Chemical Composition and Antioxidant Activity of Prokupac Grape Pomace Extract: Implications for Redox Modulation in Honey Bee Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Plant Material
2.3. Sample Preparation
2.4. Extraction Procedure
2.5. LC-MS Qualitative Analysis of Phenolic Compounds in Prokupac GP Extract
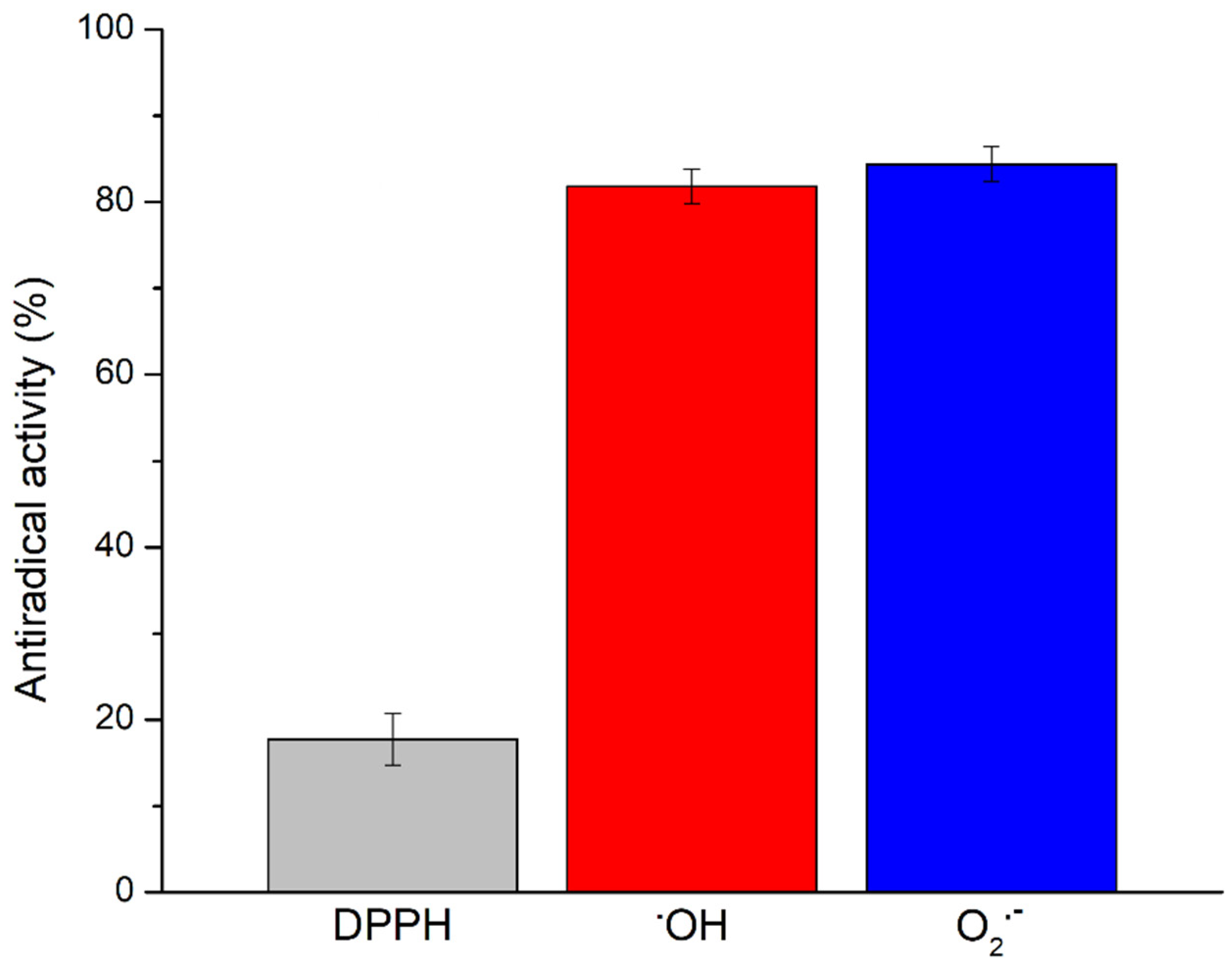
2.6. Determination of Antiradical Activity Towards DPPH Radicals
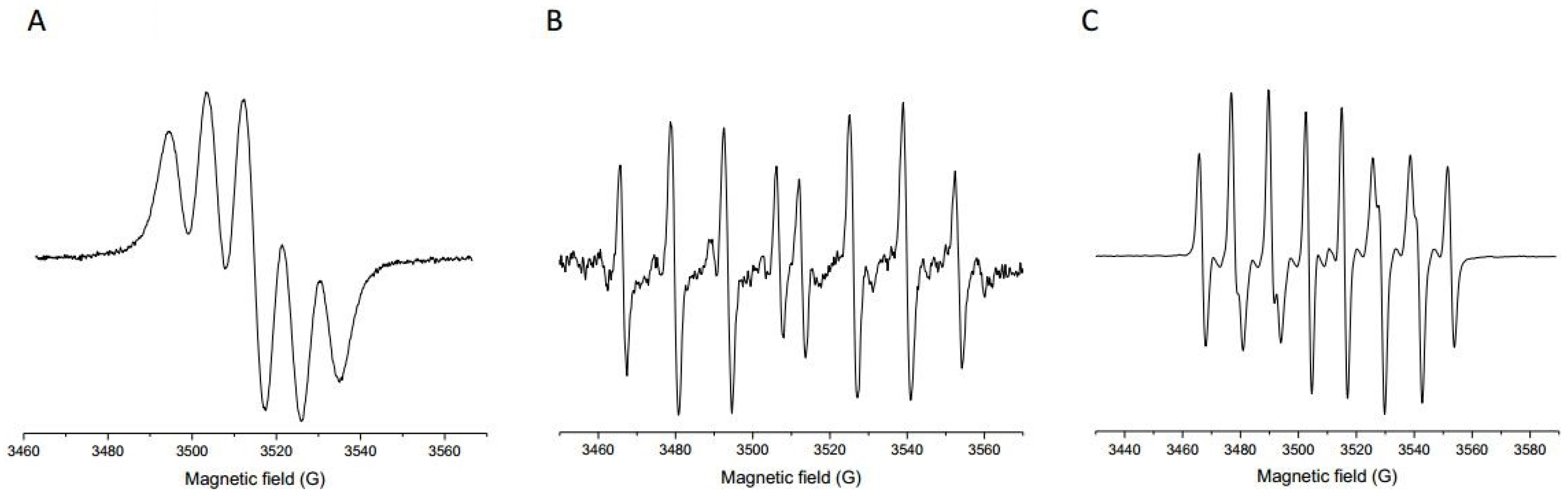
2.7. Determination of Antiradical Activity Towards Hydroxyl Radicals
2.8. Determination of Antiradical Activity Towards Superoxide Anion Radicals
2.9. Measurement of Cell Viability
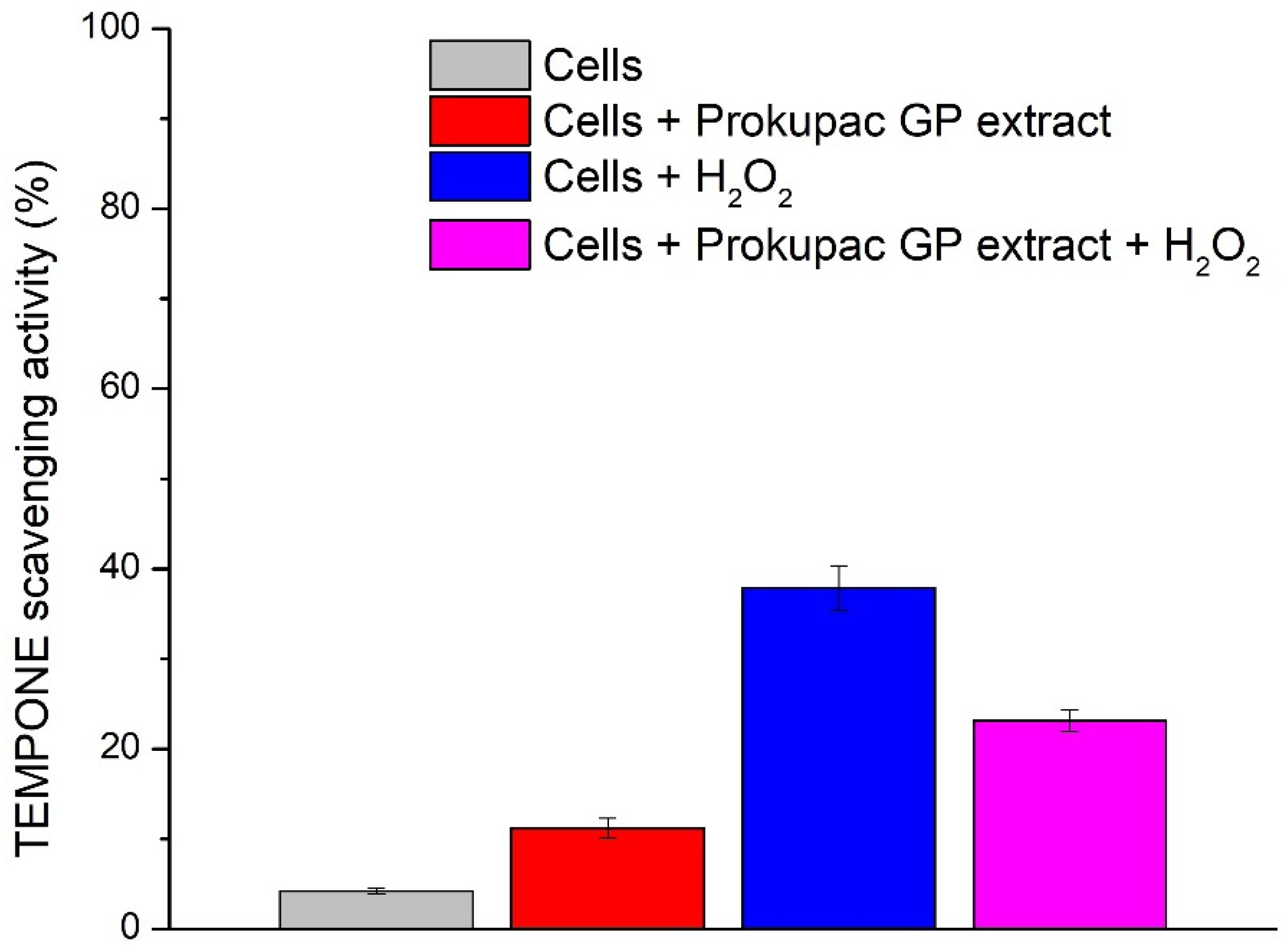
2.10. Redox Response of Apis mellifera Cells to Prokupac GP Extract Treatment
- Controls:
- ○
- Water Control: 29 µL deionized water + 1 µL TEMPONE.
- ○
- Medium Control: 27 µL Schneider’s Insect Medium + 2 µL deionized water + 1 µL TEMPONE.
- ○
- Cell Baseline: 27 µL cell suspension (100,000 cells) + 2 µL deionized water + 1 µL TEMPONE.
- Grape Extract Effects:
- ○
- Extract in Water: 28 µL deionized water + 1 µL grape extract + 1 µL TEMPONE.
- ○
- Extract in Medium: 27 µL Schneider’s Insect Medium + 1 µL grape extract + 1 µL deionized water + 1 µL TEMPONE.
- ○
- Extract with Cells: 27 µL cell suspension + 1 µL grape extract + 1 µL deionized water + 1 µL TEMPONE.
- Cells Under Oxidative Stress:
- ○
- Cell + H2O2: 27 µL cell suspension + 1 µL diluted H2O2 + 1 µL deionized water + 1 µL TEMPONE.
- ○
- Cell + Extract + H2O2: 27 µL cell suspension + 1 µL grape extract + 1 µL diluted H2O2 + 1 µL TEMPONE.
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Profiling of Prokupac GP Extract
3.2. Antiradical Activity of Prokupac GP Extract
3.3. Cell Viability Measurement Results
3.4. Redox Modulation in Apis Mellifera Cells by Prokupac GP Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, Z.; Glavinic, U.; Ristanic, M.; Aleksic, N.; Jovanovic, N.; Vejnovic, B.; Stevanovic, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Margaoan, R.; Papa, G.; Nicolescu, A.; Cornea-Cipcigan, M.; Kösoğlu, M.; Topal, E.; Negri, I. Environmental pollution effect on honey bees and their derived products: A comprehensive analysis. Environ. Sci. Pollut. Res. 2025, 32, 10370–10391. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Schwarz, R.S.; Vejnovic, B.; Evans, J.D.; Irwin, R.E.; Glavinic, U.; Stanimirovic, Z. Species-specific diagnostics of Apis mellifera trypanosomatids: A nine-year survey (2007–2015) for trypanosomatids and microsporidians in Serbian honey bees. J. Invertebr. Pathol. 2016, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Taric, E.; Glavinic, U.; Stevanovic, J.; Vejnovic, B.; Aleksic, N.; Dimitrijevic, V.; Stanimirovic, Z. Occurrence of honey bee (Apis mellifera L.) pathogens in commercial and traditional hives. J. Apicult. Sci. 2019, 58, 433–443. [Google Scholar] [CrossRef]
