Regulatory Mechanisms of Phenolic Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review
Abstract
1. Introduction
2. Regulatory Mechanisms of Phenolic Acids on MASLD
2.1. Hydroxycinnamic Acids
2.1.1. Cinnamic Acid
2.1.2. p-Coumaric Acid
2.1.3. Caffeic Acid
2.1.4. Ferulic Acid
2.1.5. Chlorogenic Acid
2.2. Hydroxybenzoic Acids
2.2.1. Protocatechuic Acid
2.2.2. Vanillic Acid
2.2.3. Gallic Acid
3. Bioavailability of Phenolic Acids
4. Safety Evaluation of Phenolic Acids
5. The Current Found and Limitations of Phenolic Acids in MASLD
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ACSF3 | Acyl-CoA synthetase family member 3 |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| APAP | Acetaminophen/N-acetyl-p-aminophenol |
| ATGL | Adipose triglyceride lipase |
| BAs | Bile acids |
| BCAAs | Branched-chain amino acids |
| BSEP | Bile salt export pump |
| CaA | Caffeic acid |
| CAPE | Caffeic acid phenethyl ester |
| CAT | Catalase |
| CA9 | Carbonic anhydrase IX |
| CCI | Constriction injury |
| CD36 | Cluster of differentiation 36 |
| CGA | Chlorogenic acid |
| CiA | Cinnamic acid |
| COX-2 | Cyclooxygenase-2 |
| CPT1α | Carnitine palmitoyltransferase-1 alpha |
| CYP7A1 | Cholesterol 7-α hydroxylase |
| DSS | Dextran sulfate sodium |
| ECM | Extracellular matrix |
| ED50 | Median effective dose |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated protein kinase |
| EVs | Extracellular vesicles |
| FA | Ferulic acid |
| FASN | Fatty acid synthase |
| FATP2 | Fatty acid transport protein 2 |
| FFA | Free fatty acid |
| FGF | Fibroblast growth factor |
| FXR | Farnesoid X receptor |
| GA | Gallic acid |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT-2 | Glucose transporter-2 |
| HBAs | Hydroxybenzoic acids |
| HCAs | Hydroxycinnamic acids |
| HCC | Hepatocellular carcinoma |
| HCHF | High-carbohydrate, high-fat diet group |
| HCs | Hepatocytes |
| HDL | High-density lipoprotein |
| HFD | High fat diet |
| HFFD | High-fat, high-fructose diet |
| HFS | 20% fructose in drinking water plus 4% sodium chloride in the diet |
| HGP | Hepatic glucose production |
| HO-1 | Heme oxygenase-1 |
| HS | Heat stress |
| HSCs | Hepatic stellate cells |
| HSP70 | Heat shock protein 70 |
| HUA | Hyperuricemia |
| IGFBP-2 | Insulin-like growth factor binding protein-2 |
| IHTGs | Intrahepatic triglycerides |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| INSIG2 | Insulin-induced gene 2 |
| IR | Insulin resistance |
| IRS1 | Insulin receptor substrate 1 |
| IRS2 | Insulin receptor substrate 2 |
| KCs | Kupffer cells |
| LC3B | Microtubule-associated protein 2 light chain 3 type B |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| LD50 | Median lethal dose |
| LPS | Lipopolysaccharides |
| LSECs | Liver sinusoidal endothelial cells |
| LTA | Lipoteichoic acid |
| LXRα | Liver X receptor alpha |
| MAPK | Mitogen-activated protein kinase |
| MAFLD | Metabolic Dysfunction-Associated Fatty Liver Disease |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MCDD | Methionine-choline-deficient diet |
| MCT | Monocarboxylate transporter |
| MIF | Macrophage migration inhibitory factor |
| MLCK | Myosin light chain kinase |
| MyD88 | Myeloid differentiation primary response 88 |
| NAFL | Non-alcoholic fatty liver |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor κB |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| PA | Palmitic acid |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PARP | Poly(ADP-ribose) polymerase |
| pCA | p-coumaric acid |
| PCA | Protocatechuic acid |
| PI3K | Phosphoinositide 3-kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| ROCK1 | Rho-associated kinase 1 |
| ROS | Reactive oxygen species |
| RRBE | Red rice bran extract |
| PRRs | Pattern Recognition Receptors |
| SCAP | SREBP cleavage-activating protein |
| SCD1 | Stearoyl-CoA desaturase-1 |
| SCFAs | Short-chain fatty acids |
| SHP | Small heterodimer partner |
| SIRT3 | Sirtuin 3 |
| SOD | Superoxide dismutase |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| TBARS | Thiobarbituric acid reactive substances |
| TC | Total cholesterol |
| TCA | Trans-cinnamic acid |
| TG | Triglyceride |
| TGF-β | Transforming Growth Factor-beta |
| TI | Therapeutic index |
| TJ | Tight junctions |
| TLRs | Toll-like receptors |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| TZDs | Thiazolidinediones |
| T2DM | Type 2 diabetes mellitus |
| VA | Vanillic acid |
| ZO-1 | Zonula occludens-1 |
| 5-HT | 5-hydroxytryptamine |
References
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla Bertot, L.; Adams, L.A. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188221145549. [Google Scholar] [CrossRef]
- Filipović, B.; Forbes, A.; Tepeš, B.; Dumitraşcu, D.L. Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2097435. [Google Scholar] [CrossRef]
- Xie, C.; Halegoua-DeMarzio, D. Role of Probiotics in Non-alcoholic Fatty Liver Disease: Does Gut Microbiota Matter? Nutrients 2019, 11, 2837. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut–liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal Products and the Liver: A Review of Adverse Effects and Mechanisms. Gastroenterology 2015, 148, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Mohammadhasani, K.; Vahedi Fard, M.; Mottaghi Moghaddam Shahri, A.; Khorasanchi, Z. Polyphenols improve non-alcoholic fatty liver disease via gut microbiota: A comprehensive review. Food Sci. Nutr. 2024, 12, 5341–5356. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Targher, G.; Lonardo, A.; Byrne, C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 99–114. [Google Scholar] [CrossRef]
