Serum 3-Nitrotyrosine in the Cardiovascular Disease of Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Laboratory Assessments
2.4. Carotid Ultrasound Assessment
2.5. Statistical Analysis
3. Results
3.1. Demographics and Disease-Related Data of Systemic Lupus Erythematosus Patients
3.2. Relationship Demographics and Disease-Related Data with 3-Nitrotyrosine
3.3. Multivariable Analysis of Cardiovascular Disease-Related Factors’ Association with 3-Nitrotyrosine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-NT | 3-Nitrotyrosine |
| SLE | Systemic lupus erythematosus |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| ACR | American College of Rheumatology |
| SLEDAI−2K | Systemic Lupus Erythematosus Disease Activity Index |
| SLICC-SDI | Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index |
| DORIS | Definitions of Remission in SLE |
| LLDAS | Lupus Low Disease Activity State |
| PGA | Physician global assessment |
| SCORE2 | Systematic Coronary Risk Evaluation−2 |
| HDL | High-density cholesterol |
| SCORE2-OP | Systematic Coronary Risk Evaluation−2 Older Persons |
| LDL | Low-density cholesterol |
| HOMA | Homeostatic model assessment |
| IR | Insulin resistance |
| cIMT | Carotid intima–media |
| SD | Standard deviation |
| IQR | Interquartile range |
| BMI | Body mass index |
| CRP | C-reactive protein |
| ACAs | Anticardiolipin antibodies |
| ANAs | Antinuclear antibodies |
| ENAs | Extractible nuclear antibodies |
| DMARD | Disease-modifying antirheumatic drug |
| ACVA | Acute cerebrovascular accident |
References
- Ahsan, H. 3-Nitrotyrosine: A Biomarker of Nitrogen Free Radical Species Modified Proteins in Systemic Autoimmunogenic Conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Peluffo, G.; Radi, R. Protein Tyrosine Nitration--Functional Alteration or Just a Biomarker? Free Radic. Biol. Med. 2008, 45, 357–366. [Google Scholar] [CrossRef]
- Daiber, A.; Münzel, T. Increased Circulating Levels of 3-Nitrotyrosine Autoantibodies: Marker for or Maker of Cardiovascular Disease? Circulation 2012, 126, 2371–2373. [Google Scholar] [CrossRef] [PubMed]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A Versatile Oxidative Stress Biomarker for Major Neurodegenerative Diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef]
- Pennathur, S.; Vivekanandan-Giri, A.; Locy, M.L.; Kulkarni, T.; Zhi, D.; Zeng, L.; Byun, J.; De Andrade, J.A.; Thannickal, V.J. Oxidative Modifications of Protein Tyrosyl Residues Are Increased in Plasma of Human Subjects with Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 861–866. [Google Scholar] [CrossRef]
