Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut–Liver Axis Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Design
2.2. Sample Collection
2.3. Histological and Structural Observations
2.4. Determination of Antioxidant Enzyme Activities
2.5. Gut Microbiome Analysis
2.6. Metabolomic Analysis
2.7. Correlation Analysis
2.8. Statistical Analyses
3. Results
3.1. Effects of Low-Temperature Stress on Feeding Rate and Behavior in Pelteobagrus vachelli
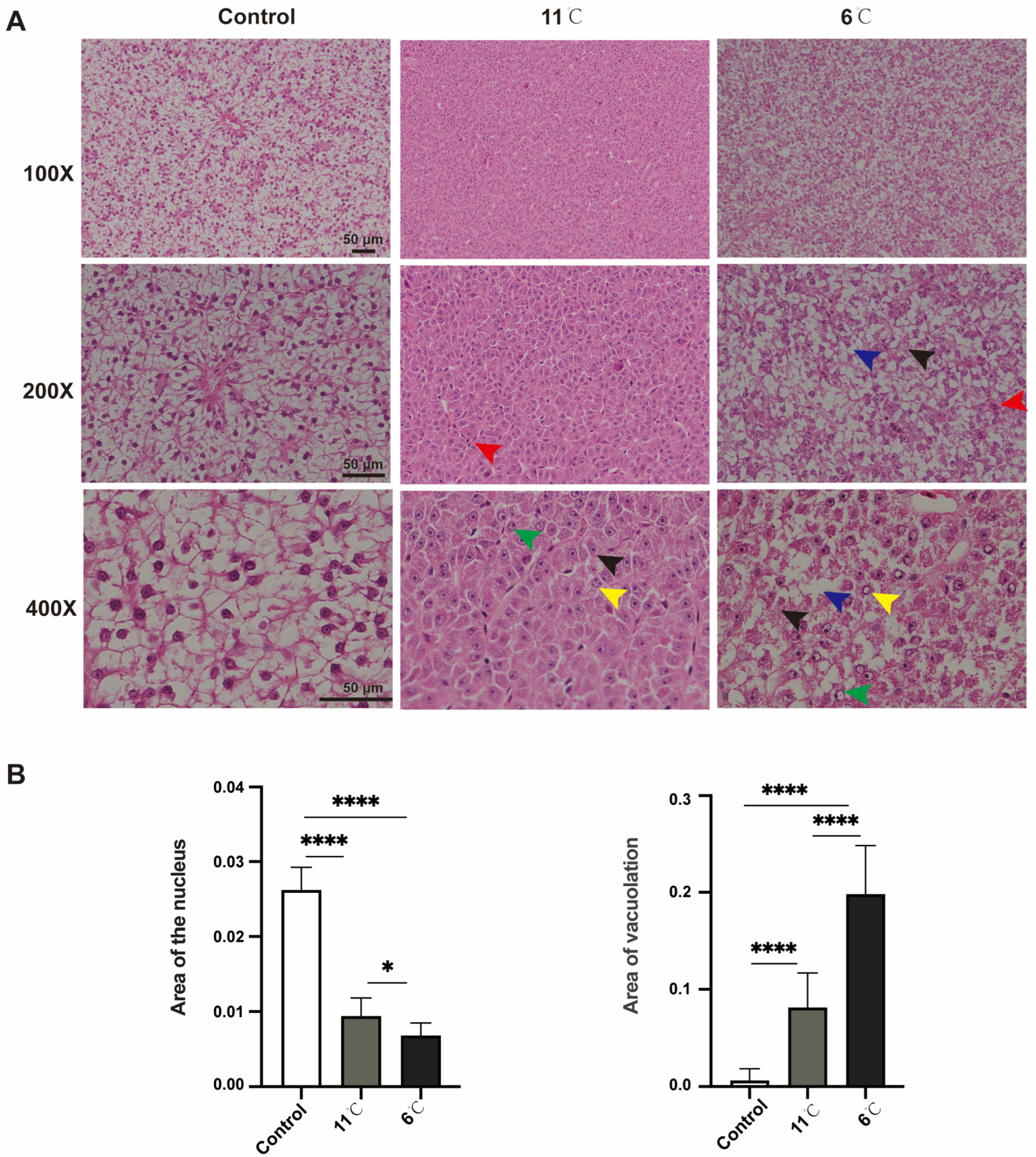
3.2. Effects of Low-Temperature Stress on Liver Histology in Pelteobagrus vachelli
3.3. Effects of Low-Temperature Stress on Antioxidant Enzymes and Innate Immunity Biochemical Parameters in Pelteobagrus vachelli
3.4. Effects of Low-Temperature Stress on Liver Metabolome in Pelteobagrus vachelli
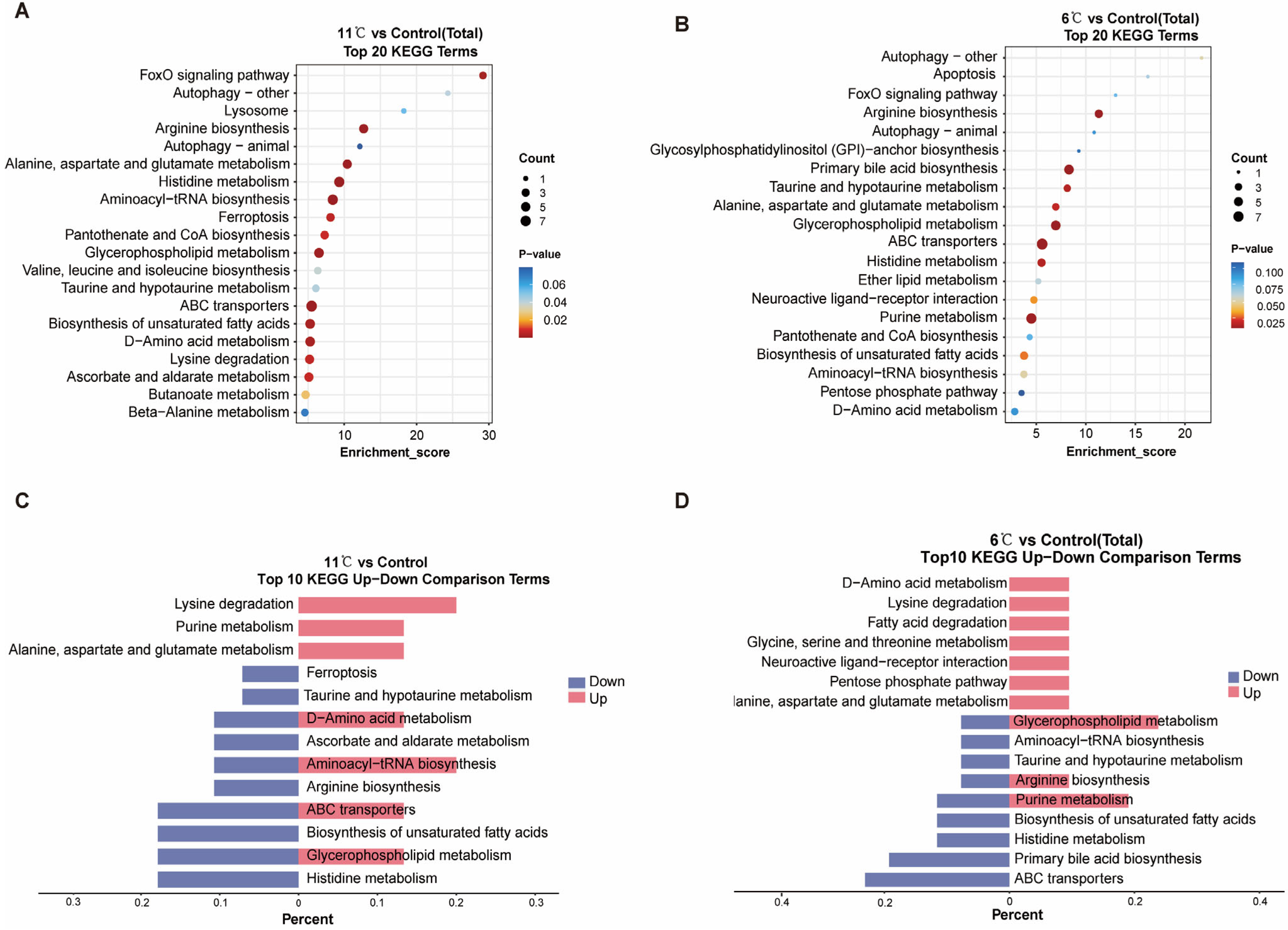
3.5. Analysis of Metabolome Function in Pelteobagrus vachelli Under Low-Temperature Stress
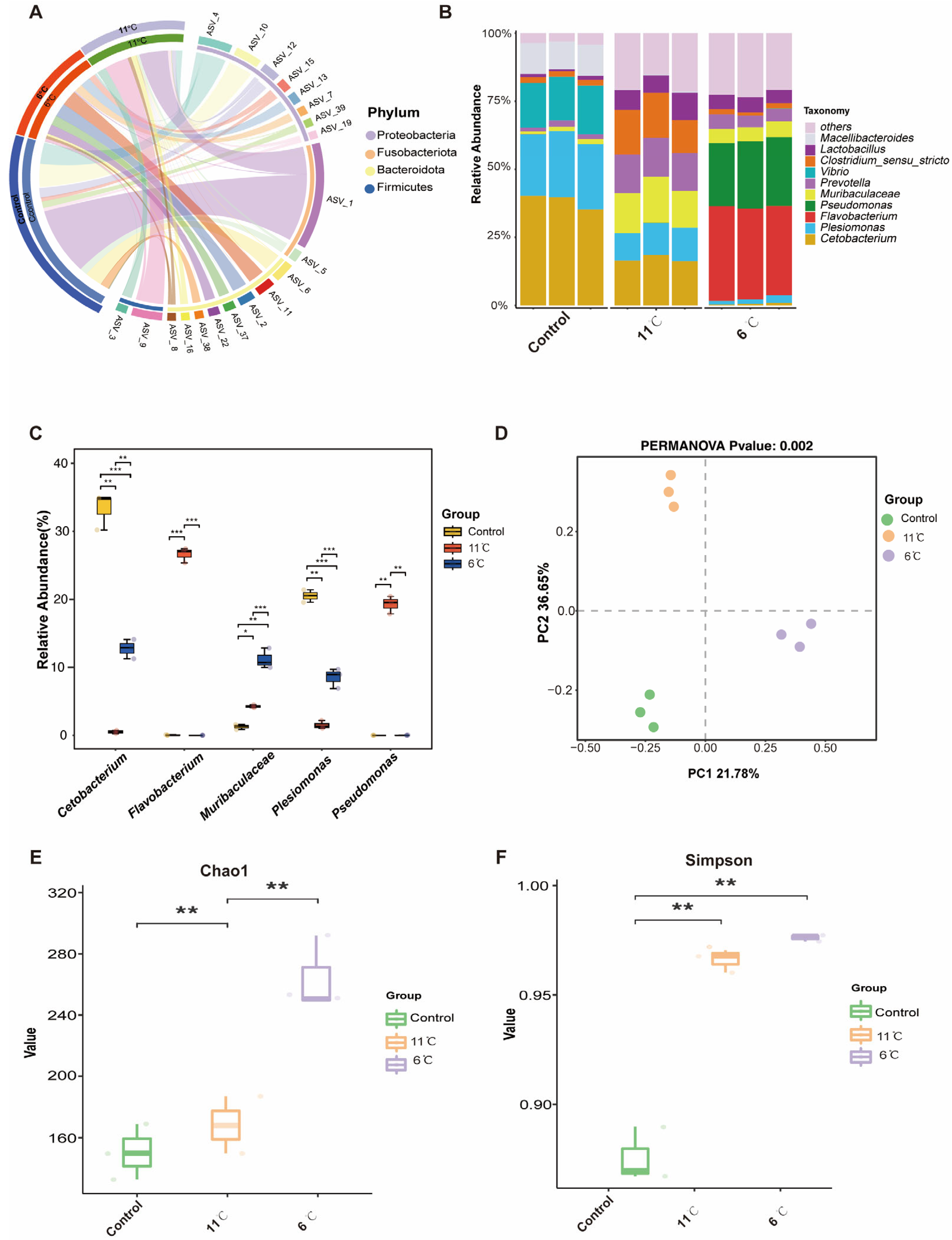
3.6. Effects of Low-Temperature Stress on Gut Microbiome in Pelteobagrus vachelli
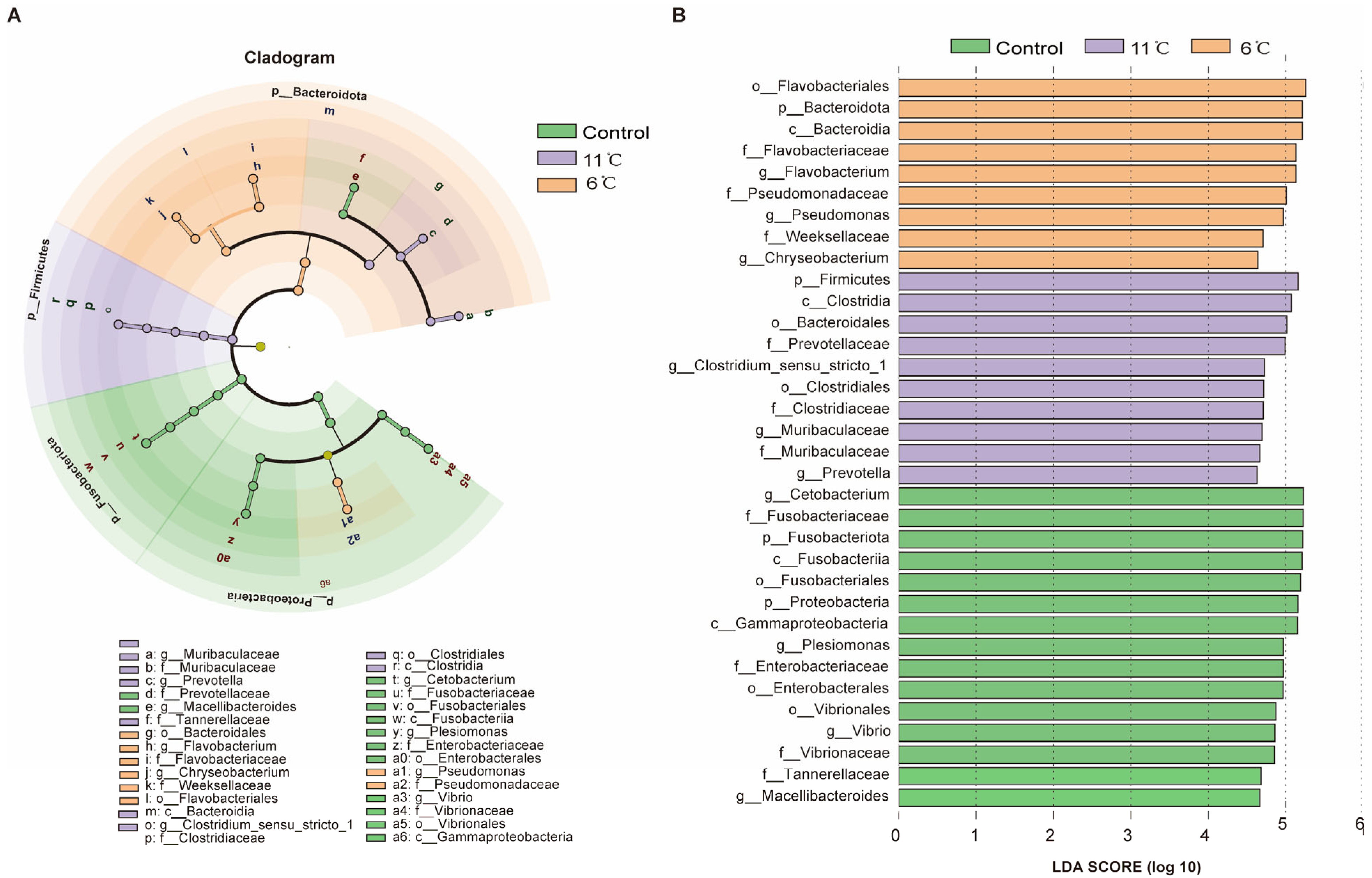
3.7. Functional Analysis of the Gut Microbiome in Pelteobagrus vachelli Under Low-Temperature Stress
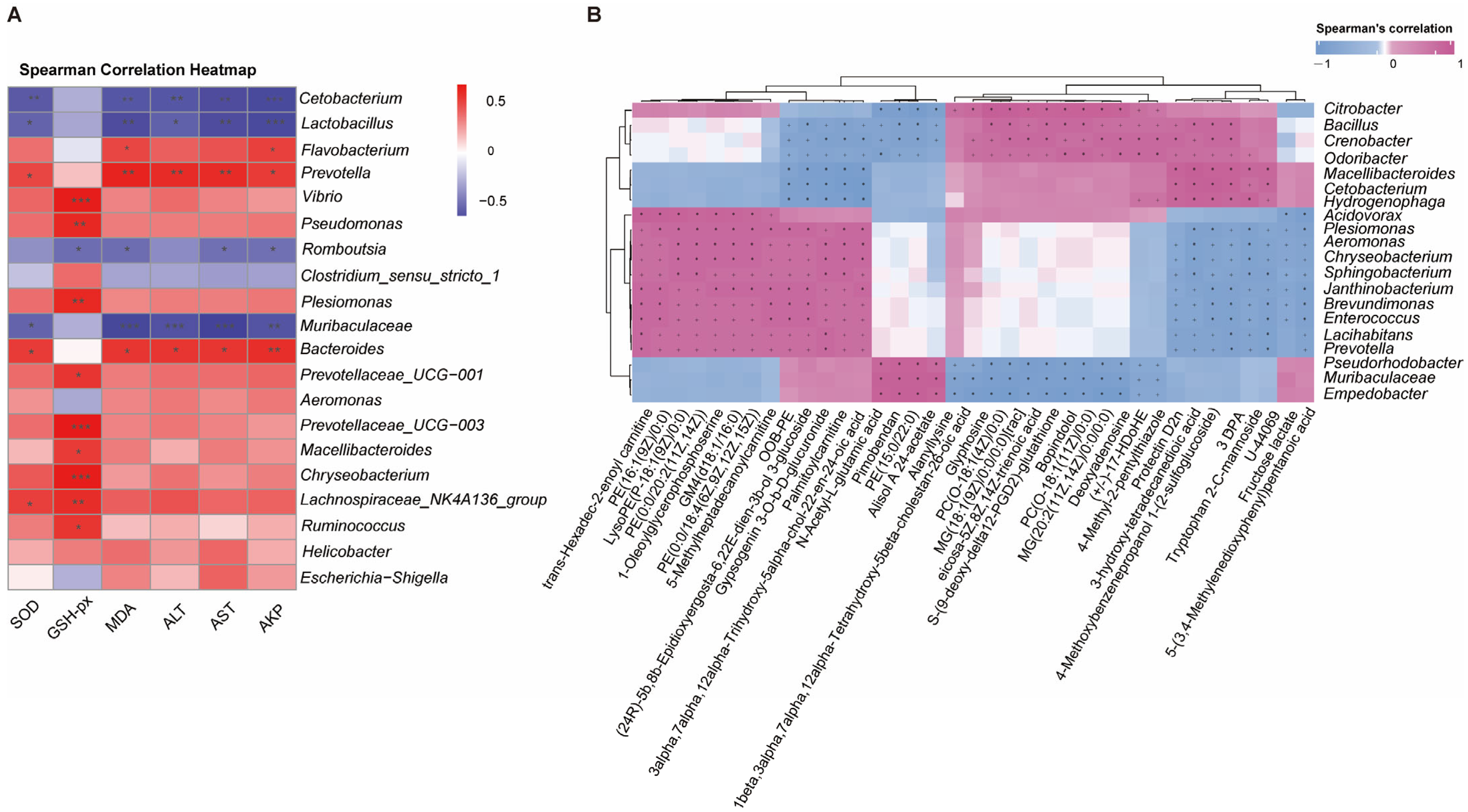
3.8. Correlation Analysis Between Liver and Intestine in Pelteobagrus vachelli
4. Discussion
4.1. Dysregulation of Hepatic Antioxidant–Immune Homeostasis Under Low-Temperature Stress
4.2. Adaptive Reprogramming of Hepatic Metabolome and Metabolic Pathways Under Low-Temperature Stress
4.3. Low-Temperature Stress Induces Structural and Functional Disruption of the Gut Microbiota
4.4. Low-Temperature Stress Perturbs Gut–Liver Axis Regulation
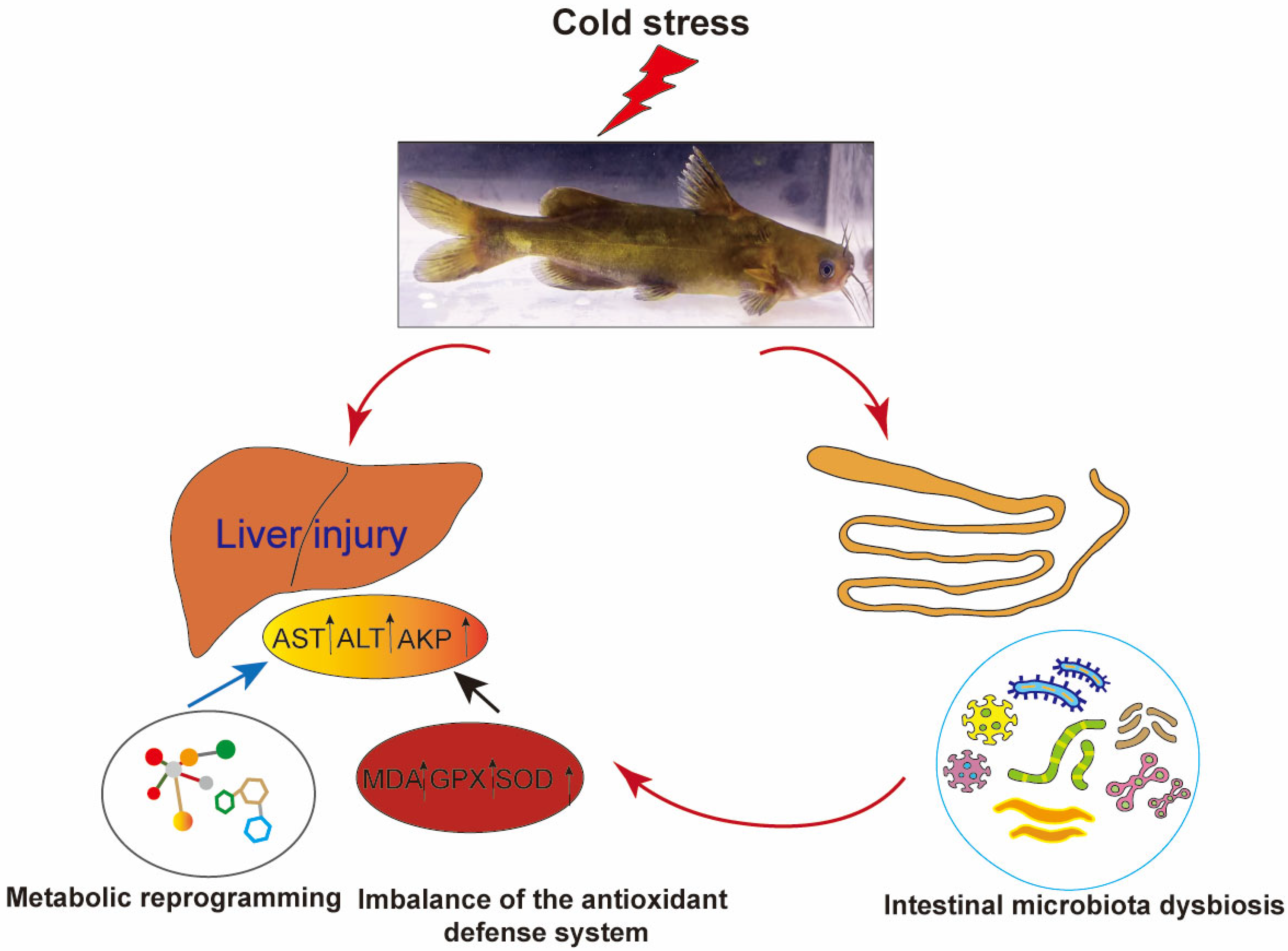
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, X.; Yu, Z.; Xu, Y.; Zhang, Y.; Mu, C.; Liu, P.; Li, J. Integrated transcriptomic and metabolomic responses in the hepatopancreas of kuruma shrimp (Marsupenaeus japonicus) under cold stress. Ecotoxicol. Environ. Saf. 2020, 206, 111360. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.A.; Morrison, W.E.; Nelson, M.W.; Stachura, M.M.; Teeters, E.J.; Griffis, R.B.; Alexander, M.A.; Scott, J.D.; Alade, L.; Bell, R.J.; et al. A Vulnerability Assessment of Fish and Invertebrates to Climate Change on the Northeast U.S. Continental Shelf. PLoS ONE 2016, 11, e0146756. [Google Scholar] [CrossRef]
