Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations
Abstract
1. Introduction
Methodology
- Structured literature search: Conducted across five major scientific databases—PubMed, Scopus, Web of Science, Embase, and ScienceDirect—to ensure broad and multidisciplinary coverage.
- Timeframe and study types: Included peer-reviewed original research articles and systematic reviews published between January 2010 and March 2024.
- Quality prioritization: Emphasis was placed on studies published in high-impact journals, particularly those indexed in Journal Citation Reports (JCR) and SCImago Journal Rank (SJR), with a focus on Q1 journals in the fields of endocrinology, pharmacology, and nutrition.
- Search strategy: Designed to reflect the multifactorial nature of type 2 diabetes mellitus (T2DM) and its interplay with oxidative stress and plant-based interventions.Keywords and Boolean operators included “type 2 diabetes mellitus”, “oxidative stress”, “plant-derived antioxidants”, “polyphenols”, “flavonoids”, “carotenoids”, “insulin resistance”, “AMP-activated protein kinase (AMPK)”, “NF-κB”, “gut microbiota”, “inflammation”, and “nutrigenomics.”
- Inclusion criteria:Experimental and clinical studies involving in vitro, in vivo, or human subjects.
- Exclusion criteria:Publications not in English.Studies lacking experimental or clinical validation.Narrative commentaries, dissertations, books, conference abstracts, or preprints.Articles considered methodologically outdated or not aligned with current pathophysiological understanding.
- Scope of the review:To critically synthesize current evidence on the mechanistic and translational role of plant-derived antioxidants in T2DM.Special focus on their ability to mitigate oxidative stress, modulate inflammation, and improve insulin signaling.Highlight key limitations (e.g., bioavailability, metabolic stability) and emerging research directions, including microbiome interactions, synergistic antioxidant strategies, and personalized nutrition.
2. Oxidative Stress, Inflammation, and Insulin Resistance in Type 2 Diabetes Mellitus
3. Classification and Bioactivity of Plant-Derived Antioxidants
3.1. Classification of Plant-Derived Antioxidants
3.1.1. Polyphenols
Flavonoids
- Quercetin: A predominant flavonol found in apples, onions, and berries, quercetin exerts multifaceted biological effects, including the regulation of glucose homeostasis through the activation of AMP-activated protein kinase (AMPK) and the facilitation of glucose transporter type 4 (GLUT4) translocation in skeletal muscle cells. Furthermore, it downregulates hepatic gluconeogenesis by inhibiting the expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), enzymes critical for glucose production [45].
- Kaempferol, also a flavonol found in leafy greens and berries, has demonstrated glucose-lowering effects and mitochondrial protective properties through the activation of PGC-1α and the inhibition of JNK phosphorylation.
- Catechins: Predominantly found in green tea, catechins, particularly epigallocatechin gallate (EGCG), have been demonstrated to exert significant antioxidant and anti-inflammatory properties. EGCG enhances insulin sensitivity by modulating the insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/Akt pathway, thereby improving glucose uptake and metabolic regulation. Additionally, catechins exhibit neuroprotective properties by modulating oxidative-stress-related pathways in neurodegenerative disorders [46].
- Anthocyanins: These pigmented flavonoids, found in berries, red grapes, and purple corn, exhibit strong antioxidant and anti-inflammatory activities. Studies have demonstrated their capacity to enhance insulin secretion from pancreatic β-cells, reduce postprandial hyperglycemia, and inhibit the activation of nuclear factor-kappa B (NF-κB), a key regulator of inflammatory responses. Moreover, anthocyanins have been implicated in modulating gut microbiota composition, fostering the proliferation of beneficial bacterial species while inhibiting pathogenic strains, thus exerting systemic metabolic benefits [47].
- Isoflavones, such as genistein from soy, have estrogen-like activity and improve insulin’s action by interacting with PPARγ and reducing oxidative damage [48].
Phenolic Acids
- Ferulic acid: Predominantly present in rice bran, oats, and wheat, ferulic acid exerts its antioxidant effects by scavenging reactive oxygen species (ROS) and enhancing the activity of endogenous antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT). Furthermore, it has been shown to modulate nitric oxide (NO) bioavailability, improving endothelial function and vascular health in metabolic disorders [50].
- Caffeic acid: Commonly found in coffee, fruits, and herbs, caffeic acid exhibits strong anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), thereby modulating systemic inflammatory responses. Moreover, it has been shown to attenuate lipid peroxidation and oxidative damage in neuronal cells, suggesting potential neuroprotective effects [51].
- Hydroxybenzoic acids, such as gallic acid and protocatechuic acid, are abundant in berries, tea, and wine. Gallic acid has been shown to protect β-cells from oxidative injury, reduce hepatic gluconeogenesis, and attenuate pro-inflammatory cytokine production by modulating MAPK and NF-κB signaling. Protocatechuic acid has been linked to improved lipid metabolism and reduced insulin resistance in animal models [52].
- Resveratrol: A naturally occurring stilbene found in grapes, red wine, and peanuts, resveratrol has garnered significant attention due to its ability to activate sirtuin 1 (SIRT1), a protein deacetylase implicated in mitochondrial function, insulin sensitivity, and longevity. Its cardioprotective effects are mediated through the enhancement of endothelial nitric oxide synthase (eNOS) activity, the reduction of oxidative stress, and the attenuation of inflammatory cascades [53].
- Lignans: These phytoestrogenic compounds, primarily found in flaxseeds and sesame seeds, exhibit antioxidative and lipid-lowering effects. Secoisolariciresinol diglucoside (SDG), a major lignan, has been shown to modulate gut microbiota metabolism, enhance short-chain fatty acid production, and reduce systemic oxidative stress markers in diabetic individuals [54].
3.1.2. Carotenoids
- β-Carotene: A provitamin A carotenoid abundant in carrots, sweet potatoes, and leafy greens, β-carotene exerts significant antioxidant effects by neutralizing reactive oxygen species (ROS), particularly singlet oxygen and lipid peroxyl radicals. In experimental models of T2DM, β-carotene supplementation has been shown to reduce oxidative stress markers, such as malondialdehyde (MDA), increase antioxidant enzyme activities, including glutathione peroxidase (GPx) and catalase (CAT), and enhance insulin sensitivity. Furthermore, β-carotene has been implicated in the inhibition of the formation of advanced glycation end-products (AGEs), which are linked to diabetic vascular complications. Comparative studies suggest that β-carotene may act synergistically with vitamin E in preserving membrane integrity and suppressing pro-inflammatory cytokine release, particularly in the context of high-fat-diet-induced insulin resistance [56].
- Lutein and zeaxanthin: These xanthophylls, found in green leafy vegetables, corn, and egg yolks, have been extensively studied for their protective effects on retinal health, especially in diabetic retinopathy. Mechanistically, both compounds reduce ROS accumulation in retinal pigment epithelial cells and inhibit nuclear factor-kappa B (NF-κB) signaling, leading to the decreased expression of pro-inflammatory mediators, such as TNF-α and IL-6. Beyond their ocular benefits, systemic administration of lutein in diabetic rodents has been associated with improved lipid metabolism, increased adiponectin levels, and the attenuation of hepatic steatosis. Clinical studies also report reductions in circulating C-reactive protein (CRP) and improvements in antioxidant capacity following lutein supplementation in patients with metabolic syndrome and T2DM [57].
3.1.3. Vitamins
- Vitamin C: As a water-soluble antioxidant, vitamin C acts by directly neutralizing a broad spectrum of ROS, including superoxide anion, hydroxyl radicals, and singlet oxygen. Importantly, it also regenerates oxidized vitamin E, thus maintaining the redox cycle between aqueous and lipid compartments. In patients with T2DM, vitamin C supplementation has been shown to reduce plasma levels of malondialdehyde (MDA), improve endothelial-dependent vasodilation, and decrease markers of systemic inflammation, such as C-reactive protein (CRP) and interleukin-6 (IL-6) [58]. Additionally, vitamin C enhances nitric oxide (NO) bioavailability and supports endothelial nitric oxide synthase (eNOS) activity, contributing to improved vascular function—a critical factor in preventing diabetic complications, such as nephropathy and retinopathy.
- Vitamin E: Vitamin E is a lipophilic antioxidant composed of eight isoforms (α-, β-, γ-, and δ-tocopherols and tocotrienols), with α-tocopherol being the most biologically active and extensively studied. It protects membrane lipids from peroxidation, interrupts lipid radical chain reactions, and modulates cellular signaling cascades. In T2DM, vitamin E has demonstrated the capacity to modulate glucose homeostasis by enhancing glucose transporter type 4 (GLUT4) translocation to the cell membrane and preserving insulin receptor substrate-1 (IRS-1) activity. Furthermore, it inhibits NF-κB activation, thereby reducing the expression of pro-inflammatory cytokines, such as TNF-α and IL-1β. Clinical trials have reported modest improvements in glycemic control and lipid profiles following high-dose α-tocopherol supplementation, although interindividual variability and baseline oxidative stress levels significantly influence the outcomes [59,60].