- Restivo, V.; Candiloro, S.; Daidone, M.; Norrito, R.; Cataldi, M.; Minutolo, G.; Caracci, F.; Fasano, S.; Ciccia, F.; Casuccio, A.; et al. Systematic Review and Meta-Analysis of Cardiovascular Risk in Rheumatological Disease: Symptomatic and Non-Symptomatic Events in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Autoimmun. Rev. 2022, 21, 102925. [Google Scholar] [CrossRef] [PubMed]
- Perl, A. Oxidative Stress in the Pathology and Treatment of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Merino de Paz, N.; García-González, M.; Gómez-Bernal, F.; Quevedo-Abeledo, J.C.; de Vera-González, A.; López-Mejias, R.; Abreu-González, P.; Martín-González, C.; González-Gay, M.; Ferraz-Amaro, I. Relationship between Malondialdehyde Serum Levels and Disease Features in a Full Characterized Series of 284 Patients with Systemic Lupus Erythematosus. Antioxidants 2023, 12, 1535. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, 16, 453–465. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Oxidative Stress in SLE T Cells, Is NRF2 Really the Target to Treat? Front. Immunol. 2021, 12, 633845. [Google Scholar] [CrossRef]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative Stress and Its Biomarkers in Systemic Lupus Erythematosus. J. Biomed. Sci. 2014, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibañez, D.; Urowltz, M.B. Systemic Lupus Erythematosus Disease Activity Index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; Hay, E.; et al. The Development and Initial Validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for Systemic Lupus Erythematosus. Arthritis Rheum. 1996, 39, 363–369. [Google Scholar] [CrossRef]
- Mosca, M.; Bombardieri, S. Assessing Remission in Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2006, 24, S99–S104. [Google Scholar]
- van Vollenhoven, R.F.; Bertsias, G.; Doria, A.; Isenberg, D.; Morand, E.; Petri, M.A.; Pons-Estel, B.A.; Rahman, A.; Ugarte-Gil, M.F.; Voskuyl, A.; et al. 2021 DORIS Definition of Remission in SLE: Final Recommendations from an International Task Force. Lupus Sci. Med. 2021, 8, e000538. [Google Scholar] [CrossRef]
- Polachek, A.; Gladman, D.D.; Su, J.; Urowitz, M.B. Defining Low Disease Activity in Systemic Lupus Erythematosus. Arthritis Care Res. 2017, 69, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- ESC Cardiovasc Risk Collaboration; SCORE2 working group. SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004-2006-2011). An Update on Behalf of the Advisory Board of the 3rd, 4th and 5th Watching the Risk Symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Maldonado, G.; Greenland, S. Simulation Study of Confounder-Selection Strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef]
