Enhancement of Hypoxia Tolerance of Gibel Carp (Carassius auratus gibelio) via a Ferroporphyrin-Rich Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Hypoxia Stress Challenge
2.2. Sample Collection
2.3. Transmission Electron Microscopy Analysis
2.4. Biochemical Analysis
2.5. Genes Expression Level Analysis of Liver
2.6. Data Processing
3. Results
3.1. The Ion Concentrations of Plasma and the Activity of Na+/K+-ATPase of Liver After Hypoxia Stress
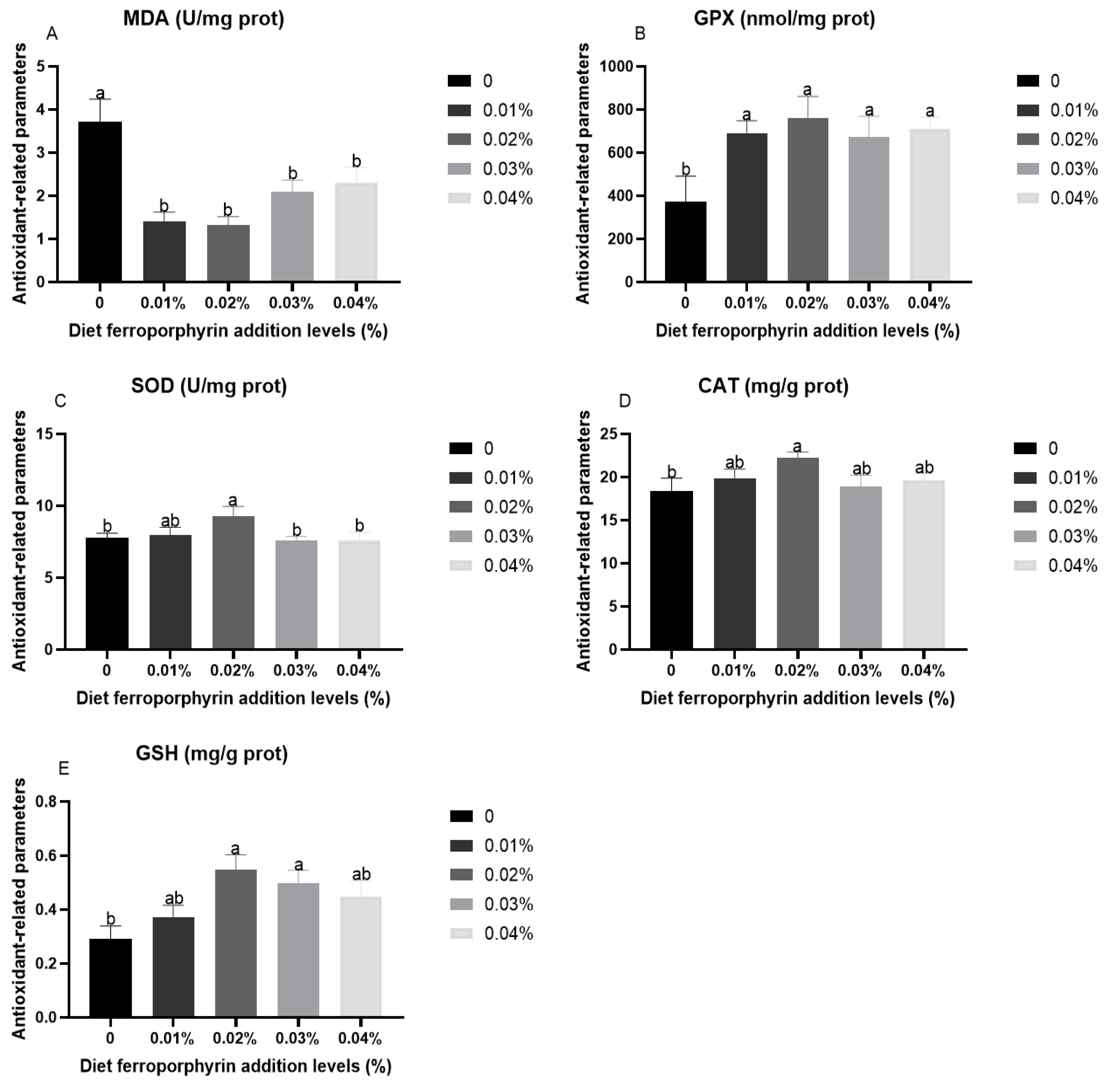
3.2. Antioxidant-Related Parameters in Liver After Hypoxia Stress
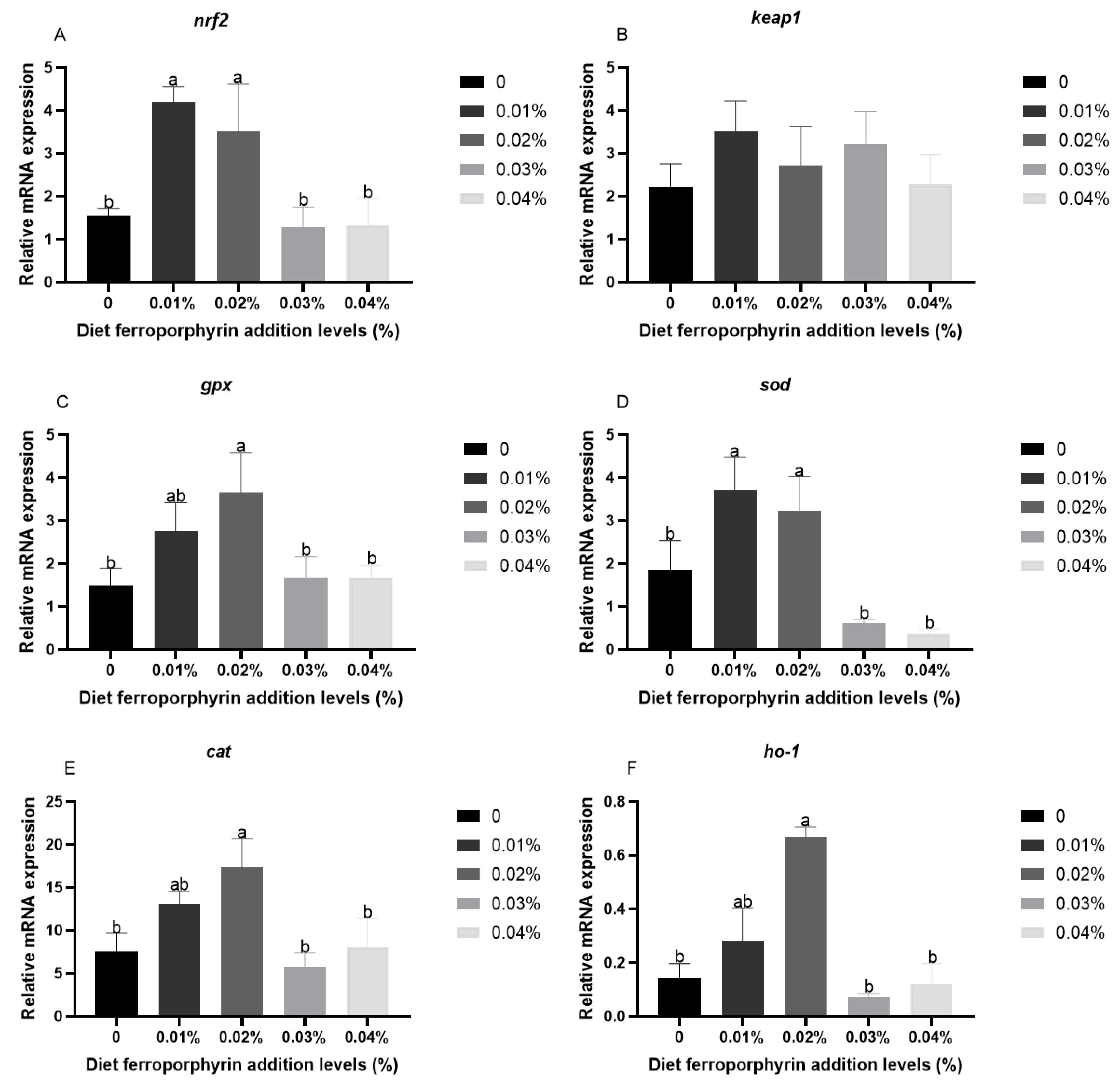
3.3. The Expression Levels of Antioxidant-Related Genes in Liver After Hypoxia Stress
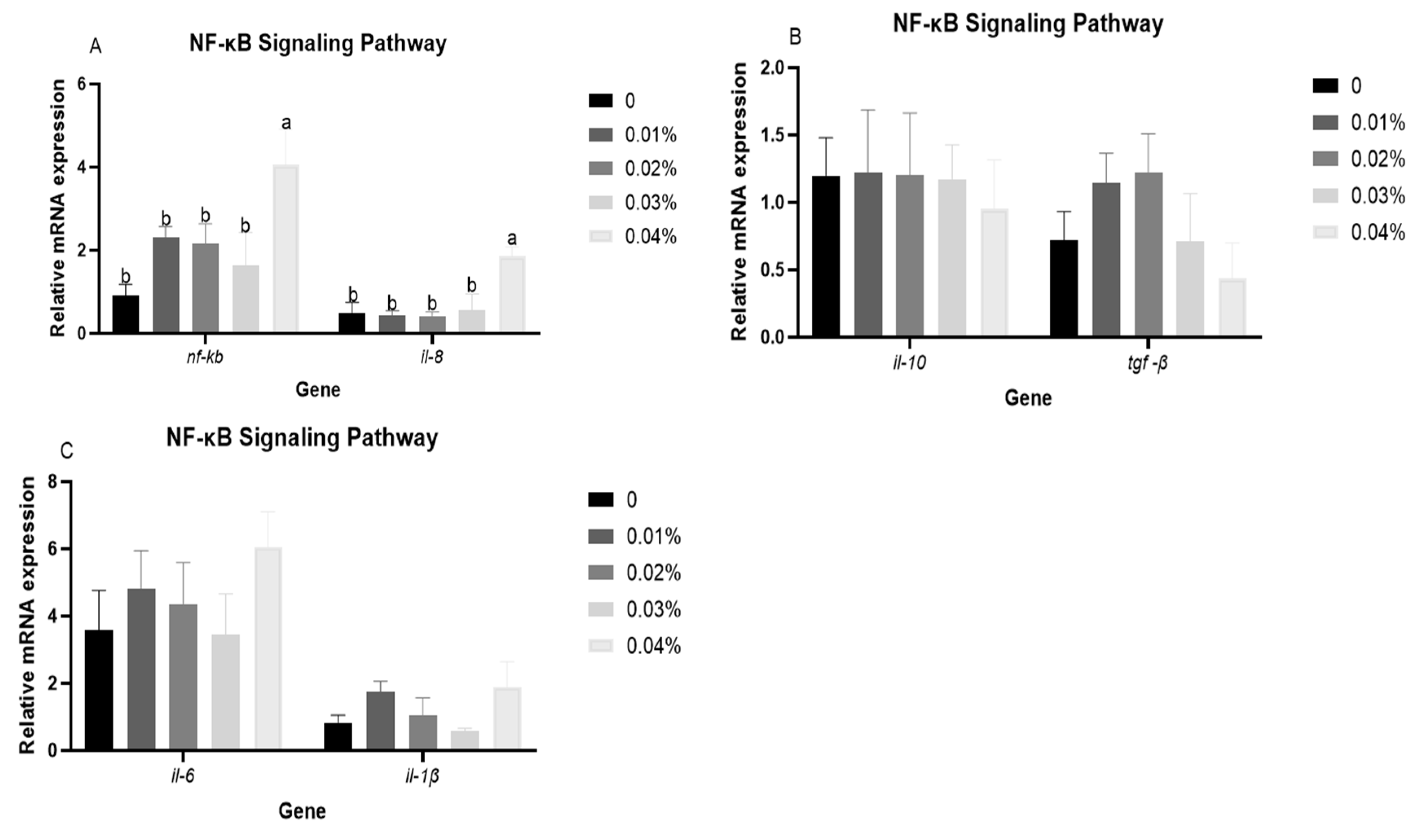
3.4. The Expression Levels of NF-κB Signalling Pathway-Related Genes in Liver After Hypoxia Stress
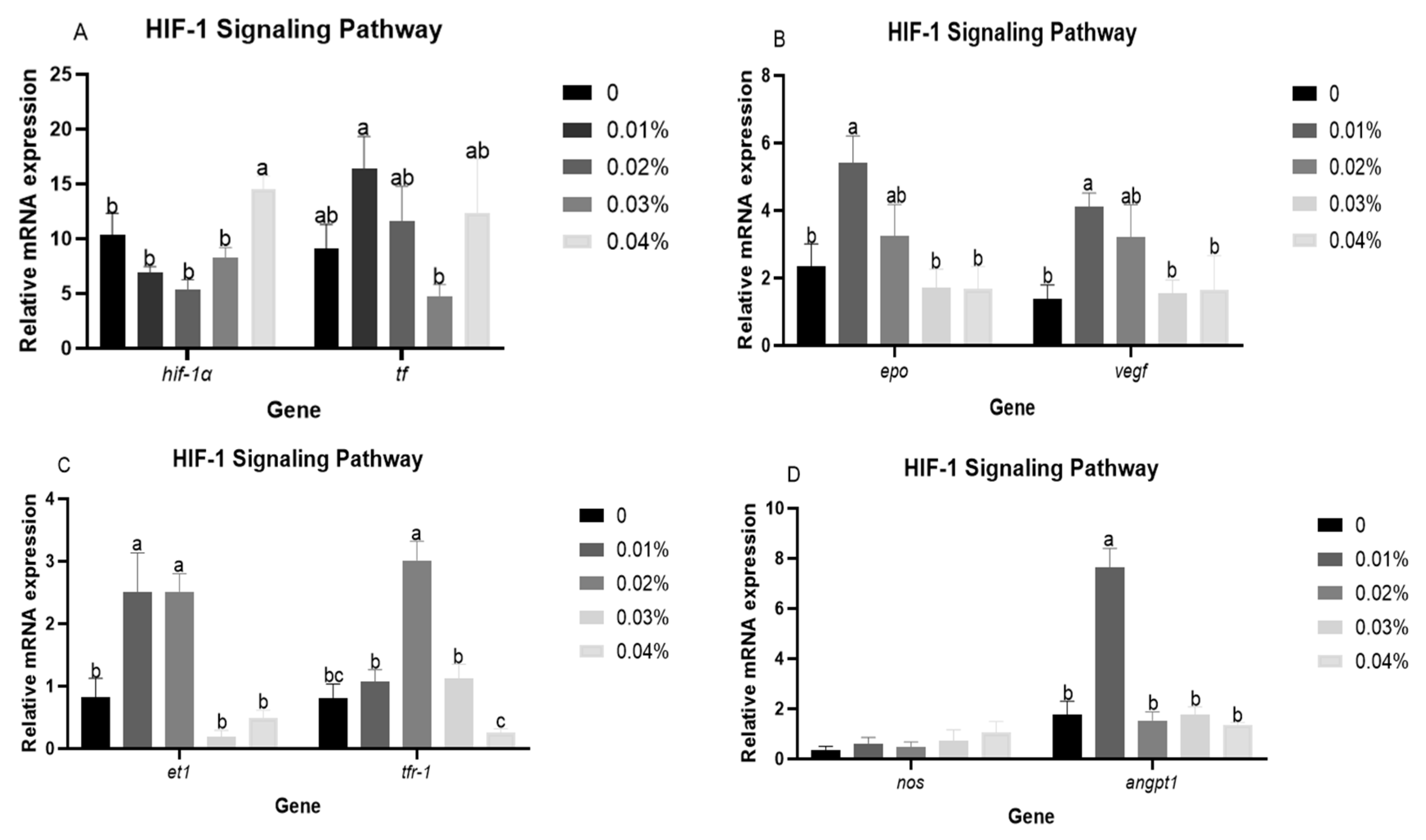
3.5. The Expression Levels of HIF-1 Signalling Pathway-Related Genes in Liver After Hypoxia Stress
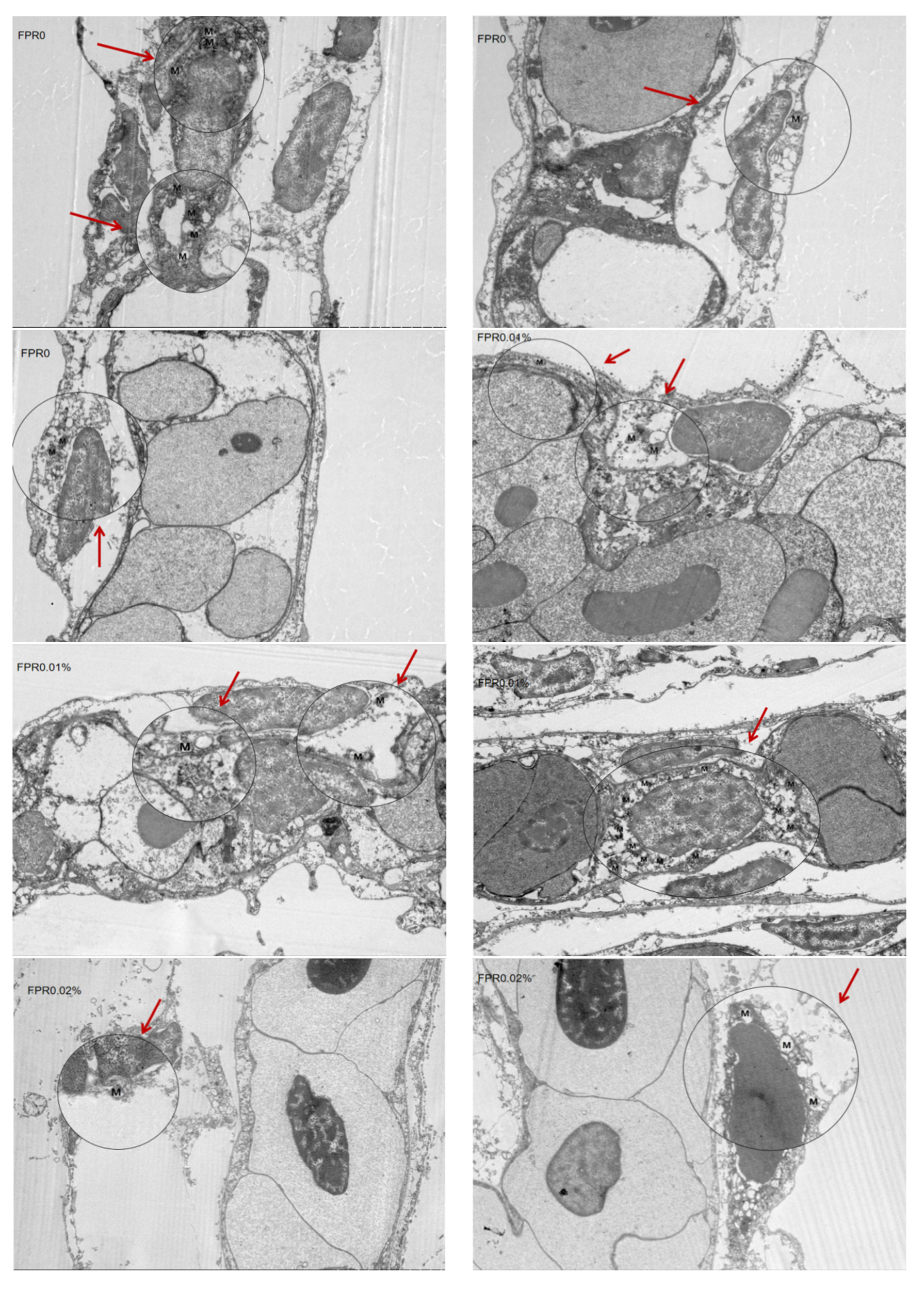
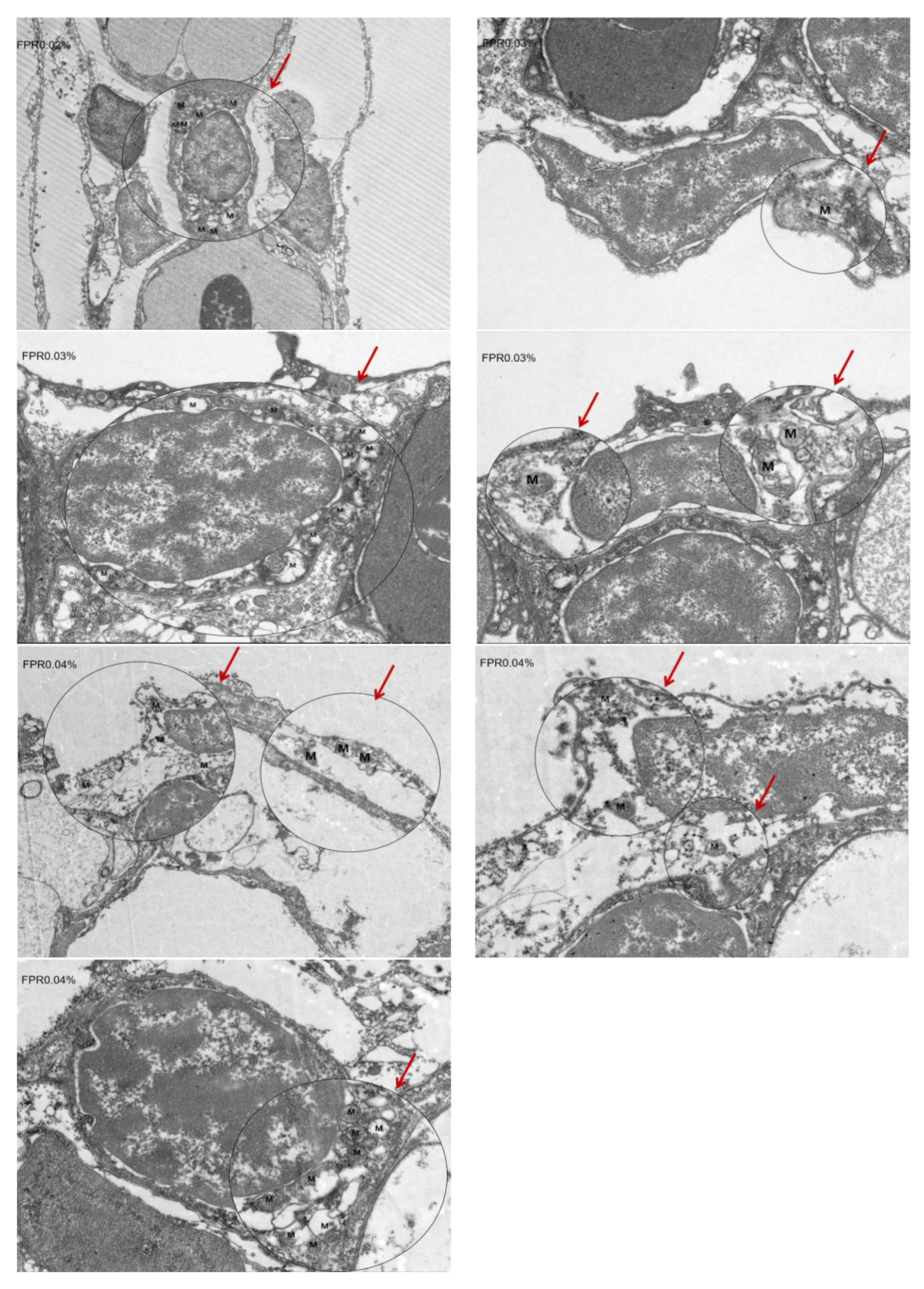
3.6. The SR of Gibel Carp and Number of Mitochondria in Gill After Hypoxia Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natarajan, R.; Fisher, B.J.; Fowler, A.A., III. Hypoxia inducible factor-1 modulates hemin-induced IL-8 secretion in microvascular endothelium. Microvasc. Res. 2007, 3, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.N.S.; Pereira, F.A. Effect of dietary iron overload on Photobacterium damselae ssp. piscicida pathogenicity in sea bass, Dicentrarchus labrax (L.). J. Fish Dis. 2004, 27, 673–676. [Google Scholar] [CrossRef]
- Wu, X.L.; Li, D.P.; Lu, J.M.; Liu, L.; Yang, Q.S.; Tang, R.; Zhang, X.; Li, L. Adaptation strategies of juvenile grass carp (Ctenopharyngodon idella) facing different dissolved oxygen concentrations in a recirculating aquaculture system. Water Biol. Secur. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Sun, H.J.; Li, J.J.; Tang, L.S.; Yang, Z. Responses of crucian carp Carassius auratus to long-term exposure to nitrite and low dissolved oxygen levels. Biochem. Syst. Ecol. 2012, 44, 224–232. [Google Scholar] [CrossRef]
- Zhou, D.S.; Wang, C.L.; Zheng, J.X.; Zhao, J.H.; Wei, S.S.; Xiong, Y.F.; Limbu, S.M.; Kong, Y.Q.; Cao, F.; Ding, Z.L. Dietary thiamine modulates carbohydrate metabolism, antioxidant status, and alleviates hypoxia stress in oriental river prawn Macrobrachium nipponense (de Haan). Fish Shellfish Immunol. 2022, 131, 42–53. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, X.D.; Qi, C.L.; Li, E.E.; Du, Z.Y.; Qin, J.G.; Chen, L.Q. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Dan, X.M.; Yan, G.J.; Zhang, A.J.; Cao, Z.D.; Fu, S.J. Effects of stable and diel-cycling hypoxia on hypoxia tolerance, postprandial metabolic response, and growth performance in juvenile qingbo (Spinibarbus sinensis). Aquaculture 2014, 428–429, 21–28. [Google Scholar] [CrossRef]
- Shen, Y.W.; You, W.W.; Luo, X.; Lu, Y.; Huang, M.Q.; Ke, C.H. An overview of the mechanisms underlying hypoxia tolerance differences in aquatic animals and their inspirations for aquaculture. Rev. Fish Biol. Fish. 2023, 33, 1223–1236. [Google Scholar] [CrossRef]