3.2. Bioactivity of Plant-Derived Antioxidants
3.2.1. Modulation of Oxidative Stress
Direct Scavenging of ROS
- Flavonoids: Quercetin and catechins interact with superoxide anions (O2−), hydroxyl radicals (OH•), and hydrogen peroxide (H2O2), effectively reducing oxidative damage to lipids, proteins, and DNA.
- Carotenoids: β-carotene and lycopene exert singlet oxygen (1O2) quenching activity, thereby protecting polyunsaturated fatty acids from peroxidation, a key process in diabetic complications, such as nephropathy and neuropathy [39].
Enhancement of Endogenous Antioxidant Enzymes
- Resveratrol: This polyphenol from grapes and red wine activates nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant defense, leading to increased expression of SOD and CAT, thereby reducing oxidative damage in pancreatic β-cells and insulin-sensitive tissues [53].
- Curcumin: The principal bioactive compound in turmeric enhances GPx activity and prevents lipid peroxidation by modulating the Nrf2/Keap1 pathway, contributing to improved glucose homeostasis and β-cell protection [61].
3.2.2. Anti-Inflammatory Effects
Inhibition of the NF-κB Pathway
- Quercetin: Suppresses NF-κB signaling in adipose tissue, reducing TNF-α and IL-6 levels and thereby improving insulin sensitivity in diabetic patients [45].
- Anthocyanins: These pigments from berries and red grapes inhibit NF-κB activation and decrease circulating C-reactive protein (CRP) levels, a key biomarker of systemic inflammation [61].
Modulation of the MAPK Pathway
- Curcumin: Attenuates inflammation by inhibiting JNK and p38 MAPK phosphorylation, reducing cytokine-mediated insulin resistance [61].
- Epigallocatechin gallate (EGCG): A catechin from green tea that downregulates ERK and JNK activation, mitigating inflammation and oxidative stress in metabolic tissues [62].
Reduction of Inflammasome Activation
- Resveratrol: Suppresses NLRP3 inflammasome activation by reducing mitochondrial ROS production, thereby attenuating inflammatory damage in insulin-sensitive tissues [53].
- Quercetin: Inhibits inflammasome activation and IL-1β release, protecting against inflammation-induced insulin resistance [45].
3.2.3. Modulation of Insulin Signaling Pathways
- Quercetin: Enhances glucose uptake in skeletal muscle by activating the IRS/PI3K/Akt pathway and promoting GLUT4 translocation to the plasma membrane, facilitating cellular glucose entry [63].
- Resveratrol: Activates AMP-activated protein kinase (AMPK), a crucial regulator of energy homeostasis, promoting glucose uptake and inhibiting hepatic gluconeogenesis, thus improving glycemic control [64].
3.2.4. Gut Microbiota Interactions
- Polyphenols: Polyphenols are metabolized into bioactive derivatives, such as urolithins and equol, which exhibit potent anti-inflammatory and antioxidant effects, further enhancing systemic metabolic health [65].
- Anthocyanins: Alter gut microbiota composition, favoring an anti-inflammatory profile, which contributes to improved insulin sensitivity and reduced metabolic endotoxemia [66].
4. Molecular Targets of Antioxidants in Diabetes
5. Resveratrol: Mechanisms and Clinical Evidence
5.1. Antioxidant Properties (Nrf2/Keap1 Pathway)
5.2. Anti-Inflammatory Effects (NF-κB and Pro-Inflammatory Cytokines)
5.3. Effects on Insulin Sensitivity (AMPK/SIRT1 and IRS/PI3K/Akt Signaling)
5.4. Mitochondrial Function and Energy Metabolism (PGC-1α Activation)
5.5. β-Cell Protection and Insulin Secretion
5.6. Clinical Evidence from Human Studies
5.6.1. Findings from Meta-Analyses and Clinical Trials
5.6.2. Effects on Glycemic Control (HbA1c, Glucose, Insulin Sensitivity)
5.6.3. Fasting Blood Glucose (FBG)
5.6.4. Glycated Hemoglobin (HbA1c)
- A 2022 meta-analysis detected a small but significant improvement in HbA1c (~0.4% absolute reduction at 3 months) with resveratrol vs. placebo [90].
- One RCT found that 3 months of resveratrol (250 mg/day), when added to standard anti-diabetic therapy, led to a statistically significant decrease in HbA1c [94].
- Another trial using 1 g/day for 45 days reported a reduction in both HbA1c and fasting glucose [79].
5.6.5. Insulin Sensitivity and HOMA-IR
- A meta-analysis of five trials (153 patients) found that resveratrol significantly lowered HOMA-IR, indicating improved insulin action (pooled decrease in HOMA-IR by ~0.5 units).
- In a placebo-controlled trial, resveratrol (1 g/day for 6 weeks) led to a ~20% reduction in fasting insulin and insulin resistance index [79].
- Another small-scale study (5 mg twice daily) observed improved insulin sensitivity and increased Akt phosphorylation in platelets, a surrogate for insulin signaling activity [89].
5.7. Impact on Lipid Profile and Cardiovascular Parameters
- Meta-analyses indicate that resveratrol (particularly at higher doses) was associated with significantly lower systolic and diastolic blood pressure compared to placebo [90].
- Pooled data indicate an average systolic BP reduction of 5–8 mmHg and a diastolic reduction of ~2–4 mmHg in resveratrol-treated diabetics [96].
- One meta-analysis found a mean systolic BP drop of 7.97 mmHg and a diastolic drop of 3.55 mmHg with resveratrol vs. the control [79].
5.8. Inflammation and Oxidative Stress Biomarkers in Patients
5.9. Dose–Response Relationships and Safety Considerations
6. Curcumin and Its Role in Insulin Sensitivity
7. Flavonoids and Metabolic Regulation: A Focus on Quercetin
8. Anthocyanins and Glycemic Control
9. Carotenoids in Diabetes Management
10. Green Tea Catechins and Insulin Resistance
11. Synergistic Effects of Antioxidant Combinations
12. Gut Microbiota and Antioxidant Interactions
13. Nutrigenomics and Personalized Antioxidant Therapy
13.1. Nutrigenomics and the Role of the Microbiome
13.2. Genetic Variability and Personalized Antioxidant Strategies
14. Plant-Derived Antioxidants in Young Adults, Older Adults, and Pregnant Women with Diabetes
14.1. Plant-Derived Antioxidants in Young Adults with Diabetes
14.2. Plant-Derived Antioxidants in Older Adults with Diabetes
14.3. Plant-Derived Antioxidants in Pregnant Women with Diabetes
15. Challenges in Bioavailability and Stability
16. Future Directions in Functional Foods and Therapeutics
17. Ethical Considerations and Regulatory Implications
- Safety and efficacy standards: Nutraceuticals and functional-food-based interventions must undergo rigorous testing to demonstrate not only efficacy but also long-term safety, particularly when targeting vulnerable populations with metabolic disorders.
- Informed consent and transparency: In precision nutrition approaches involving omics data, individuals must be fully informed about how their biological data will be used, stored, and interpreted.
- Equitable access: There is a risk that personalized interventions (e.g., microbiome profiling, metabolomic-guided nutrition) may be available only to higher-income populations. Ethical implementation should ensure these innovations do not widen health disparities.
- Data privacy and autonomy: Especially relevant in digital health tools, strict standards must be applied to protect personal health data and guarantee user autonomy over dietary or therapeutic recommendations.
- Regulatory harmonization: Coordination between food, medical, and digital health regulatory bodies is essential to establish consistent approval pathways and avoid gaps in oversight as these hybrid interventions enter the market.
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas 2025|Global Diabetes Data & Insights. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 6 May 2025).