- Przeslawski, R.; Ahyong, S.; Byrne, M.; Wörheide, G.; Hutchings, P.A.T. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Change Biol. 2008, 14, 2773–2795. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, M.; Xu, G.; Yu, H.; Jia, C.; Zhu, F.; Meng, Q.; Xu, D.; Du, S.; Zhang, D.; et al. Comprehensive analysis of histophysiology, transcriptome and metabolome tolerance mechanisms in black porgy (Acanthopagrus schlegelii) under low temperature stress. Sci. Total Environ. 2024, 927, 172318. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Pirozzi, I.; Codabaccus, B.M.; Sammut, J.; Booth, M.A. The interactive effect of dietary choline and water temperature on the liver lipid composition, histology, and plasma biochemistry of juvenile yellowtail kingfish (Seriola lalandi). Aquaculture 2021, 531, 735893. [Google Scholar] [CrossRef]
- Dellagostin, E.N.; Martins, A.W.S.; Blödorn, E.B.; Silveira, T.L.R.; Komninou, E.R.; Junior, A.S.V.; Corcini, C.D.; Nunes, L.S.; Remião, M.H.; Collares, G.L.; et al. Chronic cold exposure modulates genes related to feeding and immune system in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2022, 128, 269–278. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Wang, J.; Li, L.Y.; Han, S.L.; Limbu, S.M.; Li, D.L.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- Ge, K.; Fan, Z.; Huang, T.; Gu, W.; Wang, G.; Liu, E.; Pan, R.; Li, D.; Sun, Y.; Yao, Z.; et al. Influence of increasing acclimation temperature on growth, digestion, antioxidant capacity, liver transcriptome and intestinal microflora of Ussruri whitefish Coregonus ussuriensis Berg. Fish Shellfish. Immunol. 2024, 151, 109667. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Yang, Y.; Li, J.; Yang, X.; Li, L.; Zheng, Z.; Yang, B.; Zhang, P.; Liu, H. Effects of chronic cold stress and thermal stress on growth performance, hepatic apoptosis, oxidative stress, immune response and gut microbiota of juvenile hybrid sturgeon (Acipenser baerii female symbol x A. schrenkii male symbol). Fish Shellfish. Immunol. 2025, 157, 110078. [Google Scholar] [CrossRef]