- Oates, J.C.; Christensen, E.F.; Reilly, C.M.; Self, S.E.; Gilkeson, G.S. Prospective Measure of Serum 3-Nitrotyrosine Levels in Systemic Lupus Erythematosus: Correlation with Disease Activity. Proc. Assoc. Am. Physicians 1999, 111, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Gilkeson, G.; Cannon, C.; Oates, J.; Reilly, C.; Goldman, D.; Petri, M. Correlation of Serum Measures of Nitric Oxide Production with Lupus Disease Activity. J. Rheumatol. 1999, 26, 318–324. [Google Scholar] [PubMed]
- Wang, G.; Pierangeli, S.S.; Papalardo, E.; Ansari, G.A.S.; Khan, M.F. Markers of Oxidative and Nitrosative Stress in Systemic Lupus Erythematosus: Correlation with Disease Activity. Arthritis Rheum. 2010, 62, 2064–2072. [Google Scholar] [CrossRef]
- Morgan, P.E.; Sturgess, A.D.; Davies, M.J. Increased Levels of Serum Protein Oxidation and Correlation with Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheum. 2005, 52, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ali, R. Antibodies against Nitric Oxide Damaged Poly L-Tyrosine and 3-Nitrotyrosine Levels in Systemic Lupus Erythematosus. J. Biochem. Mol. Biol. 2006, 39, 189–196. [Google Scholar] [CrossRef]
- Khan, F.; Siddiqui, A.A.; Ali, R. Measurement and Significance of 3-Nitrotyrosine in Systemic Lupus Erythematosus. Scand. J. Immunol. 2006, 64, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized Phospholipids, Lp(a) Lipoprotein, and Coronary Artery Disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, J.Y.; Moon, J.Y.; Ahn, E.Y.; Lee, E.Y.; Lee, E.B.; Cho, K.H.; Song, Y.W. Altered Lipoproteins in Patients with Systemic Lupus Erythematosus Are Associated with Augmented Oxidative Stress: A Potential Role in Atherosclerosis. Arthritis Res. Ther. 2016, 18, 306. [Google Scholar] [CrossRef]
- Davies, M.J. Protein Nitration in the Artery Wall: A Contributor to Cardiovascular Disease? Redox Biochem. Chem. 2024, 8, 100032. [Google Scholar] [CrossRef]
| SLE patients | |
| (n = 214) | |
| 3-Nitrotyrosine, ng/mL | 5.9 (4.7–8.0) |
| Age, years | 51 ± 12 |
| Female, n (%) | 194 (91) |
| Body mass index, kg/m2 | 28 ± 6 |
| Abdominal circumference, cm | 91 ± 15 |
| Waist circumference, cm | 100 ± 15 |
| Waist to hip ratio | 0.92 ± 0.33 |
| Cardiovascular co-morbidity | |
| Smoking, n (%) | 40 (19) |
| Diabetes, n (%) | 11 (5) |
| Hypertension, n (%) | 82 (38) |
| Obesity, n (%) | 58 (27) |
| Metabolic syndrome, n (%) | 92 (44) |
| Statins, n (%) | 61 (29) |
| Aspirin, n (%) | 59 (28) |
| SCORE2, % | 2.1 (1.1–3.8) |
| SCORE2 categories | |
| Low risk | 171 (80) |
| Moderate risk | 28 (13) |
| High risk | 15 (7) |
| Carotid ultrasound | |