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Velasco, A.I.; Clemente-Suárez, V.J. Impact of Peripheral Inflammation on Blood–Brain Barrier Dysfunction and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2440. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H. Bin Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health Relevance of the Modification of Low Grade Inflammation in Ageing (Inflammageing) and the Role of Nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Portes, J.; Bullón, B.; Quiles, J.L.; Battino, M.; Bullón, P. Diabetes Mellitus and Periodontitis Share Intracellular Disorders as the Main Meeting Point. Cells 2021, 10, 2411. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative Stress and the Etiology of Insulin Resistance and Type 2 Diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Sasaki, S.; Inoguchi, T. The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications. Diabetes Metab. J. 2012, 36, 255–261. [Google Scholar] [CrossRef]
- Yáñez-Sepúlveda, R.; Olivares, R.; Ravelo, C.; Cortés-Roco, G.; Zavala-Crichton, J.P.; Hinojosa-Torres, C.; Clemente-Suárez, V.J. Use of Self-Organizing Maps for the Classification of Cardiometabolic Risk and Physical Fitness in Adolescents. Int. J. Adolesc. Youth 2024, 29, 2417903. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S. ichi Role of Advanced Glycation End Products (AGEs) and Receptor for AGEs (RAGE) in Vascular Damage in Diabetes. Exp. Gerontol. 2011, 46, 217–224. [Google Scholar] [CrossRef] [PubMed]
- de Mello, M.B.; Righi, N.C.; Schuch, F.B.; Signori, L.U.; da Silva, A.M.V. Effect of High-Intensity Interval Training Protocols on VO2max and HbA1c Level in People with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101586. [Google Scholar] [CrossRef]
- Gonzalez-Franquesa, A.; Patti, M.E. Insulin Resistance and Mitochondrial Dysfunction. Adv. Exp. Med. Biol. 2017, 982, 465–520. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Centonze, V.; Mariano, M.; Khalil, M.; D’Alessandro, A.G. Prevention of Oxidative Stress and Diseases by Antioxidant Supplementation. Med. Chem. 2022, 19, 509–537. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Aronne, L.J.; Astrup, A.; De Cabo, R.; Cantley, L.C.; Friedman, M.I.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. The Carbohydrate-Insulin Model: A Physiological Perspective on the Obesity Pandemic. Am. J. Clin. Nutr. 2021, 114, 1873–1885. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the Small Intestine, Colon and Microbiota in Determining the Metabolic Fate of Polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic Consequences of Obesity and Type 2 Diabetes: Balancing Genes and Environment for Personalized Care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef]
- Szendroedi, J.; Phielix, E.; Roden, M. The Role of Mitochondria in Insulin Resistance and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2012, 8, 92–103. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and Adipose Tissue Insulin Resistance: Malady without Mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Unwin, D.; Finucane, F. Low-Carbohydrate Diets in the Management of Obesity and Type 2 Diabetes: A Review from Clinicians Using the Approach in Practice. Int. J. Environ. Res. Public Health 2020, 17, 2557. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Reyes-García, R.; Moreno-Pérez, Ó.; Tejera-Pérez, C.; Fernández-García, D.; Bellido-Castañeda, V.; de la Torre Casares, M.L.; Rozas-Moreno, P.; Fernández-García, J.C.; Marco Martínez, A.; Escalada-San Martín, J.; et al. Document on a Comprehensive Approach to Type 2 Diabetes Mellitus. Endocrinol. Diabetes Nutr. 2019, 66, 443–458. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C. Obesity and Heart Failure with Preserved Ejection Fraction: A Paradox or Something Else? Heart Fail. Rev. 2019, 24, 379–385. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic Health, Mitochondrial Fitness, Physical Activity, and Cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Periago, M.J.; González-Barrio, R.; Hidalgo, N.; Navarro-González, I.; Gómez-Gallego, C.; Masuero, D.; Soini, E.; Vrhovsek, U.; García-Alonso, F.J. Spinach Consumption Ameliorates the Gut Microbiota and Dislipaemia in Rats with Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD). Food Funct. 2019, 10, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-Lipoic Acid: Molecular Mechanisms and Therapeutic Potential in Diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Merino del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional Modulation of the Gut–Brain Axis: A Comprehensive Review of Dietary Interventions in Depression and Anxiety Management. Metabolites 2024, 14, 549. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 1818–1892. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the Antioxidant Potential of Flavonoids as a Subgroup of Polyphenols and Partial Substitute for Synthetic Antioxidants. Avicenna J. Phytomed. 2023, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Peris-Ramos, H.C.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; David-Fernandez, S.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Personalizing Nutrition Strategies: Bridging Research and Public Health. J. Pers. Med. 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Green Tea Catechins as Brain-Permeable, Natural Iron Chelators-Antioxidants for the Treatment of Neurodegenerative Disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef]
- Ruiz-Iglesias, P.; Estruel-Amades, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Castell, M.; Pérez-Cano, F.J. Alterations in the Mucosal Immune System by a Chronic Exhausting Exercise in Wistar Rats. Sci. Rep. 2020, 10, 17950. [Google Scholar] [CrossRef]
- Jiang, T.; Dong, Y.; Zhu, W.; Wu, T.; Chen, L.; Cao, Y.; Yu, X.; Peng, Y.; Wang, L.; Xiao, Y.; et al. Underlying Mechanisms and Molecular Targets of Genistein in the Management of Type 2 Diabetes Mellitus and Related Complications. Crit. Rev. Food Sci. Nutr. 2023, 64, 11543–11555. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, Natural Sources, Dietary Intake and Pharmacokinetic Properties of Ferulic Acid: A Review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. Anti-Diabetic Effect of Hydroxybenzoic Acid Derivatives in Free Fatty Acid-Induced HepG2 Cells via MiR-1271/IRS1/PI3K/AKT/FOXO1 Pathway. J. Food Biochem. 2021, 45, e13993. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Huang, L.T.; Sheen, J.M.; Hou, C.Y.; Yeh, Y.T.; Chiang, C.P.; Lin, I.C.; Tiao, M.M.; Tsai, C.C.; Lin, Y.J.; et al. Resveratrol Treatment Improves the Altered Metabolism and Related Dysbiosis of Gut Programed by Prenatal High-Fat Diet and Postnatal High-Fat Diet Exposure. J. Nutr. Biochem. 2020, 75, 108260. [Google Scholar] [CrossRef] [PubMed]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health Effects with Consumption of the Flax Lignan Secoisolariciresinol Diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Palozza, P.; Catalano, A.; Simone, R.E.; Mele, M.C.; Cittadini, A. Effect of Lycopene and Tomato Products on Cholesterol Metabolism. Ann. Nutr. Metab. 2012, 61, 126–134. [Google Scholar] [CrossRef]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein Effect on Retina and Hippocampus of Diabetic Mice. Free Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of Vitamin C Supplementation on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials1. Am. J. Clin. Nutr. 2012, 95, 1079. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and Its Function in Membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Drevon, C.A. Absorption, Transport and Metabolism of Vitamin E. Free Radic. Res. 1991, 14, 229–246. [Google Scholar] [CrossRef]
- Li, P.; Ding, L.; Cao, S.; Feng, X.; Zhang, Q.; Chen, Y.; Zhang, N.; Qiu, F. Curcumin Metabolites Contribute to the Effect of Curcumin on Ameliorating Insulin Sensitivity in High-Glucose-Induced Insulin-Resistant HepG2 Cells. J. Ethnopharmacol. 2020, 259, 113015. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Morcillo, J.; Clemente-Suárez, V.J. Gender Differences in Body Satisfaction Perception: The Role of Nutritional Habits, Psychological Traits, and Physical Activity in a Strength-Training Population. Nutrients 2024, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, L.; Cao, L.; Zhang, Z.; Li, X.; Li, Z.; Xia, Q.; Yin, K.; Song, S.; Wang, Z.; et al. Natural Products Targeting AMPK Signaling Pathway Therapy, Diabetes Mellitus and Its Complications. Front. Pharmacol. 2025, 16, 1534634. [Google Scholar] [CrossRef]