- Xiao, W.H. The hypoxia signaling pathway and hypoxic adaptation in fishes. Sci. China Life Sci. 2015, 58, 148–155. [Google Scholar] [CrossRef]
- Mao, X.J.; Chen, W.W.; Long, X.M.; Pan, X.M.; Liu, G.Q.; Hu, W.G.; Gu, D.C.; Tan, Q.S. Effect of dietary iron (Fe) level on growth performance and health status of largemouth bass (Micropterus salmoides). Aquaculture 2024, 581, 740446. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Wang, M.M.; Guo, H.X.; Liu, W.B.; Jiang, G.Z. Metabolism, Antioxidant and Immunity in Acute and Chronic hypoxia Stress and the Improving Effect of Vitamin C in the Channel Catfish (Ictalurus Punctatus). Fish Physiol. Biochem. 2024, 50, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoo, Y.M.; Lee, B.; Jeong, S.H.; Tran, D.N.; Jeung, E.B. Melatonin mitigates the adverse effect of hypoxia during myocardial differentiation in mouse embryonic stem cells. J. Vet. Sci. 2021, 22, e54. [Google Scholar] [CrossRef] [PubMed]
- Galeana-López, J.A.; Lizárraga-Velázquez, C.E.; Hernández, C.; Leyva-López, N.; Heredia, J.B. Corn Husk Phenolics Modulate Hepatic Antioxidant Response in Nile Tilapia (Oreochromis niloticus) Exposed to Hypoxia. Molecules 2021, 26, 6161. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, D.; Xu, J.; Xu, C.X.; Dong, C.; Liu, Q.W.; Deng, S.H.; Zhao, J.; Zhang, W.; Chen, X.J. Effects of Dietary Factors on the Pharmacokinetics of 58Fe-Labeled Hemin After Oral Administration in Normal Rats and the Iron-Deficient Rats. Biol. Trace Elem. Res. 2013, 153, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders. Int. J. Mol. Sci. 2021, 22, 5509. [Google Scholar] [CrossRef]
- Foresti, R.; Goatly, H.; Green, C.J.; Motterlini, R. Role of heme oxygenase-1 in hypoxia-reoxygenation: Requirement of substrate heme to promote cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1976–H1984. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Kumar, D.; Jena, G.R.; Ram, M.; Lingaraju, M.C.; Singh, V.; Prasad, R.; Kumawat, S.; Kant, V.; Gupta, P.; Tandan, S.K.; et al. Hemin attenuated oxidative stress and inflammation to improve wound healing in diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1435–1445. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Tiwari, S. Featured article: Induction of heme oxygenase with hemin improves pericardial adipocyte morphology and function in obese Zucker rats by enhancing proteins of regeneration. Exp. Biol. Med. 2015, 240, 45–57. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhang, Y.J.; Han, L.L.; Guo, L.; Zhong, H.; Wang, J.W. Hemin ameliorates influenza pneumonia by attenuating lung injury and regulating the immune response. Int. J. Antimicrob. Agents 2017, 49, 45–52. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, W.H.; Kay, H.Y.; Kim, E.; Moon, A.; Kim, S.G. Hemin, an iron-binding porphyrin, inhibits HIF-1α induction through its binding with heat shock protein 90. Int. J. Cancer 2012, 130, 716–727. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yu, Y.; Qin, X.W.; Zeng, R.Y.; Wang, Y.Y.; Li, Z.M.; Mi, S.; Weng, S.P.; Guo, C.J.; He, J.G. Identification and functional analysis of the Mandarin fish (Siniperca chuatsi) hypoxia-inducible factor-1α involved in the immune response. Fish Shellfish Immunol. 2019, 92, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Miao, L.H.; Zhang, W.X.; Pan, W.J.; Liang, H.L.; Ge, X.P.; Xu, Y.S.; Liu, B.; Ren, M.C.; Zhou, Q.L.; et al. Effect of nitrite exposure on oxygen-carrying capacity and gene expression of NF-κB/HIF-1α pathway in gill of bighead carp (Aristichthys nobilis). Aquacult. Int. 2018, 26, 899–911. [Google Scholar] [CrossRef]
- Huang, Z.H.; Guan, W.L.; Wei, X.B.; Chen, R.C.; Lyu, X.M.; Zheng, G.H.; Mao, L.C. Examination of the role of hypoxia-inducible factor-1α (HIF-1α) in preventing hemocyte apoptosis in whiteleg shrimp (Litopenaeus vannamei). Aquaculture 2023, 563, 738905. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Lu, R.; Santamaria, A.; Zhou, J.C.; Ke, T.; Karganov, M.Y.; Tsatsakis, A.; Golokhvast, K.S.; Bowman, A.B.; et al. The role of hypoxia-inducible factor 1 alpha (HIF-1α) modulation in heavy metal toxicity. Arch. Toxicol. 2023, 97, 1299–1318. [Google Scholar] [CrossRef]
- Ni, Q.G.; Wang, D.; Xie, L.L.; Ge, H.X.; Dong, Z.G. Unravelling the characterization of hypoxia-inducible factor-1α (HIF-1α) and antioxidant enzymes in clam Cyclina sinensis in response to hypoxia. Aquacult. Res. 2022, 53, 5937–5945. [Google Scholar] [CrossRef]
- Hänze, J.; Eul, B.G.; Savai, R.; Krick, S.; Goyal, P.; Grimminger, F.; Seeger, W.; Rose, F. RNA interference for HIF-1α inhibits its downstream signalling and affects cellular proliferation. Biochem. Biophys. Res. Commun. 2003, 312, 571–577. [Google Scholar] [CrossRef]
- Metzen, E.; Zhou, J.; Jelkmann, W.; Fandrey, J.; Brüne, B. Nitric Oxide Impairs Normoxic Degradation of HIF-1α by Inhibition of Prolyl Hydroxylases. Mol. Biol. Cell 2003, 14, 3470–3481. [Google Scholar] [CrossRef]
- Rahman, M.S.; Thomas, P. Molecular cloning, characterization and expression of two hypoxia-inducible factor alpha subunits, HIF-1α and HIF-2α, in a hypoxia-tolerant marine teleost, Atlantic croaker (Micropogonias undulatus). Gene 2007, 396, 273–282. [Google Scholar] [CrossRef]
- Mladineo, I.; Block, B.A. Expression of Hsp70, Na+/K+ ATP-ase, HIF-1α, IL-1β and TNF-α in captive Pacific bluefin tuna (Thunnus orientalis) after chronic warm and cold exposure. J. Exp. Mar. Biol. Ecol. 2009, 374, 51–57. [Google Scholar] [CrossRef]
- Li, H.T.; Lu, L.; Wu, M.; Xiong, X.Q.; Luo, L.; Ma, Y.T.; Liu, Y. The effects of dietary extract of mulberry leaf on growth performance, hypoxia-reoxygenation stress and biochemical parameters in various organs of fish. Aquacult. Rep. 2020, 18, 100494. [Google Scholar] [CrossRef]
- Wu, L.Y.; Xu, W.J.; Li, H.Y.; Dong, B.; Geng, H.C.; Jin, J.Y.; Han, D.; Liu, H.K.; Zhu, X.M.; Yang, Y.X.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Yang, Y.; Han, D.; Jin, J.; Xie, S. Effect of substitution of dietary fishmeal by soya bean meal on different sizes of gibel carp (Carassius auratus gibelio): Nutrient digestibility, growth performance, body composition and morphometry. Aquacult. Nutr. 2016, 22, 142–157. [Google Scholar] [CrossRef]
