Administration of Ascorbic Acid Alleviates Neuronal Damage After Cerebral Ischemia in ODS Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Middle Cerebral Artery Occlusion and Reperfusion
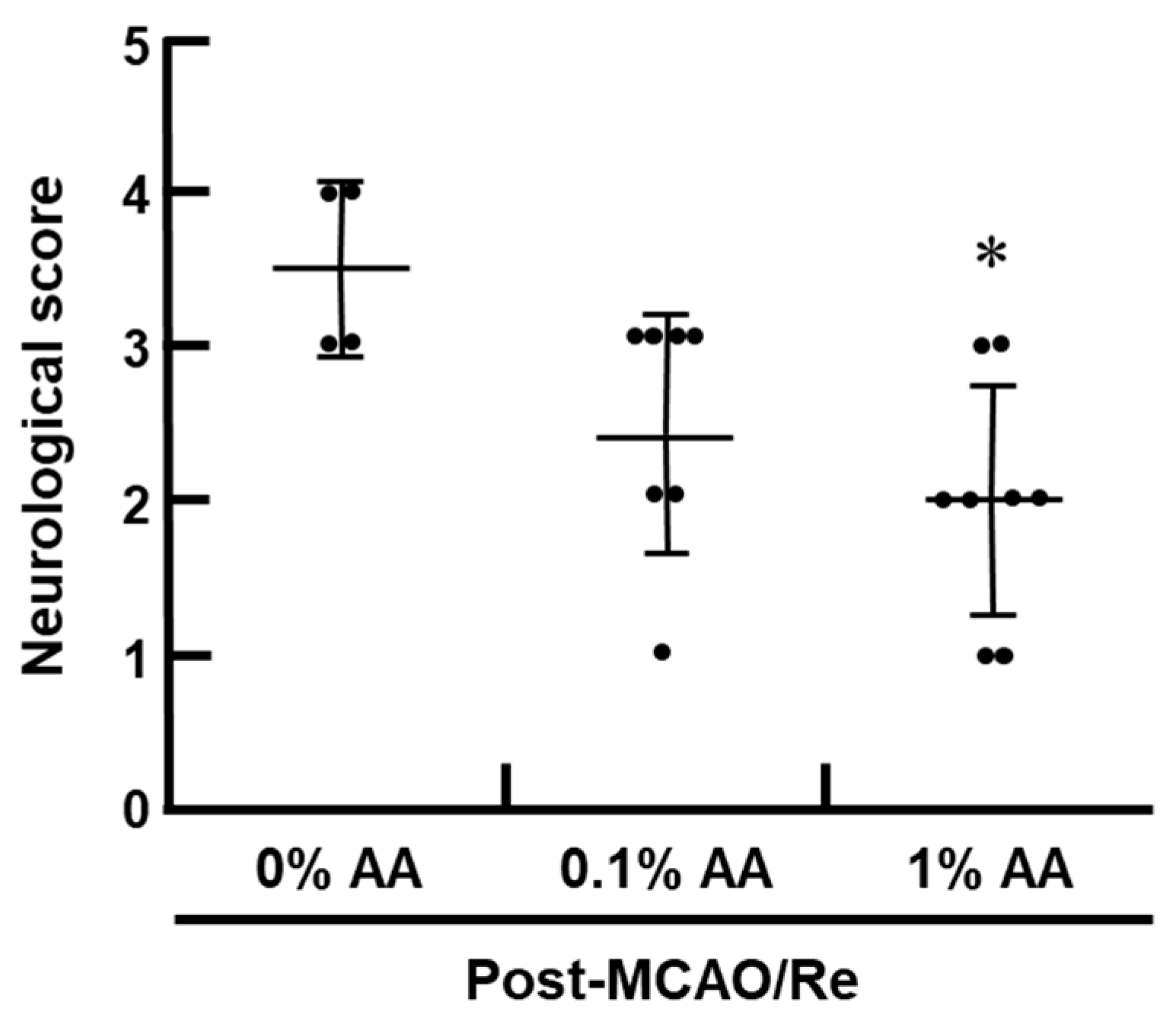
2.3. Neurological Evaluation
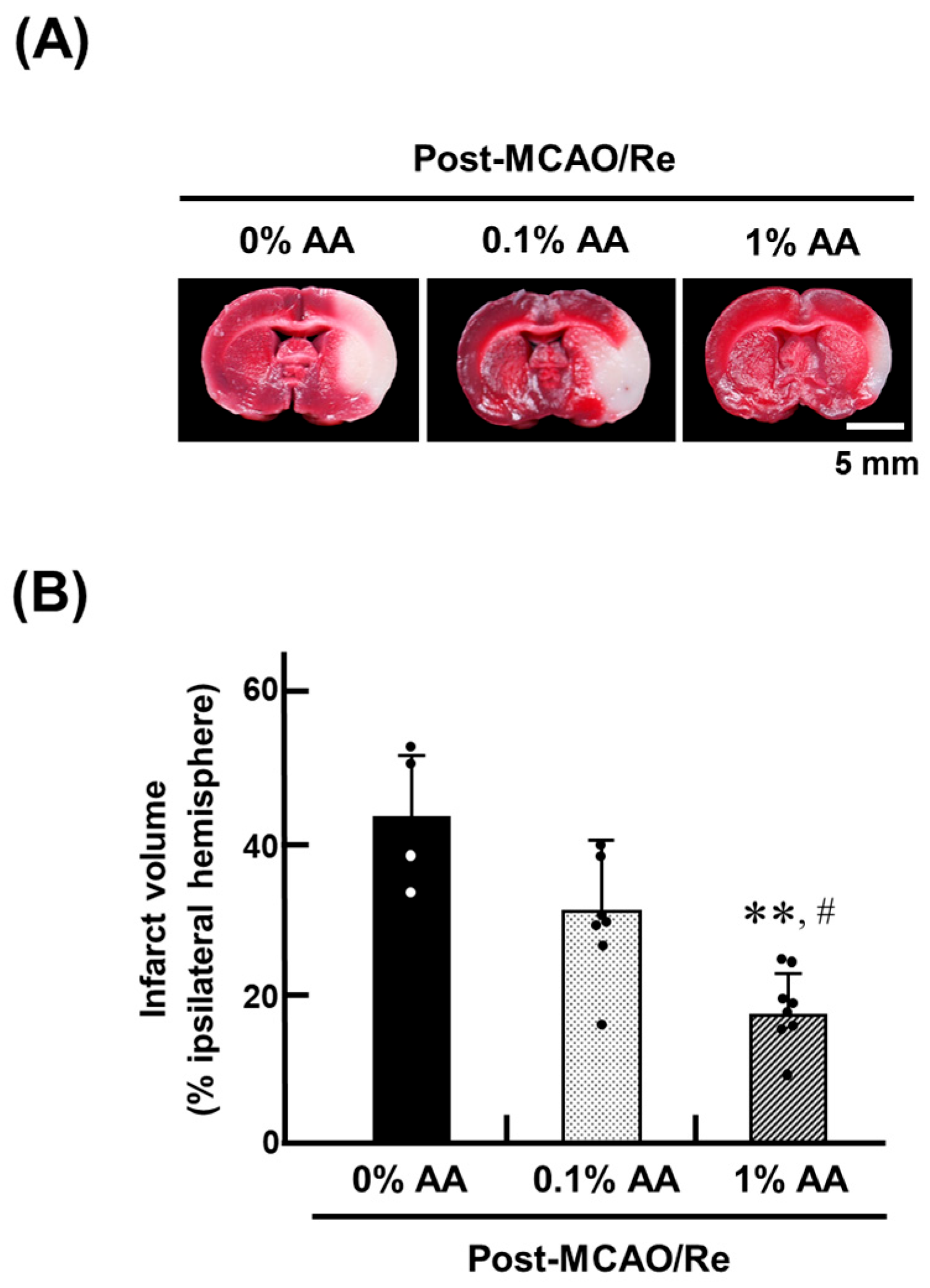
2.4. Infarct Assessment
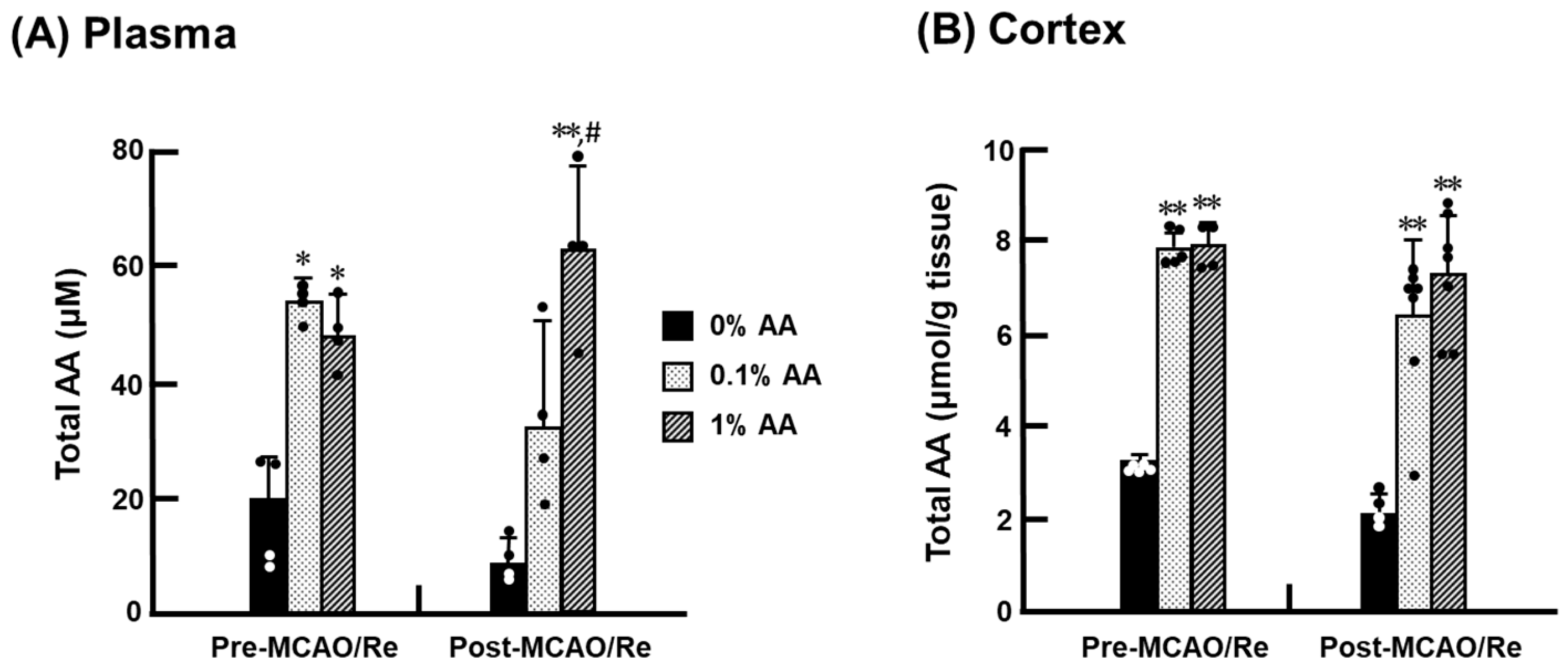
2.5. Measurement of Total AA Level
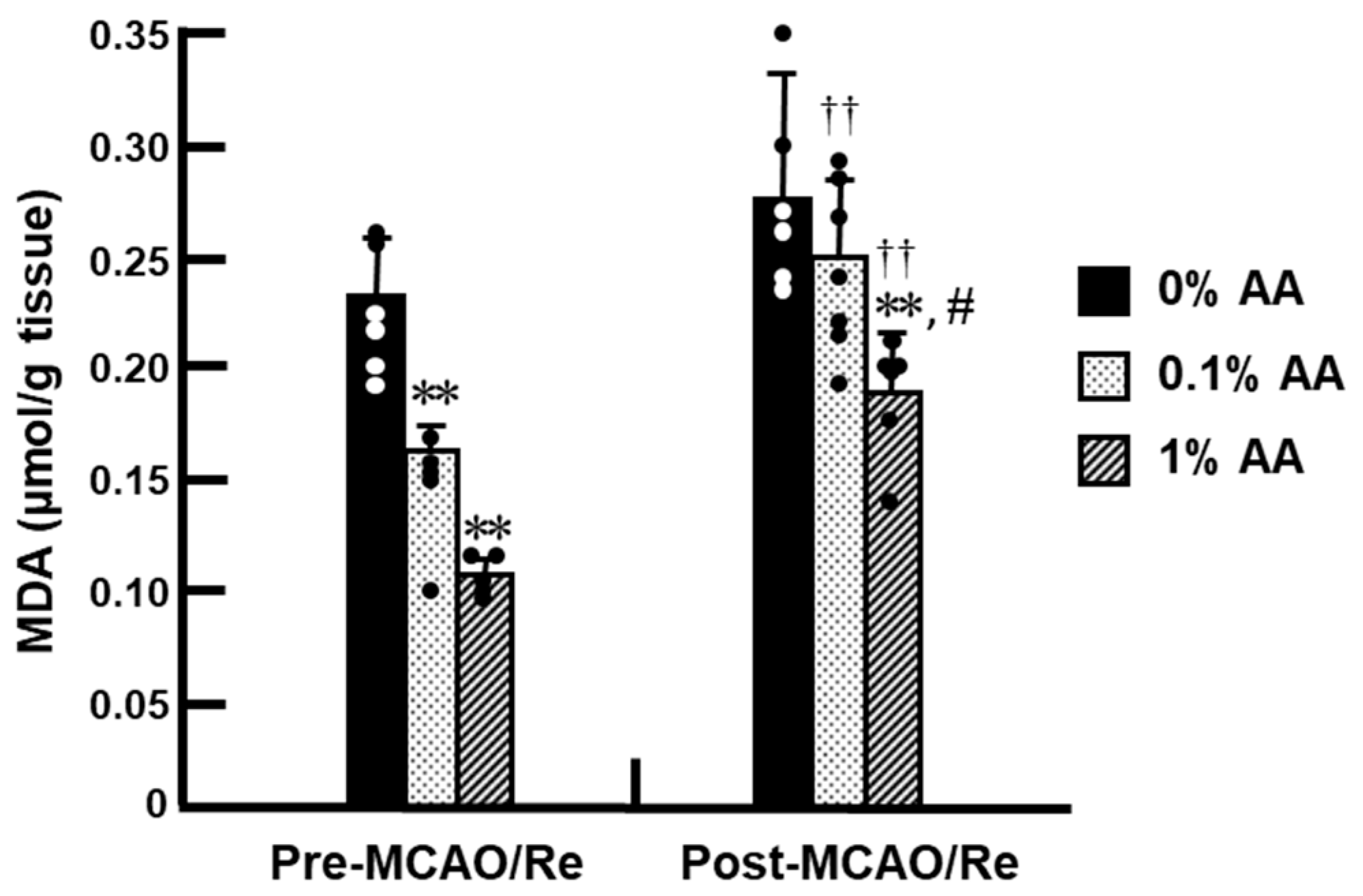
2.6. Measurement of Lipid Peroxidation Level
2.7. Detection of O2− Production in the Brain
2.8. Detection of Cell Death in the Brain
2.9. Real-Time Polymerase Chain Reaction (PCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Body Weight, Food Intake, and Water Intake
3.2. Ischemic Brain Injury and Neurological Deficits
3.3. Total AA Levels in the Cortex
3.4. Lipid Peroxidation Levels in the Cortex
3.5. O2− Production After Ischemia with Reperfusion
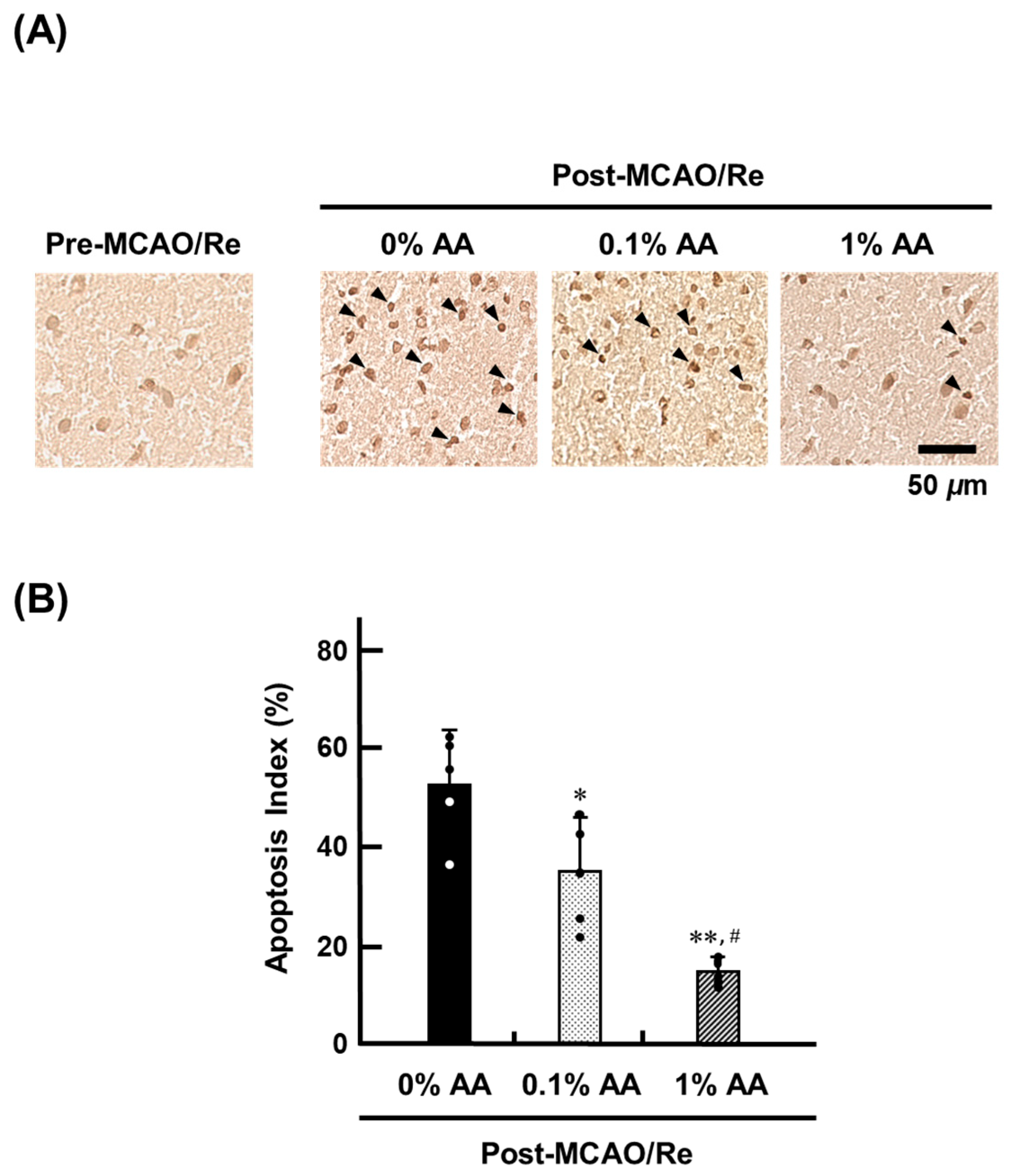
3.6. Cell Death Induced by Ischemia with Reperfusion
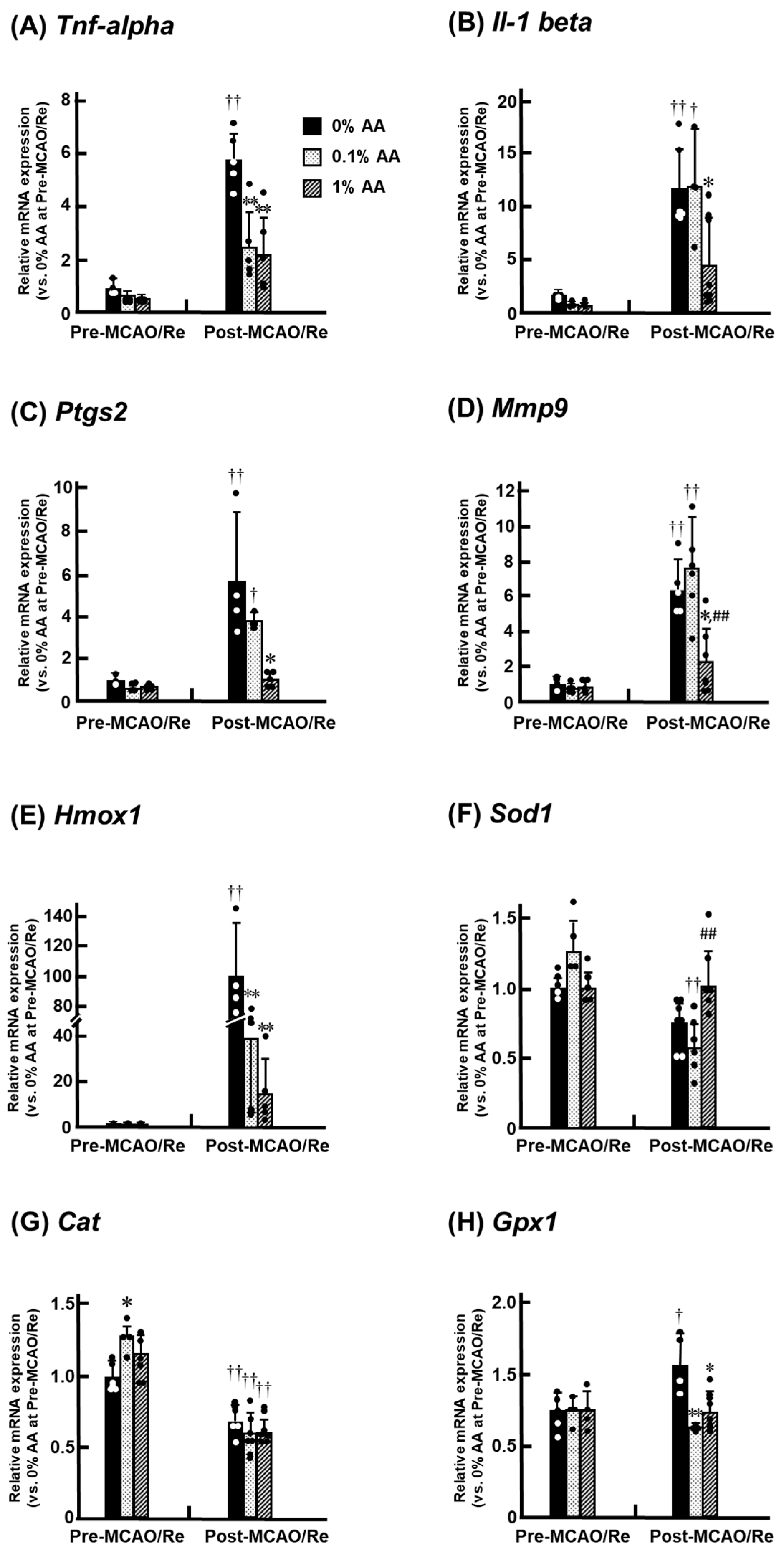
3.7. Alterations in the Expression of Inflammatory and Antioxidant Genes in the Penumbral Cortex
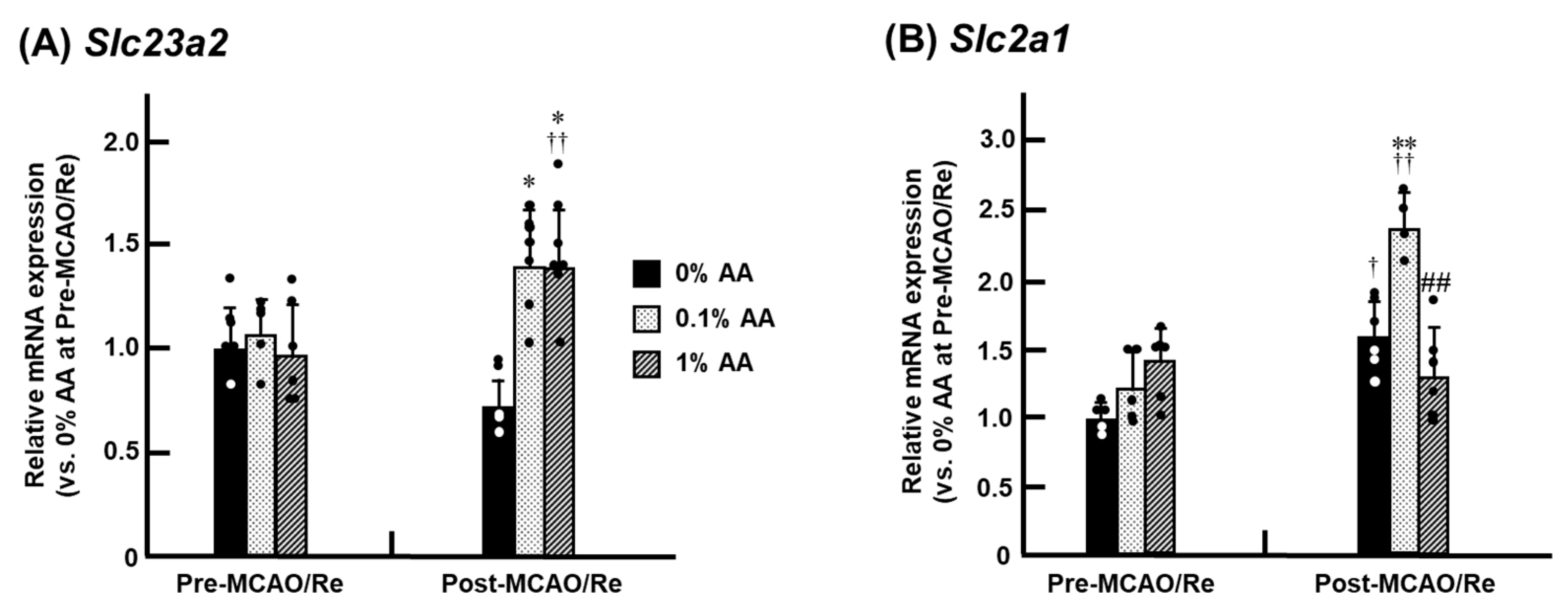
3.8. Alterations in the Expression of SVCT2 and GLUT1 mRNA in the Penumbra Cortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | L-ascorbic acid |
| BBB | blood–brain barrier |
| CAT | catalase |
| CNS | central nervous system |
| COX-2 | cyclooxygenase-2 |
| DHA | dehydroascorbic acid |
| DHE | dihydroethidium |
| GLUT1 | glucose transporter 1 |
| GLO | L-gulonolactone oxidase |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| HO-1 | heme oxygenase-1 |
| H2O2 | hydrogen peroxide |
| IL-1β | interleukin-1 beta |