- Kumar, S.; Duan, Q.; Wu, R.; Harris, E.N.; Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113869. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis in NAFLD/NASH: From pathophysiology towards diagnostic and therapeutic strategies. Mol. Asp. Med. 2024, 95, 101231. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Yang, F.; Liu, G.; Liu, J.; Zhao, W.; Ma, S.; Duan, Z. Programmed cell death and lipid metabolism of macrophages in NAFLD. Front. Immunol. 2023, 14, 1118449. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Bataller, R.; Brenner, D.A. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G949–G958. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.J.; Jain, G.; Rajagopalan, P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 2015, 24, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Yoneda, M.; Kessoku, T.; Ogawa, Y.; Maeda, S.; Sumida, Y.; Hyogo, H.; Eguchi, Y.; Wada, K.; Nakajima, A. Rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2013, 14, 21833–21857. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving Concepts in the Pathogenesis of NASH: Beyond Steatosis and Inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.; Byrne, C.D. Bidirectional Relationships and Disconnects between NAFLD and Features of the Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Kheong, C.W.; Mustapha, N.R.N.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949. [Google Scholar] [CrossRef]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2022, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Pockros, P.J.; Fuchs, M.; Freilich, B.; Schiff, E.; Kohli, A.; Lawitz, E.J.; Hellstern, P.A.; Owens-Grillo, J.; Van Biene, C.; Shringarpure, R.; et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019, 39, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Loustaud-Ratti, V.; Bureau, C.; Lawitz, E.; Abdelmalek, M.; Alkhouri, N.; Francque, S.; Girma, H.; Darteil, R.; et al. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH. J. Hepatol. 2023, 78, 479–492. [Google Scholar] [CrossRef]
- Braun, L.R.; Feldpausch, M.N.; Czerwonka, N.; Weiss, J.; Branch, K.; Lee, H.; Martinez-Salazar, E.L.; Torriani, M.; Sponseller, C.A.; Grinspoon, S.K.; et al. Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 4176–4186. [Google Scholar] [CrossRef]
- Podszun, M.C.; Alawad, A.S.; Lingala, S.; Morris, N.; Huang, W.A.; Yang, S.; Schoenfeld, M.; Rolt, A.; Ouwerkerk, R.; Valdez, K.; et al. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020, 37, 101710. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Parmar, D.; Sheikh, F.; Sarin, S.K.; Cisneros, L.; Gawrieh, S.; Momin, T.; Duseja, A.; Sanyal, A.J. Saroglitazar, a Dual PPAR α/γ Agonist, Improves Atherogenic Dyslipidemia in Patients With Non-Cirrhotic Nonalcoholic Fatty Liver Disease: A Pooled Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2597–2605. [Google Scholar] [CrossRef]
- Loomba, R.; Lutchman, G.; Kleiner, D.E.; Ricks, M.; Feld, J.J.; Borg, B.B.; Modi, A.; Nagabhyru, P.; Sumner, A.E.; Liang, T.J.; et al. Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2009, 29, 172–182. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wu, H.; Zhang, L.; Hu, X.; Li, C.; Liu, S. Noni (Morinda citrifolia L.) fruit phenolic extract supplementation ameliorates NAFLD by modulating insulin resistance, oxidative stress, inflammation, liver metabolism and gut microbiota. Food Res. Int. 2022, 160, 111732. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Z.; Xie, J.; Yu, J.; Xiong, S.; Xiang, F.; Ma, X.; Yang, C.; Lin, L. Research Progress on the Mechanism for Improving Glucose and Lipid Metabolism Disorders Using Phenolic Acid Components from Medicinal and Edible Homologous Plants. Molecules 2024, 29, 4790. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Vega, A.; Delgado, N.; Handford, M. Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. Int. J. Mol. Sci. 2022, 23, 5438. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, S.; Li, Y.; Xia, B.; Li, M.; Zhang, Z.; Shi, Z.; Peng, Q.; Li, C.; Lin, L.; et al. Phenolic acids from medicinal and edible homologous plants: A potential anti-inflammatory agent for inflammatory diseases. Front. Immunol. 2024, 15, 1345002. [Google Scholar] [CrossRef]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet- induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef]
- Solanki, N.; Patel, R. Unraveling the mechanisms of trans-cinnamic acid in ameliorating non-alcoholic fatty liver disease. Am. J. Transl. Res. 2023, 15, 5747–5756. [Google Scholar] [PubMed]
- Wu, Y.; Wang, M.; Yang, T.; Qin, L.; Hu, Y.; Zhao, D.; Wu, L.; Liu, T. Cinnamic Acid Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Hepatic Lipogenesis and Promoting Fatty Acid Oxidation. Evid. Based Complement. Altern. Med. 2021, 2021, 9561613. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.H.; Yang, T.; Qin, T.Y.; Qin, L.L.; Hu, Y.M.; Zhang, C.F.; Sun, B.J.; Ding, L.; Wu, L.L.; et al. Mechanisms for Improving Hepatic Glucolipid Metabolism by Cinnamic Acid and Cinnamic Aldehyde: An Insight Provided by Multi-Omics. Front. Nutr. 2021, 8, 794841. [Google Scholar] [CrossRef]
- Nie, W.; Yang, Y.; Li, L.; Ding, Y.; Chen, X.; Li, M.; He, N.; Ji, G.; Zhang, Y.; Kang, P.; et al. Comparison of pharmacokinetic profiles of seven major bioactive components in normal and non-alcoholic fatty liver disease (NAFLD) rats after oral administration of Ling-Gui-Zhu-Gan decoction by UPLC-MS/MS. Front. Pharmacol. 2023, 14, 1174742. [Google Scholar] [CrossRef]
