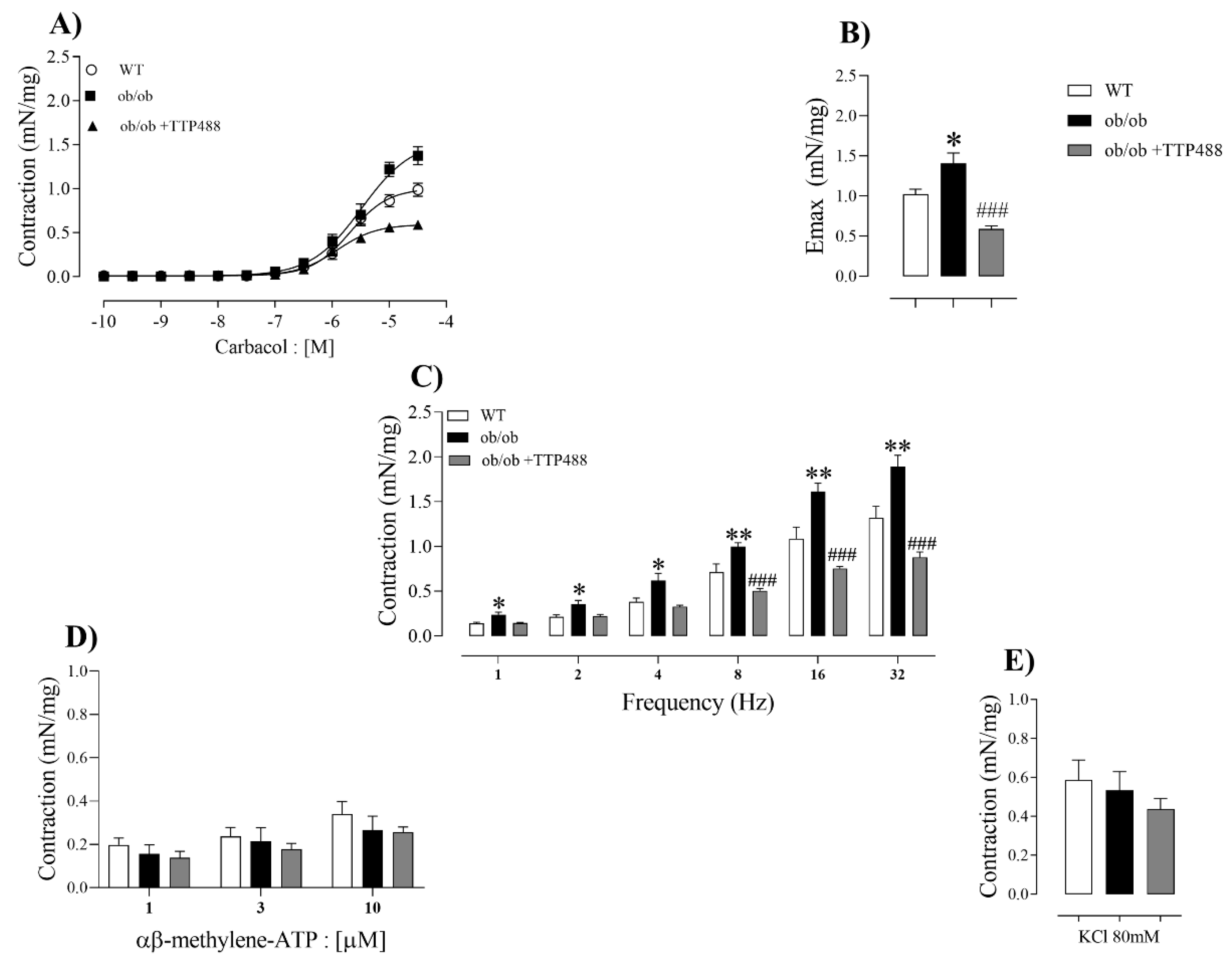
3.6. Effect of TTP488 Treatment on Ex Vivo Detrusor Contractility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-DG | 3-Deoxyglucosone |
| AGEs | Advanced glycation end products |
| CEL | N-epsilon-(1-carboxyethyl) lysine |
| CML | N-epsilon-(carboxymethyl) lysine |
| DBD | Diabetic bladder dysfunction |
| DM | Diabetes Mellitus |
| T1DM | Diabetes Mellitus phenotypes, with type 1 |
| T2DM | Diabetes Mellitus phenotypes, with type 2 |
| DOLD | 3-deoxyglucosone-derived lysine dimer |
| EFS | Electrical-field stimulation |
| F-AGES | Fluorescent AGEs |
| Glo1 | Glyoxalase 1 |
| Glo2 | Glyoxalase 2 |
| GOLD | Glyoxal-derived lysine dimer |
| GO | Glyoxal |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSH | Oxidized glutathione |
| KCL | Potassium chloride |
| MG-H1 | Methylglyoxal-derived hydroimidazolone |
| MGO | Methylglyoxal |
| MOLD | Methylglyoxal-derived lysine dimer |
| RAGE | Receptor for advanced glycation end products |
| SOD | Superoxide dismutase |
| SHG | Second harmonic generation |
References
- Tomic, D.; Morton, J.I.; Chen, L.; Salim, A.; Gregg, E.W.; Pavkov, M.E.; Arffman, M.; Balicer, R.; Baviera, M.; Boersma-van Dam, E.; et al. Lifetime risk, life expectancy, and years of life lost to type 2 diabetes in 23 high-income jurisdictions: A multinational, population-based study. Lancet Diabetes Endocrinol. 2022, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes. 2024. Available online: http://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 1 March 2025).
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Wittig, L.; Carlson, K.V.; Andrews, J.M.; Crump, R.T.; Baverstock, R.J. Diabetic Bladder Dysfunction: A Review. Urology 2019, 123, 1–6. [Google Scholar] [CrossRef]
- Daneshgari, F.; Liu, G.; Birder, L.; Hanna-Mitchell, A.T.; Chacko, S. Diabetic bladder dysfunction: Current translational knowledge. J. Urol. 2009, 182 (Suppl. S6), S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.R.; Liu, G.; Arioglu-Inan, E.; Michel, M.C. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 887–906. [Google Scholar] [CrossRef]
- Yuan, Z.; Tang, Z.; He, C.; Tang, W. Diabetic cystopathy: A review. J. Diabetes 2015, 7, 442–447. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Emerging Glycation-Based Therapeutics-Glyoxalase 1 Inducers and Glyoxalase 1 Inhibitors. Int. J. Mol. Sci. 2022, 23, 2453. [Google Scholar] [CrossRef]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, I.T.; Miskalis, A.; Nelappana, M.; Applegate, C.; Wozniak, M.; Czerwinski, A.; Kalinowski, L.; Dobrucki, L.W. Receptor for advanced glycation end-products: Biological significance and imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1935. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef]