| Carotid intima–media thickness, microns | 657 ± 144 |
| Carotid plaque, n (%) | 69 (44) |
| Laboratory data | |
| CRP, mg/dL | 1.86 (0.8–4.14) |
| Cholesterol, mg/dL | 183 ± 40 |
| Triglycerides, mg/dL | 112 ± 64 |
| HDL-cholesterol, mg/dL | 55 ± 15 |
| LDL-cholesterol, mg/dL | 106 ± 33 |
| LDL:HDL-cholesterol ratio | 2.06 ± 0.82 |
| Non-HDL-cholesterol, mg/dL | 128 ± 38 |
| Lipoprotein A, mg/dL | 32 (11–85) |
| Apolipoprotein A1, mg/dL | 157 ± 28 |
| Apolipoprotein B, mg/dL | 87 ± 23 |
| Apo B:Apo A1 ratio | 0.57 ± 0.17 |
| Atherogenic index | 3.52 ± 1.09 |
| Insulin resistance indices | |
| Glucose, mg/dL | 92 ± 17 |
| Insulin, µU/mL | 14 ± 17 |
| C-peptide, ng/mL | 2.4 ± 1.69 |
| HOMA2-IR | 1.10 (0.70–1.80) |
| HOMA2-S% | 93 (5–150) |
| HOMA2-B%-C-peptide | 122 (95–153) |
| SLE-related data | |
| Disease duration, years | 18 ± 11 |
| CRP, mg/dL | 1.9 (0.8–4.1) |
| SLICC-DI | 1 (0–1) |
| SLICC-DI >=1, n (%) | 113 (53) |
| SLEDAI−2K | 2 (0–4) |
| SLEDAI categories, n (%) | |
| No activity, n (%) | 80 (37) |
| Mild, n (%) | 111 (52) |
| Moderate to high, n (%) | 23 (11) |
| DORIS, n (%) | 139 (65) |
| LLDAS, n (%) | 146 (68) |
| Auto-antibody profile | |
| Anti-DNA positive, n (%) | 160 (75) |
| Anti-ENA positive, n (%) | 151 (72) |
| Anti-SSA, n (%) | 75 (35) |
| Anti-SSB, n (%) | 33 (15) |
| Anti-Sm, n (%) | 39 (18) |
| Anti-RNP, n (%) | 63 (30) |
| Anti-ribosome | 25 (12) |
| Anti-nucleosome | 44 (21) |
| Anti-histone | 42 (20) |
| Antiphospholipid syndrome, n (%) | 37 (17) |
| Antiphospholipid autoantibodies, n (%) | 69 (32) |
| Lupus anticoagulant, n (%) | 48 (23) |
| ACA IgM, n (%) | 29 (14) |
| ACA IgG, n (%) | 29 (14) |
| Anti beta2 glycoprotein IgM, n (%) | 20 (10) |
| Anti beta2 glycoprotein IgG, n (%) | 21 (10) |
| Current prednisone, n (%) | 74 (35) |
| Prednisone, mg/day | 5 (2.5–5) |
| Hydroxychloroquine, n (%) | 154 (72) |
| Methotrexate, n (%) | 28 (13) |
| Mycophenolate mofetil, n (%) | 26 (12) |
| Azathioprine, n (%) | 20 (9) |
| Rituximab, n (%) | 5 (2) |
| Belimumab, n (%) | 27 (13) |
| 1/3-Nitrotyrosine × 1000, ng/mL | ||||
|---|---|---|---|---|
| Beta Coef. (95% CI), p | ||||
| Univariable | Multivariable | |||
| Age, years | 0.06 (−0.72–0.84) | 0.88 | ||
| Female | −22 (−53–10) | 0.18 | ||
| Body mass index, kg/m2 | 1.53 (0.03–3.03) | 0.046 | ||
| Abdominal circumference, cm | 0.42 (−0.21–1.06) | 0.19 | ||
| Waist circumference, cm | 0.51 (−0.12–1.14) | 0.16 | ||
| Waist to hip ratio | −0.55 (−28.82–27.72) | 0.97 | ||
| Cardiovascular co-morbidity | ||||
| Smoking | 4 (−19–29) | 0.69 | ||
| Diabetes | −16 (−58–26) | 0.45 | ||
| Hypertension | 2 (−17–21) | 0.86 | ||
| Obesity | 21 (0.42–42) | 0.046 | ||