- Jiang, J.G.; Luo, Q.; Li, S.S.; Tan, T.Y.; Xiong, K.; Yang, T.; Xiao, T.B. Cinnamic acid regulates the intestinal microbiome and short-chain fatty acids to treat slow transit constipation. World J. Gastrointest. Pharmacol. Ther. 2023, 14, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Lee, S.; Lee, J.H.; Park, J.W. Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2018, 121, 131–139. [Google Scholar] [CrossRef]
- Moradi, M.; Farbood, Y.; Mard, S.A.; Dianat, M.; Goudarzi, G.; Khorsandi, L.; Seyedian, S.S. p-Coumaric acid has pure anti-inflammatory characteristics against hepatopathy caused by ischemia-reperfusion in the liver and dust exposure. Iran. J. Basic Med. Sci. 2023, 26, 164–175. [Google Scholar] [CrossRef]
- Goodarzi, G.; Tehrani, S.S.; Panahi, G.; Bahramzadeh, A.; Meshkani, R. Combination therapy of metformin and p-coumaric acid mitigates metabolic dysfunction associated with obesity and nonalcoholic fatty liver disease in high-fat diet obese C57BL/6 mice. J. Nutr. Biochem. 2023, 118, 109369. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, N.; Gao, X.; Huang, W.; Wang, H.; Bao, Y.; Ge, X.; Tao, X.; Sheng, L.; Li, H. The identification of material basis of Si Miao Formula effective for attenuating non-alcoholic fatty liver disease. J. Ethnopharmacol. 2024, 318, 116988. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, X.; Lei, F.; Sun, H.; Jiang, J.; Xing, D.; Du, L. Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules 2023, 28, 4614. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liu, Z.; Henderson, A.; Lee, K.; Hostetter, J.; Wannemuehler, M.; Hendrich, S. Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium-induced colitis by caffeic acid in mice. Exp. Biol. Med. 2009, 234, 605–616. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jazvinšćak Jembrek, M. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ge, K.; Mu, J.; Rong, J.; Zhang, L.; Wang, B.; Wan, J.; Xia, G. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016, 8, 4205–4214. [Google Scholar]
- Senaphan, K.; Kukongviriyapan, U.; Sangartit, W.; Pakdeechote, P.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E.; Kukongviriyapan, V. Ferulic Acid Alleviates Changes in a Rat Model of Metabolic Syndrome Induced by High-Carbohydrate, High-Fat Diet. Nutrients 2015, 7, 6446–6464. [Google Scholar] [CrossRef]
- Liang, Y.; Qi, J.; Yu, D.; Wang, Z.; Li, W.; Long, F.; Ning, S.; Yuan, M.; Zhong, X. Ferulic Acid Alleviates Lipid and Bile Acid Metabolism Disorders by Targeting FASN and CYP7A1 in Iron Overload-Treated Mice. Antioxidants 2024, 13, 1277. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.N.; Li, X.; Shi, H.M.; Zheng, Y.C.; Chang, S.Q.; Gao, L.; Zhao, G.P. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, J.; Li, J.; Zhang, Y.; Liu, F.; El-Omar, E.; Han, L.; Bian, J.; Gong, L.; et al. Ferulic acid attenuates high-fat diet-induced hypercholesterolemia by activating classic bile acid synthesis pathway. Front. Nutr. 2022, 9, 976638. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, Q.; Zhang, Y.; Liu, F.; Gong, L.; Han, L.; Wang, M. Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet. Nutrients 2022, 14, 2530. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, F.; Xu, L.; Yin, P.; Li, D.; Mei, C.; Jiang, L.; Ma, Y.; Xu, J. Protective Effects of Ferulic Acid against Heat Stress-Induced Intestinal Epithelial Barrier Dysfunction In Vitro and In Vivo. PLoS ONE 2016, 11, e0145236. [Google Scholar] [CrossRef]
- Gu, X.; Wei, M.; Hu, F.; Ouyang, H.; Huang, Z.; Lu, B.; Ji, L. Chlorogenic acid ameliorated non-alcoholic steatohepatitis via alleviating hepatic inflammation initiated by LPS/TLR4/MyD88 signaling pathway. Chem. Biol. Interact. 2023, 376, 110461. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.G.; Fei, Y.Q.; Wang, Y.; Wang, W.Y.; Wang, Z. Chlorogenic Acid Alleviates Colon Mucosal Damage Induced by a High-Fat Diet via Gut Microflora Adjustment to Increase Short-Chain Fatty Acid Accumulation in Rats. Oxid. Med. Cell. Longev. 2021, 2021, 3456542. [Google Scholar] [CrossRef]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS. Front. Immunol. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Yang, T.; Feng, G.; Tan, H.; Piao, X.; Chen, D.; Zhang, Y.; Jiao, W.; Chen, Y.; et al. Chlorogenic Acid Alleviates Chronic Stress-Induced Intestinal Damage by Inhibiting the P38MAPK/NF-κB Pathway. J. Agric. Food Chem. 2023, 71, 9381–9390. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhu, Y.; Liu, Y.; Tao, Y.; Lin, Y.; Lai, S.; Liang, Z.; Chen, Y.; Chen, Y.; Wang, L. Moderation of gut microbiota and bile acid metabolism by chlorogenic acid improves high-fructose-induced salt-sensitive hypertension in mice. Food Funct. 2022, 13, 6987–6999. [Google Scholar] [CrossRef]
- Li, H.; Xi, Y.; Xin, X.; Feng, Q.; Hu, Y. Geniposide plus chlorogenic acid reverses non-alcoholic steatohepatitis via regulation of gut microbiota and bile acid signaling in a mouse model in vivo. Front. Pharmacol. 2023, 14, 1148737. [Google Scholar] [CrossRef] [PubMed]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Moradi Sarabi, M. Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef] [PubMed]
- Sukhithasri, V.; Nisha, N.; Biswas, L.; Anil Kumar, V.; Biswas, R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol. Res. 2013, 168, 396–406. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Kamiya, T.; Kawada, N. Recent updates on the role of the gut-liver axis in the pathogenesis of NAFLD/NASH, HCC, and beyond. Hepatol. Commun. 2023, 7, e0241. [Google Scholar] [CrossRef]
- Fianchi, F.; Liguori, A.; Gasbarrini, A.; Grieco, A.; Miele, L. Nonalcoholic Fatty Liver Disease (NAFLD) as Model of Gut–Liver Axis Interaction: From Pathophysiology to Potential Target of Treatment for Personalized Therapy. Int. J. Mol. Sci. 2021, 22, 6485. [Google Scholar] [CrossRef]
