Impact of a Formulation Containing Chaga Extract, Coenzyme Q10, and Alpha-Lipoic Acid on Mitochondrial Dysfunction and Oxidative Stress: NMR Metabolomic Insights into Cellular Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Standards
2.2. Formulation of RP-25
2.3. UHPLC-HRMS/MS Analysis of RP-25 Formulation
2.4. Coenzyme Q10 Analysis
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Exposure of Cells to RP-25
2.8. Measurement of Mitochondrial Membrane Potential
2.9. Measurement of Intracellular ROS Production
2.10. Sample Extraction
2.11. NMR Spectra Acquisition
2.12. Statistical Analysis
3. Results
3.1. UHPLC-HRMS/MS Analysis
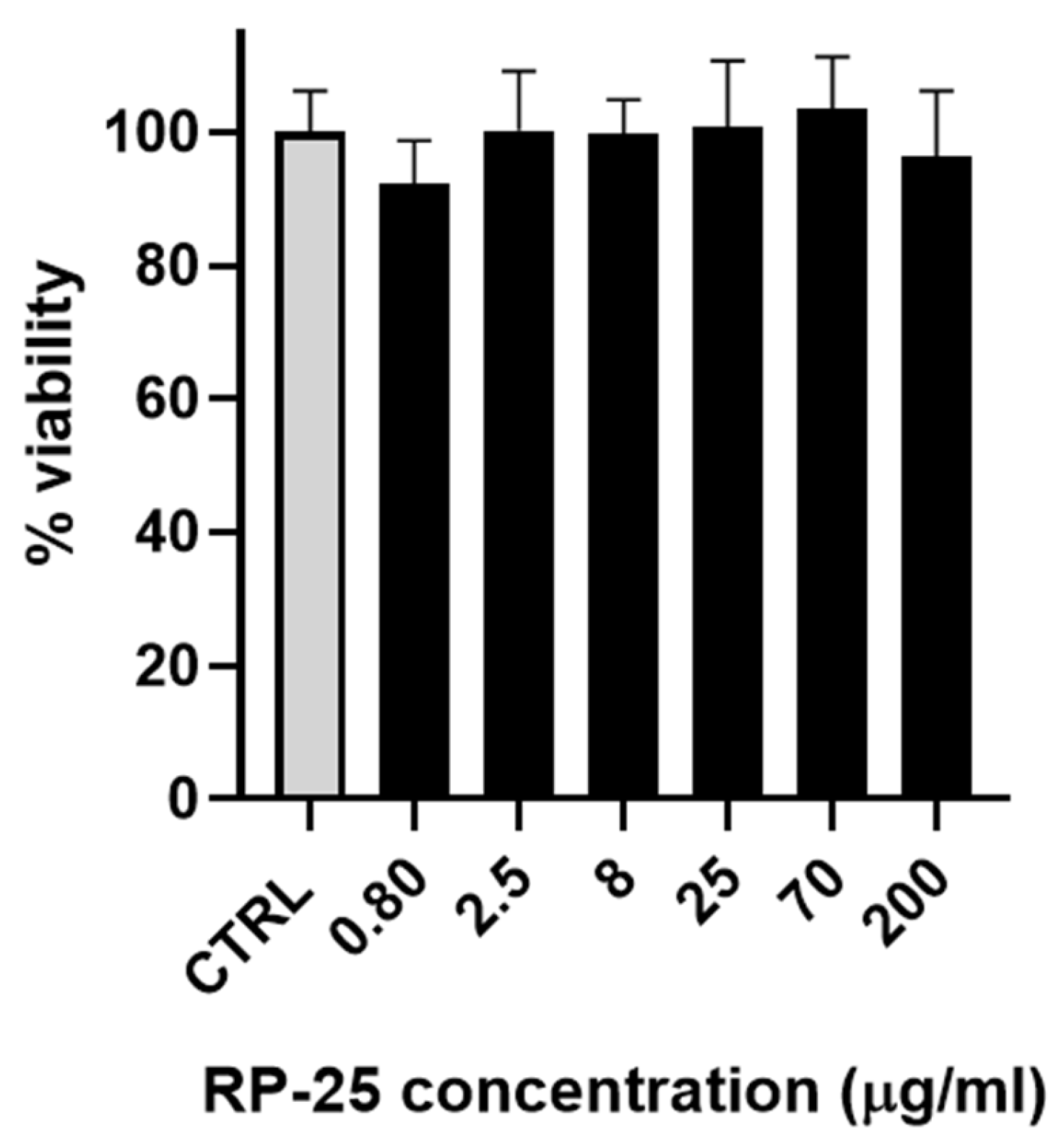
3.2. Cell Viability
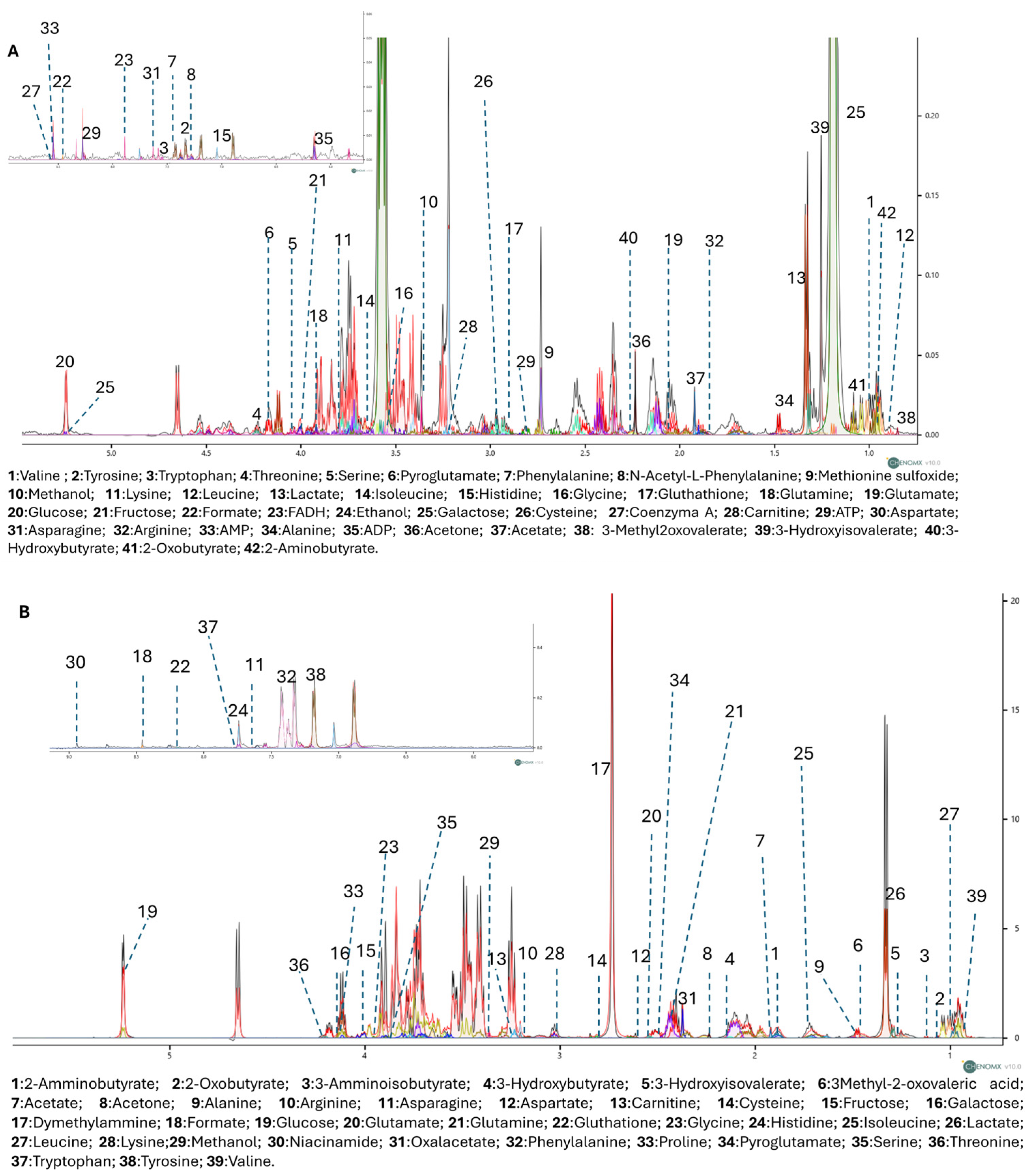
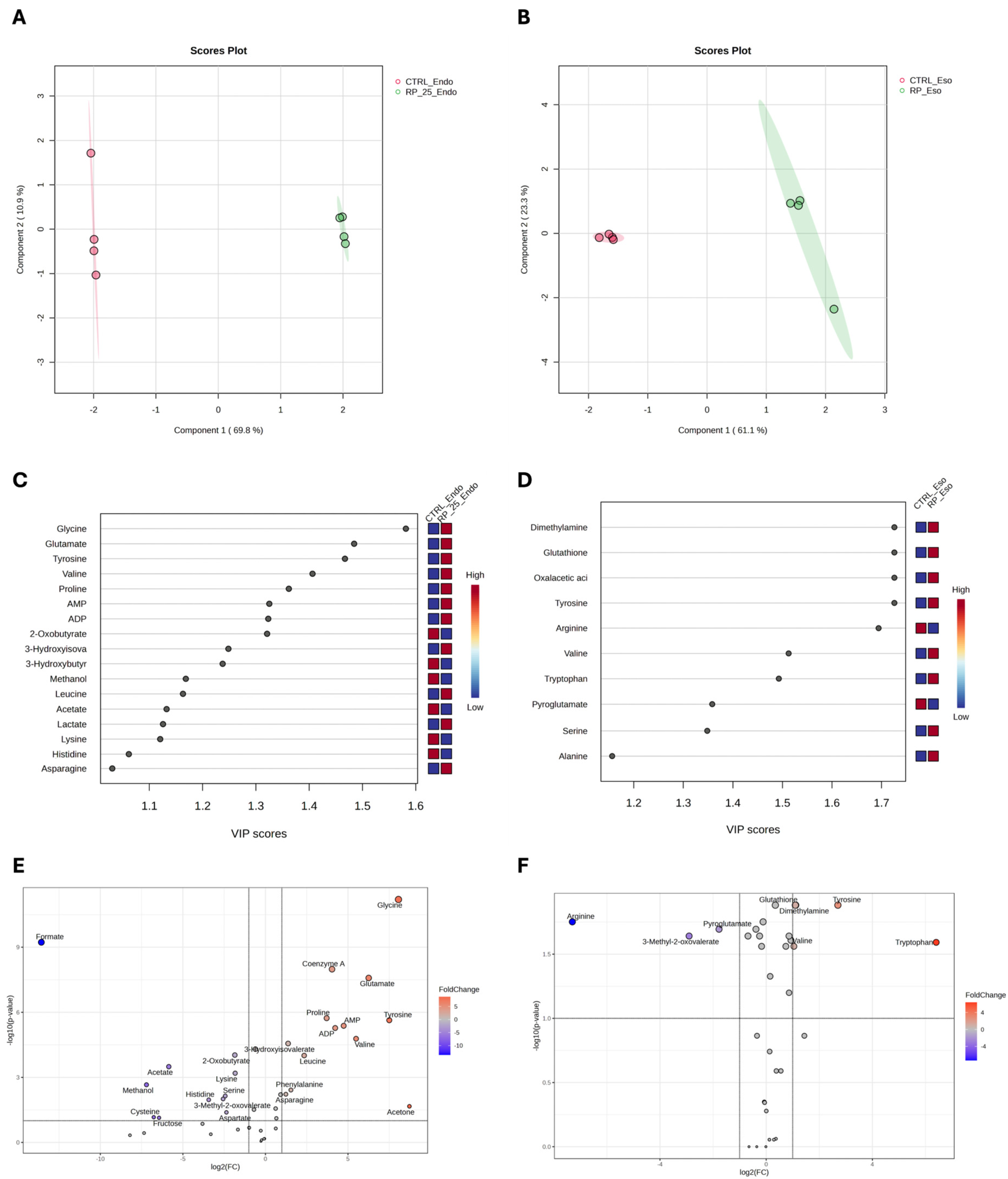
3.3. Untargeted 1H-NMR Metabolomics Analysis
3.4. Enrichment Pathway Analysis
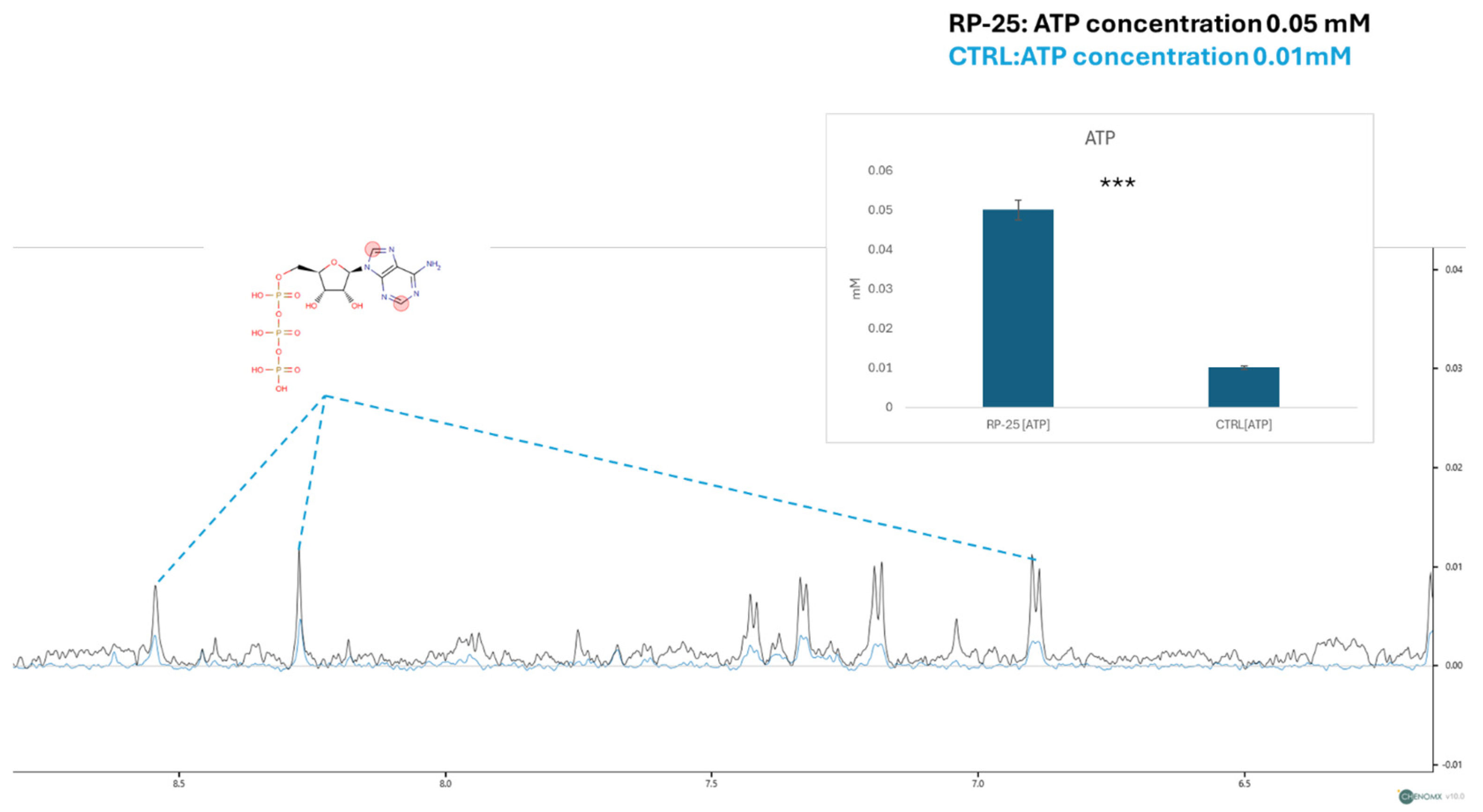
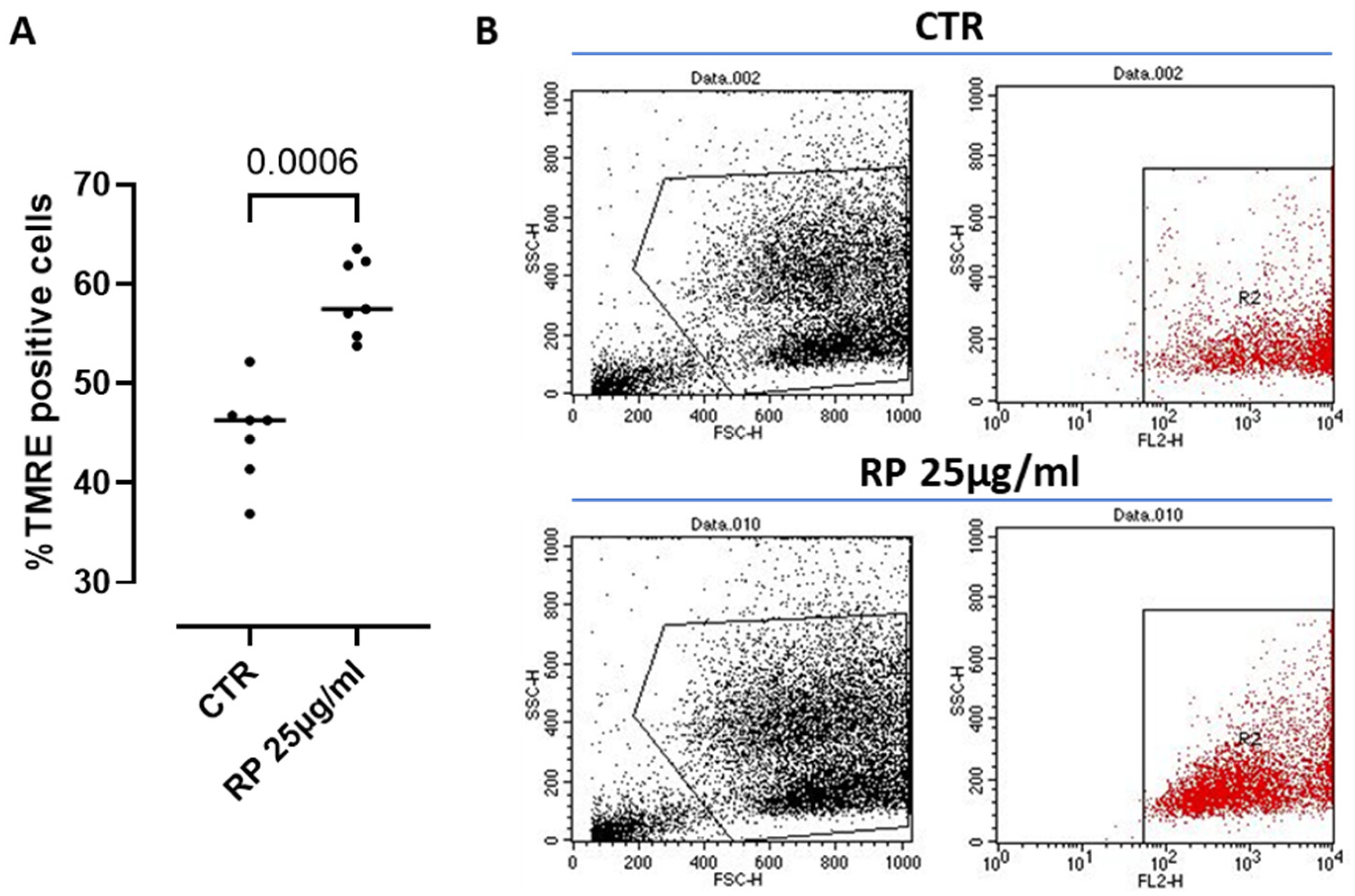
3.5. ATP Concentration and Mitochondrial Membrane Potential Analysis
4. Discussion
4.1. RP-25 Formulation
4.2. Impact on Mitochondrial Function and Energy Metabolism
4.3. Amino Acid Metabolism
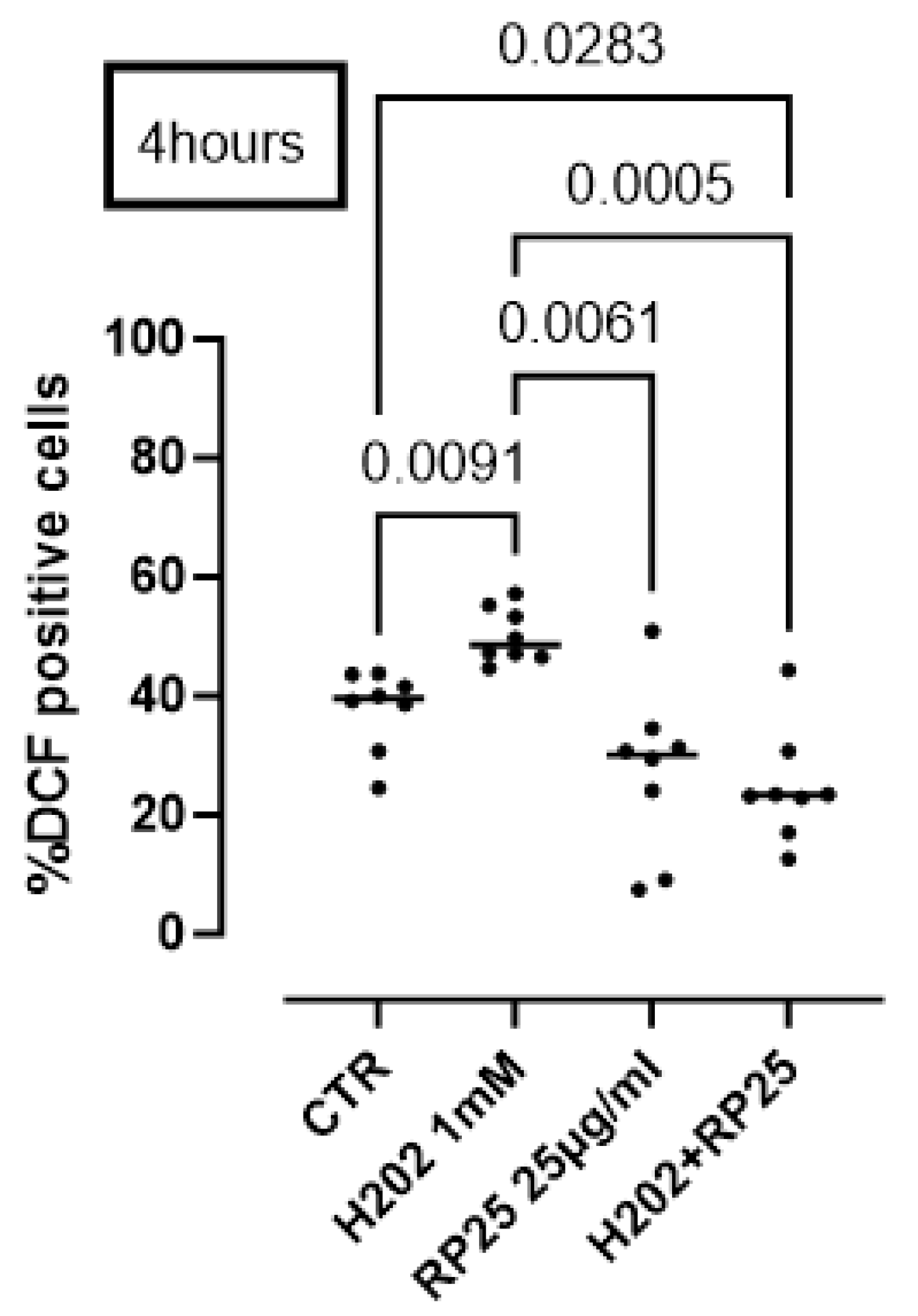
4.4. Oxidative Stress and Redox Balance
4.5. Potential Therapeutic Implications for Mitochondrial Diseases and Fibromyalgia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2024, 21, e00292. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Jain, S.M.; Blot-Chabaud, M.; Pathak, S.; Banerjee, A.; Rawat, S.; Sharma, N.R.; Duttaroy, A.K. Mitochondrial dysfunction and its association with age-related disorders. Front. Physiol. 2024, 15, 1384966. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, R.; Zhang, Y.; Zhao, Y.; Liu, Q.; Wang, J.; Yan, X.; Su, J. Attenuating mitochondrial dysfunction-derived reactive oxygen species and reducing inflammation: The potential of Daphnetin in the viral pneumonia crisis. Front. Pharmacol. 2024, 15, 1477680. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Mishra, V.K.; Munalisa, R.; Parveen, F.; Ali, S.F.; Akter, K.; Ahmed, T.; Ho, T.-J.; Huang, C.Y. Mechanistic insight of mitochondrial dysfunctions in cardiovascular diseases with potential biomarkers. Mol. Cell. Toxicol. 2024, 20, 441–463. [Google Scholar] [CrossRef]
