The Antioxidant Properties of Extracts of Cuscuta spp. Depend on the Parasite and the Host Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Polyphenol Determination
2.3. ABTS Radical Scavenging Assay
2.4. HPLC-MS Analysis
3. Results
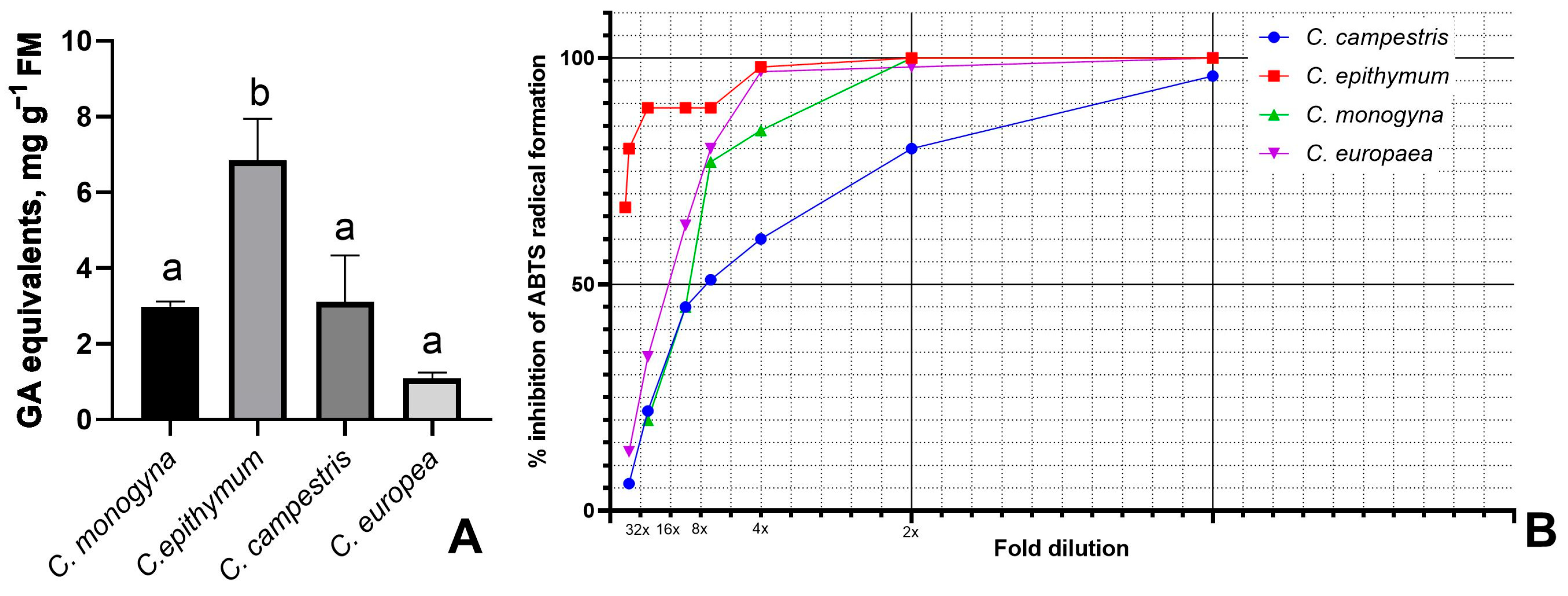
3.1. Total Polyphenolics and Antioxidant Activity
3.2. Flavonoid Content of Cuscuta Campestris in Relation to the Host Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Těšitel, J. Functional biology of parasitic plants: A review. Plant Ecol. Evol. 2016, 149, 5–20. [Google Scholar] [CrossRef]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Těšitel, J.; Li, A.-R.; Knotková, K.; McLellan, R.; Bandaranayake, P.C.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, C.; Gong, X.; Yang, M.; Ji, M.; Jiang, L.; Leonti, M.; Yao, R.; Li, M. The genus Orobanche as food and medicine: An ethnopharmacological review. J. Ethnopharmacol. 2020, 263, 113154. [Google Scholar] [CrossRef]
- Kleszken, E.; Timar, A.V.; Memete, A.R.; Miere, F.; Vicas, S.I. On overview of bioactive compounds, biological and pharmacological effects of mistletoe (Viscum Album L). Pharmacophore 2022, 13, 10–26. [Google Scholar] [CrossRef]
- Adesina, S.K.; Illoh, H.; Johnny, I.I.; Jacobs, I.E. African mistletoes (Loranthaceae); ethnopharmacology, chemistry and medicinal values: An update. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 161–170. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, Z.; Miao, J.; Wang, Z.; Li, Q.; Chai, X.; Li, M. The genus Cynomorium in China: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2013, 147, 1–15. [Google Scholar] [CrossRef]
- Puttipan, R.; Okonogi, S. Antioxidant activity of Rafflesia kerrii flower extract. Drug Discov. Ther. 2014, 8, 18–24. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Sinha, N.R. Cuscuta species: Model organisms for haustorium development in stem holoparasitic plants. Front. Plant Sci. 2022, 13, 1086384. [Google Scholar] [CrossRef]