- Wang, B.; Mao, H.; Zhao, J.; Liu, Y.; Wang, Y.; Du, X. Influences of oxygen and temperature interaction on the antibacterial activity, antioxidant activity, serum biochemical indices, blood indices and growth performance of crucian carp. Peerj 2023, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Rong, Z.; Zhao, X.; Gao, X.; Hou, Z.; Zhang, L.; Khor, W.; Xu, Y.; Chen, L.; Wu, C. Transcriptome analysis reveals hypoxic response key genes and modules as well as adaptive mechanism of crucian carp (Carassius auratus) gill under hypoxic stress. Front. Immunol. 2025, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, L.; Liang, H.L.; Ren, M.C.; Mi, H.F.; Huang, D.Y.; Gu, J.Z. Effects of Dietary Ferroporphyrin Supplementation on Growth Performance, Antioxidant Capacity, Immune Response, and Oxygen-Carrying Capacity in Gibel Carp (Carassius Auratus Gibelio). Animals 2024, 14, 3104. [Google Scholar] [CrossRef]
- Yang, K.C.; Qi, X.Z.; He, M.S.; Song, K.G.; Luo, F.; Qu, X.Y.; Wang, G.X.; Ling, F. Dietary supplementation of salidroside increases immune response and disease resistance of Gibel Carp (Carassius auratus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 106, 1–7. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Q.; Wang, R.; Sun, K.; Li, S.; Lin, G.; Lei, P.; Xu, H. Effect of dietary poly-γ-glutamic acid on growth, digestive enzyme activity, antioxidant capacity, and TOR pathway gene expression of gibel carp (Carassius auratus gibelio). Aquacult. Rep. 2022, 27, 101412. [Google Scholar] [CrossRef]
- Gu, Y.P.; Chen, K.; Xi, B.W.; Xie, J.; Bing, X.W. Protective effects of paeonol against lipopolysaccharide-induced liver oxidative stress and inflammation in gibel carp (Carassius auratus gibelio). Comp. Biochem. Physiol. C 2022, 257, 109339. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Suwannalers, P.; Aeksiri, N.; Ratanasut, K.; Chitmanat, C.; Inyawilert, W.; Phromkunthong, W.; Kaneko, G. Effects of dietary red pepper extracts on growth, hematology, pigmentation, disease resistance, and growth- and immune-related gene expressions of goldfish (Carassius auratus). Anim. Feed Sci. Technol. 2023, 301, 115658. [Google Scholar] [CrossRef]
- Obirikorang, K.A.; Acheampong, J.N.; Duodu, C.P.; Skov, P.V. Growth, metabolism and respiration in Nile tilapia (Oreochromis niloticus) exposed to chronic or periodic hypoxia. Comp. Biochem. Phys. A 2020, 248, 110768. [Google Scholar] [CrossRef]
- Qin, F.J.; Shi, M.M.; Yuan, H.X.; Yuan, L.X.; Lu, W.H.; Zhang, J.; Tong, J.; Song, X.H. Dietary nano-selenium relieves hypoxia stress and, improves immunity and disease resistance in the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2016, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Zhang, L.; Liang, H.L.; Huang, D.Y.; Ren, M.C. Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo). Antioxidants 2024, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, H.G.; Wei, Y.L.; Liang, M.Q. Effects of acute hypoxia on nutrient metabolism and physiological function in turbot, Scophthalmus maximus. Fish Physiol. Biochem. 2024, 50, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Shuang, L.; Chen, S.L.; Ren, C.; Su, X.L.; Xu, X.N.; Zheng, G.D.; Zou, S.M. Effects of hypoxia and reoxygenation on oxidative stress, histological structure, and apoptosis in a new hypoxia-tolerant variety of blunt snout bream (Megalobrama amblycephala). Comp. Biochem. Phys. A 2023, 278, 111358. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Jiang, X.Y.; Kong, X.H.; Di, G.L.; Nie, G.X.; Li, X.J. Effects of hypoxia on lysozyme activity and antioxidant defences in the kidney and spleen of Carassius auratus. Aquac. Res. 2017, 48, 223–235. [Google Scholar] [CrossRef]
- Shi, Q.C.; Yu, C.Q.; Zhu, D.S.; Li, S.K.; Wen, X.B. Effects of dietary Sargassum horneri on resisting hypoxia stress, which changes blood biochemistry, antioxidant status, and hepatic HSP mRNA expressions of juvenile black sea bream Acanthopagrus schlegelii. J. Appl. Phycol. 2020, 32, 3457–3466. [Google Scholar] [CrossRef]
- Yu, H.; Ge, X.P.; Zhang, L.; Chen, X.R.; Ren, M.C.; Liang, H.L. Transcriptome analysis reveals the feeding response and oxidative stress in juvenile Micropterus salmoides fed a low-fish-meal diet with enzyme-hydrolysed intestinal mucosa proteinsubstitution. Aquaculture 2023, 570, 739441. [Google Scholar] [CrossRef]
- Cooper, R.U.; Clough, L.M.; Farwell, M.A.; West, T.L. Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J. Exp. Mar. Biol. Ecol. 2002, 279, 1–20. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Wu, H.; Xiao, Q.; Fu, H.M.; Luo, J.; Zhou, J.; Zhao, L.L.; Wang, Y.; Yang, S.Y.; et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2017, 67, 449–458. [Google Scholar] [CrossRef]
- Wu, L.Y.; Li, H.Y.; Xu, W.J.; Dong, B.; Geng, H.C.; Jin, J.Y.; Han, D.; Liu, H.K.; Zhu, X.M.; Yang, Y.X.; et al. Emodin alleviates acute hypoxia-induced apoptosis in gibel carp (Carassius gibelio) by upregulating autophagy through modulation of the AMPK/mTOR pathway. Aquaculture 2022, 548, 737689. [Google Scholar] [CrossRef]
- Liang, H.L.; Mokrani, A.; Ji, K.; Ge, X.P.; Ren, M.C.; Xie, J.; Liu, B.; Xi, B.W.; Zhou, Q.L. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2018, 73, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Qureshi, H.A.; Al-Sultan, A.I.; Yacoubi, M.T.; Ali, A.A. Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology 2009, 257, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, M.T.; Hua, X.J.; Duan, K.X.; Lian, G.H.; Sun, L.; Tang, L.J.; Xu, Y.G.; Liu, M.; Li, Y.J. The role of infectious hematopoietic necrosis virus (IHNV) proteins in the modulation of NF-κB pathway during IHNV infection. Fish Shellfish Immunol. 2017, 63, 500–506. [Google Scholar] [CrossRef]
- Nam, S.E.; Haque, M.N.; Shin, Y.K.; Park, H.S.; Rhee, J.S. Constant and intermittent hypoxia modulates immunity, oxidative status, and blood components of red seabream and increases its susceptibility to the acute toxicity of red tide dinoflagellate. Fish Shellfish Immunol. 2020, 105, 286–296. [Google Scholar] [CrossRef]