| MCAO/Re | middle cerebral artery occlusion and reperfusion |
| MDA | malondialdehyde |
| MMP-9 | matrix metalloproteinase-9 |
| NF-κB | nuclear factor kappa-B |
| O2− | superoxide |
| ODS rat | osteogenic disorder Shionogi rat |
| PCR | quantitative real-time polymerase chain reaction |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SVCT2 | sodium-dependent vitamin C transporter 2 |
| TBARS | thiobarbituric acid-reactive substances |
| TNF-α | tumor necrosis factor-alpha |
| TTC | 2,3,5-triphenyl tetrazolium chloride |
| TUNEL | terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling |
| VE | vitamin E |
References
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 121, pp. 247–270. [Google Scholar]
- Crichton, R.R.; Charloteaux-Wauters, M. Iron transport and storage. Eur. J. Biochem. 1987, 164, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Yagi, K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1203s–1208s. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Hudes, E.S.; Tice, J.A. Relation of serum ascorbic acid to mortality among US adults. J. Am. Coll. Nutr. 2001, 20, 255–263. [Google Scholar] [CrossRef]
- Hampl, J.S.; Taylor, C.A.; Johnston, C.S. Vitamin C deficiency and depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Am. J. Public. Health 2004, 94, 870–875. [Google Scholar] [CrossRef]
- Frei, B.; Birlouez-Aragon, I.; Lykkesfeldt, J. Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit. Rev. Food Sci. Nutr. 2012, 52, 815–829. [Google Scholar] [CrossRef]
- Harrison, F.E.; Green, R.J.; Dawes, S.M.; May, J.M. Vitamin C distribution and retention in the mouse brain. Brain Res. 2010, 1348, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Saito, A.; Maier, C.M.; Narasimhan, P.; Nishi, T.; Song, Y.S.; Yu, F.; Liu, J.; Lee, Y.S.; Nito, C.; Kamada, H.; et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 2005, 31, 105–116. [Google Scholar] [CrossRef]