- Marino, Y.; Inferrera, F.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Role of mitochondrial dysfunction and biogenesis in fibromyalgia syndrome: Molecular mechanism in central nervous system. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2024, 1870, 167301. [Google Scholar] [CrossRef]
- Inferrera, F.; Marino, Y.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Impaired mitochondrial quality control in fibromyalgia: Mechanisms involved in skeletal muscle alteration. Arch. Biochem. Bio. Phys. 2024, 758, 110083. [Google Scholar] [CrossRef]
- Macchi, C.; Giachi, A.; Fichtner, I.; Pedretti, S.; Puttini, P.S.; Mitro, N.; Corsini, A.; Ruscica, M.; Gualtierotti, R. Mitochondrial function in patients affected with fibromyalgia syndrome is impaired and correlates with disease severity. Sci. Rep. 2024, 14, 30247. [Google Scholar] [CrossRef]
- Castaldo, G.; Marino, C.; Atteno, M.; D’Elia, M.; Pagano, I.; Grimaldi, M.; Conte, A.; Molettieri, P.; Santoro, A.; Napolitano, E.; et al. Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment. Nutrients 2024, 16, 1620. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P.; Domingo, J.C.; Castro-Marrero, J. Mitochondrial dysfunction and coenzyme Q10 supplementation in post-viral fatigue syndrome: An overview. Int. J. Mol. Sci. 2024, 25, 574. [Google Scholar] [CrossRef]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-lipoic acid: An antioxidant with anti-aging properties for disease therapy. Curr. Med. Chem. 2025, 32, 23–54. [Google Scholar] [CrossRef]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2024, 15, 144–161. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, M.; Marino, C.; Celano, R.; Napolitano, E.; D’Ursi, A.M.; Russo, M.; Rastrelli, L. Impact of a Withania somnifera and Bacopa monnieri Formulation on SH-SY5Y Human Neuroblastoma Cells Metabolism Through NMR Metabolomic. Nutrients 2024, 16, 4096. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.; Colarusso, C.; Popolo, A.; Pinto, A.; Sorrentino, R. IL-1α and IL-1β-producing macrophages populate lung tumor lesions in mice. Oncotarget 2016, 7, 58181. [Google Scholar] [CrossRef]