- Yang, Z.T.; Wang, L.; Wong, S.M.; Yue, G.H. The HIF1 an gene and its association with hypoxia tolerance in the Asian seabass. Gene 2020, 731, 144341. [Google Scholar] [CrossRef]
- Chen, X.; Feng, W.R.; Yan, F.Y.; Li, W.J.; Xu, P.; Tang, Y.K. Alteration of antioxidant status, glucose metabolism, and hypoxia signal pathway in Eirocheir sinensis after acute hypoxic stress and reoxygenation. Comp. Biochem. Phys. C 2023, 268, 109604. [Google Scholar] [CrossRef]
- Nie, H.T.; Wang, H.M.; Jiang, K.Y.; Yan, X.W. Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress: Potential HIF and NF-κB crosstalk in immune responses in clam. BMC Genom. 2020, 21, 318. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, S.; Zhu, X.Y.; Qiu, J.X.; Deng, G.Z.; Qiu, C.W. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J. Cell. Mol. Med. 2020, 24, 8430–8440. [Google Scholar] [CrossRef]
- Shi, X.W.; Gao, F.; Zhao, X.L.; Pei, C.; Zhu, L.; Zhang, J.; Li, C.; Li, L.; Kong, X.H. Role of HIF in Fish Inflammation. Fish Shellfish Immunol. 2023, 143, 109222. [Google Scholar] [CrossRef]
- Ferla, K.L.; Reimann, C.; Jelkmann, W.; Hellwig-Bürgel, T. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-κB. FASEB J. 2002, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, L.M.; Manzon, R.G. Hypoxia alters the expression of hif-1a mRNA and downstream HIF-1 response genes in embryonic and larval lake whitefish (Coregonus clupeaformis). Comp. Biochem. Phys. A 2019, 230, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Tapryal, N.; Mukherjee, R.; Kumar, R.; Mukhopadhyay, C.K. Insulin promotes iron uptake in human hepatic cell by regulating transferrin receptor-1 transcription mediated by hypoxia inducible factor-1. BBA-Mol. Basis Dis. 2013, 1832, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhao, Q.Q.; Li, E.C.; Zhao, D.X.; Sun, S.M. Role of hypoxia in the behaviour, physiology, immunity and response mechanisms of crustaceans: A review. Rev. Aquac. 2022, 14, 676–687. [Google Scholar] [CrossRef]
- Falfushynska, H.; Sokolova, I.M. Intermittent hypoxia differentially affects metabolic and oxidative stress responses in two species of cyprinid fish. Biol. Open 2023, 12, bio060069. [Google Scholar] [CrossRef]
- Filice, M.; Gattuso, A.; Imbrogno, S.; Mazza, R.; Amelio, D.; Caferro, A.; Agnisola, C.; Icardo, J.M.; Cerra, M.C. Functional, structural, and molecular remodelling of the goldfish (Carassius auratus) heart under moderate hypoxia. Fish Physiol. Biochem. 2024, 50, 667–685. [Google Scholar] [CrossRef]
- Yu, X.X.; Zhang, Y.R.; Li, S.S.; Zheng, G.D.; Zou, S.M. Effects of hypoxia on the gill morphological structure, apoptosis and hypoxia-related gene expression in blunt snout bream (Megalobrama amblycephala). Fish Physiol. Biochem. 2023, 49, 939–949. [Google Scholar] [CrossRef]
- Steffen, J.B.M.; Sokolov, E.P.; Bock, C.; Sokolova, I.M. Combined effects of salinity and intermittent hypoxia on mitochondrial capacity and reactive oxygen species efflux in the Pacific oyster, Crassostrea gigas. J. Exp. Biol. 2023, 226, jeb246164. [Google Scholar] [CrossRef]