- Abban, S.; Smith, B.; Corona, M.; Cook, S.C.; Evans, J.D.; Chen, Y.; Alburaki, M. Prevalence and distribution of Varroa destructor and Nosema spp. in symptomatic honey bee colonies across the USA from 2015 to 2022. Sci. Rep. 2024, 14, 1726. [Google Scholar] [CrossRef]
- Prouty, C.; Jack, C.; Sagili, R.; Ellis, J.D. Evaluating the efficacy of common treatments used for Vairimorpha (Nosema) spp. Control. Appl. Sci. 2023, 13, 1303. [Google Scholar] [CrossRef]
- Warner, S.; Pokhrel, L.R.; Akula, S.M.; Ubah, C.S.; Richards, S.L.; Jensen, H.; Kearney, G.D. A scoping review on the effects of Varroa mite (Varroa destructor) on global honey bee decline. Sci. Total Environ. 2024, 906, 167492. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Gong, H.R.; Huang, S.K.; Sohr, A.; Hu, F.L.; Chen, Y.P. Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and deformed wing virus. Vet. Microbiol. 2015, 177, 1–6. [Google Scholar] [CrossRef]
- Straub, L.; Williams, G.R.; Vidondo, B.; Khongphinitbunjong, K.; Retschnig, G.; Schneeberger, A.; Chantawannakul, P.; Dietemann, V.; Neumann, P. Neonicotinoids and ectoparasitic mites synergistically impact honeybees. Sci. Rep. 2019, 9, 8159. [Google Scholar] [CrossRef]
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. PeerJ 2019, 7, e6325. [Google Scholar] [CrossRef] [PubMed]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions between thiamethoxam and deformed wing virus can drastically impair flight behavior of honey bees. Front. Microbiol. 2020, 11, 766. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J. Agrochemicals interact synergistically to increase bee mortality. Nature 2021, 596, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Nakarada, Đ.; Glavinić, U.; Ristanić, M.; Popović, M.; Stevanović, J.; Stanimirović, Z.; Mojović, M. Bridging the buzz: In vivo EPR imaging unlocking the secrets of honey bee health. J. Exp. Zool. Part A 2024, 341, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Frizzera, D.; Del Fabbro, S.; Ortis, G.; Zanni, V.; Bortolomeazzi, R.; Nazzi, F.; Annoscia, D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie 2020, 51, 594–608. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Ellis, J.D. Reviewing the efficacy of pollen substitutes as a management tool for improving the health and productivity of western honey bee (Apis mellifera) colonies. Front. Sustain. Food Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- Güneşdoğdu, M.; Sarıoğlu-Bozkurt, A.; Şekeroğlu, A.; Abacı, S.H. Changes in vitellogenin, abdominal lipid content, and hypopharyngeal gland development in honey bees fed diets with different protein sources. Insects 2024, 15, 215. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Glavinic, U.; Jovanovic, N.M.; Dominikovic, N.; Lakic, N.; Ćosić, M.; Stevanovic, J.; Stanimirovic, Z. Potential of wormwood and oak bark-based supplement in health improvement of Nosema ceranae-infected honey bees. Animals 2024, 14, 1195. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Ilic, T.; Stevanovic, J.; Stanimirovic, Z. Effects of plant-based supplement on oxidative stress of honey bees (Apis mellifera) infected with Nosema ceranae. Animals 2023, 13, 3543. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Stevanovic, J.; Ristanic, M.; Vejnovic, B.; Dolasevic, S.; Stanimirovic, Z. A field trial to demonstrate the potential of a vitamin B diet supplement in reducing oxidative stress and improving hygienic and grooming behaviors in honey bees. Insects 2025, 16, 36. [Google Scholar] [CrossRef]
- Dolasevic, S.; Stevanovic, J.; Aleksic, N.; Glavinic, U.; Deletic, N.; Mladenovic, M.; Stanimirovic, Z. The effect of diet types on some quality characteristics of artificially reared Apis mellifera queens. J. Apicult. Res. 2020, 59, 115–123. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Jelisić, S.; Vejnović, B.; Niketić, M.; Stevanović, J. Diet supplementation helps honey bee colonies in combat infections by enhancing their hygienic behaviour. Acta Vet. 2022, 72, 145–166. [Google Scholar] [CrossRef]
- McMenamin, A.; Weiss, M.; Meikle, W.; Ricigliano, V. Efficacy of a microalgal feed additive in commercial honey bee colonies used for crop pollination. ACS Agric. Sci. Technol. 2023, 3, 748–759. [Google Scholar] [CrossRef]
- Shadmehr, S.; Chamani, M.; Tajabadi, N.; Sadeghi, A.A.; Seidavi, A. Effect of dietary supplement on the reproductive traits, products and behavioral characteristics of the European honey bee (Apis mellifera). J. Apicult. Res. 2025, 64, 657–665. [Google Scholar] [CrossRef]
- Ghasemi, V.; Nehzati Paghaleh, G.; Torabi, E.; Arzanforoosh, Z.S.; Bideshki, R.; Kalanaky, S.; Fakharzadeh, S.; Hafizi, M.; Nazaran, M.H. Effects of advanced chelate-based mineral supplement on adult worker honey bees’ (Apis mellifera L.) survival, nutritional indices, hypopharyngeal glands’ growth, energy reserve contents, and tolerance to dimethoate. J. Apicult. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Martínez-Ávila, G.C. Grape pomace—Advances in its bioactivity, health benefits, and food applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Lopes, J.D.; Madureira, J.; Margaça, F.M.; Cabo Verde, S. Grape pomace: A review of its bioactive phenolic compounds, health benefits, and applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Machado, T.O.; Portugal, I.; de AC Kodel, H.; Droppa-Almeida, D.; Lima, M.D.; Fathi, F.; Oliveira, M.B.P.P.; de Albuquerque-Júnior, R.L.C.; Dariva, C.; Souto, E.B. Therapeutic potential of grape pomace extracts: A review of scientific evidence. Food Biosci. 2024, 60, 104210. [Google Scholar] [CrossRef]