- Liu, S.; Tian, F.; Qi, D.; Qi, H.; Wang, Y.; Xu, S.; Zhao, K. Physiological, metabolomic, and transcriptomic reveal metabolic pathway alterations in Gymnocypris przewalskii due to cold exposure. BMC Genom. 2023, 24, 545. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics. Sci. Total Environ. 2018, 640, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, Y.H.; Ai, C.X.; Zhang, H.; Huang, Y.C.; Zou, W.G. Different cold tolerances among three strains of large yellow croaker: Related to antioxidant defense and energy metabolism. Fish Physiol. Biochem. 2023, 49, 471–486. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, C.; Wei, C.; Zhong, K.; Gao, Y.; Li, P.; Qu, Z.; Bao, Z.; Wang, B.; Hu, J. The Effects of Acute Temperature Changes on Transcriptomic Responses in the Liver of Leopard Coral Groupers (Plectropomus leopardus). Antioxidants 2025, 14, 223. [Google Scholar] [CrossRef]
- Rojas, I.; Caballero-Solares, A.; Vadboncoeur, E.; Sandrelli, R.M.; Hall, J.R.; Clow, K.A.; Parrish, C.C.; Rise, M.L.; Swanson, A.K.; Gamperl, A.K. Prolonged Cold Exposure Negatively Impacts Atlantic Salmon (Salmo salar) Liver Metabolism and Function. Biology 2024, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, J.; Zhu, J.; Wang, Y.; Zhang, Y.; Li, Y.; Xu, S.; Yan, X.; Zhang, D. Transcriptome, antioxidant enzymes and histological analysis reveal molecular mechanisms responsive to long-term cold stress in silver pomfret (Pampus argenteus). Fish Shellfish. Immunol. 2022, 121, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, P.; Wen, H.; Xue, M.; Wang, Q.; He, J.; He, C.; Su, S.; Li, J.; Yu, F.; et al. Hypothermia-mediated oxidative stress induces immunosuppression, morphological impairment and cell fate disorder in the intestine of freshwater drum, Aplodinotus grunniens. Aquaculture 2023, 11, 1657. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, J.; Xia, Y.; Li, X.; Liu, Y.; Liu, P.F. Response mechanism of gut microbiome and metabolism of European seabass (Dicentrarchus labrax) to temperature stress. Sci. Total Environ. 2022, 813, 151786. [Google Scholar] [CrossRef]
- Frontiers Production Office. Erratum: The effect of dietary lactic acid bacteria on intestinal microbiota and immune responses of crucian carp (Carassius auratus) under water temperature decrease. Front. Microbiol. 2022, 13, 976726. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Li, H.; Yin, X.; Wang, P.; Qu, X.; Gao, Y.; Li, W.; Chu, Z. Modulation of Antioxidant Enzymes, Heat Shock Protein, and Intestinal Microbiota of Large Yellow Croaker (Larimichthys crocea) Under Acute Cold Stress. Front. Mar. Sci. 2021, 8, 725899. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Xu, P.; Tang, Y.; Su, S.; Liu, G.; Wu, N.; Xue, M.; Yu, F.; Feng, W.; et al. Hypothermia-Mediated Apoptosis and Inflammation Contribute to Antioxidant and Immune Adaption in Freshwater Drum, Aplodinotus grunniens. Antioxidants 2022, 11, 1657. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, H.; Wang, G.; Sun, Y.; Wang, L. Energy consumption and intestinal microbiome disorders of yellow catfish (Pelteobagrus fulvidraco) under cold stress. Front. Physiol. 2022, 13, 985046. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Sang, M.; Liu, S.; Yan, H.; Zhang, B.; Chen, S.; Wu, B.; Ma, T.; Jiang, H.; Zhao, P.; Sun, G.; et al. Synergistic detoxification efficiency and mechanism of triclocarban degradation by a bacterial consortium in the liver-gut-microbiota axis of zebrafish (Danio rerio). J. Hazard. Mater. 2024, 470, 134178. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Sun, Y.; Fu, K.; Zhang, Y.; Lei, L.; Men, J.; Guo, Y.; Wu, S.; Han, J.; Zhou, B. Effects of glyphosate exposure on gut-liver axis: Metabolomic and mechanistic analysis in grass carp (Ctenopharyngodon idellus). Sci. Total Environ. 2023, 902, 166062. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.; Lin, C.; He, L.; Wei, L. Histological alterations, oxidative stress, and inflammatory response in the liver of swamp eel (Monopterus albus) acutely exposed to copper. Fish Physiol. Biochem. 2021, 47, 1865–1878. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liang, X.; Yang, Z.; Peng, Y.; Zhang, Y.; Ning, X.; Zhang, K.; Ji, J.; Wang, T.; et al. Blood redistribution preferentially protects vital organs under hypoxic stress in Pelteobagrus vachelli. Aquat. Toxicol. 2023, 258, 106498. [Google Scholar] [CrossRef]
- Gong, G.; Ke, W.; Liao, Q.; Xiong, Y.; Hu, J.; Mei, J. A chromosome-level genome assembly of the darkbarbel catfish Pelteobagrus vachelli. Sci. Data 2023, 10, 598. [Google Scholar] [CrossRef]
- Taj, S.; Han, Q.; Wu, X.; Yin, H.; Tian, L.; Yang, H.; Liu, Y.; Huang, J. Effects of Dietary Protein-to-Energy Ratios on Growth, Immune Response, Antioxidative Capacity, Liver and Intestinal Histology, and Growth-Related Gene Expression in Hybrid Yellow Catfish (Pelteobagrus fulvidraco female symbol x Pelteobagrus vachelli male symbol). Aquac. Nutr. 2023, 2023, 9106332. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Xu, J.; Pei, X.; Wu, Z.; Wang, T.; Yin, S. iTRAQ analysis of liver immune-related proteins from darkbarbel catfish (Pelteobagrus vachelli) infected with Edwardsiella ictaluri. Fish Shellfish. Immunol. 2019, 87, 695–704. [Google Scholar] [CrossRef]
