Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Bioavailability of Mg Forms
3. Methods
3.1. Inclusion Criteria
3.2. Exclusion Criteria
3.3. Study Selection, Data Collection, and Extraction
3.4. Meta-Analysis of Data
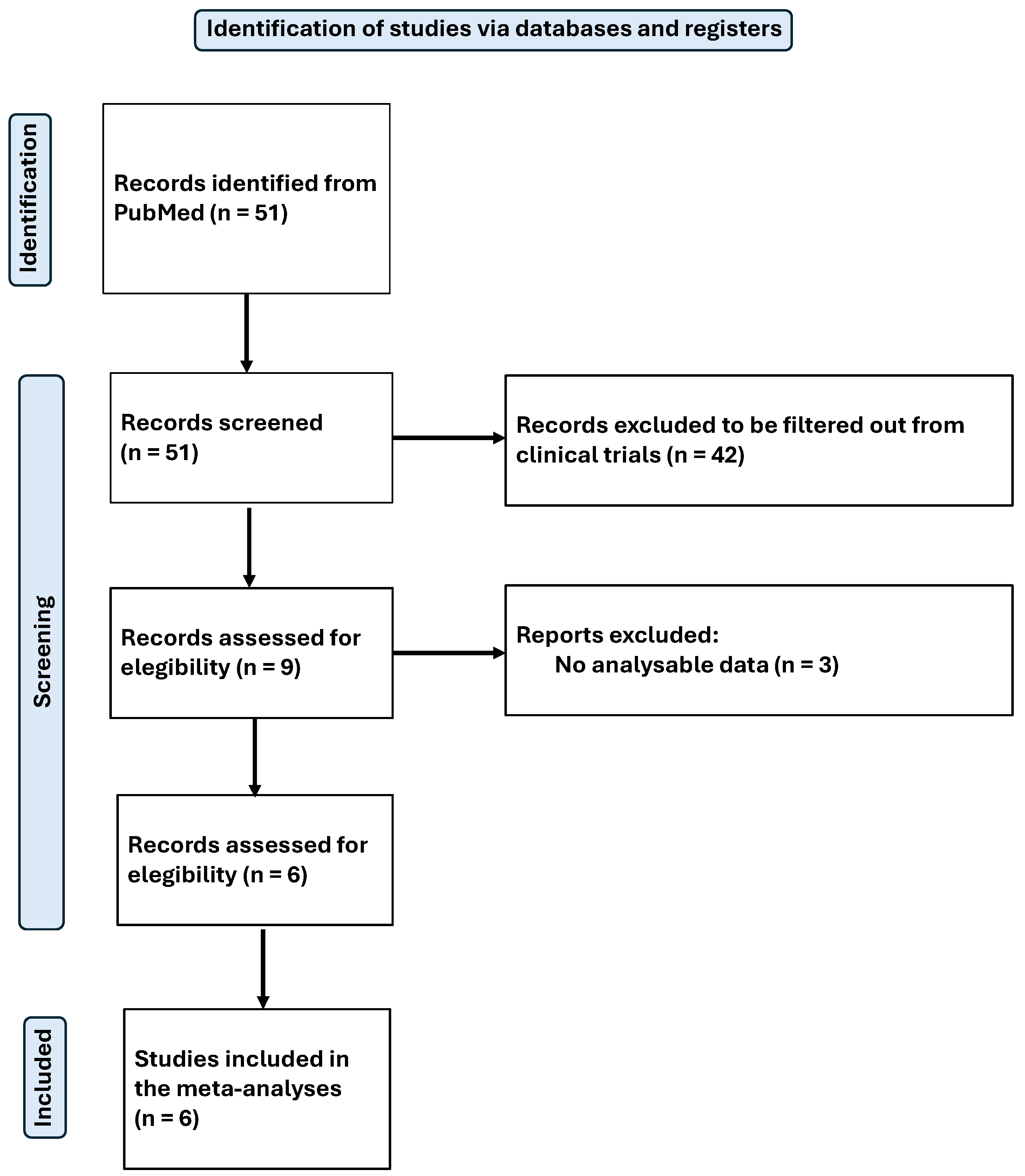
4. Results
4.1. Results of Systematic Review
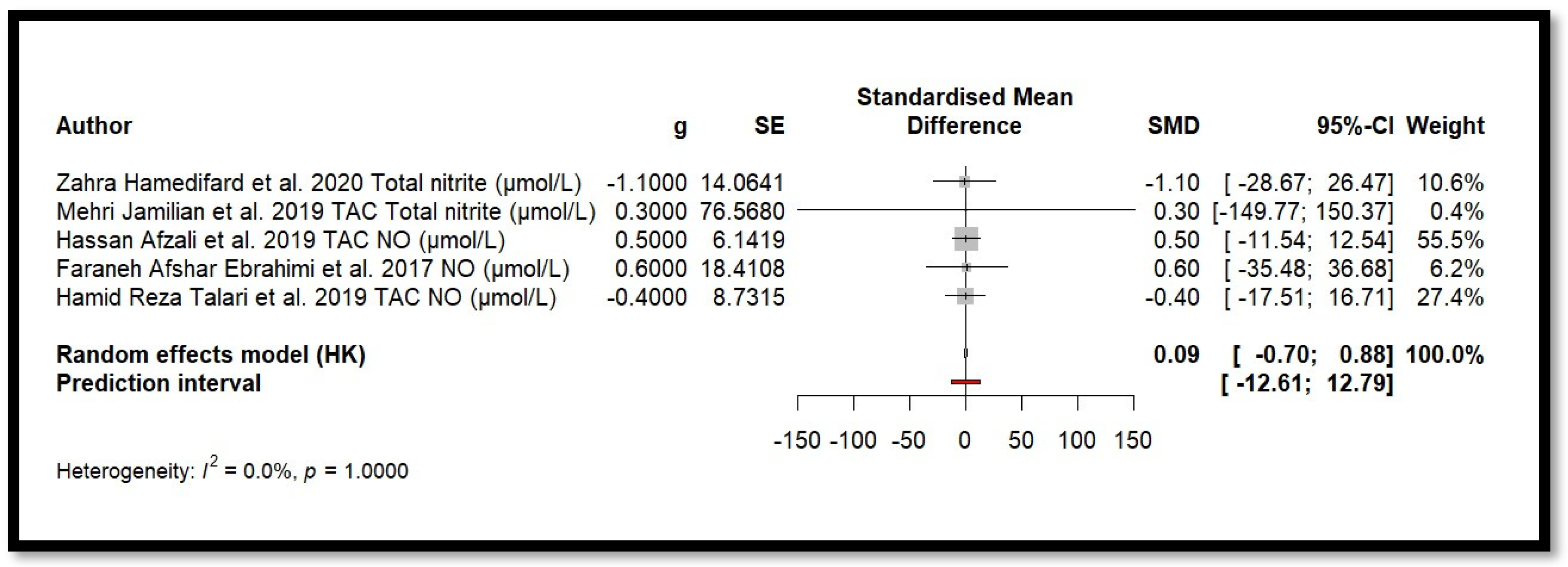
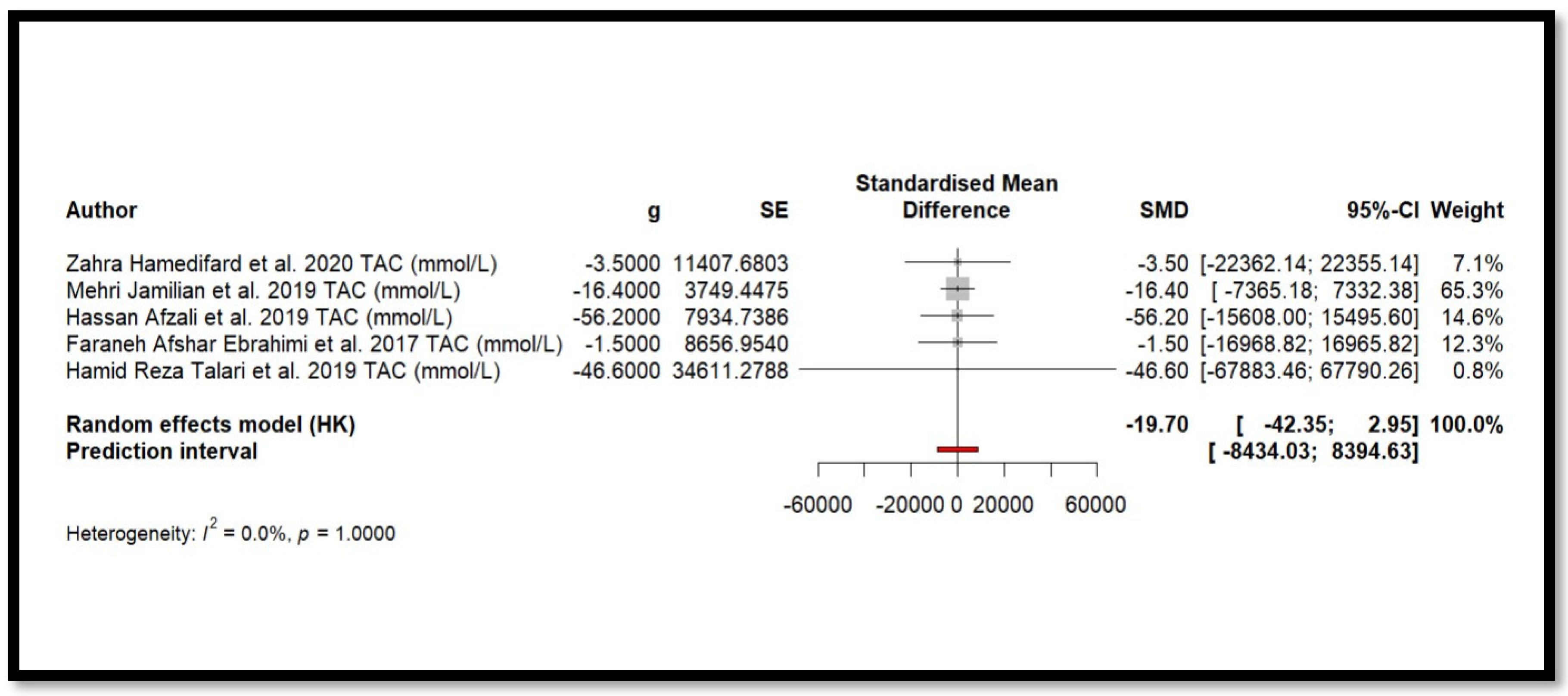