- Pascual, G.; Silva, D.; Vargas, M.; Aranda, M.; Cañumir, J.A.; López, M.D. Dietary supplement of grape wastes enhances honeybee immune system and reduces deformed wing virus (DWV) load. Antioxidants 2023, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.J.; Spivak, M.S.; Kurtti, T.J. A cell line resource derived from honey bee (Apis mellifera) embryonic tissues. PLoS ONE 2013, 8, e69831. [Google Scholar] [CrossRef]
- Nakarada, Đ.J.; Marković, S.Z.; Popović, M.D.; Dimitrijević, M.S.; Rakić, A.A.; Mojović, M.D. Redox properties of grape wine skin extracts from the Šumadija region: An electron paramagnetic resonance study. Hosp. Pharmacol. Int. Multidiscip. J. 2021, 8, 1004–1013. [Google Scholar] [CrossRef]
- Stojković, D.; Gašić, U.; Uba, A.I.; Zengin, G.; Rajaković, M.; Stevanović, M.; Drakulić, D. Chemical profiling of Anthriscus cerefolium (L.) Hoffm., biological potential of the herbal extract, molecular modeling and KEGG pathway analysis. Fitoterapia 2024, 177, 106115. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Fermo, P.; Comite, V.; Sredojević, M.; Ćirić, I.; Gašić, U.; Mutić, J.; Baošić, R.; Tešić, Ž. Elemental analysis and phenolic profiles of selected Italian wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Bajić, S.S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Dikalov, S.; Kirilyuk, I.; Grigor’ev, I. Spin trapping of O-, C-, and S-centered radicals and peroxynitrite by 2H-imidazole-1-oxides. Biochem. Biophys. Res. Commun. 1996, 218, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, L.; Xiao, R.; Qiu, S.; Cao, J.; Fu, Y.; Wang, Z. New evidence for the involvement of superoxide and singlet oxygen in UV-activated peroxydisulfate system under acidic conditions. Chem. Eng. J. 2025, 505, 159531. [Google Scholar] [CrossRef]
- Phillips, H.J. Dye exclusion tests for cell viability. In Tissue Culture: Methods and Applications; Kruse, P.F., Patterson, M.K., Eds.; Academic Press: Cambridge, MA, USA, 1973; pp. 406–408. [Google Scholar]