| Dyslipidemia | 0.59 (−18.94–20.12) | 0.95 | ||
| Statins | 5 (−16–26) | 0.65 | ||
| Aspirin | −3 (−24–18) | 0.81 | ||
| SLE-related data | ||||
| Disease duration, years | 1.04 (0.21–1.87) | 0.015 | 0.93 (0.09–1.77) | 0.030 |
| CRP, mg/dl | −0.059 (−0.19–0.08) | 0.40 | ||
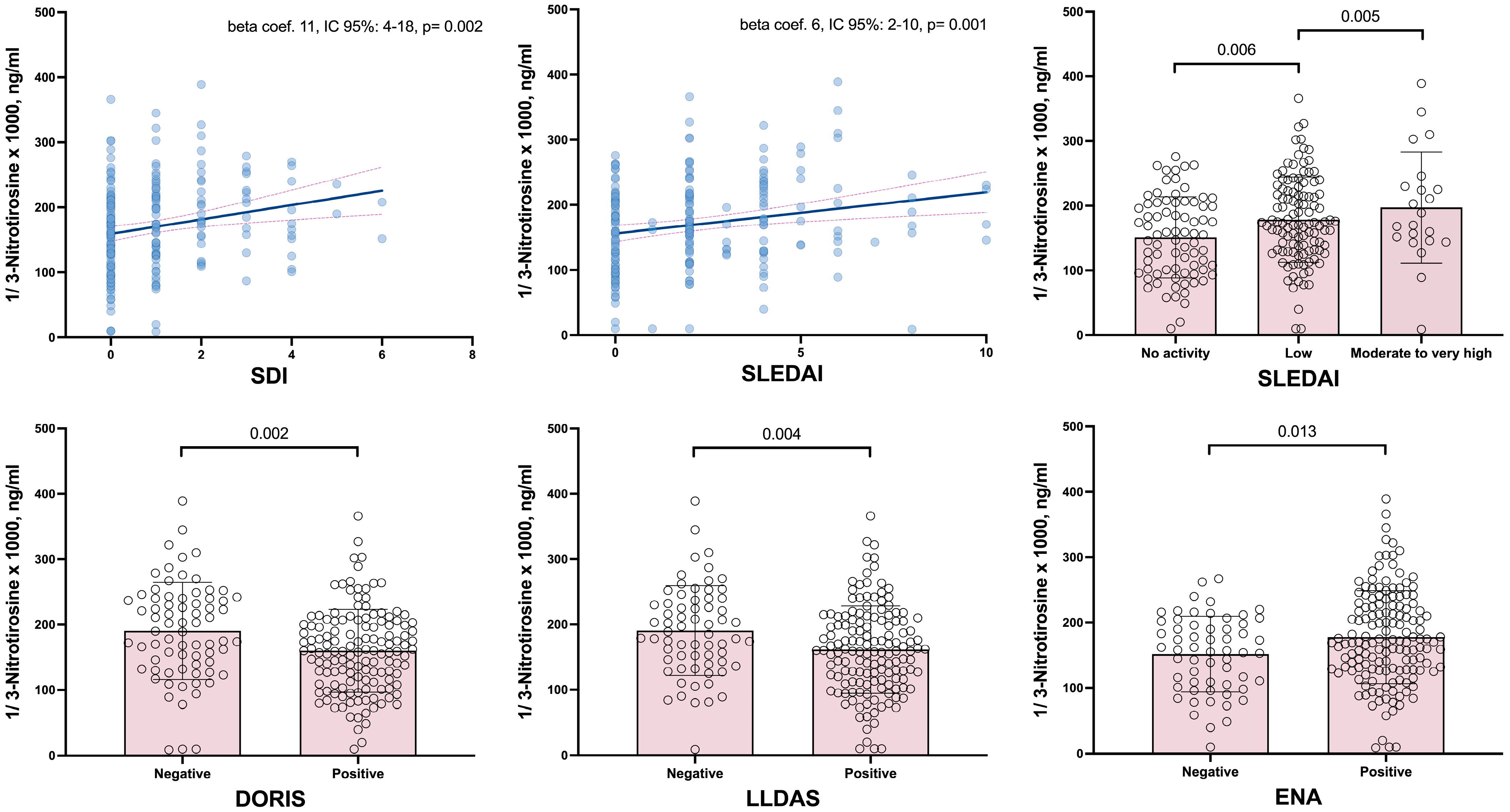
| SLICC-DI | 11 (4–18) | 0.002 | 10 (3–17) | 0.006 |
| SLICC-DI >=1 | 20 (11–48) | 0.002 | 27 (9–45) | 0.004 |
| SLEDAI−2K | 6 (2–10) | 0.001 | 6. (3–10) | 0.001 |
| SLEDAI categories | ||||
| No activity | ref. | Ref. | ||
| Mild | 28 (8–47) | 0.006 | 27 (8–47) | 0.006 |
| Moderate to very high | 46 (14–78) | 0.005 | 46 (15–78) | 0.004 |
| DORIS | −30 (−50–(−11)) | 0.002 | −28 (−47–(−9)) | 0.004 |
| LLDAS | −30 (−49–(−9)) | 0.004 | −27 (−47–(−7)) | 0.008 |
| Auto-antibody profile | ||||
| Anti-DNA positive | 20 (−0.75–42) | 0.059 | 18 (−3–40) | 0.089 |
| Anti-ENA positive | 26 (5–47) | 0.013 | 27 (6–47) | 0.011 |
| Anti-SSA | 7 (−12–27) | 0.73 | ||
| Anti-SSB | 18 (−8–45) | 0.17 | 21 (−5–47) | 0.12 |
| Anti-Sm | 17 (−7–41) | 0.16 | 14 (−10–38) | 0.26 |
| Anti-RNP | 26 (6–46) | 0.011 | 25 (5–46) | 0.013 |
| Anti-ribosome | 36 (7–64) | 0.014 | 36 (8–64) | 0.012 |
| Anti-nucleosome | 31 (8–54) | 0.008 | 31 (8–53) | 0.008 |
| Anti-histone | 13 (−10–36) | 0.28 | ||
| Antiphospholipid syndrome | −8 (−33–16) | 0.50 | ||
| Antiphospholipid autoantibodies | −12 (−34–10) | 0.29 | ||
| Lupus anticoagulant | −29 (−51–(−7)) | 0.010 | −34 (−56–(−13)) | 0.002 |
| ACA IgM | −12 (−39–14) | 0.36 | ||
| ACA IgG | 4 (−23–31) | 0.79 | ||
| Anti beta2 glycoprotein IgM | −13 (−44–19) | 0.43 | ||
| Anti beta2 glycoprotein IgG | 2 (−29–33) | 0.91 | ||
| Current prednisone | 12 (−8–31) | 0.24 | ||
| Prednisone, mg/day | 2 (−3–6) | 0.54 | ||
| Hydroxychloroquine | −1 (−21–20) | 0.96 | ||
| Methotrexate | 4 (−23–32) | 0.76 | ||
| Mycophenolate mofetil | −4 (−33–25) | 0.77 | ||
| Azathioprine | 11 (−21–42) | 0.51 | ||
| Rituximab | 14 (−48–75) | 0.44 | ||
| Belimumab | 22 (−5–50) | 0.11 | 25 (−3–53) | 0.077 |
| 1/3-Nitrotyrosine × 1000, ng/mL | ||||
|---|---|---|---|---|
| Beta coef. (95% CI), p | ||||
| Univariable | Multivariable | |||
| Lipid profile | ||||
| Cholesterol, mg/dL | 0.2 (−0.058–0.42) | 0.14 | 0.2 (−0.04–0.4) | 0.097 |
| Triglycerides, mg/dL | 0.2 (0.06–0.4) | 0.009 | 0.2 (0.03–0.4) | 0.024 |
| HDL-cholesterol, mg/dL | −0.6 (−1.2–0.09) | 0.089 | −0.5 (−1–0.2) | 0.17 |
| LDL-cholesterol, mg/dL | 0.2 (−0.05–0.5) | 0.14 | 0.2 (−0.02–0.5) | 0.070 |
| LDL:HDL-cholesterol ratio | 16 (5–27) | 0.007 | 15 (4–27) | 0.009 |
| Non-HDL-cholesterol, mg/dL | 0.3 (0.04–0.6) | 0.023 | 0.3 (−0.05–0.6) | 0.021 |