- White, B.C.; Sullivan, J.M.; DeGracia, D.J.; O’Neil, B.J.; Neumar, R.W.; Grossman, L.I.; Rafols, J.A.; Krause, G.S. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000, 179, 1–33. [Google Scholar] [CrossRef]
- Siesjö, B.K.; Zhao, Q.; Pahlmark, K.; Siesjö, P.; Katsura, K.; Folbergrová, J. Glutamate, calcium, and free radicals as mediators of ischemic brain damage. Ann. Thorac. Surg. 1995, 59, 1316–1320. [Google Scholar] [CrossRef]
- Iwata, N.; Okazaki, M.; Kamiuchi, S.; Hibino, Y. Protective effects of oral administrated ascorbic acid against oxidative stress and neuronal damage after cerebral ischemia/reperfusion in diabetic rats. J. Health Sci. 2010, 56, 20–30. [Google Scholar] [CrossRef]
- Iwata, N.; Okazaki, M.; Xuan, M.; Kamiuchi, S.; Matsuzaki, H.; Hibino, Y. Orally administrated ascorbic acid suppresses neuronal damage and modifies expression of SVCT2 and GLUT1 in the brain of diabetic rats with cerebral ischemia-reperfusion. Nutrients 2014, 6, 1554–1577. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, J.Y.; Wu, M.H.; Hu, M.L. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic. Biol. Med. 2020, 155, 29–36. [Google Scholar] [CrossRef]
- Berger, U.V.; Lu, X.C.M.; Liu, W.; Tang, Z.; Slusher, B.S.; Hediger, M.A. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J. Neurochem. 2003, 86, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Nishikimi, M.; Ozawa, T.; Yagi, K. A missense mutation of L-gulono-gamma-lactone oxidase causes the inability of scurvy-prone osteogenic disorder rats to synthesize L-ascorbic acid. J. Biol. Chem. 1992, 267, 21973–21976. [Google Scholar] [CrossRef]