- Zeng, S.; Schnabl, B. Roles for the mycobiome in liver disease. Liver Int. 2022, 42, 729–741. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Lewoń, D.; Cierzniak, A.; Okulus, M.; Gliszczyńska, A. Phospholipid Derivatives of Cinnamic Acid Restore Insulin Sensitivity in Insulin Resistance in 3T3-L1 Adipocytes. Nutrients 2021, 13, 3619. [Google Scholar] [CrossRef]
- Tiegs, G.; Horst, A.K. TNF in the liver: Targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef]
- Crespo, J.; Cayón, A.; Fernández-Gil, P.; Hernández-Guerra, M.; Mayorga, M.; Domínguez-Díez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Kim, H.M.; Jeong, H.J. p-coumaric acid, an active ingredient of Panax ginseng, ameliolates atopic dermatitis-like skin lesions through inhibition of thymic stromal lymphopoietin in mice. J. Ginseng Res. 2021, 45, 176–182. [Google Scholar] [CrossRef]
- Li, Y.H.; Wu, J.X.; He, Q.; Gu, J.; Zhang, L.; Niu, H.Z.; Zhang, X.W.; Zhao, H.T.; Xu, J.Y.; Qin, L.Q. Amelioration of radiation-induced liver damage by p-coumaric acid in mice. Food Sci. Biotechnol. 2022, 31, 1315–1323. [Google Scholar] [CrossRef]
- Cao, B.; Zeng, M.N.; Hao, F.X.; Hao, Z.Y.; Zhang, Z.K.; Liang, X.W.; Wu, Y.Y.; Zhang, Y.H.; Feng, W.S.; Zheng, X.K. P-coumaric acid ameliorates Aβ25–35-induced brain damage in mice by modulating gut microbiota and serum metabolites. Biomed. Pharmacother. 2023, 168, 115825. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Wang, J.; Wang, F.; Mao, J. P-coumaric Acid: Advances in Pharmacological Research Based on Oxidative Stress. Curr. Top. Med. Chem. 2024, 24, 416–436. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef] [PubMed]
- van Dalem, J.; Driessen, J.H.M.; Burden, A.M.; Stehouwer, C.D.A.; Klungel, O.H.; de Vries, F.; Brouwers, M. Thiazolidinediones and Glucagon-Like Peptide-1 Receptor Agonists and the Risk of Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology 2021, 74, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases-Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. The Gut-Vascular Barrier as a New Protagonist in Intestinal and Extraintestinal Diseases. Int. J. Mol. Sci. 2023, 24, 1470. [Google Scholar] [CrossRef]
- Mohr, A.E.; Crawford, M.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: Considering structural variation. FEBS Lett. 2022, 596, 849–875. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural Compounds for Counteracting Nonalcoholic Fatty Liver Disease (NAFLD): Advantages and Limitations of the Suggested Candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.; de Sousa, D.P. Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, J.R.; Rizwanullah, M. Ferulic Acid: A Comprehensive Review. Cureus 2024, 16, e68063. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Bilodeau, S.; Greten, T.F.; Wang, X.W.; Trinchieri, G. The gut-liver axis: Host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 2022, 8, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; AnjomShoa, M.; Rafieian-Kopaei, M. Ferulic acid ameliorates ulcerative colitis in a rat model via the inhibition of two LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways and thus alleviating the inflammatory, oxidative and apoptotic conditions in the colon tissue. Inflammopharmacology 2023, 31, 2587–2597. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; He, L.; Yang, C.; He, J.; Hua, Y.; Guo, J.; Liu, S. Ferulic Acid Alleviates Hepatic Lipid Accumulation and Inflammation by Improving Proximal and Distal Intestinal Barriers in NAFLD Mice. Tohoku J. Exp. Med. 2023, 260, 149–163. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Xing, G.; Meng, L.; Cao, S.; Liu, S.; Wu, J.; Li, Q.; Huang, W.; Zhang, L. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022, 23, e52280. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Zhao, J. Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Yu Cai Lim, M.; Kiat Ho, H. Pharmacological modulation of cholesterol 7α-hydroxylase (CYP7A1) as a therapeutic strategy for hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef] [PubMed]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. BBA Mol. Cell Biol. Lipids 2012, 1821, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure–activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Wei, Z.; Xue, Y.; Xue, Y.; Cheng, J.; Lv, G.; Chu, L.; Ma, Z.; Guan, S. Ferulic acid attenuates non-alcoholic steatohepatitis by reducing oxidative stress and inflammation through inhibition of the ROCK/NF-κB signaling pathways. J. Pharmacol. Sci. 2021, 147, 72–80. [Google Scholar] [CrossRef]
- Nwafor, E.O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y.; et al. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Wang, J.M.; Chen, R.X.; Zhang, L.L.; Ding, N.N.; Liu, C.; Cui, Y.; Cheng, Y.X. In vivo protective effects of chlorogenic acid against triptolide-induced hepatotoxicity and its mechanism. Pharm. Biol. 2018, 56, 626–631. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Henry, Z.; Meadows, V.; Guo, G.L. FXR and NASH: An avenue for tissue-specific regulation. Hepatol. Commun. 2023, 7, e0127. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van Rooijen, K.S.; Magnúsdóttir, S.; Ramos Pittol, J.M.; Willemsen, E.C.L.; de Zoete, M.R.; Baars, M.J.D.; Stege, P.B.; Colliva, C.; Pellicciari, R.; et al. Ablation of liver Fxr results in an increased colonic mucus barrier in mice. JHEP Rep. 2021, 3, 100344. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Meyer-Gerspach, A.C.; Blaser, L.S.; Jeker, L.; Steinert, R.E.; Heim, M.H.; Beglinger, C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS ONE 2014, 9, e87488. [Google Scholar] [CrossRef] [PubMed]