| Lipoprotein A, mg/dL | 0.02 (−0.1–0.2) | 0.70 | ||
| Apolipoprotein A1, mg/dL | −0.3 (−0.6–0.06) | 0.11 | −0.2 (−0.6–0.09) | 0.15 |
| Apolipoprotein B, mg/dL | 0.5 (0.06–0.9) | 0.025 | 0.5 (0.05–0.9) | 0.029 |
| Apo B:Apo A1 ratio | 80 (27–134) | 0.004 | 76 (22–130) | 0.006 |
| Atherogenic index | 15 (6–24) | 0.001 | 14 (5–23) | 0.002 |
| Insulin resistance indices | ||||
| Glucose, mg/dL | 0.1 (−0.4–0.7) | 0.65 | ||
| C-peptide, ng/mL | 2 (−4–7) | 0.59 | ||
| Insulin, µU/mL | 0.2 (−0.4–0.8) | 0.57 | ||
| HOMA2-IR | 2 (−4–7) | 0.53 | ||
| HOMA2-S% | −0.005 (−0.1–0.1) | 0.93 | ||
| HOMA2-B%-C-peptide | −0.01 (−0.2–0.2) | 0.91 | ||
| Carotid ultrasound | ||||
| cIMT, microns | −0.3 (−0.1–0.05) | 0.44 | ||
| Carotid plaque | 15 (−6–36) | 0.17 | 14 (−7–36) | 0.20 |
| SCORE2 calculator | 0.4 (−2–3) | 0.77 | ||
| Low risk | ref. | ref. | ||
| Moderate risk | −0.5 (−28–27) | 0.97 | −6 (−34–22) | 0.67 |
| High risk | 44 (6–83) | 0.025 | 37 (−2–76) | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quevedo-Abeledo, J.C.; Hernández-Díaz, M.; García-González, M.; Gómez-Bernal, F.; Almeida-Santiago, C.; Heras-Recuero, E.; de Vera-González, A.; González-Delgado, A.; Abreu-González, P.; Tejera-Segura, B.; et al. Serum 3-Nitrotyrosine in the Cardiovascular Disease of Patients with Systemic Lupus Erythematosus. Antioxidants 2025, 14, 739. https://doi.org/10.3390/antiox14060739
Quevedo-Abeledo JC, Hernández-Díaz M, García-González M, Gómez-Bernal F, Almeida-Santiago C, Heras-Recuero E, de Vera-González A, González-Delgado A, Abreu-González P, Tejera-Segura B, et al. Serum 3-Nitrotyrosine in the Cardiovascular Disease of Patients with Systemic Lupus Erythematosus. Antioxidants. 2025; 14(6):739. https://doi.org/10.3390/antiox14060739
Chicago/Turabian StyleQuevedo-Abeledo, Juan C., Marta Hernández-Díaz, María García-González, Fuensanta Gómez-Bernal, Cristina Almeida-Santiago, Elena Heras-Recuero, Antonia de Vera-González, Alejandra González-Delgado, Pedro Abreu-González, Beatriz Tejera-Segura, and et al. 2025. "Serum 3-Nitrotyrosine in the Cardiovascular Disease of Patients with Systemic Lupus Erythematosus" Antioxidants 14, no. 6: 739. https://doi.org/10.3390/antiox14060739
APA StyleQuevedo-Abeledo, J. C., Hernández-Díaz, M., García-González, M., Gómez-Bernal, F., Almeida-Santiago, C., Heras-Recuero, E., de Vera-González, A., González-Delgado, A., Abreu-González, P., Tejera-Segura, B., Martín-González, C., González-Gay, M. Á., & Ferraz-Amaro, I. (2025). Serum 3-Nitrotyrosine in the Cardiovascular Disease of Patients with Systemic Lupus Erythematosus. Antioxidants, 14(6), 739. https://doi.org/10.3390/antiox14060739