- Mizushima, Y.; Harauchi, T.; Yoshizaki, T.; Makino, S. A rat mutant unable to synthesize vitamin C. Experientia 1984, 40, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Horio, F.; Ozaki, K.; Yoshida, A.; Makino, S.; Hayashi, Y. Requirement for ascorbic acid in a rat mutant unable to synthesize ascorbic acid. J. Nutr. 1985, 115, 1630–1640. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Gess, B.; Sevimli, S.; Strecker, J.K.; Young, P.; Schäbitz, W.R. Sodium-dependent vitamin C transporter 2 (SVCT2) expression and activity in brain capillary endothelial cells after transient ischemia in mice. PLoS ONE 2011, 6, e17139. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C transport and its role in the central nervous system. Sub-Cell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef]
- Bradley, D.W.; Emery, G.; Maynard, J.E. Vitamin C in plasma: A comparative study of the vitamin stabilized with trichloroacetic acid or metaphosphoric acid and the effects of storage at −70 degrees, −20 degrees, 4 degrees, and 25 degrees on the stabilized vitamin. Clin. Chim. Acta 1973, 44, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Okazaki, M.; Kasahara, C.; Kamiuchi, S.; Suzuki, F.; Iizuka, H.; Hibino, Y. Protective effects of a water-soluble extract from culture medium of Ganoderma lucidum mycelia against neuronal damage after cerebral ischemia/reperfusion in diabetic rats. J. Jpn. Soc. Nutr. Food Sci. 2008, 61, 119–127. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, S.; Gao, Y.; Wang, R.; Wu, Q.; Wu, H.; Luo, T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res. Bull. 2016, 121, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Martinovich, G.G.; Golubeva, E.N.; Martinovich, I.V.; Cherenkevich, S.N. Redox regulation of calcium signaling in cancer cells by ascorbic acid involving the mitochondrial electron transport chain. J. Biophys. 2012, 2012, 921653. [Google Scholar] [CrossRef]
- Sharma, P.; Morgan, P.D. Ascorbate reduces superoxide production and improves mitochondrial respiratory chain function in human fibroblasts with electron transport chain deficiencies. Mitochondrion 2001, 1, 191–198. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.C.; May, J.M. GSH is required to recycle ascorbic acid in cultured liver cell Lines. Antioxid. Redox Signal. 2001, 3, 1089–1097. [Google Scholar] [CrossRef]
- Beyer, R.E. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 1994, 26, 349–358. [Google Scholar] [CrossRef]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef]
- Hughes, R.E.; Hurley, R.J.; Jones, P.R. The retention of ascorbic acid by guinea-pig tissues. Br. J. Nutr. 1971, 26, 433–438. [Google Scholar] [CrossRef]
- Sinclair, P.R.; Gorman, N.; Sinclair, J.F.; Walton, H.S.; Bement, W.J.; Lambrecht, R.W. Ascorbic acid inhibits chemically induced uroporphyria in ascorbate-requiring rats. Hepatology 1995, 22, 565–572. [Google Scholar]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Kawade, N.; Murai, A.; Suzuki, W.; Takeuchi, K.; Kondo, M.; Kobayashi, M.; Horio, F. Ascorbic acid deficiency induces hepatic and intestinal expression of inflammation-related genes irrespective of the presence or absence of gut microbiota in ODS rats. J. Nutr. Biochem. 2020, 86, 108485. [Google Scholar] [CrossRef]