- Demidova, T.Y.; Lobanova, K.G.; Oinotkinova, O.S. Gut microbiota is a factor of risk for obesity and type 2 diabetes. Ter. Arkh. 2020, 92, 97–104. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022, 59, 6834–6856. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Wang, X.; Kim, K.; Bartolome, A.; Dongiovanni, P.; Yates, K.P.; Valenti, L.; Carrer, M.; Sadowski, T.; et al. Hepatocyte TLR4 triggers inter-hepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci. Transl. Med. 2021, 13, eabe1692. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Tan, H.; Zhen, W.; Bai, D.; Liu, K.; He, X.; Ito, K.; Liu, Y.; Li, Y.; Zhang, Y.; Zhang, B.; et al. Effects of dietary chlorogenic acid on intestinal barrier function and the inflammatory response in broilers during lipopolysaccharide-induced immune stress. Poult. Sci. 2023, 102, 102623. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Wu, N.; Li, J.; Xi, N.; Xu, M.; Wu, F.; Fu, Q.; Yan, G.; Liu, Y.; et al. Chlorogenic acid attenuates deoxynivalenol-induced apoptosis and pyroptosis in human keratinocytes via activating Nrf2/HO-1 and inhibiting MAPK/NF-κB/NLRP3 pathways. Biomed. Pharmacother. 2024, 170, 116003. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Luo, Y.; Li, Y.; He, J. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. 2018, 9, 4968–4978. [Google Scholar] [CrossRef]
- Song, L.; Wu, T.; Zhang, L.; Wan, J.; Ruan, Z. Chlorogenic acid improves the intestinal barrier by relieving endoplasmic reticulum stress and inhibiting ROCK/MLCK signaling pathways. Food Funct. 2022, 13, 4562–4575. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Ajiboye, T.O. Protocatechuic acid protects against menadione-induced liver damage by up-regulating nuclear erythroid-related factor 2. Drug Chem. Toxicol. 2020, 43, 567–573. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, S.H. Protocatechuic acid protects hepatocytes against hydrogen peroxide-induced oxidative stress. Curr. Res. Food Sci. 2022, 5, 222–227. [Google Scholar] [CrossRef]
- Sun, R.; Kang, X.; Zhao, Y.; Wang, Z.; Wang, R.; Fu, R.; Li, Y.; Hu, Y.; Wang, Z.; Shan, W.; et al. Sirtuin 3-mediated deacetylation of acyl-CoA synthetase family member 3 by protocatechuic acid attenuates non-alcoholic fatty liver disease. Br. J. Pharmacol. 2020, 177, 4166–4180. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Zhao, Q.; Ding, Y.; Zhang, B.; Kong, L. Protocatechuic acid inhibits proliferation, migration and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 969–976. [Google Scholar] [CrossRef]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef]
- Tan, J.; Hu, R.; Gong, J.; Fang, C.; Li, Y.; Liu, M.; He, Z.; Hou, D.X.; Zhang, H.; He, J.; et al. Protection against Metabolic Associated Fatty Liver Disease by Protocatechuic Acid. Gut Microbes 2023, 15, 2238959. [Google Scholar] [CrossRef] [PubMed]
- Zeinalian Boroujeni, Z.; Khorsandi, L.; Zeidooni, L.; Badiee, M.S.; Khodayar, M.J. Protocatechuic Acid Protects Mice Against Non-Alcoholic Fatty Liver Disease by Attenuating Oxidative Stress and Improving Lipid Profile. Rep. Biochem. Mol. Biol. 2024, 13, 218–230. [Google Scholar] [CrossRef]
- Taqvi, S.; Ahmed Bhat, E.; Sajjad, N.; Sabir, J.S.M.; Qureshi, A.; Rather, I.A.; Rehman, S. Protective effect of vanillic acid in hydrogen peroxide-induced oxidative stress in D.Mel-2 cell line. Saudi J. Biol. Sci. 2021, 28, 1795–1800. [Google Scholar] [CrossRef]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Wu, J.S.; Chen, C.W.; Kuo, P.L.; Chien, H.M.; Wang, Y.T.; Shen, S.C. Protective Effect of Vanillic Acid against Hyperinsulinemia, Hyperglycemia and Hyperlipidemia via Alleviating Hepatic Insulin Resistance and Inflammation in High-Fat Diet (HFD)-Fed Rats. Nutrients 2015, 7, 9946–9959. [Google Scholar] [CrossRef]
- Obydah, W.O.; Shaker, G.A.; Samir, S.M.; El Bassiony, S.F.; Abd El Moneim, H.A. Effect of vanillic acid and exercise training on fatty liver and insulin resistance in rats: Possible role of fibroblast growth factor 21 and autophagy. Physiol. Int. 2021, 108, 412–426. [Google Scholar] [CrossRef]
- Qin, L.; Tan, J.; Lv, X.; Zhang, J. Vanillic acid alleviates liver fibrosis through inhibiting autophagy in hepatic stellate cells via the MIF/CD74 signaling pathway. Biomed. Pharmacother. 2023, 168, 115673. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L.; Feng, G.; Bao, W.; Wang, Y.; Huang, Y.; Chen, T.; Chen, J.; Cao, X.; You, K.; et al. Vanillic acid restores homeostasis of intestinal epithelium in colitis through inhibiting CA9/STIM1-mediated ferroptosis. Pharmacol. Res. 2024, 202, 107128. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Yang, L.; Zhao, W.; Liu, Z.; Wang, E.; Wang, J. Phytochemical gallic acid alleviates nonalcoholic fatty liver disease via AMPK-ACC-PPARa axis through dual regulation of lipid metabolism and mitochondrial function. Phytomedicine 2023, 109, 154589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Q.; Yang, X.; Zhang, Z.; Wang, D.; Hu, D.; Huang, Y.; Sheng, J.; Wang, X. Gallic Acid Can Promote Low-Density Lipoprotein Uptake in HepG2 Cells via Increasing Low-Density Lipoprotein Receptor Accumulation. Molecules 2024, 29, 1999. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Alternat. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Kendrick, A.A.; Choudhury, M.; Rahman, S.M.; McCurdy, C.E.; Friederich, M.; Van Hove, J.L.; Watson, P.A.; Birdsey, N.; Bao, J.; Gius, D.; et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 2011, 433, 505–514. [Google Scholar] [CrossRef]