- Costea, M.; Stefanović, S. Cuscuta jepsonii (Convolvulaceae): An invasive weed or an extinct endemic? Am. J. Bot. 2009, 96, 1744–1750. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Guo, D.a. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass. Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass. Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Concordance between antioxidant activities and flavonol contents in different extracts and fractions of Cuscuta chinensis. Food Chem. 2008, 108, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sánchez, I.J.; Garza-Ortiz, A. Is there a secondary/specialized metabolism in the genus Cuscuta and which is the role of the host plant? Phytochem. Rev. 2019, 18, 1299–1335. [Google Scholar] [CrossRef]
- Assyov, B.; Petrova, A. Conspectus of the Vascular Plants in Bulgaria; Bulgarian Biodiversity Foundation: Sofia, Bulgaria, 2012; pp. 1–489. [Google Scholar]
- Stoyanov, K.; Raycheva, T.; Cheschmedzhiev, I. Key to the Native and Foreign Vascular Plants in Bulgaria; Agricultural University Plovdiv Academic Press: Plovdiv, Bulgaria, 2021. [Google Scholar]
- Dimitrova, V.; Tashev, A. Medicinal Plants of Bulgaria. Curr. Perspect. Med. Aromat. Plants 2019, 2, 29–39. [Google Scholar] [CrossRef][Green Version]
- Stojanov, N.; Kitanov, B. Wild Useful Plants in Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1960. [Google Scholar]
- Zhelev, Z.D.; Stanilova, S.A.; Carpenter, B.G. Isolation, Partial Characterization, and Complement-Inhibiting Activity of a New Glycoprotein from Cuscuta europea. Biochem. Biophys. Res. Commun. 1994, 202, 186–194. [Google Scholar] [CrossRef]
- Perveen, S.; Bukhari, I.H.; Kousar, S.; Rehman, J. Antimicrobial, antioxidant and minerals evaluation of Cuscuta europea and Cuscuta reflexa collected from different hosts and exploring their role as functional attribute. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 43–49. [Google Scholar]
- Chabra, A.; Monadi, T.; Azadbakht, M.; Haerizadeh, S.I. Ethnopharmacology of Cuscuta epithymum: A comprehensive review on ethnobotany, phytochemistry, pharmacology and toxicity. J. Ethnopharmacol. 2019, 231, 555–569. [Google Scholar] [CrossRef]
- Teofanova, D.; Lozanova, Y.; Lambovska, K.; Pachedjieva, K.; Tosheva, A.; Odjakova, M.; Zagorchev, L. Cuscuta spp. populations as potential reservoirs and vectors of four plant viruses. Phytoparasitica 2022, 50, 555–566. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Thitilertdecha, N.; Rakariyatham, N. Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci. Hortic. 2011, 129, 247–252. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Chakarova, B.; Zagorchev, L.; Pachedjieva, K.; Tosheva, A.; Zagorcheva, T.; Rusanov, K.; Teofanova, D. Analysis of Variations in the Flavonoid Profiles of Cuscuta campestris and Cuscuta epithymum in Bulgaria as a Potential Chemotaxonomical Marker. Plants 2025, 14, 1220. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Barath, K.; Csiky, J. Host range and host choice of Cuscuta species in Hungary. Acta Bot. Croat. 2012, 71, 215–227. [Google Scholar] [CrossRef]
- Zagorchev, L.I.; Petrova, V.P.; Albanova, I.; Georgieva, K.P.; Sarić-Krsmanović, M.; Muscolo, A.; Teofanova, D.R. Salinity modulates crop plants suitability as hosts for Cuscuta campestris parasitism. J. Saudi Soc. Agric. Sci. 2022, 21, 324–330. [Google Scholar] [CrossRef]
- Jafari, E.; Bahmanzadegan, A.; Ghanbarian, G.; Rowshan, V. Antioxidant activity and total phenolic content from aerial parts of three Cuscuta species. Anal. Chem. Lett. 2015, 5, 377–384. [Google Scholar] [CrossRef]
- Snyder, A.M.; Clark, B.M.; Bungard, R.A. Light-dependent conversion of carotenoids in the parasitic angiosperm Cuscuta reflexa L. Plant Cell Environ. 2005, 28, 1326–1333. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef]
- Kumar, K.; Amir, R. The effect of a host on the primary metabolic profiling of Cuscuta campestris’ main organs, haustoria, stem and flower. Plants 2021, 10, 2098. [Google Scholar] [CrossRef]
- Guo, C.; Qin, L.; Ma, Y.; Qin, J. Integrated metabolomic and transcriptomic analyses of the parasitic plant Cuscuta japonica Choisy on host and non-host plants. BMC Plant Biol. 2022, 22, 393. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi Halat, D.; Krayem, M.; Khaled, S.; Younes, S. A focused insight into thyme: Biological, chemical, and therapeutic properties of an indigenous Mediterranean herb. Nutrients 2022, 14, 2104. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Ramezan, D.; Farrokhzad, Y.; Zargar, M.; Stybayev, G.; Kipshakbayeva, G.; Baitelenova, A. An Insight into Cuscuta campestris as a Medicinal Plant: Phytochemical Variation of Cuscuta campestris with Various Host Plants. Agriculture 2023, 13, 770. [Google Scholar] [CrossRef]