- Kawade, N.; Suzuki, W.; Kobayashi, M.; Ohno, T.; Murai, A.; Horio, F. Low Ascorbic Acid Intake Induces Inflammatory Changes in Intestine and Liver of ODS Rats. J. Nutr. Sci. Vitaminol. 2022, 68, 481–487. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Taheri, S.; Yang, Y.; Sood, R.; Grossetete, M.; Estrada, E.Y.; Fiebich, B.L.; Rosenberg, G.A. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J. Pharmacol. Exp. Ther. 2007, 323, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, S.; Zhang, C.; Meng, F.; Wu, W.; Hu, R.; Hadass, O.; Lehmidi, T.; Blair, G.J.; Lee, M.; et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol. Neurodegener. 2012, 7, 21. [Google Scholar] [CrossRef]
- Okazaki, M.; Hibino, Y.; Iwata, N.; Matsuzaki, H.; Nakano, R.; Kamiuchi, S.; Kasahara, C.; Suzuki, F.; Iizuka, H. Diabetes-Mediated Exacerbation of Neuronal Damage and Inflammation After Cerebral Ischemia in Rat: Protective Effects of Water-Soluble Extract from Culture Medium of Ganoderma lucidum Mycelia. In Advances in the Preclinical Study of Ischemic Stroke; Balestrino, M., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar][Green Version]
- Choi, Y.K.; Kim, Y.M. Beneficial and Detrimental Roles of Heme Oxygenase-1 in the Neurovascular System. Int. J. Mol. Sci. 2022, 23, 7041. [Google Scholar] [CrossRef]
- Imuta, N.; Hori, O.; Kitao, Y.; Tabata, Y.; Yoshimoto, T.; Matsuyama, T.; Ogawa, S. Hypoxia-mediated induction of heme oxygenase type I and carbon monoxide release from astrocytes protects nearby cerebral neurons from hypoxia-mediated apoptosis. Antioxid. Redox Signal. 2007, 9, 543–552. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme oxygenase 1 in the nervous system: Does it favor neuronal cell survival or induce neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M.; Ali, U.; Mansell, A.; Hertzog, P.J. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke 2006, 37, 1533–1538. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M.; Flentjar, N.J.; de Haan, J.; Hertzog, P.; Iannello, R.C.; Kola, I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J. Neurochem. 2001, 78, 1389–1399. [Google Scholar] [CrossRef]
- Wolf, M.S.; Manole, M.D.; New, L.A.; Chen, Y.; Soysal, E.; Kochanek, P.M.; Bayır, H.; Clark, R.S.B. Ascorbate deficiency confers resistance to hippocampal neurodegeneration after asphyxial cardiac arrest in juvenile rats. Pediatr. Res. 2022, 91, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Seno, T.; Inoue, N.; Matsui, K.; Ejiri, J.; Hirata, K.; Kawashima, S.; Yokoyama, M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J. Vasc. Res. 2004, 41, 345–351. [Google Scholar] [CrossRef]
- Salazar, K.; Espinoza, F.; Cerda-Gallardo, G.; Ferrada, L.; Magdalena, R.; Ramírez, E.; Ulloa, V.; Saldivia, N.; Troncoso, N.; Oviedo, M.J.; et al. Svct2 overexpression and ascorbic acid uptake increase cortical neuron differentiation, which is dependent on vitamin c recycling between neurons and astrocytes. Antioxidants 2021, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.R.; Praditi, C.; Phillips, E.; Vissers, M.C.M.; Robinson, B.A.; Dachs, G.U.; Wiggins, G.A.R. Role of Sodium-Dependent Vitamin C Transporter-2 and Ascorbate in Regulating the Hypoxic Pathway in Cultured Glioblastoma Cells. J. Cell. Biochem. 2025, 126, e30658. [Google Scholar] [CrossRef]
- Parker, W.H.; Qu, Z.C.; May, J.M. Ascorbic acid transport in brain microvascular pericytes. Biochem. Biophys. Res. Commun. 2015, 458, 262–267. [Google Scholar] [CrossRef]