- Song, Y.M.; Wu, M.Y.; Pang, Y.Y.; Song, X.Z.; Shi, A.; Shi, X.L.; Niu, C.; Cheng, Y.X.; Yang, X.Z. Effects of melatonin feed on the changes of hemolymph immune parameters, antioxidant capacity, and mitochondrial functions in Chinese mitten crab (Eriocheir sinensis) caused by acute hypoxia. Aquaculture 2021, 535, 736374. [Google Scholar] [CrossRef]
- Hao, T.S.; Yu, J.L.; Wu, Z.D.; Jiang, J.; Gong, L.L.; Wang, B.J.; Guo, H.Z.; Zhao, H.B.; Lu, B.; Engelender, S.; et al. Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion. Nat. Commun. 2023, 14, 4105. [Google Scholar] [CrossRef]
- Shen, H.T.; Liang, P.; Qiu, S.H.; Zhang, B.; Wang, Y.L.; Lv, P. The role of Na+, K+-ATPase in the hypoxic vasoconstriction in isolated rat basilar artery. Vasc. Pharmacol. 2016, 81, 53–60. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhu, J.; Xu, G.C.; Zhang, L.; Chen, X.R.; Wang, Y.L.; Ren, M.C.; Liang, H.L. Sodium chloride alleviates oxidative stress and physiological responses induced by extreme winter cold in genetically improved farmed tilapia (GIFT; Oreochromis niloticus). Sci. Total Environ. 2023, 904, 166800. [Google Scholar] [CrossRef] [PubMed]
- Dada, L.A.; Chandel, N.S.; Ridge, K.M.; Pedemonte, C.; Bertorello, A.M.; Sznajder, J.I. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J. Clin. Investig. 2003, 111, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Comellas, A.P.; Dada, L.A.; Lecuona, E.; Pesce, L.M.; Chandel, N.S.; Quesada, N.; Budinger, G.R.S.; Strous, G.J.; Ciechanover, A.; Sznajder, J.I. Hypoxia-Mediated Degradation of Na,K-ATPase via Mitochondrial Reactive Oxygen Species and the Ubiquitin-Conjugating System. Circ. Res. 2006, 98, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
| Items | Methods | The Model of Commercial Kits | Tested Wavelength | Testing Equipment/Assay Kits |
|---|---|---|---|---|
| CAT | Visible light method | Model A007-1-1 | 405 nm | Assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China); Spectrophotometer (Thermo Fisher Multiskan GO, Shanghai, China). |
| GSH | Microplate method | Model A006-2-1 | 405 nm | |
| SOD | WST-1 method | Model A001-3-2 | 450 nm | |
| MDA | TBA method | Model A003-1-2 | 532 nm | |
| GPX | Colorimetric method | Model A005-1-2 | 412 nm | |
| Na+/K+-ATPase | Microplate method | Model A070-2-2 | 636 nm | |
| Na+ | Colorimetric method | Model C002-1-1 | 620 nm | |
| Cl− | Microplate method | Model C003-2-1 | 505 nm | |
| Ca2+ | Microplate method | Model C004-2-1 | 610 nm | |
| K+ | Turbidimetry assay | Model C001-1-1 | 440 nm |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession Number/Reference |
|---|---|---|---|
| β-actin | GATGATGAAATTGCCGCACTG | ACCGACCATGACGCCCTGATGT | [37] |
| keap1 | CTCCGCTGAATGCTACAA | GGTCATAACACTCCACACT | XM_026245355.1 |
| nrf2 | TACCAAAGACAAGCAGAAGAAACG | GCCTCGTTGAGCTGGTGTTTGG | [38] |
| sod | TCGGAGACCTTGGTAATGT | CGCCTTCTCATGGATCAC | JQ776518.1 |
| cat | TGAAGTTCTACACCGATGAG | CTGAGAGTGGACGAAGGA | XM_026238665.1 |
| gpx | GAAGTGAACGGTGTGAACGC | GATCCCCCATCAAGGACACG | DQ983598.1 |