- Mojović, M.D.; Spasojević, I.; Vuletić, M.M.; Vučinić, Ž.B.; Bačić, G. An EPR spin-probe and spin-trap study of the free radicals produced by plant plasma membranes. J. Serb. Chem. Soc. 2005, 70, 177–186. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.; Radovanović, A. Free radical scavenging activity and anthocyanin profile of Cabernet Sauvignon wines from the Balkan region. Molecules 2010, 15, 4213–4226. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food anthocyanins: Malvidin and its glycosides as promising antioxidant and anti-inflammatory agents with potential health benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Strugała-Danak, P.; Spiegel, M.; Hurynowicz, K.; Gabrielska, J. Interference of malvidin and its mono-and di-glucosides on the membrane—Combined in vitro and computational chemistry study. J. Funct. Foods 2022, 99, 105340. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in vitro of quercetin glycoside obtained in Beauveria bassiana culture and its interaction with liposome membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Tar. 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Masuda, S.; Furugori, M.; Esaki, S.; Kinae, N. Radioprotective effect of antioxidative flavonoids in γ-ray irradiated mice. Carcinogenesis 1994, 15, 2669–2672. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–induced acute pancreatitis: An experimental study on rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agr. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Jelisić, S.; Stanimirović, Z.; Ristanić, M.; Nakarada, Đ.; Mojović, M.; Bošnjaković, D.; Glavinić, U. The potential of Agaricus bisporus in mitigating pesticide-induced oxidative stress in honey bees infected with Nosema ceranae. Life 2024, 14, 1498. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Vlaicu, P.A.; Turcu, R.P.; Panaite, T.D. Studies on antioxidant activities of grape pomace using in vitro, ex vivo, and in vivo models. J. Food Meas. Charact. 2023, 17, 121–128. [Google Scholar] [CrossRef]
- Pantelić, M.; Dabić Zagorac, D.; Gašić, U.; Jović, S.; Bešlić, Z.; Todić, S.; Natić, M. Phenolic profiles of Serbian autochthonous variety ‘Prokupac’ and monovarietal international wines from the Central Serbia wine region. Nat. Prod. Res. 2017, 32, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Carlson, E.A.; Lucas, H.M.; Melathopoulos, A.P.; Sagili, R.R. Field rates of Sivanto™ (flupyradifurone) and Transform® (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLoS ONE 2020, 15, e0233033. [Google Scholar] [CrossRef] [PubMed]
- Pal, E.; Almasri, H.; Paris, L.; Diogon, M.; Pioz, M.; Cousin, M.; Sené, D.; Tchamitchian, S.; Tavares, D.A.; Delbac, F.; et al. Toxicity of the pesticides imidacloprid, difenoconazole and glyphosate alone and in binary and ternary mixtures to winter honey bees: Effects on survival and antioxidative defenses. Toxics 2022, 10, 104. [Google Scholar] [CrossRef]