- Selma, M.V.; Tomás-Barberán, F.A.; Romo-Vaquero, M.; Cortés-Martín, A.; Espín, J.C. Understanding Polyphenols’ Health Effects Through the Gut Microbiota. In Dietary Polyphenols: Their Metabolism and Health Effects; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 497–531. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Park, S.; Park, S.-Y. Can Antioxidants Be Effective Therapeutics for Type 2 Diabetes? Yeungnam Univ. J. Med. 2020, 38, 83. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Md Sayem, A.S.; Arya, A.; Karimian, H.; Krishnasamy, N.; Hasamnis, A.A.; Hossain, C.F. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef]
- Das, L.; Vinayak, M. Long Term Effect of Curcumin in Restoration of Tumour Suppressor P53 and Phase-II Antioxidant Enzymes via Activation of Nrf2 Signalling and Modulation of Inflammation in Prevention of Cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef]
- Tantiwong, P.; Shanmugasundaram, K.; Monroy, A.; Ghosh, S.; Li, M.; DeFronzo, R.A.; Cersosimo, E.; Sriwijitkamol, A.; Mohan, S.; Musi, N. NF-ΚB Activity in Muscle from Obese and Type 2 Diabetic Subjects under Basal and Exercise-Stimulated Conditions. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E794. [Google Scholar] [CrossRef]
- Sotty, J.; Kluza, J.; De Sousa, C.; Tardivel, M.; Anthérieu, S.; Alleman, L.Y.; Canivet, L.; Perdrix, E.; Loyens, A.; Marchetti, P.; et al. Mitochondrial Alterations Triggered by Repeated Exposure to Fine (PM2.5–0.18) and Quasi-Ultrafine (PM0.18) Fractions of Ambient Particulate Matter. Environ. Int. 2020, 142, 105830. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in Glucose and Lipid Metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The Interaction among Gut Microbes, the Intestinal Barrier and Short Chain Fatty Acids. Anim. Nutr. 2021, 9, 159–174. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Zhao, Y.; Peng, K.; Li, W.; Wang, Y.; Zhang, J.; Zhou, S.; Liu, Q.; Li, X.; et al. Inhibition of JNK Phosphorylation by a Novel Curcumin Analog Prevents High Glucose-Induced Inflammation and Apoptosis in Cardiomyocytes and the Development of Diabetic Cardiomyopathy. Diabetes 2014, 63, 3497–3511. [Google Scholar] [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative Stress in Diabetes Mellitus and Its Complications: From Pathophysiology to Therapeutic Strategies. Chin. Med. J. 2024, 138, 15–27. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, Insulin Resistance, and the Metabolic Syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Ageeli Hakami, M. Diabetes and Diabetic Associative Diseases: An Overview of Epigenetic Regulations of TUG1. Saudi J. Biol. Sci. 2024, 31, 103976. [Google Scholar] [CrossRef]
- Pinto, D.S.; Skolnick, A.H.; Kirtane, A.J.; Murphy, S.A.; Barron, H.V.; Giugliano, R.P.; Cannon, C.P.; Braunwald, E.; Gibson, C.M. U-Shaped Relationship of Blood Glucose with Adverse Outcomes among Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2005, 46, 178–180. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Deniziak, M.; Sassek, M.; Szkudelski, I.; Noskowiak, W.; Szkudelski, T. Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 2469. [Google Scholar] [CrossRef]
- Palsamy, P.; Subramanian, S. Resveratrol Protects Diabetic Kidney by Attenuating Hyperglycemia-Mediated Oxidative Stress and Renal Inflammatory Cytokines via Nrf2-Keap1 Signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 719–731. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Song, Y.; Ding, N.; Lu, M.; Jia, L.; Zhao, Y.; Liu, M.; Chen, Z. Activation of NF-ΚB-Inducing Kinase in Islet β Cells Causes β Cell Failure and Diabetes. Mol. Ther. 2020, 28, 2430. [Google Scholar] [CrossRef]
- Nanjan, M.J.; Betz, J. Resveratrol for the Management of Diabetes and Its Downstream Pathologies. Eur. Endocrinol. 2014, 10, 31. [Google Scholar] [CrossRef]
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 9734738. [Google Scholar] [CrossRef]
- Abdelhaleem, I.A.; Brakat, A.M.; Adayel, H.M.; Asla, M.M.; Rizk, M.A.; Aboalfetoh, A.Y. The Effects of Resveratrol on Glycemic Control and Cardiometabolic Parameters in Patients with T2DM: A Systematic Review and Meta-Analysis. Med. Clin. 2022, 158, 576–585. [Google Scholar] [CrossRef]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The Effects of Rutin Flavonoid Supplement on Glycemic Status, Lipid Profile, Atherogenic Index of Plasma, Brain-Derived Neurotrophic Factor (BDNF), Some Serum Inflammatory, and Oxidative Stress Factors in Patients with Type 2 Diabetes Mellitus: A Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2023, 37, 271–284. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol Treatment as an Adjunct to Pharmacological Management in Type 2 Diabetes Mellitus-Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of Resveratrol on Glucose Control and Insulin Sensitivity: A Meta-Analysis of 11 Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The Effects of Resveratrol Supplementation in Patients with Type 2 Diabetes, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Oliver Chen, C.Y.; Rasmussen, H.; Kamil, A.; Du, P.; Blumberg, J.B. Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men. Nutrients 2017, 9, 130. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of Resveratrol Supplementation on Plasma Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef]
- Bazyar, H.; Javid, A.Z.; Zakerkish, M.; Yousefimanesh, H.A.; Haghighi-Zadeh, M.H. Effects of Melatonin Supplementation in Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis under Nonsurgical Periodontal Therapy: A Double-Blind Randomized Controlled Trial. J. Res. Med. Sci. 2022, 27, 52. [Google Scholar] [CrossRef]
- Ayub, H.; Islam, M.; Saeed, M.; Ahmad, H.; Al-Asmari, F.; Ramadan, M.F.; Alissa, M.; Arif, M.A.; Rana, M.U.J.; Subtain, M.; et al. On the Health Effects of Curcumin and Its Derivatives. Food Sci. Nutr. 2024, 12, 8623–8650. [Google Scholar] [CrossRef]
- Gu, Y.; Niu, Q.; Zhang, Q.; Zhao, Y. Ameliorative Effects of Curcumin on Type 2 Diabetes Mellitus. Molecules 2024, 29, 2934. [Google Scholar] [CrossRef]
- Balakumar, P.; Venkatesan, K.; Abdulla Khan, N.; Raghavendra, N.M.; Venugopal, V.; Bharathi, D.R.; Fuloria, N.K. Mechanistic Insights into the Beneficial Effects of Curcumin on Insulin Resistance: Opportunities and Challenges. Drug Discov. Today 2023, 28, 103627. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and Its Impact on the Immune System. Curr. Diabetes Rev. 2019, 16, 442–449. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Tang, Y.; Ren, H.; Hu, H. Meta-Analysis of Randomized Controlled Trials of 4 Weeks or Longer Suggest That Curcumin May Afford Some Protection against Oxidative Stress. Nutr. Res. 2018, 60, 1–12. [Google Scholar] [CrossRef]
- Zou, T.; Li, S.; Wang, B.; Wang, Z.; Liu, Y.; You, J. Curcumin Improves Insulin Sensitivity and Increases Energy Expenditure in High-Fat-Diet–Induced Obese Mice Associated with Activation of FNDC5/Irisin. Nutrition 2021, 90, 111263. [Google Scholar] [CrossRef]
- Clark, C.C.T.; Ghaedi, E.; Arab, A.; Pourmasoumi, M.; Hadi, A. The Effect of Curcumin Supplementation on Circulating Adiponectin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2819–2825. [Google Scholar] [CrossRef]
- Lee, S.J.; Chandrasekran, P.; Mazucanti, C.H.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Dietary Curcumin Restores Insulin Homeostasis in Diet-Induced Obese Aged Mice. Aging 2022, 14, 225–239. [Google Scholar] [CrossRef]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-Inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-Related Diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, S.; Askari, G.; Iraj, B.; Sathyapalan, T.; Guest, P.C.; Bagherniya, M.; Sahebkar, A. Effect of Curcumin on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review of Randomized Clinical Trials. Adv. Exp. Med. Biol. 2021, 1291, 139–149. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-KB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin Improves Nonalcoholic Fatty Liver by Ameliorating Inflammation, Oxidative Stress, and Lipid Metabolism in Db/Db Mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed. Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Bellavite, P.; Fazio, S.; Affuso, F. A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention. Molecules 2023, 28, 4491. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, X.; Mu, J.; Zou, Y.; Xu, L.; Chen, J.; Zhu, X.; Li, B.; Zeng, Z.; Wu, X.; et al. Quercetin Induces MGMT+ Glioblastoma Cells Apoptosis via Dual Inhibition of Wnt3a/β-Catenin and Akt/NF-ΚB Signaling Pathways. Phytomedicine 2023, 118, 154933. [Google Scholar] [CrossRef]