- Sanders, K.A.; Delker, D.A.; Huecksteadt, T.; Beck, E.; Wuren, T.; Chen, Y.; Zhang, Y.; Hazel, M.W.; Hoidal, J.R. RAGE is a Critical Mediator of Pulmonary Oxidative Stress, Alveolar Macrophage Activation and Emphysema in Response to Cigarette Smoke. Sci. Rep. 2019, 9, 231. [Google Scholar] [CrossRef]
- Galì, A.; Mucciardi, G.; Butticè, S.; Subba, E.; D’Amico, C.; Lembo, F.; Magno, C. Correlation Between Advanced Glycation End-Products, Lower Urinary Tract Symptoms and Bladder Dysfunctions in Patients with type 2 Diabetes Mellitus. Low. Urin. Tract. Symptoms 2017, 9, 15–20. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Medeiros, M.L.; Ghezzi, A.C.; Dos Santos, G.A.; Mello, G.C.; Mónica, F.Z.; Antunes, E. Evidence that methylglyoxal and receptor for advanced glycation end products are implicated in bladder dysfunction of obese diabetic ob/ob mice. Am. J. Physiol. Renal Physiol. 2023, 325, F436–F447. [Google Scholar] [CrossRef] [PubMed]
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J Prev Alzheimers Dis. 2018, 5, 149–154. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Ministry of Science, Technology and Innovation. National Council for the Control of Animal Experimentation. Brazilian guide for the production, maintenance or use of animals in teaching or scientific research activities. In National Council for the Control of Animal Experimentation, 1st ed.; Ministry of Science, Technology and Innovation: Brasília, Brazil, 2023; p. 1107. [Google Scholar]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Du Sert, N.P.; Bamsey, I.; Bate, S.T.; Berdoy, M.; Clark, R.A.; Cuthill, I.C.; Fry, D.; Karp, N.A.; Macleod, M.; Moon, L.; et al. The Experimental Design Assistant. Nat. Methods 2017, 14, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhu, L.; Li, T.; Chen, J.; Fan, B.; Ji, W.; Zhang, C.; Cai, X.; Hu, C.; Sun, X.; et al. Azeliragon inhibits PAK1 and enhances the therapeutic efficacy of AKT inhibitors in pancreatic cancer. Eur. J. Pharmacol. 2023, 948, 175703. [Google Scholar] [CrossRef]
- Amato, A.A.; Rajagopalan, S.; Lin, J.Z.; Carvalho, B.M.; Figueira, A.C.; Lu, J.; Ayers, S.D.; Mottin, M.; Silveira, R.L.; Souza, P.C.; et al. GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain. J. Biol. Chem. 2012, 287, 28169–28179. [Google Scholar] [CrossRef]
- Jandova, J.; Wondrak, G.T. Genomic GLO1 deletion modulates TXNIP expression, glucose metabolism, and redox homeostasis while accelerating human A375 malignant melanoma tumor growth. Redox Biol. 2021, 39, 101838. [Google Scholar] [CrossRef]
- Liu, Y.; Keikhosravi, A.; Mehta, G.S.; Drifka, C.R.; Eliceiri, K.W. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol. Biol. 2017, 1627, 429–451. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Hill, W.G.; Zeidel, M.L.; Bjorling, D.E.; Vezina, C.M. Void spot assay: Recommendations on the use of a simple micturition assay for mice. Am. J. Physiol. Renal Physiol. 2018, 315, F1422–F1429. [Google Scholar] [CrossRef]