- Terlizzi, M.; Colarusso, C.; Di Maio, U.; Bagnulo, A.; Pinto, A.; Sorrentino, R. Antioxidant and antimicrobial properties of Pelargonium sidoides DC and lactoferrin combination. Biosci. Rep. 2020, 40, BSR20203284. [Google Scholar] [CrossRef]
- Jing, X.; Yu, F.; Lin, W. Monitoring cysteine level changes under LPS or H2O2 induced oxidative stress using a polymer-based ratiometric fluorescent probe. Anal. Chim. Acta 2021, 1174, 338738. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692. [Google Scholar] [CrossRef]
- McKay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts Magn. Reson. Part A 2011, 38A, 197–220. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated 460 to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 10–14. [Google Scholar] [CrossRef]
- Kumar, N.; Hoque, M.A.; Sugimoto, M. Robust volcano plot: Identification of differential metabolites in the presence of 464 outliers. BMC Bioinform. 2018, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for 466 metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, gkae253. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shahidi, F. Qualitative analysis of secondary metabolites of chaga mushroom (Inonotus obliquus): Phenolics, fatty acids, and terpenoids. J. Food Bioact. 2022, 17, 56–72. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of pH and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (Inonotus obliquus) mushroom. Antioxidants 2021, 10, 1322. [Google Scholar] [CrossRef]
- Liao, Z.; Alrosan, M.; Alu’datt, M.H.; Tan, T. C 10-hydroxy decanoic acid, trans-10-hydroxy-2-decanoic acid, and sebacic acid: Source, metabolism, and potential health functionalities and nutraceutical applications. J. Food Sci. 2024, 89, 3878–3893. [Google Scholar] [CrossRef]
- Tanaka, R.; Toyoshima, M.; Yamada, T. New lanostane-type triterpenoids, inonotsutriols D, and E, from Inonotus obliquus. Phytochem. Lett. 2011, 4, 328–332. [Google Scholar] [CrossRef]
- Rakuša, Ž.T.; Kristl, A.; Roškar, R. Quantification of reduced and oxidized coenzyme Q10 in supplements and medicines by HPLC-UV. Anal. Methods 2020, 12, 2580–2589. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. Ampk: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Bjørklund, G.; Mujawdiya, P.K.; Semenova, Y.; Piscopo, S.; Peana, M. Coenzyme Q10 in aging and disease. Crit. Rev. Food Sci. Nutr. 2024, 64, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.H. Mitochondrial dysfunction in aging and diseases of aging. Biology 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Ji, Q.; Fan, Z.; Liu, Z.; Liu, M.; Yang, Y. Efficacy and Optimal Dose of Coenzyme Q10 Supplementation on Inflammation-Related Biomarkers: A GRADE-Assessed Systematic Review and Updated Meta-Analysis of Randomized Controlled Trials. Mol. Nutr. Food Res. 2023, 67, 2200800. [Google Scholar] [CrossRef]
- Campisi, L.; La Motta, C. The use of the Coenzyme Q10 as a food supplement in the management of fibromyalgia: A critical review. Antioxidants 2022, 11, 1969. [Google Scholar] [CrossRef]
- Podda, M.; Zollner, T.M.; Grundmann-Kollmann, M.; Thiele, J.J.; Packer, L.; Kaufmann, R. Activity of alpha-lipoic acid in the protection against oxidative stress in skin. Oxid. Antioxid. Cutan. Biol. 2000, 29, 43–51. [Google Scholar]
- Tülüce, Y.; Osmanoğlu, D.; Rağbetli, M.Ç.; Altındağ, F. Protective action of curcumin and alpha-lipoic acid, against experimental ultraviolet-A/B induced dermal-injury in rats. Cell Biochem. Biophys. 2024, 82, 1–12. [Google Scholar] [CrossRef]
- Superti, F.; Russo, R. Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits. Antioxidants 2024, 13, 1228. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rybczyńska-Tkaczyk, K.; Tobiasz, J.; Yelnytska-Stawasz, V.; Pomianek, T.; Gmiński, J. Biological and anticancer properties of Inonotus obliquus extracts. Process Biochem. 2018, 73, 180–187. [Google Scholar] [CrossRef]
- Kou, R.W.; Han, R.; Gao, Y.Q.; Li, D.; Yin, X.; Gao, J.M. Anti-neuroinflammatory polyoxygenated lanostanoids from Chaga mushroom Inonotus obliquus. Phytochemistry 2021, 184, 112647. [Google Scholar] [CrossRef]
- Chang, Y.; Bai, M.; Xue, X.B.; Zou, C.X.; Huang, X.X.; Song, S.J. Isolation of chemical compositions as dietary antioxidant supplements and neuroprotectants from Chaga mushroom (Inonotus obliquus). Food Biosci. 2022, 47, 101623. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, Y.S.; Jang, Y.W.; Jung, J.Y.; Yun, B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bio. Med. Chem. Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Li, J.; Shen, Z.C.; Lin, X.F.; Chen, A.Q.; Wang, Y.F.; Gong, E.-S.; Liu, D.; Zou, Q.; Wang, X.Y. Advances in health-promoting effects of natural polysaccharides: Regulation on Nrf2 antioxidant pathway. Front. Nutr. 2023, 10, 1102146. [Google Scholar] [CrossRef]