- Ke, X.; Chen, Z.; Wang, X.; Kang, H.; Hong, S. Quercetin Improves the Imbalance of Th1/Th2 Cells and Treg/Th17 Cells to Attenuate Allergic Rhinitis. Autoimmunity 2023, 56, 2189133. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin Ameliorates Diabetic Kidney Injury by Inhibiting Ferroptosis via Activating Nrf2/HO-1 Signaling Pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef]
- Dini, S.; Zakeri, M.; Ebrahimpour, S.; Dehghanian, F.; Esmaeili, A. Quercetin-conjugated Superparamagnetic Iron Oxide Nanoparticles Modulate Glucose Metabolism-Related Genes and MiR-29 Family in the Hippocampus of Diabetic Rats. Sci. Rep. 2021, 11, 8618. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Sharma, A.; Singh, T.R.; Udayabanu, M. Quercetin Ameliorates Chronic Unpredicted Stress-Mediated Memory Dysfunction in Male Swiss Albino Mice by Attenuating Insulin Resistance and Elevating Hippocampal GLUT4 Levels Independent of Insulin Receptor Expression. Horm. Behav. 2017, 89, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, Z.; ShahaniPour, K.; Monajemi, R.; Ahadi, A.M. Effect of Quercetin and Abelmoschus esculentus (L.) Moench on Lipids Metabolism and Blood Glucose through AMPK-α in Diabetic Rats (HFD/STZ). J. Food Biochem. 2022, 46, e14506. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-Hydrogel Embedded with Quercetin and Oleic Acid as a New Formulation in the Treatment of Diabetic Foot Ulcer: A Pilot Study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
- Khorshidi, M.; Moini, A.; Alipoor, E.; Rezvan, N.; Gorgani-Firuzjaee, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. The Effects of Quercetin Supplementation on Metabolic and Hormonal Parameters as Well as Plasma Concentration and Gene Expression of Resistin in Overweight or Obese Women with Polycystic Ovary Syndrome. Phytother. Res. 2018, 32, 2282–2289. [Google Scholar] [CrossRef]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A Potential Polydynamic Drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Terao, J. Factors Modulating Bioavailability of Quercetin-Related Flavonoids and the Consequences of Their Vascular Function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative Protective Activities of Quercetin, Quercetin-3-Glucoside, and Rutin in Alcohol-Induced Liver Injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the Treatment of Diabetes: A Comprehensive and Latest Review. Front. Endocrinol. 2022, 13, 990299. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic Uses of Epicatechin in Diabetes and Cancer. Vet. World 2017, 10, 869. [Google Scholar] [CrossRef]
- Fuhr, U.; Klittich, K.; Staib, A. Inhibitory Effect of Grapefruit Juice and Its Bitter Principal, Naringenin, on CYP1A2 Dependent Metabolism of Caffeine in Man. Br. J. Clin. Pharmacol. 1993, 35, 431–436. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Belinchón-deMiguel, P.; Ramos-Campo, D.J.; Curiel-Regueros, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. The Interplay of Sports and Nutrition in Neurological Health and Recovery. J. Clin. Med. 2024, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef]
- Oppedisano, F.; Spagnoletta, A.; Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Franco-San Sebastián, D.; Alaniz-Monreal, S.; Rabadán-Chávez, G.; Vázquez-Manjarrez, N.; Hernández-Ortega, M.; Gutiérrez-Salmeán, G. Anthocyanins: Potential Therapeutic Approaches towards Obesity and Diabetes Mellitus Type 2. Molecules 2023, 28, 1237. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the Biological Activity of Anthocyanins and Polyphenols in Blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Lu, J.; Sun, Y.; Cui, Y. Research Progress of Proanthocyanidins and Anthocyanidins. Phytother. Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium Ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000745. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, L.; Yu, J. Effects of Blueberry Anthocyanins on Retinal Oxidative Stress and Inflammation in Diabetes through Nrf2/HO-1 Signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Sommella, E.; Cianciarulo, D.; Ostacolo, C.; Manfra, M.; Di Sarno, V.; Musella, S.; Russo, M.; Messore, A.; Parrino, B.; et al. Polyphenolic Extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” Exerts Anti-Inflammatory and Antioxidant Effects via NF-KB and Nrf-2 Activation in Murine Macrophages. Nutrients 2018, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of Dietary Anthocyanins on Biomarkers of Glycemic Control and Glucose Metabolism: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Food Res. Int. 2020, 137, 109379. [Google Scholar] [CrossRef]
- Zheng, F.; Xue, H.; Wang, B.X.; Wu, M.Y.; Chen, D.X.; Yue, H.; Wen, L.K.; He, Y. Identification of Stabilization of Malvid Anthocyanins and Antioxidant Stress Activation via the AMPK/SIRT1 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 9934646. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Ye, X.; Chen, W.; Huang, X.F.; Yan, F.J.; Deng, S.G.; Zheng, X.D.; Shan, P.F. Anti-Diabetic Effect of Anthocyanin Cyanidin-3-O-Glucoside: Data from Insulin Resistant Hepatocyte and Diabetic Mouse. Nutr. Diabetes 2024, 14, 7. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224. [Google Scholar] [CrossRef]
- Kozłowska, A.; Nitsch-Osuch, A. Anthocyanins and Type 2 Diabetes: An Update of Human Study and Clinical Trial. Nutrients 2024, 16, 1674. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Tian, J.; Xin, G.; Liu, L.; Sun, X.; Li, B. Advanced Approaches for Improving Bioavailability and Controlled Release of Anthocyanins. J. Control. Release 2022, 341, 285–299. [Google Scholar] [CrossRef]
- Ansari, M.H.R.; Saher, S.; Parveen, R.; Khan, W.; Khan, I.A.; Ahmad, S. Role of Gut Microbiota Metabolism and Biotransformation on Dietary Natural Products to Human Health Implications with Special Reference to Biochemoinformatics Approach. J. Tradit. Complement. Med. 2022, 13, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry-Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Zhuang, H.; Yang, Z.; Jiang, G.; Liu, Z. Promoting Intestinal IgA Production in Mice by Oral Administration with Anthocyanins. Front. Immunol. 2022, 13, 826597. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of Anthocyanins and Consequent Effects on the Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends in Foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Committee, A.D.A.P.P. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF Diabetes Atlas: Global Estimates of Undiagnosed Diabetes in Adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress-Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High-Serum Carotenoids Associated with Lower Risk for Developing Type 2 Diabetes among Japanese Subjects: Mikkabi Cohort Study. BMJ Open Diabetes Res. Care 2015, 3, e000147. [Google Scholar] [CrossRef] [PubMed]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes Mellitus and Serum Carotenoids: Findings of a Population-Based Study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.M.V. Diabetes Mellitus and Serum Carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef]
- Russell, R.M.; Paiva, S.A.R. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of Beta-Carotene Fortified Synbiotic Food on Metabolic Control of Patients with Type 2 Diabetes Mellitus: A Double-Blind Randomized Cross-over Controlled Clinical Trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef]