- Oliveira, A.L.; de Oliveira, M.G.; Medeiros, M.L.; Mónica, F.Z.; Antunes, E. Metformin abrogates the voiding dysfunction induced by prolonged methylglyoxal intake. Eur. J. Pharmacol. 2021, 910, 174502. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; de Oliveira, M.G.; Mónica, F.Z.; Antunes, E. Methylglyoxal and Advanced Glycation End Products (AGEs): Targets for the Prevention and Treatment of Diabetes-Associated Bladder Dysfunction? Biomedicines 2024, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.K.; Hamadani, C.; Zeidel, M.L.; Hill, W.G. Urological complications of obesity and diabetes in males and females of three mouse models: Temporal manifestations. Am. J. Physiol. Renal Physiol. 2020, 318, F160–F174. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Barendrecht, M.M. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol. Ther. 2008, 117, 297–312. [Google Scholar] [CrossRef]
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Nobe, K.; Yamazaki, T.; Tsumita, N.; Hashimoto, T.; Honda, K. Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway. J. Pharmacol. Exp. Ther. 2009, 328, 940–950. [Google Scholar] [CrossRef]
- Saito, M.; Okada, S.; Kazuyama, E.; Satoh, I.; Kinoshita, Y.; Satoh, K. Pharmacological properties, functional alterations and gene expression of muscarinic receptors in young and old type 2 Goto-Kakizaki diabetic rat bladders. J. Urol. 2008, 180, 2701–2705. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Hayashi, Y.; Yoshida, A.; Yoshida, S.; Ito, Y.; Yamaguchi, K.; Yamada, S.; Takahashi, S. Concomitant alteration in number and affinity of P2X and muscarinic receptors are associated with bladder dysfunction in early stage of diabetic rats. Int. Urol. Nephrol. 2018, 50, 451–458. [Google Scholar] [CrossRef]
- Leiria, L.O.; Sollon, C.; Calixto, M.C.; Lintomen, L.; Mónica, F.Z.; Anhê, G.F.; De Nucci, G.; Zanesco, A.; Grant, A.D.; Antunes, E. Role of PKC and CaV1.2 in detrusor overactivity in a model of obesity associated with insulin resistance in mice. PLoS ONE 2012, 7, e48507. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of RAGE and Advances in Small-Molecule Inhibitors. Int. J. Mol. Sci. 2021, 22, 6904. [Google Scholar] [CrossRef]
- Mahavadi, S.; Sriwai, W.; Manion, O.; Grider, J.R.; Murthy, K.S. Diabetes-induced oxidative stress mediates upregulation of RhoA/Rho kinase pathway and hypercontractility of gastric smooth muscle. PLoS ONE 2017, 12, e0178574. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulos, A.; Rosado, C.J.; Thomas, M.C. Dicarbonyl-mediated AGEing and diabetic kidney disease. J. Nephrol. 2020, 33, 909–915. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. RAGE and glyoxalase in kidney disease. Glycoconj. J. 2016, 33, 619–626. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31 Pt 6, 1343–1348. [Google Scholar] [CrossRef]