- Gitzinger, M.; Kemmer, C.; Fluri, D.A.; El-Baba, M.D.; Weber, W.; Fussenegger, M. The food additive vanillic acid controls transgene expression in mammalian cells and mice. Nucleic Acids Res. 2012, 40, e37. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ(1–42)-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Shekari, S.; Khonsha, F.; Rahmati-Yamchi, M.; Nejabati, H.R.; Mota, A. Vanillic Acid and Non-Alcoholic Fatty Liver Disease: A Focus on AMPK in Adipose and Liver Tissues. Curr. Pharm. Des. 2021, 27, 4686–4692. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Espinosa, A.; Rodríguez, A.; Barriga, A.; Palomino-Calderón, A.; Romero, N.; Troncoso, R.H.; Aubourg, S.P. Effect of Structured Phenolic Lipids with EPA/DHA and Gallic Acid against Metabolic-Associated Fatty Liver Disease (MAFLD) in Mice. Molecules 2022, 27, 7702. [Google Scholar] [CrossRef]
- Chao, J.; Cheng, H.Y.; Chang, M.L.; Huang, S.S.; Liao, J.W.; Cheng, Y.C.; Peng, W.H.; Pao, L.H. Gallic Acid Ameliorated Impaired Lipid Homeostasis in a Mouse Model of High-Fat Diet-and Streptozotocin-Induced NAFLD and Diabetes through Improvement of β-oxidation and Ketogenesis. Front. Pharmacol. 2020, 11, 606759. [Google Scholar] [CrossRef]
- Chao, J.; Huo, T.I.; Cheng, H.Y.; Tsai, J.C.; Liao, J.W.; Lee, M.S.; Qin, X.M.; Hsieh, M.T.; Pao, L.H.; Peng, W.H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.B.; Choi, C.S.; Samuel, V.T.; Liu, Z.-X.; Zhang, D.; Wang, A.; Zhang, X.-M.; Cline, G.W.; Yu, X.X.; Geisler, J.G.; et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Investig. 2006, 116, 817–824. [Google Scholar] [CrossRef]
- Gang, X.; Kaixun, H.; Jun, Z. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef]
- Choung, S.; Kim, J.M.; Joung, K.H.; Lee, E.S.; Kim, H.J.; Ku, B.J. Epidermal growth factor receptor inhibition attenuates non-alcoholic fatty liver disease in diet-induced obese mice. PLoS ONE 2019, 14, e0210828. [Google Scholar] [CrossRef]
- Sun, D.Q.; Liu, W.Y.; Wu, S.J.; Zhu, G.Q.; Braddock, M.; Zhang, D.C.; Shi, K.Q.; Song, D.; Zheng, M.H. Increased levels of low-density lipoprotein cholesterol within the normal range as a risk factor for nonalcoholic fatty liver disease. Oncotarget 2016, 7, 5728–5737. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, H.; Mard, S.A.; Sarkaki, A.; Goudarzi, G.; Khorsandi, L. Gallic acid protects the liver against NAFLD induced by dust exposure and high-fat diet through inhibiting oxidative stress and repressing the inflammatory signaling pathways NF-kβ/TNF-α/IL-6 in Wistar rats. Avicenna J. Phytomed. 2021, 11, 527–540. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Bioavailability of hydroxycinnamates: A brief review of in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- Konishi, Y.; Hitomi, Y.; Yoshioka, E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J. Agric. Food Chem. 2004, 52, 2527–2532. [Google Scholar] [CrossRef]
- Konishi, Y.; Hitomi, Y.; Yoshida, M.; Yoshioka, E. Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. J. Agric. Food Chem. 2005, 53, 4740–4746. [Google Scholar] [CrossRef]
- Islam, M.; Shehzadi, N.; Salman, M.; Zahid, F.; Khan, H.M.; Amjad, S.; Khan, M.T.; Danish, M.Z.; Bukhari, N.I.; Hussain, K. Pharmacokinetics of caffeic acid from methanol seed extract of Syzygium cumini L in rats. Trop. J. Pharm. Res. 2016, 15, 363–369. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, W.; Zhang, Y.; Wang, Q.; Qin, C.; Du, S.; Huang, L.; Ye, F.; Chen, L.; Zheng, T. Pharmacokinetics, Bioavailability, and Tissue Distribution Study of Angoroside C and Its Metabolite Ferulic Acid in Rat Using UPLC-MS/MS. Front. Pharmacol. 2018, 9, 1186. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Santiago-Osorio, E.; Sánchez-Flores, N.; Salazar-Aguilar, S.; Soto-Hernández, R.M.; Riviello-Flores, M.L.; Macías-Zaragoza, V.M.; Aguiñiga-Sánchez, I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules 2024, 29, 1439. [Google Scholar] [CrossRef]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of microbial metabolites of dietary polyphenols in rats: Is the brain their target destination? ACS Chem. Neurosci. 2015, 6, 1341–1352. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef]
- Sayavongsa, P.; Cooper, M.L.; Jackson, E.M.; Harris, L.; Ziegler, T.R.; Hibbert, J.M. Vanillic acid excretion can be used to assess compliance with dietary supplements. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2007, 2, e134–e137. [Google Scholar] [CrossRef][Green Version]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Kishida, K.; Matsumoto, H. Urinary excretion rate and bioavailability of chlorogenic acid, caffeic acid, p-coumaric acid, and ferulic acid in non-fasted rats maintained under physiological conditions. Heliyon 2019, 5, e02708. [Google Scholar] [CrossRef]
- Wang, S.J.; Zeng, J.; Yang, B.K.; Zhong, Y.M. Bioavailability of caffeic acid in rats and its absorption properties in the Caco-2 cell model. Pharm. Biol. 2014, 52, 1150–1157. [Google Scholar] [CrossRef]
- Lira, A.B.; Montenegro, C.A.; de Oliveira, K.M.; de Oliveira Filho, A.A.; da Paz, A.R.; de Araújo, M.O.; de Sousa, D.P.; de Almeida, C.L.F.; da Silva, T.G.; Lima, C.; et al. Isopropyl Caffeate: A Caffeic Acid Derivative-Antioxidant Potential and Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 6179427. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.A. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J. Appl. Toxicol. 1984, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Choudhari, A.S.; Pandita, S.; Islam, M.A.; Raina, P.; Kaul-Ghanekar, R. Cinnamaldehyde, Cinnamic Acid, and Cinnamyl Alcohol, the Bioactives of Cinnamomum cassia Exhibit HDAC8 Inhibitory Activity: An In vitro and In silico Study. Pharmacogn. Mag. 2017, 13 (Suppl. 3), S645–S651. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Cheon, S.S.; Khan, S.; O’Brien, P.J. Acute toxicity of caffeic acid in mice and rats. Toxicol. Lett. 1994, 70, 1–8. [Google Scholar]