- Tanruean, K.; Poolprasert, P.; Kumla, J.; Suwannarach, N.; Lumyong, S. Bioactive compounds content and their biological properties of acetone extract of Cuscuta reflexa Roxb. grown on various host plants. Nat. Prod. Res. 2019, 33, 544–547. [Google Scholar] [CrossRef]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front. Plant Sci. 2015, 6, 45. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta–host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Li, J.; Zhuang, H.; Sun, H.; Xu, Y.; Qi, J.; Zhang, J.; Lei, Y.; Qin, Y.; Sun, G. Stem parasitic plant Cuscuta australis (dodder) transfers herbivory-induced signals among plants. Proc. Natl. Acad. Sci. USA 2017, 114, E6703–E6709. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]


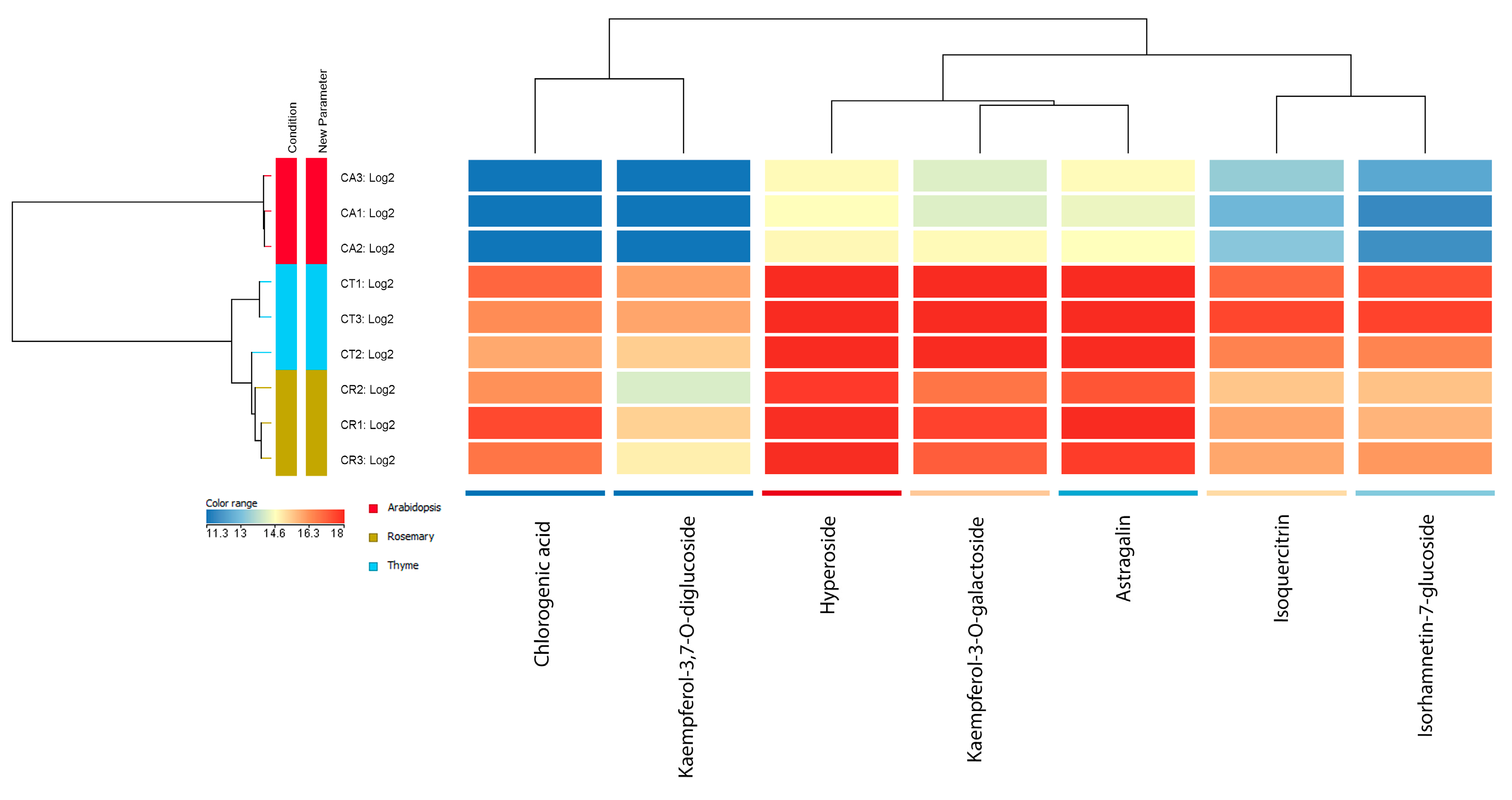
| Peak | tR (min) | Identity | [M-H]− m/z |
|---|---|---|---|
| 1 | 3.15 | Chlorogenic acid | 353.0858 |
| 2 | 4.85 | Kaempferol-3,7-O-diglucoside | 609.1439 |
| 3 | 8.59 | Quercetin-3-O-galactoside (Hyperoside) | 463.0853 |
| 4 | 8.94 | Kaempferol-3-O-galactoside | 447.0902 |
| 5 | 10.17 | Quercetin-3-O-glucoside (Isoquercitrin) | 463.0852 |
| 6 | 10.36 | Astragalin | 447.0902 |
| 7 | 10.66 | Isorhamnetin-7-glucoside | 477.1005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozanova, V.; Teofanova, D.; Chakarova, B.; Rusanov, K.; Pachedjieva, K.; Tosheva, A.; Zagorcheva, T.; Zagorchev, L. The Antioxidant Properties of Extracts of Cuscuta spp. Depend on the Parasite and the Host Species. Antioxidants 2025, 14, 761. https://doi.org/10.3390/antiox14070761
Lozanova V, Teofanova D, Chakarova B, Rusanov K, Pachedjieva K, Tosheva A, Zagorcheva T, Zagorchev L. The Antioxidant Properties of Extracts of Cuscuta spp. Depend on the Parasite and the Host Species. Antioxidants. 2025; 14(7):761. https://doi.org/10.3390/antiox14070761
Chicago/Turabian StyleLozanova, Vanina, Denitsa Teofanova, Bilyana Chakarova, Krasimir Rusanov, Kalina Pachedjieva, Anita Tosheva, Tzvetelina Zagorcheva, and Lyuben Zagorchev. 2025. "The Antioxidant Properties of Extracts of Cuscuta spp. Depend on the Parasite and the Host Species" Antioxidants 14, no. 7: 761. https://doi.org/10.3390/antiox14070761
APA StyleLozanova, V., Teofanova, D., Chakarova, B., Rusanov, K., Pachedjieva, K., Tosheva, A., Zagorcheva, T., & Zagorchev, L. (2025). The Antioxidant Properties of Extracts of Cuscuta spp. Depend on the Parasite and the Host Species. Antioxidants, 14(7), 761. https://doi.org/10.3390/antiox14070761