- Angelini, G.; Russo, S.; Carli, F.; Infelise, P.; Panunzi, S.; Bertuzzi, A.; Caristo, M.E.; Lembo, E.; Calce, R.; Bornstein, S.R.; et al. Dodecanedioic acid prevents and reverses metabolic-associated liver disease and obesity and ameliorates liver fibrosis in a rodent model of diet-induced obesity. FASEB J. 2024, 38, e70202. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Alalykina, E.S.; Sergeeva, T.N.; Ananyan, M.A.; Cherenkov, I.A.; Sergeev, V.G. A Water-soluble form of dihydroquercetin reduces LPS-induced astrogliosis, vascular remodeling, and mRNA VEGF-A levels in the substantia nigra of aged rats. J. Neurosci. Neurol. Disord. 2024, 8, 14–19. [Google Scholar]
- Liu, Z.; Qiu, D.; Yang, T.; Su, J.; Liu, C.; Su, X.; Li, A.; Sun, P.; Li, J.; Yan, L.; et al. Research progress of dihydroquercetin in the treatment of skin diseases. Molecules 2023, 28, 6989. [Google Scholar] [CrossRef]
- Abadi, A.; Crane, J.D.; Ogborn, D.; Hettinga, B.; Akhtar, M.; Stokl, A.; MacNeil, L.; Safdar, A.; Tarnopolsky, M.; Alisi, A. Supplementation with α-lipoic acid, CoQ10, and vitamin E augments running performance and mitochondrial function in female mice. PLoS ONE 2013, 8, e60722. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–49. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.D.; Tejero, J.R.; Lara, A.M.; Sepulveda, I.; Díaz, A.H.; Carvajal, M.; Cotán, D.; Alcázar, J.S. AB1379 Microbiota and mitochondrial imbalance in fibromyalgia: A pilot study. Ann. Rheum. Dis. 2023, 82, 1919. [Google Scholar] [CrossRef]
- Castaldo, G.; Marino, C.; D’Elia, M.; Grimaldi, M.; Napolitano, E.; D’Ursi, A.M.; Rastrelli, L. The Effectiveness of the Low-Glycemic and Insulinemic (LOGI) Regimen in Maintaining the Benefits of the VLCKD in Fibromyalgia Patients. Nutrients 2024, 16, 4161. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Glonek, T. Intracellular ATP concentration and implication for cellular evolution. Biology 2021, 10, 1166. [Google Scholar] [CrossRef]