- Liu, Y.; Qiu, S.; Wang, L.; Zhang, N.; Shi, Y.; Zhou, H.; Liu, X.; Shao, L.; Liu, X.; Chen, J.; et al. Reproductive and developmental toxicity study of caffeic acid in mice. Food Chem. Toxicol. 2019, 123, 106–112. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-x.; Guo, S.-d.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Peng, C.-C.; Hsieh, C.-L.; Wang, H.-E.; Chung, J.-Y.; Chen, K.-C.; Peng, R.Y. Ferulic acid is nephrodamaging while gallic acid is renal protective in long term treatment of chronic kidney disease. Clin. Nutr. 2012, 31, 405–414. [Google Scholar] [CrossRef]
- Liu, B.; Cao, L.; Zhang, L.; Yuan, X.; Zhao, B. Preparation, Phytochemical Investigation, and Safety Evaluation of Chlorogenic Acid Products from Eupatorium adenophorum. Molecules 2016, 22, 67. [Google Scholar] [CrossRef]
- Amano, Y.; Honda, H.; Nukada, Y.; Ikeda, N.; Yamane, M.; Nakano, K.; Kameyama, A.; Morita, O. Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays. Pharmaceuticals 2019, 12, 110. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.C.; Panchal, S.S. Safety Assessment of Vanillic Acid: Subacute Oral Toxicity Studies in Wistar Rats. Turk. J. Pharm. Sci. 2020, 17, 432–439. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Truzzi, F.; Valerii, M.C.; Tibaldi, C.; Zhang, Y.; Abduazizova, V.; Spisni, E.; Dinelli, G. Are Supplements Safe? Effects of Gallic and Ferulic Acids on In Vitro Cell Models. Nutrients 2020, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7268. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Z.; Zhang, L.; Li, X.; Xu, B.; Xiao, Y.; Shi, X.; Zhang, H.; Liao, T.; Wang, P. Vanillic Acid Reduces Pain-Related Behavior in Knee Osteoarthritis Rats Through the Inhibition of NLRP3 Inflammasome-Related Synovitis. Front. Pharmacol. 2020, 11, 599022. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]








| Agent (Trial Name) | Primary Mechanism | Most Frequent Adverse Events | Trial Number | Ref. |
|---|---|---|---|---|
| Silymarin | Antioxidant | No obvious adverse events | NCT02006498 | [30] |
| Fucoxanthin | Bioactive dietary supplement | No obvious adverse events | NCT02875392 | [31] |
| Aspirin | Cox inhibitor | Upper respiratory infection, gastrointestinal disorders | NCT04031729 | |
| Obeticholic Acid | FXR agonist | Pruritus, fatigue, gastrointestinal disorders | NCT02633956 | [32] |
| EDP-305 | FXR agonist | Pruritus, fatigue | NCT03421431 | [33] |
| EYP001a | FXR agonist | Pruritus, fatigue | NCT03812029 | [34] |
| Pitavastatin | HMG-CoA reductase inhibitor | The risk of diabetes, gastrointestinal disorders | NCT02290106 | [35] |
| Vitamin E | Lipid-soluble antioxidant | Iron deficiency or anemia | NCT01792115 | [36] |
| Saroglitazar Magnesium | PPAR α/δ Agonist | Pruritus | NCT03863574 | [37] |
| Metformin | Suppresses HGP | Weight loss | NCT00063232 | [38] |
| HCAs | Structure | Effects | Model | Dose | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|
| Cinnamic acid (C9H8O2) |  | Anti-inflammatory | HFD-induced hyperlipidemia in rats | 30 mg/kg PO for 49 days | ↓ Serum leptin, pancreatic lipase | [51] |
| HFFD-induced MASLD in rats | 10, 20, 40 mg/kg PO for 70 days | ↓ TNF-α | [52] | |||
| Hepatoprotection | HFD-induced hyperlipidemia in rats | 30 mg/kg PO for 49 days | ↓ TC, TG, LDL-C | [51] | ||
| OA-induced HepG2 | 12.5, 25, 50, 100, 200 μM for 24 h | ↓ TG, FFA, IHTG | [53] | |||
| db/db mice | 20 mg/kg PO for 28 days | |||||
| db/db mice | 20 mg/kg PO for 28 days | ↑ ATP1B1, LDLR, 5-HT ↓ TG, FFA | [54] | |||
| HFD-induced MASLD in rats | 23 g/kg PO for 56 days | ↑ MDR1, NTCP, OATP1A2, OCT1, MATE1 ↓ TC, HDL-C | [55] | |||
| Intestinal barrier integrity | Loperamide-induced STC in mice | 40, 80 mg/kg PO for 28 days | ↑ Firmicutes, SCFAs, goblet cells | [56] | ||
| p-coumaric acid (C9H8O3) | 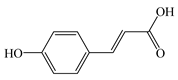 | Antioxidant | APAP-induced hepatotoxicity in mice | 50 mg/kg PO for 7 days | ↓ ROS | [57] |
| Anti-inflammatory | APAP-induced hepatotoxicity in mice | 50 mg/kg PO for 7 days | ↓ MAPK, NF-κB, TNF-α, IL-1β, IL-6 | [57] | ||
| Dust + LPT rats and Dust + IR rats | 100 mg/kg IP for 42 days | ↓ NF-κB, TNF-α, IL-1β, IL-6 | [58] | |||
| HFD-induced MASLD in rats | 200 mg/kg PO for 70 days | ↓ NF-κB, TNF-α | [59] | |||
| HFHS-induced MASLD in mice | 50 mg/kg PO for 84 days | ↓ TNF-α, IL-6, IL-8, insulin, adiponectin, serum leptin | [60] | |||
| Hepatoprotection | Dust + LPT rats and Dust + IR rats | 100 mg/kg IP for 42 days | ↓ TC, TG, LDL-C, HDL-C | [58] | ||
| HFD-induced MASLD in rats | 200 mg/kg PO for 70 days | ↓ TG | [59] | |||
| Triton WR1339-induced hyperlipidemia HepG2 | 10 μg/mL | ↑ AMPK, PPARα/γ ↓ TC, TG | [61] | |||
| Triton WR1339-induced hyperlipidemia in mice | 100 mg/kg PO | |||||
| Caffeic acid (C9H8O4) | 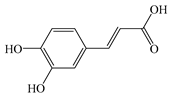 | Anti-inflammatory | DSS-induced colitis in mice | 179 mg/kg PO 14 days | ↑ IL-4, CYP4B1 ↓ L-17, iNOS | [62] |
| DSS-induced colitis in mice | 1 mM PO for 23 days | ↓ NF-κB, IL-6, TNF-α, IFNγ | [63] | |||
| Hepatoprotection | Alloxan-induced type 1 diabetic in mice | 50 mg IP for 7 days | ↓ TC, TG, LDL-C, HDL-C | [64] | ||
| Ferulic acid (C10H10O4) |  | Antioxidant | APAP-induced inflammation in mice | 10, 30, 100 mg/kg PO 3 times | ↓ SOD, CAT | [65] |
| HCHF-induced metabolic syndrome in rats | 30, 60 mg/kg PO 42 days | ↑ HO-1, HSP70, Akt ↓ COX-2 | [66] | |||
| Iron-induced mice | 50 mg/kg PO 16 days | ↓ ROS | [67] | |||