- Qiang, J.; Zhong, C.Y.; Bao, J.W.; Liang, M.; Liang, C.; Li, H.X.; He, J.; Xu, P. The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae (Tachysurus fulvidraco female symbol x Pseudobagrus vachellii male symbol). J. Therm. Biol. 2019, 86, 102436. [Google Scholar] [CrossRef]
- Zheng, T.; Tao, Y.; Lu, S.; Qiang, J.; Xu, P. Integrated Transcriptome and 16S rDNA Analyses Reveal That Transport Stress Induces Oxidative Stress and Immune and Metabolic Disorders in the Intestine of Hybrid Yellow Catfish (Tachysurus fulvidraco female symbol x Pseudobagrus vachellii male symbol). Antioxidants 2022, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Shi, Q.; Shangguan, Y.; Chen, C.; Zhu, J.; Dong, Z.; Hong, X.; Liu, X.; Wei, C.; Zhu, X.; et al. Molecular Response and Metabolic Reprogramming of the Spleen Coping with Cold Stress in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Antioxidants 2025, 14, 217. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, W.; Zhao, R.; Gu, C.; Shen, H.; Li, H.; Wang, L.; Cheng, J.; Wan, X. Integrated physiological, transcriptome, and metabolome analyses of the hepatopancreas of Litopenaeus vannamei under cold stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101196. [Google Scholar] [CrossRef]
- Wu, S.M.; Liu, J.H.; Shu, L.H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef]
- Xu, D.; Wu, J.; Sun, L.; Qin, X.; Fan, X.; Zheng, X. Combined stress of acute cold exposure and waterless duration at low temperature induces mortality of shrimp Litopenaeus vannamei through injuring antioxidative and immunological response in hepatopancreas tissue. J. Therm. Biol. 2021, 100, 103080. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, G.; Gentile, A.; Soriano, S.; Novelli, A.; Lenti, M.V.; Di Sabatino, A. Glutathione: Pharmacological aspects and implications for clinical use in non-alcoholic fatty liver disease. Front. Med. 2023, 10, 1124275. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. 2020, 12, eaaz2841. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, K.; Xu, C.; Jiang, Y.; Shan, J.; Zhang, Z.; Cai, J. Cypermethrin induces apoptosis, autophagy and inflammation via ERS-ROS-NF-kappaB axis in hepatocytes of carp (Cyprinus carpio). Pestic. Biochem. Physiol. 2023, 196, 105625. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, X.; Li, C.; Wu, J.; Sun, L.; Qin, X.; Fan, X. Insights into the response mechanism of Litopenaeus vannamei exposed to cold stress during live transport combining untargeted metabolomics and biochemical assays. J. Therm. Biol 2022, 104, 103200. [Google Scholar] [CrossRef]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.; Ren, X.; Yuan, J.; Mao, L. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish. Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017, 1, 3–9. [Google Scholar] [CrossRef]
- Wu, G.; Baumeister, R.; Heimbucher, T. Molecular Mechanisms of Lipid-Based Metabolic Adaptation Strategies in Response to Cold. Cells 2023, 12, 1353. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Li, B.; Ding, L.; Wei, X.; Wang, P.; Chen, Z.; Han, S.; Huang, T.; Wang, B.; et al. Physiological responses to heat stress in the liver of rainbow trout (Oncorhynchus mykiss) revealed by UPLC-QTOF-MS metabolomics and biochemical assays. Ecotoxicol. Environ. Saf. 2022, 242, 113949. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef]
- Castro, R.E.; Sola, S.; Ramalho, R.M.; Steer, C.J.; Rodrigues, C.M. The bile acid tauroursodeoxycholic acid modulates phosphorylation and translocation of bad via phosphatidylinositol 3-kinase in glutamate-induced apoptosis of rat cortical neurons. J. Pharmacol. Exp. Ther. 2004, 311, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, H.; Zhu, W.; Jiang, Q.; Dong, Z.; Wang, L. Integrated Analysis of Transcriptome and Metabolome in the Brain After Cold Stress of Red Tilapia During Overwintering. Int. J. Mol. Sci. 2024, 25, 13372. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, L.; Ma, R.; Wang, J.; Du, L. FoxO signaling and mitochondria-related apoptosis pathways mediate tsinling lenok trout (Brachymystax lenok tsinlingensis) liver injury under high temperature stress. Int. J. Biol. Macromol. 2023, 251, 126404. [Google Scholar] [CrossRef]
- Ren, J.; Long, Y.; Liu, R.; Song, G.; Li, Q.; Cui, Z. Characterization of Biological Pathways Regulating Acute Cold Resistance of Zebrafish. Int. J. Mol. Sci. 2021, 22, 3028. [Google Scholar] [CrossRef]