- Schneider, L.; Giordano, S.; Zelickson, B.R.; Johnson, M.S.; Benavides, G.A.; Ouyang, X.; Fineberg, N.; Darley-Usmar, V.M.; Zhang, J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free. Radic. Biol. Med. 2011, 51, 2007–2017. [Google Scholar] [CrossRef]
- Evinova, A.; Baranovicova, E.; Hajduchova, D.; Dibdiakova, K.; Baranova, I.; Racay, P.; Strnadel, J.; Pecova, R.; Halasova, E.; Pokusa, M. The impact of ATP-sensitive potassium channel modulation on mitochondria in a Parkinson’s disease model using SH-SY5Y cells depends on their differentiation state. J. Bioenerg. Biomembr. 2024, 56, 347–360. [Google Scholar] [CrossRef]
- Bon, L.I.; Maksimovich, N.Y.; Burak, I.N. Amino Acids that Play an Important Role in the Functioning of the Nervous System Review. Clin. Trails Clin. Res. 2023, 2, 520–2644. [Google Scholar]
- Mooradian, A.D.; Haas, M.J. Role of Thyroid Hormone in Neurodegenerative Disorders of Older People. Cells 2025, 14, 140. [Google Scholar] [CrossRef]
- Gasmi, A.; Nasreen, A.; Menzel, A.; Gasmi Benahmed, A.; Pivina, L.; Noor, S.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 2022, 28, 210. [Google Scholar] [CrossRef]
- He, W.; Wu, G. Metabolism of amino acids in the brain and their roles in regulating food intake. Amino Acids Nutr. Health Amino Acids Syst. Funct. Health 2020, 1265, 167–185. [Google Scholar]
- Orsucci, D.; Mancuso, M.; Ienco, E.C.; LoGerfo, A.; Siciliano, G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr. Med. Chem. 2011, 18, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Markiewicz, L.; Kabzinski, J.; Odrobina, D.; Majsterek, I. Potential of redox therapies in neurodegenerative disorders. Front. Biosci. 2017, 9, 214–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ahuja, A.; Pathak, S. Potential Role of Oxidative Stress in the Pathophysiology of Neurodegenerative Disorders. Comb. Chem. High Throughput Screen. 2024, 27, 2043–2061. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.R.; Winter, M.G.; Duerkop, B.A.; Spiga, L.; de Carvalho, T.F.; Zhu, W.; Gillis, C.C.; Büttner, L.; Smoot, M.P.; Behrendt, C.L.; et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 2017, 21, 208–219. [Google Scholar] [CrossRef]
- Phang, J.M. Proline metabolism in cell regulation and cancer biology: Recent advances and hypotheses. Antioxid. Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef]