- Sakai, S.; Shichita, T. Inflammation and neural repair after ischemic brain injury. Neurochem. Int. 2019, 130, 104316. [Google Scholar] [CrossRef]

| Groups | Body Weight (g) | Food Intake (g/Day) | Water Intake (mL/Day) | EstimatedAA Intake (mg) |
|---|---|---|---|---|
| 0% AA | 244.8 ± 9.8 | 16.1 ± 3.1 | 34.0 ± 7.6 | ― |
| 0.1% AA | 259.1 ± 8.9 | 21.8 ± 2.4 ** | 36.2 ± 8.1 | 36.2 ± 8.1 |
| 1% AA | 242.1 ± 18.0 | 20.5 ± 2.1 ** | 46.5 ± 12.8 | 465 ± 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, N.; Ogawa, N.; Imai, T.; Ridzuan, S.S.B.; Kamiuchi, S.; Matsuzaki, H.; Xuan, M.; Yuan, B.; Okazaki, M.; Hibino, Y. Administration of Ascorbic Acid Alleviates Neuronal Damage After Cerebral Ischemia in ODS Rats. Antioxidants 2025, 14, 773. https://doi.org/10.3390/antiox14070773
Iwata N, Ogawa N, Imai T, Ridzuan SSB, Kamiuchi S, Matsuzaki H, Xuan M, Yuan B, Okazaki M, Hibino Y. Administration of Ascorbic Acid Alleviates Neuronal Damage After Cerebral Ischemia in ODS Rats. Antioxidants. 2025; 14(7):773. https://doi.org/10.3390/antiox14070773
Chicago/Turabian StyleIwata, Naohiro, Naoto Ogawa, Tom Imai, Siti Sabirah Binti Ridzuan, Shinya Kamiuchi, Hirokazu Matsuzaki, Meiyan Xuan, Bo Yuan, Mari Okazaki, and Yasuhide Hibino. 2025. "Administration of Ascorbic Acid Alleviates Neuronal Damage After Cerebral Ischemia in ODS Rats" Antioxidants 14, no. 7: 773. https://doi.org/10.3390/antiox14070773
APA StyleIwata, N., Ogawa, N., Imai, T., Ridzuan, S. S. B., Kamiuchi, S., Matsuzaki, H., Xuan, M., Yuan, B., Okazaki, M., & Hibino, Y. (2025). Administration of Ascorbic Acid Alleviates Neuronal Damage After Cerebral Ischemia in ODS Rats. Antioxidants, 14(7), 773. https://doi.org/10.3390/antiox14070773