- Glavinic, U.; Blagojevic, J.; Ristanic, M.; Stevanovic, J.; Lakic, N.; Mirilovic, M.; Stanimirovic, Z. Use of thymol in Nosema ceranae control and health improvement of infected honey bees. Insects 2022, 13, 574. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Skowronek, P.; Strachecka, A. Cannabidiol (CBD) supports the honeybee worker organism by activating the antioxidant system. Antioxidants 2023, 12, 279. [Google Scholar] [CrossRef]
| No | Compound Name | tR, min | Molecular Formula, [M ± H]± | Calculated Mass, m/z | Exact Mass, m/z | Δ mDa | MS2 Fragments, (% Base Peak) | Relative Content, % |
|---|---|---|---|---|---|---|---|---|
| Flavonoid glycosides | ||||||||
| 1 | Myricetin 3-O-glucuronide | 6.69 | C21H19O14+ | 495.07696 | 495.07114 | 5.82 | 319.04129 (100) | 0.06 |
| 2 | Myricetin 3-O-glucoside | 6.70 | C21H21O13+ | 481.09779 | 481.09215 | 5.64 | 153.01657 (8), 217.04716 (3), 245.04181 (4), 273.03644 (3), 319.04123 (100) | 0.64 |
| 3 | Myricetin 3,4′-di-O-glucoside | 6.71 | C27H31O18+ | 643.15052 | 643.14488 | 5.64 | 153.01663 (3), 319.04135 (100), 481.09253 (46) | 0.10 |
| 4 | Laricitrin 3-O-glucoside | 6.96 | C22H23O13+ | 495.11334 | 495.10778 | 5.57 | 245.04189 (3), 301.03110 (4), 318.03412 (7), 333.05667 (100) | 0.67 |
| 5 | Kaempferol 3-O-glucoside | 7.01 | C21H21O11+ | 449.10787 | 449.10273 | 5.13 | 153.01622 (4), 287.05197 (100) | 0.06 |
| 6 | Syringetin 3-O-(6”-O-acetyl)-glucoside | 7.48 | C25H27O14+ | 551.13956 | 551.13314 | 6.42 | 347.07251 (100) | 0.08 |
| Flavonoid aglycones | ||||||||
| 7 | Epicatechin | 6.40 | C15H15O6+ | 291.08634 | 291.08292 | 3.42 | 91.05327 (10), 119.04826 (11), 123.04277 (79), 139.03754 (100), 147.04240 (18), 165.05298 (11) | 0.11 |
| 8 | Myricetin | 6.71 | C15H11O8+ | 319.04487 | 319.04112 | 3.75 | 153.01646 (23), 217.0462 (10), 245.04092 (5), 273.03641 (4), 319.04129 (100) | 0.41 |
| 9 | Eriodictyol | 7.34 | C15H13O6+ | 289.07079 | 289.06748 | 3.31 | 107.0479 (95), 149.02167 (70), 153.01648 (100), 215.06883 (46), 217.12192 (14), 243.06279 (50) | 0.05 |
| 10 | Luteolin | 7.55 | C15H11O6+ | 287.05504 | 287.05180 | 3.24 | 123.00639 (18), 139.05305 (7), 183.02797 (7), 287.05179 (100) | 1.19 |
| 11 | Quercetin | 7.83 | C15H11O7+ | 303.04996 | 303.04658 | 3.38 | 137.02168 (8), 153.01657 (15), 229.04723 (9), 257.04175 (3), 303.04657 (100) | 0.16 |
| 12 | Kaempferol | 8.21 | C15H11O6+ | 287.05504 | 287.05181 | 3.24 | 121.02708 (11), 137.02194 (3), 153.01660 (16), 287.05185 (100) | 0.10 |
| 13 | Isorhametin | 8.28 | C16H13O7+ | 317.06561 | 317.06203 | 3.58 | 153.01654 (25), 217.04721 (7), 229.04698 (7), 245.04184 (6), 302.03873 (8), 317.06192 (100) | 0.07 |
| 14 | Daidzein | 9.04 | C15H11O4+ | 255.06519 | 255.06232 | 2.86 | 129.03270 (9), 153.01711 (22), 255.06216 (100) | 0.03 |
| Anthocyanins and pyranoanthocyanins | ||||||||