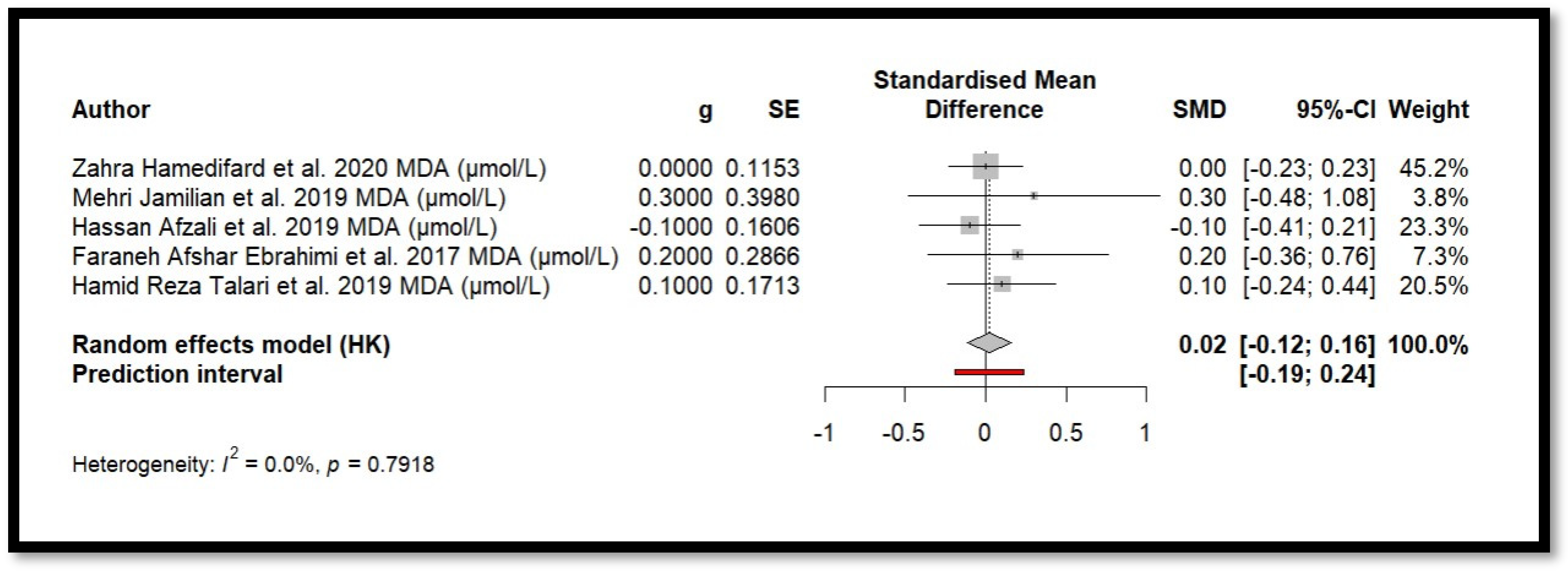
4.2. Impact of Mg on Inflammation and Oxidative Stress
4.3. Effect on Glucose Metabolism and Lipid Profile
4.4. Cardiovascular Health and Vascular Function
4.5. Liver Function and Protection Against Oxidative Damage
4.6. Bone Health and Mineral Metabolism
4.7. Impact on Auditory Health and Neuromuscular Function
4.8. Effect on Anxiety and Mood