| Anti-inflammatory | APAP-induced inflammation in mice | 10, 30, 100 mg/kg PO 3 times | ↓ p38MAPK, NF-κB, TLR4, IL-1β | [65] | ||
| CCI-induced mice | 100 mg/kg PO 21 days | ↓ TLR4, NF-κB, IL-1β, IL-6 | [68] | |||
| HFD-induced mice | 100 mg/kg PO 84 days | ↓ NF-κB, TLR4 | [69] | |||
| Hepatoprotection | APAP-induced inflammation in mice | 10, 30, 100 mg/kg PO 3 times | ↓ CYP2E1 | [65] | ||
| HFD-induced mice | 100 mg/kg PO 84 days | ↑ SCFAs ↓ LPS | [69] | |||
| HFD-induced mice | 100 mg/kg PO 84 days | ↑ CYP7A1, BAs ↓ TC | [70] | |||
| Iron-induced mice | 50 mg/kg PO 16 days | ↓ FASN, CYP7A1, TC, BAs | [67] | |||
| HFD-induced mice | 100 mg/kg PO 84 days | ↑ PPARα | [71] | |||
| Intestinal barrier integrity | HS-induced IEC-6 cells | 1, 5, 10, 20, 50, 100, 200, 500 μM for 48 h | ↑ ZO-1, occludi, E-cadherin | [72] | ||
| HS-induced rats | 50 mg/kg PO 7 days | |||||
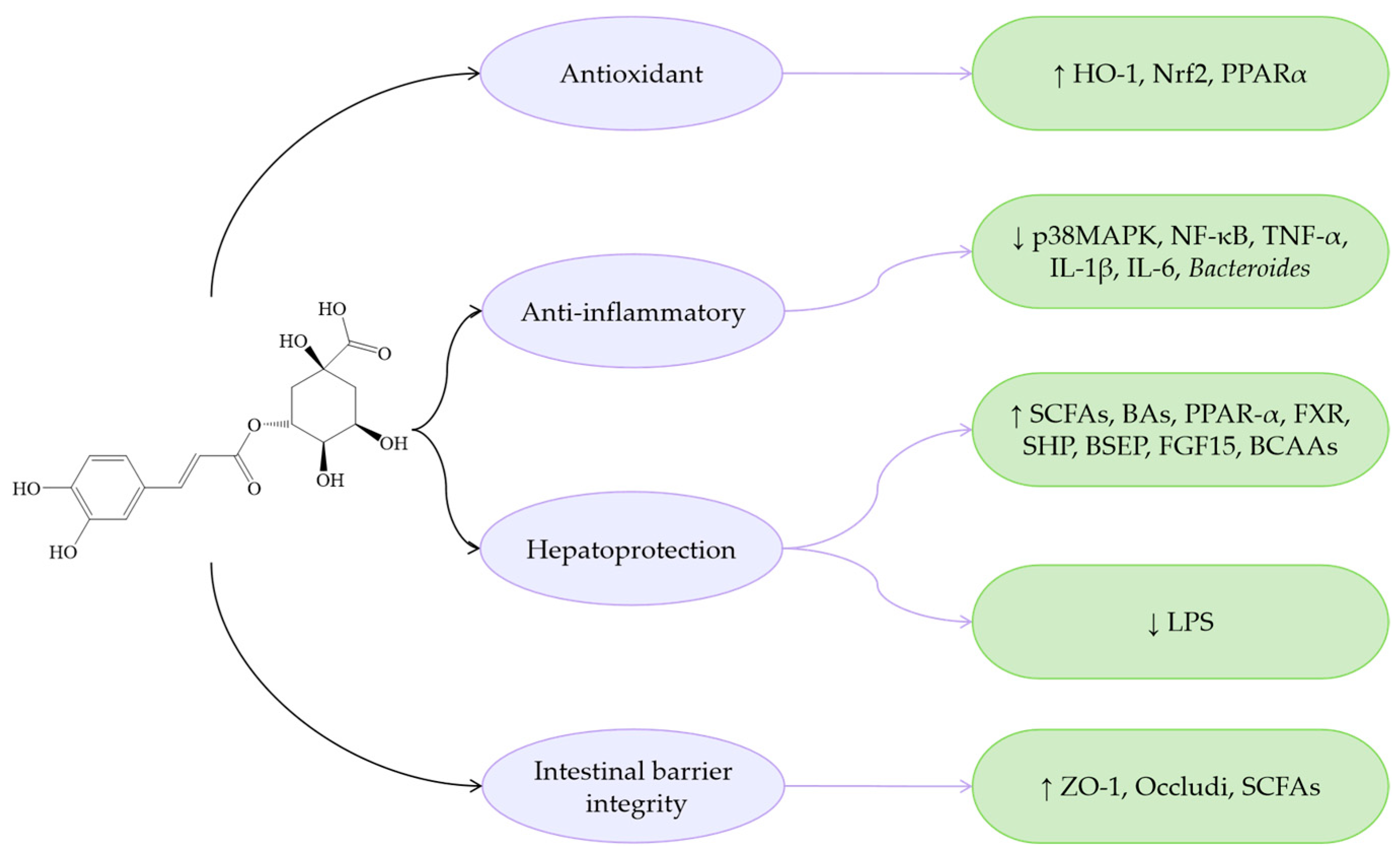
| Chlorogenic acid (C16H18O9) | 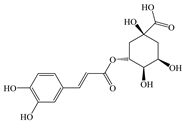 | Antioxidant | MCDD-induced MASLD in mice | 30, 60 mg/kg PO 28 days | ↑ Nrf2, PPARα | [73] |
| Weaned piglets | 1000 mg/kg | ↑ Nrf2, HO-1 | [74] | |||
| Anti-inflammatory | HFD-induced colitis in mice | 100 mg/kg PO 105 days | ↓ NF-κB, TLR4 | [75] | ||
| LPS-induced inflammation in mice | 12.5, 25, 50 mg/kg IP | ↓ NF-κB, TNF-α, IL-1β, IL-6 | [76] | |||
| Indomethacin-induced mice | 50 mg/kg PO 12 days | ↓ Bacteroides, LPS | [77] | |||
| MCDD-induced MASLD in mice | 30, 60 mg/kg PO 28 days | ↓ TLR4 | [73] | |||
| Weaned piglets | 1000 mg/kg | ↓ NF-κB, TLR4, IL-1β, IL-6 | [74] | |||
| Chronic stress-induced rats | 100 mg/kg PO | ↓ p38MAPK, NF-κB | [78] | |||
| HUA-induced mice | 30, 60 mg/kg PO 19 days | ↓ IL-1β, IL-6 | [79] | |||
| Hepatoprotection | HFS-induced mice | 50, 100, 200 mg/kg PO | ↑ FXR, BAs | [80] | ||
| HFD-induced MASLD in mice | 1.34 mg/kg PO 28 days | ↑ FXR, SHP, BSEP, FGF15 | [81] | |||
| HFD-induced colitis in mice | 100 mg/kg PO 105 days | ↑ PPAR-α, BCAAs, SCFAs ↓ LPS | [75] | |||
| MCDD-induced MASLD in mice | 30, 60 mg/kg PO 28 days | ↑ PPARα ↓ LPS | [73] | |||
| Intestinal barrier integrity | HUA-induced mice | 30, 60 mg/kg PO 19 days | ↑ ZO-1, occludi, SCFAs | [79] |
| HBAs | Structure | Effects | Model | Dose | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|
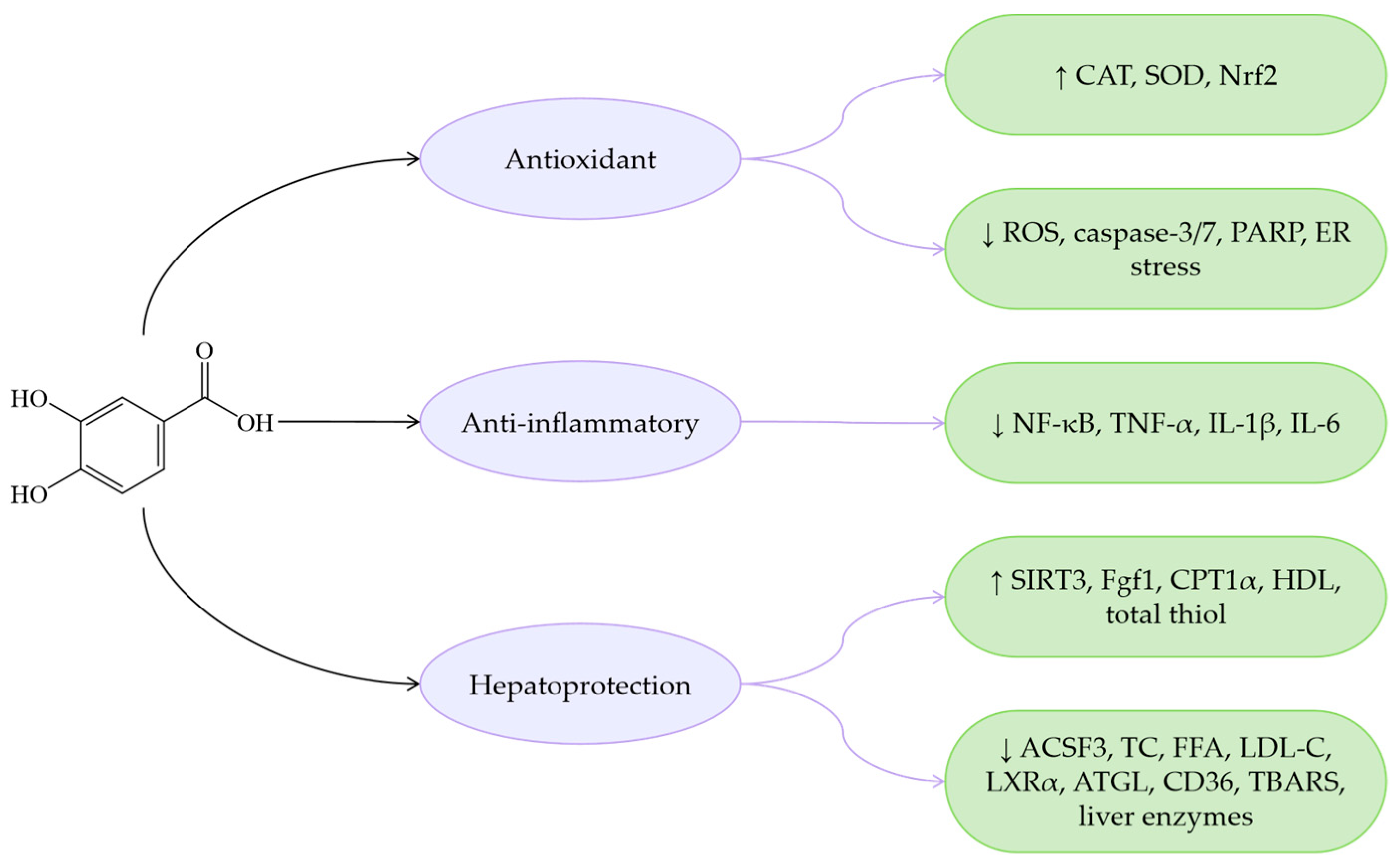
| Protocatechuic acid (C7H6O4) | 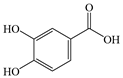 | Antioxidant | Menadione-induced hepatotoxicity in rats | 10, 20 mg/kg PO 7 days | ↑ CAT, SOD, Nrf2 | [150] |
| HepG2 | 0, 25, 50 μM for 24 h | ↓ ROS, caspase-3/7, PARP, ER stress | [151] | |||
| HFD-induced MASLD in mice | 30 mg/kg PO 56 days | ↓ ROS | [152] | |||
| HFD-induced MASLD in rats | 10, 20 mg/kg PO 56 days | |||||
| Anti-inflammatory | Synovial membrane tissues | 5, 10, 20, 40 μM for 24, 48 h | ↓ NF-κB, TNF-α, IL-1β, IL-6 | [153] | ||
| Hepatoprotection | HFD-induced MASLD in mice | 30 mg/kg PO 56 days | ↑ SIRT3 ↓ ACSF3 | [152] | ||
| HFD-induced MASLD in rats | 10, 20 mg/kg PO 56 days | |||||
| HFD-induced MASLD in mice | 0.25, 0.5, 1 g/kg RRBE PO 84 days | ↓ TC, FFA, LDL-C, LXRα, ATGL, CD36 | [154] | |||
| HFD-induced MASLD in mice | HFD containing 0.4% of PCA for 84 days | ↑ FGF1, CPT1α ↓ Enterococcus faecalis | [155] | |||
| HFD-induced MASLD in mice | HFD containing 200 mg/kg/20 mL of PCA for 42 days | ↑ HDL, total thiol ↓ TC, TBARS, liver enzymes | [156] | |||
| Vanillic acid (C8H8O4) |  | Antioxidant | H2O2-induced D.Mel-2 cell | 125, 250 μg/mL 24 h | ↑ CAT, SOD ↓ ROS | [157] |
| Anti-inflammatory | IL-1β, TNF-α-induced chondrocytes | 1 μM 72 h | ↓ NF-κB, IL-1β, TNF-α | [158] | ||
| Hepatoprotection | FL83B | 20 μL 6.25 ng/mL | ↑ PI3K, GLUT-2, phosphorylated ACC | [159] | ||
| HFD-induced mice | 30 mg/kg PO 28 days | |||||
| HFD-induced MASLD in rats | 50 mg/kg PO 56 days | ↑ FGF21 ↓ TC, TG, P62 | [160] | |||
| CCl4 and olive oil-induced liver fibrosis in rats | 5, 20 mg/kg PO 56 days | ↓ MIF, CD74, LC3B | [161] | |||
| Intestinal barrier integrity | Weaned piglets | 4000 mg/kg PO 21 days | ↑ Oclaudin, Firmicutes/Bacteroidetes ratio ↓ LPS | [162] | ||
| DSS-induced colitis in mice | 12.5, 25, 50 mg/kg PO 7 days | ↑ SREBP1, SCD1 | [163] | |||
| Gallic acid (C7H6O5) |  | Anti-inflammatory | IL-1β-induced HIEC-6 | 20, 40, 60 mg/mL 48 h | ↑ IL-4, IL-10 | [164] |
| TNBS-induced colitis in mice | 20, 40, 60 mg/kg PO 7 days | ↓ TGF-β, TNF-α, IL-1, IL-6, IL-12 | ||||
| Hepatoprotection | PA-induced HepG2, Hepa 1-6, RAW 264 | 50, 200 μM 24 h | ↑ AMPK ↓ SREBP-1c, LXRα, CD36, FATP2, ACCα, caspase 3/7 | [165] | ||
| OA/PA-induced HepG2, SMMC-7721 | 20 μM 24 h | ↑ AMPK, PPARα | [166] | |||
| HepG2 | 0, 10, 20, 40 μM 24 h | ↑ EGFR-ERK1/2, LDLR | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Shen, C.; Di, H.; Wang, Y.; Guan, F. Regulatory Mechanisms of Phenolic Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Antioxidants 2025, 14, 760. https://doi.org/10.3390/antiox14070760
Zhang S, Shen C, Di H, Wang Y, Guan F. Regulatory Mechanisms of Phenolic Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Antioxidants. 2025; 14(7):760. https://doi.org/10.3390/antiox14070760
Chicago/Turabian StyleZhang, Shengyu, Congcong Shen, Han Di, Yanhong Wang, and Feng Guan. 2025. "Regulatory Mechanisms of Phenolic Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review" Antioxidants 14, no. 7: 760. https://doi.org/10.3390/antiox14070760
APA StyleZhang, S., Shen, C., Di, H., Wang, Y., & Guan, F. (2025). Regulatory Mechanisms of Phenolic Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Antioxidants, 14(7), 760. https://doi.org/10.3390/antiox14070760