| 16 | Delphinidin 3-O-glucoside | 5.98 | C21H21O12+ | 465.10288 | 465.09737 | 5.51 | 229.04707 (4), 303.04663 (100), 465.09833 (7) | 3.34 |
| 17 | Cyanidin 3-O-glucoside | 6.18 | C21H21O11+ | 449.10787 | 449.10260 | 5.27 | 287.05188 (100) | 0.67 |
| 18 | Petunidin 3-O-glucoside | 6.25 | C22H23O12+ | 479.11843 | 479.11283 | 5.60 | 245.04167 (4), 274.04401 (7), 302.03854 (16), 317.06171 (100), 479.11267 (8) | 2.77 |
| 19 | Peonidin 3-O-glucoside | 6.43 | C22H23O11+ | 463.12352 | 463.11824 | 5.27 | 258.04932 (8), 286.04398 (19), 301.06711 (100), 463.11801 (9) | 5.94 |
| 20 | Malvidin 3-O-glucoside | 6.44 | C23H25O12+ | 493.13418 | 493.12901 | 5.17 | 242.05501 (5), 270.04877 (4), 287.05194 (6), 315.04642 (12), 331.07739 (100), 493.12900 (11) | 37.93 |
| 21 | Delphinidin 3-O-(6″-O-acetyl)-glucoside | 6.57 | C23H23O13+ | 507.11334 | 507.10763 | 5.72 | 257.04181 (3), 303.04630 (100), 507.1066 (19) | 0.16 |
| 22 | Delphinidin 3,5-di-O-glucoside | 6.63 | C27H31O17+ | 627.15560 | 627.14923 | 6.37 | 303.04630 (100), 465.09827 (41), 627.15192 (19) | 0.06 |
| 23 | Malvidin 3-O-glucoside-pyruvic acid (Vitisin A) | 6.65 | C26H25O14+ | 561.12391 | 561.11712 | 6.79 | 383.03549 (11), 399.06644 (100), 561.11633 (8) | 0.11 |
| 24 | Malvidin 3-O-glucoside-acetaldehyde (Vitisin B) | 6.69 | C25H25O12+ | 517.13418 | 517.12800 | 6.18 | 266.05453 (7), 294.04871 (3), 311.05157 (4), 339.04639 (20), 355.07745 (100), 517.12842 (12) | 0.05 |
| 25 | Cyanidin 3-O-(6″-O-acetyl)-glucoside | 6.72 | C23H23O12+ | 491.11843 | 491.11284 | 5.59 | 287.05200 (100) | 0.10 |
| 26 | Petunidin 3-O-(6″-O-acetyl)-glucoside | 6.75 | C24H25O13+ | 521.12909 | 521.12297 | 6.12 | 274.04404 (6), 302.03873 (20), 317.06186 (100), 521.12262 (26) | 0.62 |
| 27 | Malvidin 3-O-(6″-O-acetyl)-glucoside-pyruvic acid | 6.80 | C28H27O15+ | 603.13447 | 603.12963 | 4.84 | 310.04498 (5), 338.03796 (3), 355.04129 (4), 383.03543 (11), 399.06668 (100), 603.12836 (21) | 0.03 |
| 28 | Malvidin 3-O-(6″-O-acetyl)-glucoside | 6.90 | C25H27O13+ | 535.14464 | 535.13868 | 5.97 | 242.05484 (4), 270.04877 (4), 287.05176 (6), 315.04575 (14), 331.07773 (100), 535.13867 (31) | 12.24 |
| 29 | Peonidin 3-O-(6″-O-acetyl)-glucoside | 6.90 | C24H25O12+ | 505.13418 | 505.12832 | 5.86 | 286.04416 (22), 301.06726 (100), 505.12970 (23) | 1.99 |
| 30 | Delphinidin 3-O-(6″-O-p-coumaroyl)-glucoside | 6.99 | C30H27O14+ | 611.13956 | 611.13474 | 4.82 | 303.04639 (100), 611.13330 (46) | 0.29 |
| 31 | Malvidin 3-O-(6″-O-caffeoyl)-glucoside | 7.06 | C32H31O15+ | 655.16579 | 655.15982 | 5.96 | 287.05182 (3), 315.04565 (4), 331.07724 (100), 655.15771 (47) | 0.70 |
| 32 | Cyanidin 3-O-(6″-O-p-coumaroyl)-glucoside | 7.11 | C30H27O13+ | 595.14464 | 595.13782 | 6.82 | 287.05185 (100), 595.13727 (31) | 0.23 |