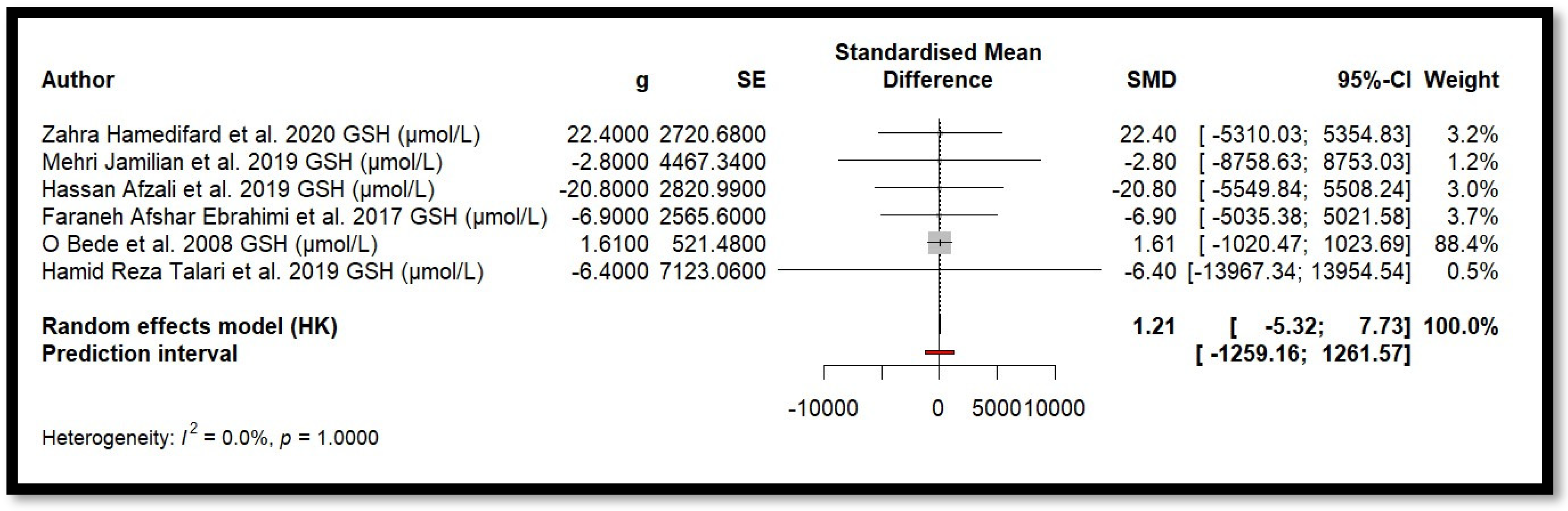
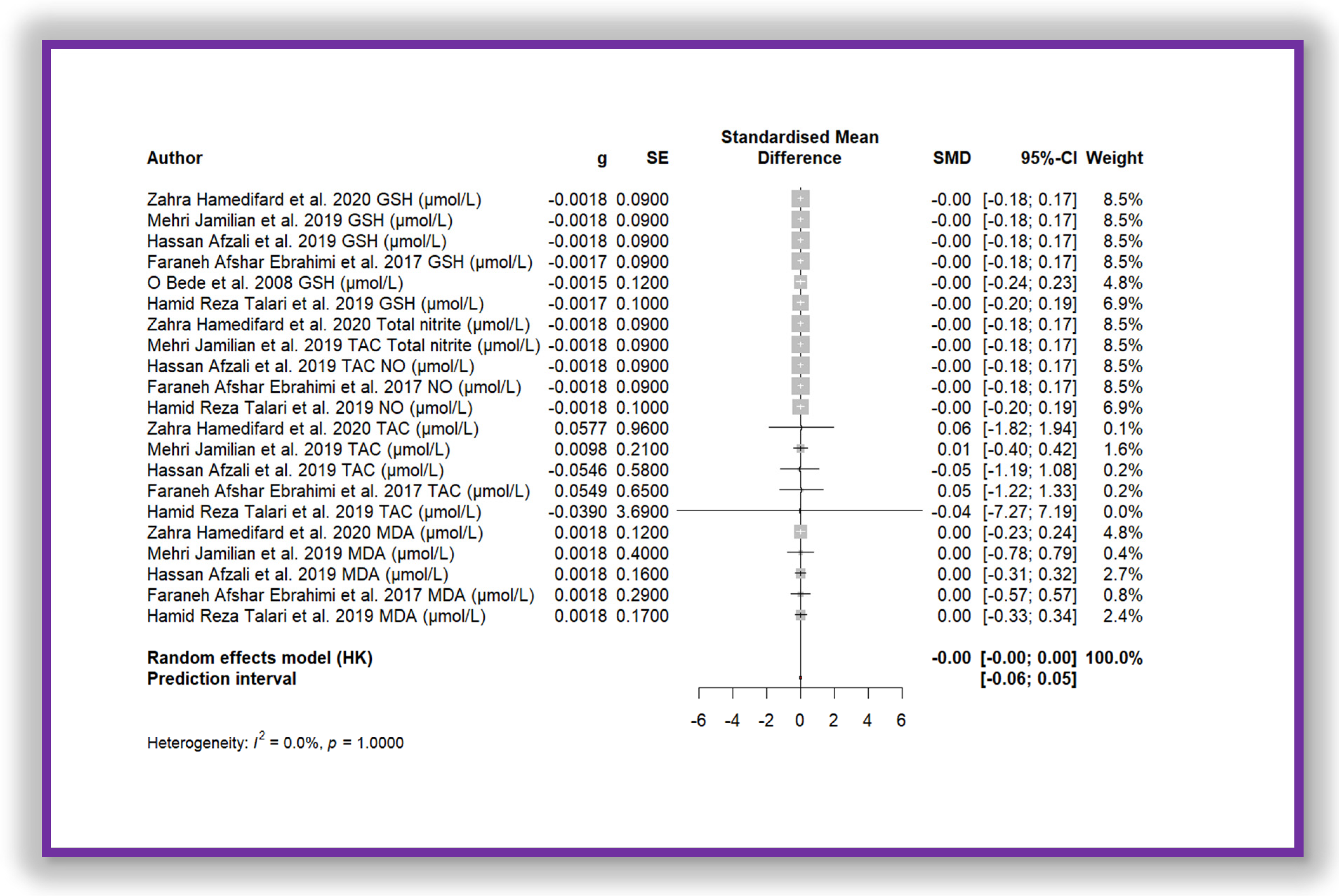
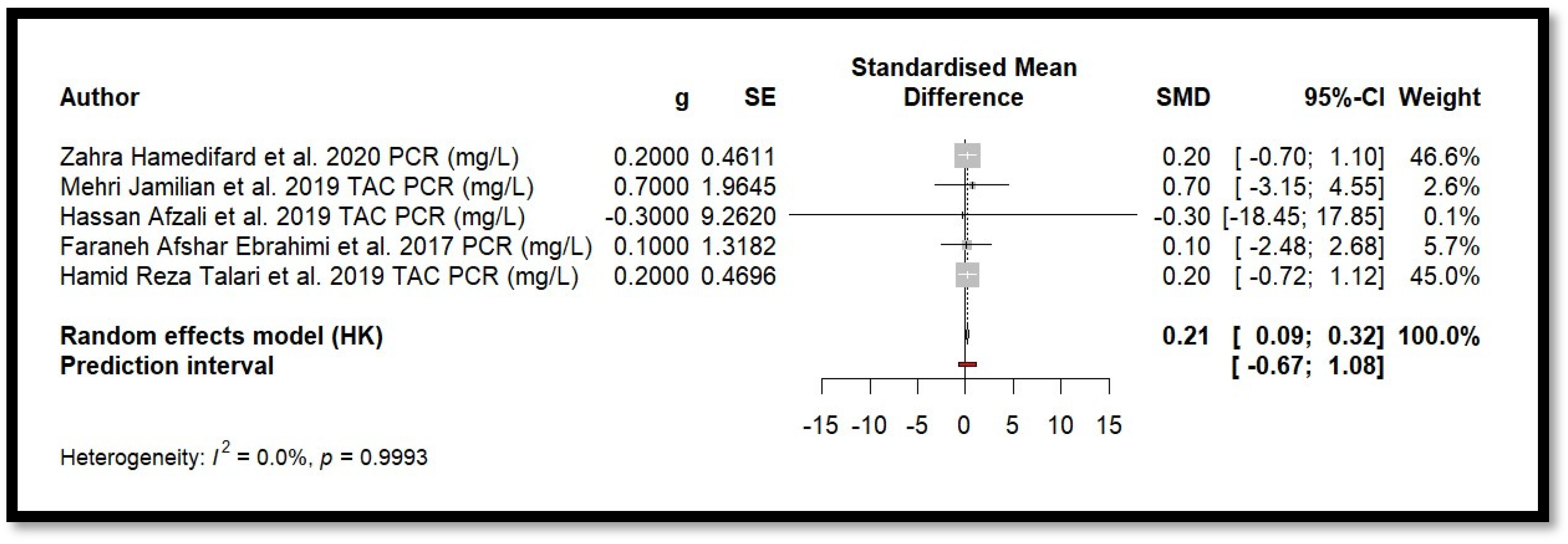
4.9. Results of Meta-Analysis
5. Discussion
Strengths, Limitations, and Future Considerations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- U.S. Department of Health & Human Services. Mg Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/#en2 (accessed on 7 February 2025).