- Ärnlöv, J.; Zethelius, B.; Risérus, U.; Basu, S.; Berne, C.; Vessby, B.; Alfthan, G.; Helmersson, J. Serum and Dietary β-Carotene and α-Tocopherol and Incidence of Type 2 Diabetes Mellitus in a Community-Based Study of Swedish Men: Report from the Uppsala Longitudinal Study of Adult Men (ULSAM) Study. Diabetologia 2009, 52, 97–105. [Google Scholar] [CrossRef]
- van Steenwijk, H.P.; Bast, A.; de Boer, A. The Role of Circulating Lycopene in Low-Grade Chronic Inflammation: A Systematic Review of the Literature. Molecules 2020, 25, 4378. [Google Scholar] [CrossRef]
- Vincent, H.K.; Bourguignon, C.M.; Weltman, A.L.; Vincent, K.R.; Barrett, E.; Innes, K.E.; Taylor, A.G. Effects of Antioxidant Supplementation on Insulin Sensitivity, Endothelial Adhesion Molecules, and Oxidative Stress in Normal-Weight and Overweight Young Adults. Metabolism 2009, 58, 254–262. [Google Scholar] [CrossRef]
- Yeum, K.J.; Russell, R.M. Carotenoid Bioavailability and Bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, Vitamin A, and Their Association with the Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Lampousi, A.M.; Lundberg, T.; Löfvenborg, J.E.; Carlsson, S. Vitamins C, E, and β-Carotene and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100211. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ozaki, M.; Miyashita, M.; Fukazawa, M.; Nakaoka, T.; Wakisaka, T.; Matsui, Y.; Hibi, M.; Osaki, N.; Shibata, S. Effects of Timing of Acute Catechin-Rich Green Tea Ingestion on Postprandial Glucose Metabolism in Healthy Men. J. Nutr. Biochem. 2019, 73, 108221. [Google Scholar] [CrossRef]
- Cremonini, E.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin in the Control of Glucose Homeostasis: Involvement of Redox-Regulated Mechanisms. Free Radic. Biol. Med. 2019, 130, 478–488. [Google Scholar] [CrossRef]
- Ito, A.; Matsui, Y.; Takeshita, M.; Katashima, M.; Goto, C.; Kuriki, K. Gut Microbiota-Mediated Associations of Green Tea and Catechin Intakes with Glucose Metabolism in Individuals without Type 2 Diabetes Mellitus: A Four-Season Observational Study with Mediation Analysis. Arch. Microbiol. 2023, 205, 191. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Hussein, O.E.; Mahmoud, A.M. Pathophysiological Mechanisms of Diabetic Cardiomyopathy and the Therapeutic Potential of Epigallocatechin-3-Gallate. Biomed. Pharmacother. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E. Curative Effect of Catechin Isolated from Elaeagnus Umbellata Thunb. Berries for Diabetes and Related Complications in Streptozotocin-Induced Diabetic Rats Model. Molecules 2020, 26, 137. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Hayashibara, K.; Nagano, T.; Ikeda, M.; Yuan, S.; Ueda, S.; Shirai, Y.; Yoshida, K.I.; Ashida, H. Epigallocatechin Gallate Induces GLUT4 Translocation in Skeletal Muscle through Both PI3K- and AMPK-Dependent Pathways. Food Funct. 2018, 9, 4223–4233. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; de Pascual-Teresa, S. Effects of Polyphenol-Rich Foods on Chronic Diseases. Nutrients 2023, 15, 4134. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Zhao, M.; Wang, J.; Wang, Z. Hawthorn Polyphenols, D-Chiro-Inositol, and Epigallocatechin Gallate Exert a Synergistic Hypoglycemic Effect. J. Food Biochem. 2021, 45, e13771. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin Mitigates High-Fructose-Associated Insulin Resistance by Modulating Redox Signaling and Endoplasmic Reticulum Stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Feng, Z.; Liu, J.; Liu, J.; Long, J. (−)-Epigallocatechin-3-Gallate Attenuated Myocardial Mitochondrial Dysfunction and Autophagy in Diabetic Goto–Kakizaki Rats. Free Radic. Res. 2014, 48, 898–906. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Hu, X.; Xu, Q.; Zhang, Y.; Liu, H.; Diao, Y.; Zhang, X.; Li, L.; Yu, J.; et al. Epigallocatechin-3-Gallate Prevents Inflammation and Diabetes -Induced Glucose Tolerance through Inhibition of NLRP3 Inflammasome Activation. Int. Immunopharmacol. 2021, 93, 107412. [Google Scholar] [CrossRef]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and Quercetin Exhibit in Vitro Antioxidant Effect, Improve Biochemical Parameters Related to Metabolic Syndrome, and Decrease Cellular Genotoxicity in Humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef]
- Takahashi, M.; Ozaki, M.; Tsubosaka, M.; Kim, H.K.; Sasaki, H.; Matsui, Y.; Hibi, M.; Osaki, N.; Miyashita, M.; Shibata, S. Effects of Timing of Acute and Consecutive Catechin Ingestion on Postprandial Glucose Metabolism in Mice and Humans. Nutrients 2020, 12, 565. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Cheng, Z.; Shu, Y.; Li, X.; Li, Y.; Zhou, S.; Liu, H. Evaluation of Potential Cardiotoxicity of Ammonia: L-Selenomethionine Inhibits Ammonia-Induced Cardiac Autophagy by Activating the PI3K/AKT/MTOR Signaling Pathway. Ecotoxicol. Environ. Saf. 2022, 233, 113304. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Pedersen, J.Z.; Incerpi, S.; Belli, S.; Sakib, R.; Rossi, M. Interaction between Vitamins C and E When Scavenging the Superoxide Radical Shown by Hydrodynamic Voltammetry and DFT. Biophysica 2024, 4, 310–326. [Google Scholar] [CrossRef]
- Huang, H.Y.; Appel, L.J.; Croft, K.D.; Miller, E.R.; Mori, T.A.; Puddey, I.B. Effects of Vitamin C and Vitamin E on in Vivo Lipid Peroxidation: Results of a Randomized Controlled Trial. Am. J. Clin. Nutr. 2002, 76, 549–555. [Google Scholar] [CrossRef]
- Hamilton, I.M.J.; Gilmore, W.S.; Benzie, I.F.F.; Mulholland, C.W.; Strain, J.J. Interactions between Vitamins C and E in Human Subjects. Br. J. Nutr. 2000, 84, 261–267. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and Cardiovascular Disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Siddiqui, I.A.; Panackal, J.E.; Mintie, C.A.; Ahmad, N. Quercetin–Resveratrol Combination for Prostate Cancer Management in TRAMP Mice. Cancers 2020, 12, 2141. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Hsiao, Y.F.; Huang, S.C.; Cheng, S.B.; Hsu, C.C.; Huang, Y.C. Glutathione and Selenium Supplementation Attenuates Liver Injury in Diethylnitrosamine-Induced Hepatocarcinogenic Mice by Enhancing Glutathione-Related Antioxidant Capacities. Int. J. Mol. Sci. 2024, 25, 11339. [Google Scholar] [CrossRef]
- Goda, K.; Muta, K.; Yasui, Y.; Oshida, S.I.; Kitatani, K.; Takekoshi, S. Selenium and Glutathione-Depleted Rats as a Sensitive Animal Model to Predict Drug-Induced Liver Injury in Humans. Int. J. Mol. Sci. 2019, 20, 3141. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Ekramzadeh, M.; Hejazi, N. Efficacy of Supplementary Vitamins C and E on Anxiety, Depression and Stress in Type 2 Diabetic Patients: A Randomized, Single-Blind, Placebo-Controlled Trial. Pak. J. Biol. Sci. 2013, 16, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Acuña-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and Mitochondrial Function. Life Sci. 2004, 75, 765–790. [Google Scholar] [CrossRef]
- López-Burillo, S.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Manchester, L.C.; Reiter, R.J. Melatonin, Xanthurenic Acid, Resveratrol, EGCG, Vitamin C and α-Lipoic Acid Differentially Reduce Oxidative DNA Damage Induced by Fenton Reagents: A Study of Their Individual and Synergistic Actions. J. Pineal Res. 2003, 34, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-Microbiota Interactions and Personalized Nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Boronat, A.; Rodriguez-Morató, J.; Serreli, G.; Fitó, M.; Tyndale, R.F.; Deiana, M.; De La Torre, R. Contribution of Biotransformations Carried Out by the Microbiota, Drug-Metabolizing Enzymes, and Transport Proteins to the Biological Activities of Phytochemicals Found in the Diet. Adv. Nutr. 2021, 12, 2172–2189. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Malczewski, A.B.; Ketheesan, N.; Coward, J.I.G.; Navarro, S. Enhancing Checkpoint Inhibitor Therapy in Solid Tissue Cancers: The Role of Diet, the Microbiome & Microbiome-Derived Metabolites. Front. Immunol. 2021, 12, 624434. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Yang, R.; Chen, F.; Liao, Y.; Zhu, Z.; Wu, Z.; Sun, X.; Wang, L. Effects of Berberine on the Gastrointestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 10, 588517. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Cho, D.; Lee, J.H.; Choi, J.K.; Park, M.; Kim, W. Green Tea Encourages Growth of Akkermansia Muciniphila. J. Med. Food 2020, 23, 841–851. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, J.; Yu, B.; Liu, Z.; Chen, H.; He, J.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; et al. Gut Microbiota Absence and Transplantation Affect Growth and Intestinal Functions: An Investigation in a Germ-Free Pig Model. Anim. Nutr. 2021, 7, 295–304. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Kumar Gangwar, S.; Nair Devanarayanan, T.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakara, A. Interplay of Dietary Antioxidants and Gut Microbiome in Human Health: What Has Been Learnt Thus Far? J. Funct. Foods 2023, 100, 105365. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Kassem, N.M.; Abdelmegid, Y.A.; El-Sayed, M.K.; Sayed, R.S.; Abdel-Aalla, M.H.; Kassem, H.A. Nutrigenomics and Microbiome Shaping the Future of Personalized Medicine: A Review Article. J. Genet. Eng. Biotechnol. 2023, 21, 134. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, S. Nutrigenomics and Nutrigenetics: New Insight in Disease Prevention and Cure. Indian J. Clin. Biochem. 2017, 32, 371. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Allayee, H.; Gerszten, R.E.; Ideraabdullah, F.; Kris-Etherton, P.M.; Ordovás, J.M.; Rimm, E.B.; Wang, T.J.; Bennett, B.J. Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Genet. 2016, 9, 291–313. [Google Scholar] [CrossRef]
- Niforou, A.; Konstantinidou, V.; Naska, A. Genetic Variants Shaping Inter-Individual Differences in Response to Dietary Intakes-A Narrative Review of the Case of Vitamins. Front. Nutr. 2020, 7, 558598. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking Dietary Patterns with Gut Microbial Composition and Function. Gut Microbes 2016, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiu, P.; Zhou, X.; Yang, X.; Bi, C.; Li, S.; Su, W.; Pan, Y.; Tao, W.; Wu, X.; et al. Akkermansia Muciniphila Exerts a Protective Effect on the Development of Abdominal Aortic Aneurysm by Inhibiting Inflammation. J. Funct. Foods 2025, 127, 106718. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M.S. Evidence for Greater Production of Colonic Short-Chain Fatty Acids in Overweight than Lean Humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Qiu, H.; Ma, Y.; Hou, N.; Zhang, J.; Kan, C.; Han, F.; Sun, X.; Shi, J. The Complex Link between the Gut Microbiome and Obesity-Associated Metabolic Disorders: Mechanisms and Therapeutic Opportunities. Heliyon 2024, 10, e37609. [Google Scholar] [CrossRef]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal Microbiota Transplantation in Metabolic Syndrome: History, Present and Future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Cooper, G.M. The Central Role of Enzymes as Biological Catalysts; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Elsamanoudy, A.; Mohamed Neamat-Allah, M.; Hisham Mohammad, F.; Hassanien, M.; Nada, H. The Role of Nutrition Related Genes and Nutrigenetics in Understanding the Pathogenesis of Cancer. J. Microsc. Ultrastruct. 2016, 4, 115. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Behrens, K.A.; Jania, L.A.; Snouwaert, J.N.; Nguyen, M.T.; Moy, S.S.; Tikunov, A.P.; Macdonald, J.M.; Koller, B.H. Beyond Detoxification: Pleiotropic Functions of Multiple Glutathione S-Transferase Isoforms Protect Mice against a Toxic Electrophile. PLoS ONE 2019, 14, e0225449. [Google Scholar] [CrossRef]
- Mishra, U.N.; Jena, D.; Sahu, C.; Devi, R.; Kumar, R.; Jena, R.; Irondi, E.A.; Rout, S.; Tiwari, R.K.; Lal, M.K.; et al. Nutrigenomics: An Inimitable Interaction amid Genomics, Nutrition and Health. Innov. Food Sci. Emerg. Technol. 2022, 82, 103196. [Google Scholar] [CrossRef]
- Omer, R.E.; Verhoef, L.; Van’t Veer, P.; Idris, M.O.; Kadaru, A.M.Y.; Kampman, E.; Bunschoten, A.; Kok, F.J. Peanut Butter Intake, GSTM1 Genotype and Hepatocellular Carcinoma: A Case-Control Study in Sudan. Cancer Causes Control 2001, 12, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ordovas, J.M. Genetic Influences on Blood Lipids and Cardiovascular Disease Risk: Tools for Primary Prevention. Am. J. Clin. Nutr. 2009, 89, 1509S–1517S. [Google Scholar] [CrossRef] [PubMed]
- Bakker, G.C.M.; Van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.P.; Kooistra, T.; Van Ommen, B.; Hendriks, H.F.J. An Antiinflammatory Dietary Mix Modulates Inflammation and Oxidative and Metabolic Stress in Overweight Men: A Nutrigenomics Approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef]
- Casanova, A.G.; López-Hernández, F.J.; Vicente-Vicente, L.; Morales, A.I.; Di Pietro, N.; Bonomini, M.; Husi, H. Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease? Antioxidants 2021, 10, 1669. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Frank, G.; Smeriglio, A.; Trombetta, D.; Colica, C.; Cianci, R.; De Lorenzo, A.; Di Renzo, L. Antioxidant-Enriched Diet on Oxidative Stress and Inflammation Gene Expression: A Randomized Controlled Trial. Genes 2023, 14, 206. [Google Scholar] [CrossRef]
- Kwon, G.; Gibson, K.M.; Bi, L. Editorial Commentary on the Special Issue “Antioxidant Therapy for Cardiovascular Diseases”—Cutting-Edge Insights into Oxidative Stress and Antioxidant Therapy in Cardiovascular Health. Antioxidants 2024, 13, 1034. [Google Scholar] [CrossRef]
- Al Balushi, H.; Ahmed, J.; Ahuja, L.K.; Barkha, F.; Shafeeq, M.I.; Baluch, A.B.; Altinkaynak, Y.; Abdallah, S.; Islam, H.; Islam, R.; et al. Evaluating the Efficacy of Antioxidant Therapy in Enhancing the Quality of Life of Chronic Pancreatitis Patients: A Systematic Review. Cureus 2024, 16, e57402. [Google Scholar] [CrossRef]
- Birk, R. Nutrigenetics of Antioxidant Enzymes and Micronutrient Needs in the Context of Viral Infections. Nutr. Res. Rev. 2021, 34, 174–184. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef]
- Sotoudeh, G.; Abshirini, M.; Bagheri, F.; Siassi, F.; Koohdani, F.; Aslany, Z. Higher Dietary Total Antioxidant Capacity Is Inversely Related to Prediabetes: A Case-Control Study. Nutrition 2018, 46, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.L.; Bowden, R.G.; Willoughby, D.S. Cassia Cinnamon Supplementation Reduces Peak Blood Glucose Responses but Does Not Improve Insulin Resistance and Sensitivity in Young, Sedentary, Obese Women. J. Diet. Suppl. 2016, 13, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Chang, P.S.; Chen, H.W.; Lee, P.F.; Chang, Y.C.; Tseng, C.Y.; Lin, P.T. Ubiquinone Supplementation with 300 Mg on Glycemic Control and Antioxidant Status in Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Antioxidants 2020, 9, 823. [Google Scholar] [CrossRef]
- Shrivastav, D.; Dabla, P.K.; Sharma, J.; Viswas, A.; Mir, R. Insights on Antioxidant Therapeutic Strategies in Type 2 Diabetes Mellitus: A Narrative Review of Randomized Control Trials. World J. Diabetes 2023, 14, 919. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Järvinen, R.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Salleh, N.H.; Zulkipli, I.N.; Mohd Yasin, H.; Ja’Afar, F.; Ahmad, N.; Wan Ahmad, W.A.N.; Ahmad, S.R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid.-Based Complement. Altern. Med. 2021, 2021, 5570939. [Google Scholar] [CrossRef]
- Tesoriere, L.; Miyazawa, T.; Abe, C.; Carpentero Burdeos, G.; Matsumoto, A.; Toda, M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals 2022, 2, 181–204. [Google Scholar] [CrossRef]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef]
- Gervasi, T.; Barreca, D.; Laganà, G.; Mandalari, G. Health Benefits Related to Tree Nut Consumption and Their Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 5960. [Google Scholar] [CrossRef]
- Asp, M.L.; Collene, A.L.; Norris, L.E.; Cole, R.M.; Stout, M.B.; Tang, S.Y.; Hsu, J.C.; Belury, M.A. Time-Dependent Effects of Safflower Oil to Improve Glycemia, Inflammation and Blood Lipids in Obese, Post-Menopausal Women with Type 2 Diabetes: A Randomized, Double-Masked, Crossover Study. Clin. Nutr. 2011, 30, 443–449. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef] [PubMed]
- Appiah, C.A.; Ngounda, J.; Boakye-Yiadom, M.; Mills-Robertson, F.C.; Nel, M.; Johnson, R.; Walsh, C. Bridelia Ferruginea Tea Consumption Improves Antioxidant Status in Individuals Living with Type 2 Diabetes. Diabetology 2025, 6, 6. [Google Scholar] [CrossRef]