- Ge, G.; Long, Y.; Shi, L.; Ren, J.; Yan, J.; Li, C.; Li, Q.; Cui, Z. Transcriptomic profiling revealed key signaling pathways for cold tolerance and acclimation of two carp species. BMC Genom. 2020, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, S.; Zhang, X.; Zhou, J.; Yan, X.; Liu, H.; Liang, J.; Luo, L.; Zhou, D.; Yin, Z. Gamma-Glutamylcysteine alleviates ethanol-induced hepatotoxicity via suppressing oxidative stress, apoptosis, and inflammation. J. Food Biochem. 2022, 46, e14318. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef]
- Fan, L.; Wang, L.; Wang, Z. Proteomic characterization of the hepatopancreas in the Pacific white shrimp Litopenaeus vannamei under cold stress: Revealing the organism homeostasis mechanism. Fish Shellfish. Immunol. 2019, 92, 438–449. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Ussery, D.W.; Nielsen, J.; Nookaew, I. A closer look at bacteroides: Phylogenetic relationship and genomic implications of a life in the human gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhou, W.; Xie, Y.; Li, Y.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Ran, C.; Zhou, Z. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 2021, 543, 736943. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Alnabhani, Z.; Montcuquet, N.; Biaggini, K.; Dussaillant, M.; Roy, M.; Ogier-Denis, E.; Madi, A.; Jallane, A.; Feuilloley, M.; Hugot, J.P.; et al. Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1beta expression through hematopoietic NOD2 signaling. Inflamm. Bowel Dis. 2015, 21, 543–555. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-kappaB signaling. J. Recept. Signal Transduct. 2021, 41, 294–303. [Google Scholar] [CrossRef]
- Gu, T.; Kong, M.; Duan, M.; Chen, L.; Tian, Y.; Xu, W.; Zeng, T.; Lu, L. Cu exposure induces liver inflammation via regulating gut microbiota/LPS/liver TLR4 signaling axis. Ecotoxicol. Environ. Saf. 2024, 278, 116430. [Google Scholar] [CrossRef]
- Blesl, A.; Stadlbauer, V. The Gut-Liver Axis in Cholestatic Liver Diseases. Nutrients 2021, 13, 1018. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-Resident Lactobacilli Activate Hepatic Nrf2 and Protect Against Oxidative Liver Injury. Cell Metab. 2020, 31, 956–968 e955. [Google Scholar] [CrossRef]