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal Magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Harvard, T.H.; Chan School of Public Health. Magnesium. 2023. Available online: https://nutritionsource.hsph.harvard.edu/Magnesium/ (accessed on 7 February 2025).
- Aal-Hamad, A.H.; Al-Alawi, A.M.; Kashoub, M.S.; Falhammar, H. Hypermagnesemia in Clinical Practice. Medicina 2023, 59, 1190. [Google Scholar] [CrossRef]
- Gröber, U. Magnesium and Drugs. Int. J. Mol. Sci. 2019, 20, 2094. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Pardo, M.R.; Garicano Vilar, E.; San Mauro Marín, I.; Camina Martín, M.A. Bioavailability of Magnesium food supplements: A systematic review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- Aniebo Umoh, E.; Obembe, A.O.; Ikpi, D.E.; Ekpenyong Eniang-Esien, O.; Okon Asuquo, J.; Effiom-Ekaha, O.O. Effect of chronic administration of Magnesium supplement (Magnesium glycinate) on male albino wistar rats’ intestinal (Ileum) motility, body weight changes, food and water intake. Heliyon 2023, 9, e19042. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Q.; Li, S.; Dai, F.; Qian, W.; Hewlings, S.; Yan, T.; Wang, Y. A Magtein®, Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Magnesium citrate found more bioavailable than other Magnesium preparations in a randomised, double-blind study. Magnes. Res. 2003, 16, 183–191. [Google Scholar] [PubMed]
- Ranade, V.V.; Somberg, J.C. Bioavailability and pharmacokinetics of Magnesium after administration of Magnesium salts to humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.; Kizildag, S.; Yuksel, O.; Hosgorler, F.; Yuce, Z.; Guvendi, G.; Kandis, S.; Karakilic, A.; Koc, B.; Uysal, N. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol. Trace Elem. Res. 2019, 192, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef]
- Schuette, S.A.; Lashner, B.A.; Janghorbani, M. Bioavailability of Magnesium diglycinate vs. Magnesium oxide in patients with ileal resection. J. Parenter. Enteral Nutr. 1994, 18, 430–435. [Google Scholar] [CrossRef]
- Shrivastava, P.; Choudhary, R.; Nirmalkar, U.; Singh, A.; Shree, J.; Vishwakarma, P.K.; Bodakhe, S.H. Magnesium taurate attenuates progression of hypertension and cardiotoxicity against cadmium chloride-induced hypertensive albino rats. J. Tradit. Complement. Med. 2018, 9, 119–123. [Google Scholar] [CrossRef]
- Houston, M. The role of Magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- Huypens, P.; Pillai, R.; Sheinin, T.; Schaefer, S.; Huang, M.; Odegaard, M.L.; Ronnebaum, S.M.; Wettig, S.D.; Joseph, J.W. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 2011, 54, 135–145. [Google Scholar] [CrossRef]
- Kumar, A.; Mehan, S.; Tiwari, A.; Khan, Z.; Das Gupta, G.; Narula, A.S.; Samant, R. Magnesium (Mg2+): Essential Mineral for Neuronal Health: From Cellular Biochemistry to Cognitive Health and Behavior Regulation. Curr. Pharm. Des. 2024, 30, 3074–3107. [Google Scholar] [CrossRef]
- Liao, W.; Wei, J.; Liu, C.; Luo, H.; Ruan, Y.; Mai, Y.; Yu, Q.; Cao, Z.; Xu, J.; Zheng, D.; et al. Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis. Neural. Regen. Res. 2024, 19, 2281–2289. [Google Scholar] [CrossRef]
- Fu, C.; Huang, L.; Lian, C.; Yue, J.; Lin, P.; Xu, L.; Lai, W.; Gao, C.; Li, C.; Long, Y. Effects of long-term Magnesium L-threonate supplementation on neuroinflammation, demyelination and blood-brain barrier integrity in mice with neuromyelitis optica spectrum disorder. Brain Res. 2025, 1846, 149234. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Lynch, T.; Hooper, S.; Shrestha, A.; Rosendale, D.; Gu, J. Magnesium-L-threonate improves sleep quality and daytime functioning in adults with self-reported sleep problems: A randomized controlled trial. Sleep Med. X 2024, 8, 100121. [Google Scholar] [CrossRef]
- Gröber, U.; Werner, T.; Vormann, J.; Kisters, K. Myth or Reality-Transdermal Mg? Nutrients 2017, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Hicks, M.A.; Tyagi, A. Magnesium Sulfate. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554553 (accessed on 10 February 2025).
- Firoz, M.; Graber, M. Bioavailability of US commercial Magnesium preparations. Magnes. Res. 2001, 14, 257–262. [Google Scholar] [PubMed]
- MedlinePlus. Magnesium Oxide. 2024. Available online: https://medlineplus.gov/druginfo/meds/a601074.html (accessed on 10 February 2025).
- Baker, W.L.; Kluger, J.; White, C.M.; Dale, K.M.; Silver, B.B.; Coleman, C.I. Effect of Magnesium L-lactate on blood pressure in patients with an implantable cardioverter defibrillator. Ann. Pharmacother. 2009, 43, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Dogterom, P.; Fu, C.; Legg, T.; Chiou, Y.J.; Brandon, S. The absolute bioavailability and the effect of food on a new Magnesium lactate dihydrate extended-release caplet in healthy subjects. Drug Dev. Ind. Pharm. 2018, 44, 1481–1487. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 87869, Magnesium L-aspartate. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium-L-aspartate (accessed on 18 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11029, Magnesium Carbonate. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium-Carbonate (accessed on 18 February 2025).
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. Biomedicine 2016, 6, 20. [Google Scholar] [CrossRef]
- López-Baltanás, R.; Rodríguez-Ortiz, M.E.; Díaz-Tocados, J.M.; Martinez-Moreno, J.M.; Membrives, C.; Rodelo-Haad, C.; de Mier, M.V.P.R.; Rodríguez, M.; Canalejo, A.; Almadén, Y.; et al. Dietary Magnesium Supplementation Decreases Oxidative Stress, Inflammation, and Vascular Dysfunction in an Experimental Model of Metabolic Syndrome with Renal Failure. Antioxidants 2023, 12, 283. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Piuri, G.; Maier, J.A. Magnesium: A Defense Line to Mitigate Inflammation and Oxidative Stress in Adipose Tissue. Antioxidants 2024, 13, 893. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.5. The Cochrane Training. 2024. Available online: https://training.cochrane.org/handbook/current (accessed on 12 February 2025).
- Centre for Reviews and Dissemination, University of York. Systematic Reviews CRD’s Guidance for Undertaking Reviews in Health Care; University of York: York, UK, 2009. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 12 February 2025).
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024; Available online: https://jbi-global-wiki.refined.site/space/MANUAL (accessed on 22 April 2025).