- Inacio, M.D.; Costa, M.C.; Lima, T.F.O.; Figueiredo, I.D.; Motta, B.P.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Pentoxifylline mitigates renal glycoxidative stress in obese mice by inhibiting AGE/RAGE signaling and increasing glyoxalase levels. Life Sci. 2020, 258, 118196. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Chen, L.; Li, J.; Zhang, H.; Guo, X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxid. Med. Cell Longev. 2019, 2019, 4628962. [Google Scholar] [CrossRef]
- Moreira, A.P.; Vizuete, A.F.K.; Zin, L.E.F.; de Marques, C.O.; Pacheco, R.F.; Leal, M.B.; Gonçalves, C.A. The Methylglyoxal/RAGE/NOX-2 Pathway is Persistently Activated in the Hippocampus of Rats with STZ-Induced Sporadic Alzheimer’s Disease. Neurotox. Res. 2022, 40, 395–409. [Google Scholar] [CrossRef]
- Trayhurn, P.; Arch, J.R.S. Is energy expenditure reduced in obese mice with mutations in the leptin/leptin receptor genes? J. Nutr. Sci. 2020, 9, e23. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef]
- Kim, T.; Spiegel, D.A. The unique reactivity of N-phenacyl-derived thiazolium salts toward α-dicarbonyl compounds. Rejuvenation Res. 2013, 16, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Toprak, C.; Yigitaslan, S. Alagebrium and Complications of Diabetes Mellitus. Eurasian J. Med. 2019, 51, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Watson, A.; Jandeleit-Dahm, K. Inhibitors of Advanced Glycation End Product (AGE) Formation and Accumulation. In Handbook of Experimental Pharmacology; Springers: Berlin/Heidelberg, Germany, 2021; pp. 395–423. [Google Scholar]
- Aragonès, G.; Rowan, S.G.; Francisco, S.; Yang, W.; Weinberg, J.; Taylor, A.; Bejarano, E. Glyoxalase System as a Therapeutic Target against Diabetic Retinopathy. Antioxidants 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Tran, G.B.; Nguyen, C.T. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 2020, 98, 59–69. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Jakuš, V.; Sándorová, E.; Kalninová, J.; Krahulec, B. Monitoring of glycation, oxidative stress and inflammation in relation to the occurrence of vascular complications in patients with type 2 diabetes mellitus. Physiol. Res. 2014, 63, 297–309. [Google Scholar] [CrossRef]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase 1 Modulation in Obesity and Diabetes. Antioxid. Redox Signal. 2019, 30, 354–374. [Google Scholar] [CrossRef]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Romaldi, B.; Laudadio, E.; Galeazzi, R.; Antognelli, C.; Armeni, T. Glyoxalase 2: Towards a Broader View of the Second Player of the Glyoxalase System. Antioxidants 2022, 11, 2131. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-β1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.D.; Harmon, M.B.; Stewart, J.A., Jr. The Impact of Diabetic Conditions and AGE/RAGE Signaling on Cardiac Fibroblast Migration. Front. Cell Dev. Biol. 2020, 8, 112. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Medeiros, M.L.; de Oliveira, M.G.; Teixeira, C.J.; Mónica, F.Z.; Antunes, E. Enhanced RAGE Expression and Excess Reactive-Oxygen Species Production Mediates Rho Kinase-Dependent Detrusor Overactivity After Methylglyoxal Exposure. Front. Physiol. 2022, 13, 860342. [Google Scholar] [CrossRef] [PubMed]
- Dudenkova, V.V.; Shirmanova, M.V.; Lukina, M.M.; Feldshtein, F.I.; Virkin, A.; Zagainova, E.V. Examination of Collagen Structure and State by the Second Harmonic Generation Microscopy. Biochemistry 2019, 84 (Suppl. S1), S89–S107. [Google Scholar] [CrossRef] [PubMed]
- Bancelin, S.; Aimé, C.; Gusachenko, I.; Kowalczuk, L.; Latour, G.; Coradin, T.; Schanne-Klein, M.C. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat. Commun. 2014, 5, 4920. [Google Scholar] [CrossRef]
- Raub, C.B.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010, 6, 4657–4665. [Google Scholar] [CrossRef]
- Hwang, J.; San, B.H.; Turner, N.J.; White, L.J.; Faulk, D.M.; Badylak, S.F.; Li, Y.; Yu, S.M. Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 2017, 53, 268–278. [Google Scholar] [CrossRef]
- Hoy, R.C.; D’Erminio, D.N.; Krishnamoorthy, D.; Natelson, D.M.; Laudier, D.M.; Illien-Jünger, S.; Iatridis, J.C. Advanced glycation end products cause RAGE-dependent annulus fibrosus collagen disruption and loss identified using in situ second harmonic generation imaging in mice intervertebral disk in vivo and in organ culture models. JOR Spine 2020, 3, e1126. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.L.; Medeiros, M.L.; Campos, A.T.P.; Cesar, C.L.; Mónica, F.Z.; Antunes, E. The RAGE Inhibitor TTP488 (Azeliragon) Improves Diabetic Bladder Dysfunction in Leptin-Deficient Obese Mice. Antioxidants 2025, 14, 793. https://doi.org/10.3390/antiox14070793
Oliveira AL, Medeiros ML, Campos ATP, Cesar CL, Mónica FZ, Antunes E. The RAGE Inhibitor TTP488 (Azeliragon) Improves Diabetic Bladder Dysfunction in Leptin-Deficient Obese Mice. Antioxidants. 2025; 14(7):793. https://doi.org/10.3390/antiox14070793
Chicago/Turabian StyleOliveira, Akila Lara, Matheus Leite Medeiros, Antonio Thiago Pereira Campos, Carlos Lenz Cesar, Fabiola Zakia Mónica, and Edson Antunes. 2025. "The RAGE Inhibitor TTP488 (Azeliragon) Improves Diabetic Bladder Dysfunction in Leptin-Deficient Obese Mice" Antioxidants 14, no. 7: 793. https://doi.org/10.3390/antiox14070793
APA StyleOliveira, A. L., Medeiros, M. L., Campos, A. T. P., Cesar, C. L., Mónica, F. Z., & Antunes, E. (2025). The RAGE Inhibitor TTP488 (Azeliragon) Improves Diabetic Bladder Dysfunction in Leptin-Deficient Obese Mice. Antioxidants, 14(7), 793. https://doi.org/10.3390/antiox14070793