- Pietzke, M.; Meiser, J.; Vazquez, A. Formate metabolism in health and disease. Mol. Metab. 2020, 33, 23–37. [Google Scholar] [CrossRef]
- Szrok-Jurga, S.; Turyn, J.; Hebanowska, A.; Swierczynski, J.; Czumaj, A.; Sledzinski, T.; Stelmanska, E. The role of Acyl-CoA β-oxidation in brain metabolism and neurodegenerative diseases. Int. J. Mol. Sci. 2023, 24, 13977. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Eriksson, E.K.; Agmo Hernandez, V.; Edwards, K. Effect of ubiquinone-10 on the stability of biomimetic membranes of relevance for the inner mitochondrial membrane. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1205–1215. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, K.Y.; Shim, M.S.; Choi, S.H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W.K. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef]


| N. a | RT [min]. | m/z | Formula | ppm | MS/MS | Name |
|---|---|---|---|---|---|---|
| 1 | 7.0 | 443.0696 | C32H11O3 | 1.424 | 221, 125, 80 | unknown |
| 2 | 9.7 | 741.188 | C32H37O20 | 1.053 | 300/301 | Quercetin pentose-rutinoside |
| 3 | 10.8 | 609.1457 | C27H29O16 | 1.213 | 300/301 | Rutin b |
| 4 | 12.6 | 593.1509 | C27H29O15 | 1.422 | 284/285, 255, 227 | Kaempferol rutinoside |
| 5 | 13.0 | 623.1613 | C28H31O16 | 1.057 | 315, 299, 271 | Isorhamnetin rutinoside |
| 6 | 15.0 | 253.0506 | C15H9O4 | 4.563 | Dadzein b | |
| 7 | 16.3 | 301.0353 | C15H9O7 | 3.425 | 179, 151 | Quercetin b |
| 8 | 17.3 | 205.0362 | C8H13O2S2 | 5.084 | 171, 127 | lipoic acid |
| 9 | 17.6 | 269.0456 | C15H9O5 | 4.349 | Apigenin b | |
| 10 | 22.8 | 227.1289 | C12H19O4 | 5.127 | 209, 183, 165 | Dodecenedioic acid isomer |
| 11 | 23.5 | 227.1288 | C12H19O4 | 4.686 | 209, 183,165 | Dodecenedioic acid isomer |
| 12 | 26.9 | 941.5097 | C48H77O18 | 0.713 | 439 | lanostane-type saponin |
| 13 | 27.8 | 795.4530 | C42H67O14 | 0.587 | 439 | lanostane-type saponin |
| 14 | 27.9 | 911.5001 | C47H75O17 | 0.332 | 439, 421 | lanostane-type saponin |
| 15 | 28.7 | 925.5159 | C48H77O17 | 0.435 | 423 | lanostane-type saponin |
| Hits | p-Value | FDR | |
|---|---|---|---|
| Pantothenate and CoA biosynthesis | 4 | 9.56 × 10−5 | 3.82 × 10−3 |
| Glyoxylate and dicarboxylate metabolism | 6 | 4.60 × 10−2 | 9.20 × 10−1 |