- Afshar Ebrahimi, F.; Foroozanfard, F.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: A Randomized Controlled Clinical Trial. Biol. Trace Elem. Res. 2018, 184, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Afzali, H.; Jafari Kashi, A.H.; Momen-Heravi, M.; Razzaghi, R.; Amirani, E.; Bahmani, F.; Gilasi, H.R.; Asemi, Z. The effects of Magnesium and vitamin E co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2019, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ahokas, R.A.; Sun, Y.; Bhattacharya, S.K.; Gerling, I.C.; Weber, K.T. Aldosteronism and a proinflammatory vascular phenotype: Role of Mg2+, Ca2+, and H2O2 in peripheral blood mononuclear cells. Circulation 2005, 111, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Moreno, J.C.; Flores-Muñoz, M.; Blázquez-Morales, M.S.L.; García-Rivera, M.E.; Rodríguez-Alba, J.C.; Castro-López, C.R.; Nachón-García, F.J.; Muñoz-Muñoz, V.H.; Nachón-García, M.G. The effects of non-surgical periodontal treatment plus zinc and Magnesium supplementation on oxidative stress and antioxidants enzymes in type 2 diabetes patients: A quasi-experimental study. BMC Oral Health 2024, 24, 892. [Google Scholar] [CrossRef]
- Altura, B.M.; Shah, N.C.; Jiang, X.C.; Li, Z.; Perez-Albela, J.L.; Sica, A.C.; Altura, B.T. Short-term Magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H86–H92. [Google Scholar] [CrossRef]
- Bassey, I.E.; Ikpi, D.E.; Isong, I.K.P.; Akpan, U.O.; Onyeukwu, C.C.; Nwankwo, N.P.; Udofia, I.G. Effect of combined Calcium, Magnesium, vitamin C and E supplementation on seminal parameters and serum oxidative stress markers in fructose-induced diabetic Wistar rats. Arch. Physiol. Biochem. 2022, 128, 643–650. [Google Scholar] [CrossRef]
- Bede, O.; Nagy, D.; Surányi, A.; Horváth, I.; Szlávik, M.; Gyurkovits, K. Effects of Magnesium supplementation on the glutathione redox system in atopic asthmatic children. Inflamm. Res. 2008, 57, 279–286. [Google Scholar] [CrossRef]
- Choi, Y.H.; Miller, J.M.; Tucker, K.L.; Hu, H.; Park, S.K. Antioxidant vitamins and Magnesium and the risk of hearing loss in the US general population. Am. J. Clin. Nutr. 2014, 99, 148–155. [Google Scholar] [CrossRef]
- Djurhuus, M.S.; Klitgaard, N.A.; Pedersen, K.K.; Blaabjerg, O.; Altura, B.M.; Altura, B.T.; Henriksen, J.E. Magnesium reduces insulin-stimulated glucose uptake and serum lipid concentrations in type 1 diabetes. Metabolism 2001, 50, 1409–1417. [Google Scholar] [CrossRef]
- Dou, M.; Ma, A.G.; Wang, Q.Z.; Liang, H.; Li, Y.; Yi, X.M.; Zhang, S.C. Supplementation with Magnesium and vitamin E were more effective than Magnesium alone to decrease plasma lipids and blood viscosity in diabetic rats. Nutr. Res. 2009, 29, 519–524. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Sabry, D.; Abd Al Haleem, E.N. Comparative study of antifibrotic activity of some Magnesium-containing supplements on experimental liver toxicity. Molecular Study. Drug Chem. Toxicol. 2017, 40, 47–56. [Google Scholar] [CrossRef] [PubMed]
- ElZohary, L.; Weglicki, W.B.; Chmielinska, J.J.; Kramer, J.H.; Mak, I.T. Magnesium-supplementation attenuated lipogenic and oxidative/nitrosative gene expression caused by Combination Antiretroviral Therapy (cART) in HIV-1-transgenic rats. PLoS ONE 2019, 14, e0210107. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Jalali, M.; Siassi, F.; Saadat, N.; Hosseini, M. The impact of vitamins and/or mineral supplementation on blood pressure in type 2 diabetes. J. Am. Coll. Nutr. 2004, 23, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Glombowsky, P.; da Silva, A.S.; Soldá, N.M.; Galli, G.M.; Biazus, A.H.; Campigotto, G.; Bottari, N.B.; Sousa, R.S.; Brisola, M.C.; Stefani, L.M.; et al. Mineralization in newborn calves contributes to health, improve the antioxidant system and reduces bacterial infections. Microb. Pathog. 2018, 114, 344–349. [Google Scholar] [CrossRef]
- Hamedifard, Z.; Farrokhian, A.; Reiner, Ž.; Bahmani, F.; Asemi, Z.; Ghotbi, M.; Taghizadeh, M. The effects of combined Magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis. 2020, 19, 112. [Google Scholar] [CrossRef]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of Magnesium-zinc-Calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Dolan, D.F.; Bennett, D.C.; Boxer, P.A. Nutrient plasma levels achieved during treatment that reduces noise-induced hearing loss. Transl. Res. 2011, 158, 54–70. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Gagnon, P.M.; Bennett, D.C.; Ohlemiller, K.K. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl. Res. 2011, 158, 38–53. [Google Scholar] [CrossRef]
- Liu, Y.X.; Guo, Y.M.; Wang, Z. Effect of Magnesium on reactive oxygen species production in the thigh muscles of broiler chickens. Br. Poult. Sci. 2007, 48, 84–89. [Google Scholar] [CrossRef]
- Manuel y Keenoy, B.; Moorkens, G.; Vertommen, J.; Noe, M.; Nève, J.; De Leeuw, I. Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: Effects of supplementation with Magnesium. J. Am. Coll. Nutr. 2000, 19, 374–382. [Google Scholar] [CrossRef]
- Markiewicz-Górka, I.; Zawadzki, M.; Januszewska, L.; Hombek-Urban, K.; Pawlas, K. Influence of selenium and/or Magnesium on alleviation alcohol induced oxidative stress in rats, normalization function of liver and changes in serum lipid parameters. Hum. Exp. Toxicol. 2011, 30, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- McKeever, T.M.; Scrivener, S.; Broadfield, E.; Jones, Z.; Britton, J.; Lewis, S.A. Prospective study of diet and decline in lung function in a general population. Am. J. Respir. Crit. Care Med. 2002, 165, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.S.; Severo, J.S.; de Oliveira, A.R.S.; Cruz, K.J.C.; da Silva Dias, T.M.; de Assis, R.C.; Colli, C.; do Nascimento Marreiro, D. Magnesium Status and Its Association with Oxidative Stress in Obese Women. Biol. Trace Elem. Res. 2017, 175, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Picado, C.; Deulofeu, R.; Lleonart, R.; Agustí, M.; Mullol, J.; Torra, M.; Quintó, L. Dietary micronutrients/antioxidants and their relationship with bronchial asthma severity. Allergy 2001, 56, 43–49. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, D.; Shrivastava, S.; Shukla, S. Effect of monothiol along with antioxidant against mercury-induced oxidative stress in rat. Indian J. Exp. Biol. 2007, 45, 1037–1044. [Google Scholar]
- Talari, H.R.; Zakizade, M.; Soleimani, A.; Bahmani, F.; Ghaderi, A.; Mirhosseini, N.; Eslahi, M.; Babadi, M.; Mansournia, M.A.; Asemi, Z. Effects of Magnesium supplementation on carotid intima-media thickness and metabolic profiles in diabetic haemodialysis patients: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2019, 121, 809–817. [Google Scholar] [CrossRef]
- Tatarkova, Z.; de Baaij, J.H.F.; Grendar, M.; Aschenbach, J.R.; Racay, P.; Bos, C.; Sponder, G.; Hoenderop, J.G.; Röntgen, M.; Turcanova Koprusakova, M.; et al. Dietary Mg2+ Intake and the Na+/Mg2+ Exchanger SLC41A1 Influence Components of Mitochondrial Energetics in Murine Cardiomyocytes. Int. J. Mol. Sci. 2020, 21, 8221. [Google Scholar] [CrossRef]
- Wolters, M.; Hahn, A. Plasma ubiquinone status and response to six-month supplementation combined with multivitamins in healthy elderly women--results of a randomized, double-blind, placebo-controlled study. Int. J. Vitam. Nutr. Res. 2003, 73, 207–214. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial (last updated October 2019). In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Chapter 8; Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 22 April 2025).