- van der Schaft, N.; Schoufour, J.D.; Nano, J.; Kiefte-de Jong, J.C.; Muka, T.; Sijbrands, E.J.G.; Ikram, M.A.; Franco, O.H.; Voortman, T. Dietary Antioxidant Capacity and Risk of Type 2 Diabetes Mellitus, Prediabetes and Insulin Resistance: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 853. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational Diabetes Mellitus—A Metabolic and Reproductive Disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef]
- Heshmati, S.; Moludi, J.; Nachvak, S.M.; Pirjani, R.; Heshmati, J.; Sepidarkish, M. The Association of Dietary Total Antioxidant Capacity and Gestational Diabetes: A Prospective Cohort Study from the Mothers and Their Children’s Health (MATCH). Nutr. Diabetes 2024, 14, 78. [Google Scholar] [CrossRef]
- Schiattarella, A.; Lombardo, M.; Morlando, M.; Rizzo, G. The Impact of a Plant-Based Diet on Gestational Diabetes: A Review. Antioxidants 2021, 10, 557. [Google Scholar] [CrossRef]
- Jorquera, G.; Fornes, R.; Cruz, G.; Thomas-Valdés, S. Association of Polyphenols Consumption with Risk for Gestational Diabetes Mellitus and Preeclampsia: A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 2294. [Google Scholar] [CrossRef]
- Jaworsky, K.; DeVillez, P.; Basu, A. The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials. Int. J. Environ. Res. Public Health 2023, 20, 4188. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, F.; Liu, G.; Li, M.; Voortman, T.; Tobias, D.K.; Ley, S.H.; Bhupathiraju, S.N.; Li, L.J.; Chavarro, J.E.; et al. Prepregnancy Plant-Based Diets and the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study of 14,926 Women. Am. J. Clin. Nutr. 2021, 114, 1997–2005. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Williamson, G.; Rice-Evans, C.A.; Packer, L. Absorption of quercetin glycosides. Oxidative Stress Dis. 2003, 9, 391–412. [Google Scholar]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, diet, and health: The epigenetic link. Front. Genet. 2013, 4, 1–16. [Google Scholar]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Molecular mechanisms for anti-aging by natural dietary compounds. Mol. Nutr. Food Res. 2012, 56, 88–115. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Rizzetto, L.; Tuohy, K.M. Gut microbiota and health: Connecting actors across the metabolic system. Proc. Nutr. Soc. 2019, 78, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Cipriano, P.; Riccardi, G.; Rivellese, A.A. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Jie, C.J.; Chin, K.Y.; Ima-Nirwana, S.; Kamisah, Y.; Tan, J.L.C. Precision Nutrition Unveiled: Gene–Nutrient Interactions, Microbiota Dynamics, and Lifestyle Factors in Obesity Management. Nutrients 2024, 16, 581. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss in high-fat-fed obese mice. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]

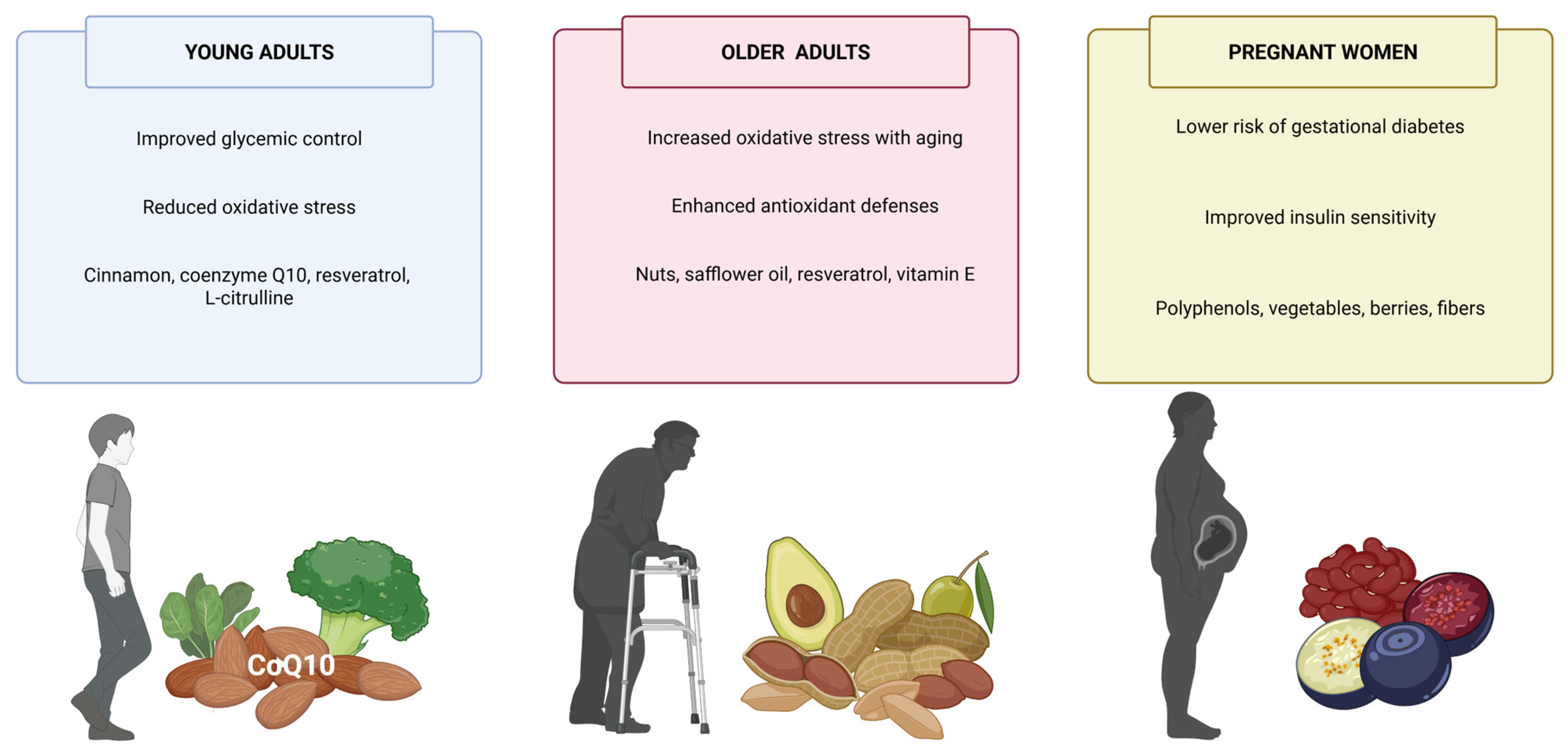
| Antioxidant Group | Representative Compounds | Primary Molecular Targets | Mechanisms of Action | Clinical Evidence | Key Limitations | Potential Applications |
|---|---|---|---|---|---|---|
| Polyphenols | Quercetin, Resveratrol, EGCG | IRS/PI3K/Akt, NF-κB, AMPK, Nrf2 | ↑ Insulin sensitivity, ↓ inflammation, ↑ antioxidant defense | Strong (RCTs and meta-analyses) | Poor bioavailability; metabolism-dependent effects | Adjunct therapy, personalized nutrition |
| Curcuminoids | Curcumin | NF-κB, JNK, AMPK, PPARγ | Anti-inflammatory, antioxidant; modulates insulin signaling | Moderate (human studies, meta-analyses) | Low solubility; variable absorption | Formulated supplements, nano-delivery systems |
| Carotenoids | β-Carotene, Lutein | Nrf2, mitochondrial ROS | ROS scavenging, ↓ lipid peroxidation, ↓ AGEs | Limited but promising | Lipophilicity; food matrix dependent | Ocular protection, vascular support |
| Vitamins | Vitamin C, Vitamin E | Nrf2, NF-κB, eNOS | Redox balance, ↑ NO, ↓ systemic inflammation | Mixed results, dose-dependent | Variable efficacy; threshold effects | Complementary antioxidant support |
| Lignans and Stilbenes | Secoisolariciresinol, Resveratrol | SIRT1, PGC-1α, NLRP3 | ↑ Mitochondrial biogenesis, ↓ inflammasome activation | Emerging evidence | Low bioavailability; population-specific response | Gut microbiota modulation, metabolic flexibility |
| Strategy/Innovation | Description | Expected Benefit | Reference |
|---|---|---|---|
| Nanoencapsulation of polyphenols | Use of liposomes, nanoemulsions, or solid lipid nanoparticles for oral delivery | Improved bioavailability and stability of antioxidants | [271,272] |
| Functional food matrices with synergistic components | Formulation of polyphenols with fibers, omega-3, or probiotics (e.g., synbiotics) | Enhanced metabolic action and gut microbiota modulation | [274,275] |
| Personalized antioxidant therapy | Tailoring interventions based on genetics, gut microbiota, and metabolic phenotype | Improved individual response and efficacy | [276,277] |
| Regulatory standardization and clinical validation | Harmonized biomarkers, health claims approval, and randomized controlled trials (RCTs) | Therapeutic legitimacy and broader clinical integration | [278] |
| Integration with pharmacologic therapy | Co-administration of functional foods with drugs like metformin | Potential for synergistic glycemic and oxidative benefits | [272] |
| Long-term cohort and intervention studies | Evaluation of functional food efficacy over months/years in diverse populations | Insight into sustainability, adherence, and real-world outcomes | [279] |
| Precision nutrition and nutrigenomics application | Genotype- and microbiome-driven customization of dietary antioxidant strategies | Optimal antioxidant selection and dose per patient | [276] |
| Combination of multiple polyphenols with complementary targets | Multi-compound formulations targeting inflammation, oxidative stress, and insulin signaling | Amplified metabolic impact through multitarget modulation | [270,273] |
| Strategy/Innovation | Current Development Stage | Estimated Time to Broad Clinical/Practical Use | Expected Implementation Horizon | Notes |
|---|---|---|---|---|
| Microbiome-targeted therapies | Early-stage clinical trials; functional food applications | 3–5 years | Short- to mid-term | Includes polyphenol–microbiota interaction studies; high translational potential |
| Precision nutrition (nutrigenomics, metabolomics) | Pilot programs and academic research | 5–10 years | Mid- to long-term | Requires omics integration, AI tools, and regulatory support |
| Nanoencapsulation of antioxidants | Preclinical and emerging commercial products | 3–5 years | Short- to mid-term | Focused on enhancing bioavailability and compound stability |
| Synergistic antioxidant combinations | Product formulation and validation in progress | 2–4 years | Short-term | May reach market rapidly via nutraceutical and food industry channels |
| Digital health integration for antioxidant-based interventions | Conceptual and pilot phases | 5–8 years | Mid-term | Dependent on digital platforms, mobile tech, and personalization tools |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Beltrán-Velasco, A.I.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Valcárcel-Martín, R.; Tornero-Aguilera, J.F. Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants 2025, 14, 725. https://doi.org/10.3390/antiox14060725
Clemente-Suárez VJ, Martín-Rodríguez A, Beltrán-Velasco AI, Rubio-Zarapuz A, Martínez-Guardado I, Valcárcel-Martín R, Tornero-Aguilera JF. Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants. 2025; 14(6):725. https://doi.org/10.3390/antiox14060725
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Alexandra Martín-Rodríguez, Ana Isabel Beltrán-Velasco, Alejandro Rubio-Zarapuz, Ismael Martínez-Guardado, Roberto Valcárcel-Martín, and José Francisco Tornero-Aguilera. 2025. "Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations" Antioxidants 14, no. 6: 725. https://doi.org/10.3390/antiox14060725
APA StyleClemente-Suárez, V. J., Martín-Rodríguez, A., Beltrán-Velasco, A. I., Rubio-Zarapuz, A., Martínez-Guardado, I., Valcárcel-Martín, R., & Tornero-Aguilera, J. F. (2025). Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants, 14(6), 725. https://doi.org/10.3390/antiox14060725