- Carbajo-Pescador, S.; Porras, D.; Garcia-Mediavilla, M.V.; Martinez-Florez, S.; Juarez-Fernandez, M.; Cuevas, M.J.; Mauriz, J.L.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis. Models Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Ding, C.; Yang, Y.; Gao, Z.; Ding, W.; Ma, J.; Li, X. Destruction of the intestinal microbiota and gut-liver axis homeostasis by microcystin-LR-induced inflammation in the common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2025, 295, 118155. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin alleviates lead-induced fatty liver in the common carps (Cyprinus carpio) via gut-liver axis. Environ. Pollut. 2023, 317, 120730. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Kaziem, A.E.; Miao, X.; Huang, S.; Cheng, D.; Xu, H.; Zhang, Z. Effects of Exposure to Different Types of Metal-Organic Framework Nanoparticles on the Gut Microbiota and Liver Metabolism of Adult Zebrafish. ACS Nano 2024, 18, 25425–25445. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.M.; Bedi, B.; Yuan, Z.; Goldberg, J.B.; Koval, M.; Hart, C.M.; Sadikot, R.T. Pseudomonas aeruginosa Induced Host Epithelial Cell Mitochondrial Dysfunction. Sci. Rep. 2019, 9, 11929. [Google Scholar] [CrossRef]
- Peng, K.Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Lessing, D.J.; Chu, W. The attenuating effects of synbiotic containing Cetobacterium somerae and Astragalus polysaccharide against trichlorfon-induced hepatotoxicity in crucian carp (Carassius carassius). J. Hazard. Mater. 2024, 461, 132621. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Duan, G.; Yang, L.; Hu, Y.; Zhou, H.; Wang, H. Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut–Liver Axis Dysregulation. Antioxidants 2025, 14, 762. https://doi.org/10.3390/antiox14070762
Liu A, Duan G, Yang L, Hu Y, Zhou H, Wang H. Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut–Liver Axis Dysregulation. Antioxidants. 2025; 14(7):762. https://doi.org/10.3390/antiox14070762
Chicago/Turabian StyleLiu, Amei, Guoqing Duan, Libo Yang, Yuting Hu, Huaxing Zhou, and Huan Wang. 2025. "Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut–Liver Axis Dysregulation" Antioxidants 14, no. 7: 762. https://doi.org/10.3390/antiox14070762
APA StyleLiu, A., Duan, G., Yang, L., Hu, Y., Zhou, H., & Wang, H. (2025). Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut–Liver Axis Dysregulation. Antioxidants, 14(7), 762. https://doi.org/10.3390/antiox14070762