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for As-sessing the Quality If Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 April 2025).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef]
- Fedele, G.; Castiglioni, S.; Trapani, V.; Zafferri, I.; Bartolini, M.; Casati, S.M.; Ciuffreda, P.; Wolf, F.I.; Maier, J.A. Impact of Inducible Nitric Oxide Synthase Activation on Endothelial Behavior under Magnesium Deficiency. Nutrients 2024, 16, 1406. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Mohammadi, H.; Talebi, S.; Ghavami, A.; Rafiei, M.; Sharifi, S.; Faghihimani, Z.; Golnaz, R.; Maryam, M.; Gholamreza, A. Effects of zinc supplementation on inflammatory biomarkers and oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2021, 68, 126857. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; He, K. Dietary Magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2015, 69, 409. [Google Scholar] [CrossRef]
- Heidari, H.; Hajhashemy, Z.; Saneei, P. A meta-analysis of effects of vitamin E supplementation alone and in combination with omega-3 or magnesium on polycystic ovary syndrome. Sci. Rep. 2022, 12, 19927. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef]
- Talebi, S.; Miraghajani, M.; Hosseini, R.; Mohammadi, H. The Effect of Oral Magnesium Supplementation on Inflammatory Biomarkers in Adults: A Comprehensive Systematic Review and Dose-response Meta-analysis of Randomized Clinical Trials. Biol. Trace Elem. Res. 2022, 200, 1538–1550. [Google Scholar] [CrossRef]
- Li, R.; Li, Z.; Huang, Y.; Hu, K.; Ma, B.; Yang, Y. The effect of Magnesium alone or its combination with other supplements on the markers of inflammation, OS and metabolism in women with polycystic ovarian syndrome (PCOS): A systematic review. Front. Endocrinol. 2022, 13, 974042. [Google Scholar] [CrossRef]
- EL-Derawi, W.A.; Naser, I.A.; Taleb, M.H.; Abutair, A.S. The Effects of Oral Magnesium Supplementation on Glycemic Response among Type 2 Diabetes Patients. Nutrients 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xiang, R.; Li, X.; Tian, H.; Li, C.; Peng, Z.; Xiang, M. Magnesium deficiency in liver cirrhosis: A retrospective study. Scand. J. Gastroenterol. 2021, 56, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Mortazavi, P.; Moradi, F.; Rohani, A.H.; Safi, S. Magnesium attenuates carbon tetrachloride-induced hepatic injury in rats. Magnes. Res. 2013, 26, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Wang, J.; Yang, C.; Lai, H. The cochlea Magnesium content is negatively correlated with hearing loss induced by impulse noise. Am. J. Otolaryngol. 2013, 34, 209–215. [Google Scholar] [CrossRef]
- Wei, X. Dietary Magnesium and Calcium intake is associated with lower risk of hearing loss in older adults: A cross-sectional study of NHANES. Front. Nutr. 2023, 10, 1101764. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, vitamin D, vitamin K2, and Magnesium supplementation and skeletal health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]

| Patients | Intervention | Comparison | Outcomes | Study Type |
|---|---|---|---|---|
| General population, rats and mice with or without pathology | Oral Mg intake or supplementation and antioxidant capacity | Physiological effect and antioxidant capacity of oral intake or supplementation with or without Mg | Physiological, biochemical, or clinical parameters | Cross-sectional, longitudinal, case-control, cohort, and controlled trials |
| Reference | Study Design | Sample Subjects and Groups | Duration of Intervention | Mg Intervention | Parameters Analysed | Results/Conclusion |
|---|---|---|---|---|---|---|
| Afshar Ebrahimi et al. 2017 [40] | Randomised clinical trial | N total = 60 (two groups). Women with polycystic ovary syndrome. | 12 weeks | CG (n = 30): Placebo. IG (n = 30): 250 mg Mg oxide + 220 mg Zn sulphate. | Mg in blood. Gene expression. | Mg + Zn reduced inflammation (hs-CRP), increased antioxidants, and decreased interleukin-1 and tumour necrosis factor-α expression. |
| Afzali et al. 2019 [41] | Randomised clinical trial | N total = 57 (2 groups). Subjects with grade 3 diabetic foot ulcer. | 12 weeks | CG (n = 28): placebo. IG (n = 29): 250 mg of Mg + 400 IU Vit. E. | Insulin, hs-CRP, and MDA in blood | Mg + Vit. E improved healing, glycaemic control, lipid profile, and inflammatory markers. |
| Ahokas et al. 2005 [42] | Quasi-experimental study | N total = 25 (5 groups) male Sprague-Dawley rats. | 4 weeks | G1. CG (n = 5): Untreated rats, no surgery. G2. ALDOST (n = 5): Uninephrectomy, standard diet (20–40 mg/kg Mg), water with 1% NaCl and 0.4% KCl and aldosterone (0.75 µg/h). G3 (n = 5): ALDOST + Mg (40–60 mg/kg). G4 (n = 5): ALDOST + amlodipine (10 mg/kg/day). G5 (n = 5): ALDOST + NAC 200 mg/kg/day. | Blood Mg and vascular inflammation. | Supplementation with Mg (G3) and NAC (G5) reduced vascular inflammation and oxidative stress. |
| Alarcón-Moreno et al. 2024 [43] | Quasi-experimental study | N total = 39 (2 groups). Subjects with non-surgical periodontal treatment and T2DM. | 30 days | CG (N = 19): Placebo. IG (N = 20): 500 mg Mg oxide + 50 mg Zn gluconate | Oxidative biomarkers (MDA and antioxidant enzymes). | Mg + Zn significantly increased SOD and CAT activity compared to non-surgical periodontal treatment alone (CG). |
| Altura et al. 2009 [44] | Quasi-experimental study | Total N = 89 (5 groups). Wistar rats. | 21 days | CG: No Mg deficiency and no treatment. G1 MgD: Mg-deficient. G2 MgD + 15 mg Mg/L in water. G3 MgD + 40 mg Mg/L in water. G4 MgD + 100 mg Mg/L in water. | Mg in serum, cell damage (fragmented DNA), lipid peroxidation, caspase-3 activation, sphingomyelin, and phosphatidylcholine. | Mg deficiency increased lipid peroxidation and DNA fragmentation; supplemented Mg, even at low doses, was protective against cardiovascular damage and oxidative stress. |
| Bassey et al. 2022 [45] | Quasi-experimental study | N total = 30 rats (5 groups). | No data | G1 (N = 6): Non-diabetic. G2 (N = 6): Untreated diabetic group. G3 (N = 6): Mg 400 mg/kg + Ca 400 mg/kg × body weight. G4 (N = 6): Vit. C 100 mg/kg + Vit. E 100 mg/kg × body weight. G5 (N = 6): Mg 400 mg/kg + Ca 400 mg/kg + Vit. C 100 mg/kg + Vit. E 100 mg/kg. They were allowed free access to food and water. | TAC, NO, MDA, and seminal quality. | Mg + Ca + Vit. C + E (G5) improved semen and antioxidant parameters; Vit. C + E (G4) had the greatest effect. |
| Bede et al. 2008 [46] | Randomised clinical trial | N total = 40 asthmatic children (from 4 to 16 years old). | 12 weeks | CG: Placebo. IG: <7 years old received 200 mg and >7 years old 290 mg Mg citrate. | -Oxidized and reduced glutathione (GSSG and GSH). Oxyhaemoglobin, methaemoglobin (metHb), and haemochromes. -Bilirubin levels. -GSH stability tests. | Mg increased GSH and reduced metHb and haemochrome in the treated group vs. placebo. |