| Tyrosine metabolism | 3 | 4.63 × 10−1 | 0.00046293 |
| Lipoic acid metabolism | 3 | 0.00052912 | 0.0035275 |
| Histidine metabolism | 3 | 0.0015907 | 0.0079534 |
| Glutathione metabolism | 5 | 0.0018923 | 0.0084101 |
| Phenylalanine metabolism | 2 | 0.0028912 | 0.0096374 |
| Phenylalanine tyrosine and tryptophan biosynthesis | 2 | 0.0028912 | 0.0096374 |
| Glycolysis/Gluconeogenesis | 2 | 0.0060371 | 0.017249 |
| Pyruvate metabolism | 2 | 0.0060371 | 0.017249 |
| Taurine and hypotaurine metabolism | 2 | 0.015772 | 0.03943 |
| Alanine aspartate and glutamate metabolism | 4 | 0.020867 | 0.049098 |
| Arginine and proline metabolism | 3 | 0.026161 | 0.038135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, M.; Marino, C.; Celano, R.; Napolitano, E.; Colarusso, C.; Sorrentino, R.; D’Ursi, A.M.; Rastrelli, L. Impact of a Formulation Containing Chaga Extract, Coenzyme Q10, and Alpha-Lipoic Acid on Mitochondrial Dysfunction and Oxidative Stress: NMR Metabolomic Insights into Cellular Energy. Antioxidants 2025, 14, 753. https://doi.org/10.3390/antiox14060753
D’Elia M, Marino C, Celano R, Napolitano E, Colarusso C, Sorrentino R, D’Ursi AM, Rastrelli L. Impact of a Formulation Containing Chaga Extract, Coenzyme Q10, and Alpha-Lipoic Acid on Mitochondrial Dysfunction and Oxidative Stress: NMR Metabolomic Insights into Cellular Energy. Antioxidants. 2025; 14(6):753. https://doi.org/10.3390/antiox14060753
Chicago/Turabian StyleD’Elia, Maria, Carmen Marino, Rita Celano, Enza Napolitano, Chiara Colarusso, Rosalinda Sorrentino, Anna Maria D’Ursi, and Luca Rastrelli. 2025. "Impact of a Formulation Containing Chaga Extract, Coenzyme Q10, and Alpha-Lipoic Acid on Mitochondrial Dysfunction and Oxidative Stress: NMR Metabolomic Insights into Cellular Energy" Antioxidants 14, no. 6: 753. https://doi.org/10.3390/antiox14060753
APA StyleD’Elia, M., Marino, C., Celano, R., Napolitano, E., Colarusso, C., Sorrentino, R., D’Ursi, A. M., & Rastrelli, L. (2025). Impact of a Formulation Containing Chaga Extract, Coenzyme Q10, and Alpha-Lipoic Acid on Mitochondrial Dysfunction and Oxidative Stress: NMR Metabolomic Insights into Cellular Energy. Antioxidants, 14(6), 753. https://doi.org/10.3390/antiox14060753