| ho-1 | GCAAACCAAGAGAAGCCACC | GGAAGTAGACGGGCTGAACC | KC758864 |
| nf-kb | GCTCTGACTGCGGTCTTATAC | GCGCTTCATCGAGGATAGTT | [39] |
| tgf-β | GTTGGCGTAATAACCAGAAGG | AACAGAACAAGTTTGTACCGATAAG | [37] |
| il-10 | AGTGAGACTGAAGGAGCTCCG | TGGCAGAATGGTGTCCAAGTA | [40] |
| il-6 | CGGAGGGGCTTAACAGGATG | GCTGGCTCAGGAATGGGTAT | DQ861993.1 |
| il-8 | ATTGGTGAAGGAATGAGTCT | CCACAGATGACCTTGACAT | KC184490.1 |
| tnf-α | CATTCCTACGGATGGCATTTACTT | CCTCAGGAATGTCAGTCTTGCAT | [37] |
| il-1β | GCGCTGCTCAACTTCATCTTG | GTGACACATTAAGCGGCTTCA C | [37] |
| hif-1α | CTGCCGATCAGTCTGTCTCC | TTTGTGGAGTCTGGACCACG | DQ306727.1 |
| epo | CGAAGTGTCAGCATACCGGA | GCAGATGACGCACTTTTCCC | KC460317.1 |
| vegf | ATCGAGCACACGTACATCCC | CCTTTGGCCTGCATTCACAC | NM_131408.3 |
| et1 | TAAAGCAGCGTCAGACAGGG | CTGCCAGCTTGTGTTTGCAT | NM_131519.1 |
| tf | CCGAGAAGATGCACGCAAAG | TGTGCATGCCTTGACCAGAT | AF518747.1 |
| tfr-1 | CTTTGTCAACGAAGTGGCTGAAT | TACCAAAGAAAATGTGGCGGAAC | XM_052542523.1 |
| angpt1 | CCAAACCTCACCAAGCAAGC | GGATTACAGTCCAGCCTCCG | XM_059556208.1 |
| nos | GGGGACCCTCCTGAAAATGG | TTCTGTCCTCAACGCTGGTG | AY644726.1 |
| FPR Addition Level (%) | Na+ (mmol/L) | K+ (mmol/L) | Ca+ (mmol/L) | Cl− (mmol/L) | Na+/K+-ATPase (U/mgprot) |
|---|---|---|---|---|---|
| 0 | 82.58 ± 0.17 b | 17.31 ± 0.39 a | 1.42 ± 0.13 a | 70.08 ± 3.81 | 0.90 ± 0.04 b |
| 0.01 | 116.60 ± 14.98 ab | 16.94 ± 0.42 ab | 1.37 ± 0.03 a | 68.07 ± 0.88 | 1.55 ± 0.15 a |
| 0.02 | 191.10 ± 40.24 a | 15.39 ± 1.15 b | 0.87 ± 0.09 b | 66.77 ± 1.74 | 1.23 ± 0.19 ab |
| 0.03 | 128.78 ± 8.85 ab | 16.83 ± 0.43 ab | 0.80 ± 0.15 b | 65.64 ± 1.50 | 0.95 ± 0.30 b |
| 0.04 | 147.51 ± 19.90 ab | 16.61 ± 0.45 ab | 1.10 ± 0.14 ab | 64.17 ± 2.60 | 0.85 ± 0.21 b |
| FPR Supplementation Level (%) | Mitochondrial Number (Per Cell) | SR (%) |
|---|---|---|
| 0 | 3.33 ± 1.86 | 23.81 ± 2.38 b |
| 0.01 | 6.00 ± 3.00 | 54.76 ± 2.38 a |
| 0.02 | 4.00 ± 2.08 | 52.38 ± 8.58 a |
| 0.03 | 4.33 ± 2.40 | 47.62 ± 2.38 a |
| 0.04 | 6.33 ± 1.76 | 47.62 ± 2.38 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Mi, H.; Wang, K.; Ren, M.; Zhang, L.; Huang, D.; Gu, J. Enhancement of Hypoxia Tolerance of Gibel Carp (Carassius auratus gibelio) via a Ferroporphyrin-Rich Diet. Antioxidants 2025, 14, 738. https://doi.org/10.3390/antiox14060738
Liang H, Mi H, Wang K, Ren M, Zhang L, Huang D, Gu J. Enhancement of Hypoxia Tolerance of Gibel Carp (Carassius auratus gibelio) via a Ferroporphyrin-Rich Diet. Antioxidants. 2025; 14(6):738. https://doi.org/10.3390/antiox14060738
Chicago/Turabian StyleLiang, Hualiang, Haifeng Mi, Kai Wang, Mingchun Ren, Lu Zhang, Dongyu Huang, and Jiaze Gu. 2025. "Enhancement of Hypoxia Tolerance of Gibel Carp (Carassius auratus gibelio) via a Ferroporphyrin-Rich Diet" Antioxidants 14, no. 6: 738. https://doi.org/10.3390/antiox14060738
APA StyleLiang, H., Mi, H., Wang, K., Ren, M., Zhang, L., Huang, D., & Gu, J. (2025). Enhancement of Hypoxia Tolerance of Gibel Carp (Carassius auratus gibelio) via a Ferroporphyrin-Rich Diet. Antioxidants, 14(6), 738. https://doi.org/10.3390/antiox14060738