| Choi et al. 2014 [47] | Prevalence and incidence study | N total = 2592 subjects (20–69 years old). | Extracted from NHANES 2001–2004 | Dietary intake of Mg (non-supplemented) determined by 24 h dietary reminder. | Dietary b-carotene, Vit. C, Vit. E, and Mg. Audiometry examination. | Higher intakes of β-carotene, Vit. C, and Mg were associated with lower (better) pure tone averaged auditory thresholds at both speech and high frequencies. |
| Djurhuus et al. 2001 [48] | Quasi-experimental study | N total = 15 subjects (2 groups). | 24 weeks | CG (N = 5): Healthy subjects. IG (N = 10): Type I diabetics received an intravenous dose of 30 mmol MgSO4 in 500 mL 0.9% NaCl, followed by 500 mg oral MgO, twice daily. | Serum Mg, muscle Mg, renal Mg excretion, serum lipids, glucose uptake, and Mg intake. | Mg supplementation increased muscle Mg by 5%, reduced cholesterol and LDL, and decreased glucose uptake. |
| Dou et al. 2009 [49] | Randomised clinical trial | N total = 98 Wistar rats with diabetes. | 8 weeks | G1 (N = 15): Control, no supplementation. G2 (N = 17): 0.5 g/kg Vit. E. G3 (N = 16): 0.6 g/kg Mg. G4 (N = 16): 1.2 g/kg Mg. G5 (N = 17): 0.5 g/kg Vit. E + 0.6 g/kg Mg. G6 (N = 17): 0.5 g/kg Vit. E + 1.2 g/kg Mg. | Oxidative stress, lipid profile, and blood viscosity. | Mg + Vit. E improved the lipid profile and reduced blood viscosity; they may affect lipid peroxidation, but did not directly influence it. |
| El-Tantawy et al. 2017 [50] | Quasi-experimental study | N total = 36 Wistar rats (5 groups). | 1 month | CG (N = 6): NOT CCl4-induced liver fibrosis, healthy rats. G1 (N = 6): CCl4 + No treatment. G2 (N = 6): CCl4 + Mg 20 mg/150 gm bw. G3 (N = 6): CCl4 + Mg 20 mg + 17 mg Sacch. cerevisiae/150 gm bw. G4 (N = 6): CCl4 + Mg 20 mg, Ca 165 mg, Vit. D3 82 IU, and 37 mg Vit. C/150 g bw. G5 (N = 6): CCl4 + Silymarin at a dose of 50 mg/kg bw. | (1) Collagen I, transforming growth factor β1, platelet-derived growth factor-C, and nuclear factor kappa-β gene expression. (2) Levels of hepatic collagen (hydroxyproline). (3) Oxidative and antioxidant stress markers: MDA, NO, and GSH. SOD and GST activities. (4) Histopathological examination. | Mg reduced liver fibrosis and oxidative stress; the G5 formulation showed the greatest inhibition of collagen I, transforming growth factor β1, and hepatic hydroxyproline. |
| ElZohary et al. 2019 [51] | Quasi-experimental study | N total = Nº not specified, rats (8 groups). | 18 weeks | G.A1: Control, normal Mg group. G.A2: Control + cART treatment. G.A3: HIV-Tg rats alone. G.A4: HIV-Tg rats + cART treatment (normal Mg). G.B1: Control + high Mg. G.B2: Control + cART treatment + high Mg. G.B3: HIV-Tg rats + high Mg. G.B4: HIV-Tg rats + cART treatment + high Mg. Groups A1–A4 were fed a Mg-normal (0.1% MgO/kg food) diet and groups B1-B4 received a Mg-supplemented (0.6% MgO/kg food) diet. | Genes related to oxidative/nitrosative stress and lipogenesis are analysed by RT-PCR in the liver. Plasma biomarkers (8-isoprostane, nitrotyrosine, RBC-GSSG) and triglyceride and cholesterol levels are also measured. | Mg supplementation in HIV-1-transgenic rats attenuated metabolic and oxidative/nitrosative stress induced by cART. It normalized antioxidant gene expression (HmOX1 and GST), reduced iNOS levels, and improved lipid profiles. |
| Farvid et al. 2004 [52] | Randomised clinical trial | N total = 69 diabetic subjects (4 groups). | 3 months | CG (N = 18): Placebo. G1 (N = 16): 200 mg Mg + 30 mg Zn. G2 (N = 18): 200 mg Vit. C + 150 mg Vit. E. G3 (N = 17): Mg + Zn + Vit. C + E (above indicated quantities). | Blood pressure and biochemical parameters such as serum potassium and MDA were measured. | In G3, there was a significant reduction of systolic (−8 mmHg, p < 0.05), diastolic (−6 mmHg, p < 0.05), and mean (−7 mmHg, p < 0.05) blood pressure; increase in serum potassium (p < 0.05); and decrease in MDA (p < 0.05). |
| Glombowsky et al. 2018 [53] | Quasi-experimental study | N total = 10 calves (2 groups). | 30 days | CG: (N = 5): Non-supplemented. IG (N = 5): Two intramuscular doses (3 mL) of a commercial mineral complex on days 2 and 14 post-birth. Each 100 mL of the product had NaGly (14 g), NaH2PO4 (20.1 g), CuCl2 (0.4 g), KCl (0.6 g), MgCl2 (2.5 g), Na2SeO3 (0.24 g), and sterile H2O. | Blood and faecal samples, body weight and body temperature, blood counts, seric biochemistry, SOD, CAT, and GPx activity. | Mineral supplementation improved antioxidant activity and reduced infections in newborn calves. |
| Hamedifard et al. 2020 [54] | Randomised clinical trial | N total = 60 subjects with coronary heart disease and T2DM (2 groups). | 12 weeks | CG (N = 30): Placebo. IG (N = 30): Oral supplementation: 250 mg of Mg oxide + 150 mg of Zn sulphate. | Blood samples (glucose, insulin, CRP, HDL, and TAC) and test for anxiety (BAI) and depression (BECK). | Mg + Zn reduced glucose, insulin, and CRP; increased HDL and TAC; and improved anxiety and depression. |
| Jamilian et al. 2019 [55] | Randomised clinical trial | N total = 60 women with gestational diabetes (2 groups). | 6 weeks | CG (N = 30): Placebo. IG (N = 30): 100 mg Mg, 4 mg Zn, 400 mg Ca, and 200 IU Vit. D supplements. | Blood samples (oxidative stress biomarkers, hs-CRP, and nitrites) and pregnancy variables. | Supplementation reduced hs-CRP and MDA, increased TAC, and decreased neonatal weight and macrosomia rate. |
| Le Prell et al. 2011 [56] | Randomised clinical trial | N total = 25 guinea pigs (2 groups). | 6 days | CG (N = 9): Saline and vegetable oil. IG (N = 16): β -carotene and vitamins C and E + 2.85 mmol/kg of Mg sulphate. | Plasma levels, noise response, and ear hair cells. | Mg + antioxidants reduced noise-induced hearing loss. |
| Le Prell et al. 2011 [57] | Quasi-experimental study | N total = 31 CBA/J mice (3 groups). | 28 days | CG (N = 16): Control diet (CD). IG-A (N = 8): CD + Mg 2656 + β-carotene 77 + ascorbic acid 2250 + α-tocopherol 863. IG-B (N = 7): CD + Mg 4500 + β -carotene 224 + ascorbic acid 3600 + α -tocopherol 2650. Quantities are indicated per mg/kg of pellet chow. | Permanent hearing threshold loss, survival of hair cells, type II fibroblasts loss, and cell density. | Antioxidant supplementation did not protect hair cells, but reduced hearing loss and showed potential in oxidative stress-related diseases. |
| Liu et al. 2007 [58] | Quasi-experimental study | N total = 96 (2 groups) male Arbor Acre broiler chickens on reactive oxygen species (ROS). | 6 weeks | CG (n = 48): Diet with 2.4 g Mg/kg dry matter. Low Mg group (n = 48): Diet with 1.2 g Mg/kg dry matter. | Oxidative stress: ROS, MDA, and GSH. Mitochondrial activity. Concentration of Mg, Fe, Ca, and fatty acids in muscle. | Low Mg increased ROS, MDA, and mitochondrial activity in muscles. |