| 33 | Petunidin 3-O-(6″-O-p-coumaroyl)-glucoside | 7.13 | C31H29O14+ | 625.15521 | 625.14977 | 5.43 | 302.03879 (12), 317.06210 (100), 625.14832 (41) | 1.02 |
| 34 | Malvidin 3-O-(6″-O-p-coumaroyl)-glucoside-pyruvic acid | 7.14 | C35H31O16+ | 707.16078 | 707.15654 | 4.24 | 399.06665 (100), 707.15253 (39) | 0.07 |
| 35 | Malvidin 3-O-(6″-O-feruloyl)-glucoside | 7.23 | C33H33O15+ | 669.18142 | 669.17678 | 4.64 | 287.05356 (3), 316.05267 (4), 331.07776 (100), 669.17291 (46) | 0.09 |
| 36 | Malvidin 3-O-(6″-O-p-coumaroyl)-glucoside | 7.27 | C32H31O14+ | 639.17086 | 639.16558 | 5.28 | 315.04639 (4), 331.07748 (100), 639.16357 (42) | 22.21 |
| 37 | Peonidin 3-O-(6″-O-p-coumaroyl)-glucoside | 7.28 | C31H29O13+ | 609.16039 | 609.15525 | 5.14 | 286.04407 (14), 301.06726 (100), 609.15338 (43) | 4.41 |
| Stilbenes | ||||||||
| 38 | Piceatanol | 6.41 | C14H11O4- | 243.06630 | 243.06328 | 3.02 | 175.07425 (6), 199.07414 (5), 201.05354 (13), 243.06316 (100) | 0.12 |
| 39 | Resveratrol 3-O-glucoside (Polydatin) | 6.58 | C20H21O8- | 389.12420 | 389.11963 | 4.57 | 227.06857 (100) | 0.39 |
| 40 | Resveratrol | 7.28 | C14H11O3- | 227.07140 | 227.06869 | 2.71 | 143.04865 (6), 159.07988 (3), 183.07947 (6), 185.05856 (21), 227.06863 (100) | 0.10 |
| 41 | Viniferin | 7.48 | C28H21O6- | 453.13440 | 453.12864 | 5.76 | 359.08707 (18), 369.10822 (19), 385.13977 (18), 411.11874 (40), 435.11868 (29), 453.12857 (100) | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavinić, U.; Nakarada, Đ.; Stevanović, J.; Gašić, U.; Ristanić, M.; Mojović, M.; Stanimirović, Z. Chemical Composition and Antioxidant Activity of Prokupac Grape Pomace Extract: Implications for Redox Modulation in Honey Bee Cells. Antioxidants 2025, 14, 751. https://doi.org/10.3390/antiox14060751
Glavinić U, Nakarada Đ, Stevanović J, Gašić U, Ristanić M, Mojović M, Stanimirović Z. Chemical Composition and Antioxidant Activity of Prokupac Grape Pomace Extract: Implications for Redox Modulation in Honey Bee Cells. Antioxidants. 2025; 14(6):751. https://doi.org/10.3390/antiox14060751
Chicago/Turabian StyleGlavinić, Uroš, Đura Nakarada, Jevrosima Stevanović, Uroš Gašić, Marko Ristanić, Miloš Mojović, and Zoran Stanimirović. 2025. "Chemical Composition and Antioxidant Activity of Prokupac Grape Pomace Extract: Implications for Redox Modulation in Honey Bee Cells" Antioxidants 14, no. 6: 751. https://doi.org/10.3390/antiox14060751
APA StyleGlavinić, U., Nakarada, Đ., Stevanović, J., Gašić, U., Ristanić, M., Mojović, M., & Stanimirović, Z. (2025). Chemical Composition and Antioxidant Activity of Prokupac Grape Pomace Extract: Implications for Redox Modulation in Honey Bee Cells. Antioxidants, 14(6), 751. https://doi.org/10.3390/antiox14060751