| Manuel y Keenoy et al. 2000 [59] | Quasi-experimental study | N total = 93 (2 groups) patients with chronic fatigue. | 3 months | G1: No Mg deficiency and no treatment group (n = 49). G2: Mg deficiency group (n = 44, 20% or more retention of intravenously infused Mg): Mg 10 mg/kg/day. | Antioxidant capacity in plasma, GSH, Vit. E, and lipid peroxidation (TBARS in non-HDL lipoproteins) | Mg supplementation in Mg-deficient patients improved Mg stores, increased Vit. E, and reduced oxidative stress. |
| Markiewicz-Górka et al. 2011 [60] | Randomised clinical trial | N total = 40 mice (5 groups). | 3 months | G0 CG (n = 8): No alcohol intoxication. G1 ethanol group (n = 8): Intoxicated with alcohol (15% ethanol in drinking water). G2 Et + Mg group (n = 8): Intoxicated with alcohol + Mg (100 mg/L water). G3 Et + Se group (n = 8): Intoxicated with alcohol + Se (0.4 mg/L water). G4 Et + Mg + Se group (n = 8): Intoxicated by alcohol + Mg + Se. | Oxidative stress: Serum TAS, GPx activity in liver, reduced/oxidised glutathione (GSH/GSSG) ratio in liver. Lipid peroxidation. Liver histopathology. | Group Et + Mg + Se reduced liver damage and improved TAS and GPx. |
| McKeever et al. 2002 [61] | Cohort study | 2633 adults (1991) and 1346 adults (2000). | No data | Cross-sectional (comparing data from 1991 and 2000) and longitudinal (analysing the change in lung function (FEV1) between the two years) analyses were performed. | Lung function was measured using forced expiratory volume in 1 s (FEV1). Dietary intake of Mg and Vit. C via the food frequency questionnaire. | Higher Vit. C and Mg intake → better lung function (FEV1) in cross-sectional analyses. At 9-year analysis → only Vit. C was associated with less lung function loss. Each 100 mg/day of Vit. C → reduced FEV1 decline by 50.8 mL (95% CI: 3.8–97.9). Mg had no impact on long-term FEV1 decline. |
| Morais et al. 2016 [62] | Prevalence and incidence study | N total = 83 women. | No data | CG (n = 52): Women with BMI = 18.5 and 24.9 kg/m2. Obese group (n = 31): Women with BMI = 30 and 39.9 kg/m2. Dietary Mg calculated by 3-day log. | Dietary intake of Mg. Blood Mg concentration (plasma and erythrocyte). Oxidative stress and lipid peroxidation: TBARS. | Obese women had higher oxidative stress and lipid peroxidation (p < 0.05). Positive correlation between erythrocyte Mg levels and TBARS in the obese group (p = 0.021). |
| Picado et al. 2001 [63] | Case–control study | Total N = 239 subjects. | No data | GC (n = 121): Healthy subjects. Group with asthma (n = 118): Patients with asthma diagnosis. Comparison between groups: - Asthmatics vs. healthy subjects. - Different levels of asthma severity. | Blood levels (plasma and serum) and dietary intake (food frequency questionnaire) of Vit. C, E, A, Mg, Zn, and Se. Antioxidant activity: GSH-Px. Asthma severity according to four severity groups. | There were no differences in the levels of vitamins (C, E, and A), Se, Mg, and Zn between the two groups. Asthma severity was not related to diet or levels of these micronutrients. Patients with severe asthma had lower GSH-Px activity. This suggests that more severe asthmatics may have lower antioxidant capacity. |
| Singh et al. 2007 [64] | Quasi-experimental study | N total = 48 Sprague-Dawley adult rats (8 groups). | No data | Group I (n = 6): Served as normal control (vehicle only). Group II (n = 6): Treated as experimental control (dimethyl mercury (DMM) 10 mg/kg, po, once only). Group III (n = 6): DMM + NAC + Zn. Group IV (n = 6): DMM + NAC + Se. Group V (n = 6): DMM + NAC + Mg. Group VI (n = 6): DMM + DPA + Zn. Group VII (n = 6): DMM + DPA + Se. Group VIII (n = 6): DMM + DPA + Mg. | Biomarkers of liver and kidney damage: AST, ALT, ALP, LDH, GGT, bilirubin, and creatinine. Oxidative stress: Lipid peroxidation and GSH. Acetylcholinesterase activity. Efficacy of treatments with chelators and supplements. | NAC + Se and DPA + Mg treatments were the most effective in reducing oxidative damage and restoring biochemical parameters. |
| Talari et al. 2019 [65] | Randomised clinical trial | N total = 54 patients with diabetes on haemodialysis (HD). | 24 weeks | CG (n = 27): Placebo. IG (n = 27): Supplementation with Mg oxide 250 mg/d. | Carotid intima-media thickness. Glycaemic control: Serum insulin levels, insulin resistance index, percentage of glycated haemoglobin, and insulin sensitivity index. Cardiometabolic risk markers: Total cholesterol, LDL, hs-CRP, MDA, and TAC. | Mg supplementation for 24 weeks in patients with diabetes on haemodialysis improved arterial health, better controlled glucose, reduced inflammation and oxidative stress, and improved lipid profile. |
| Tatarkova et al. 2020 [66] | Quasi-experimental study | N total = 20 mice (4 groups). | 2 weeks | G1 (n = 5): Normal Mg diet and SLC41A1 +/+ genotype (normal control). G2 (n = 5): Low Mg diet and SLC41A1 +/+ genotype (low Mg diet control). G3 (n = 5): Normal Mg diet and SLC41A1-/- genotype (knockout genotype with normal diet). G4 (n = 5): Low Mg diet and SLC41A1-/- genotype (knockout genotype with low Mg diet). | Krebs cycle enzymes: Aconitate hydratase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase. Electronic transport chain complexes: CI, CII, CIII, CIV, and CV. | Dietary Mg content and the functionality of the SLC41A1 exchanger influence energy production in cardiac mitochondria, affecting the activity of key enzymes in the Krebs cycle and the electron transport chain. |
| Wolters and Hahn 2003 [67] | Randomised clinical trial | N total = 220 older women (two groups). | 6 months | CG (n = 109): Placebo. IG (n = 111): Supplementation of coenzyme Q10 + multivitamins + Se + Mg (50 mg) | Serum concentrations of coenzyme Q10. | Supplementation increased serum coenzyme Q10 levels by 106% in the intervention group and 31% in the control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepeda, V.; Ródenas-Munar, M.; García, S.; Bouzas, C.; Tur, J.A. Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation. Antioxidants 2025, 14, 740. https://doi.org/10.3390/antiox14060740
Cepeda V, Ródenas-Munar M, García S, Bouzas C, Tur JA. Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation. Antioxidants. 2025; 14(6):740. https://doi.org/10.3390/antiox14060740
Chicago/Turabian StyleCepeda, Violeta, Marina Ródenas-Munar, Silvia García, Cristina Bouzas, and Josep A. Tur. 2025. "Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation" Antioxidants 14, no. 6: 740. https://doi.org/10.3390/antiox14060740
APA StyleCepeda, V., Ródenas-Munar, M., García, S., Bouzas, C., & Tur, J. A. (2025). Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation. Antioxidants, 14(6), 740. https://doi.org/10.3390/antiox14060740








