Role of Redox-Induced Protein Modifications in Spermatozoa in Health and Disease
Abstract
1. Introduction
2. Thiol Oxidation (Sulfenic, Sulfinic and Sulfonic Acid)
3. S-Nitrosylation and Tyrosine Nitration
4. Protein S-Glutathionylation
5. Protein S-CoAlation
6. Protein Carbonylation and Lipid Peroxidation
7. Protein S-Sulfhydration
8. Methionine Oxidation
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| H2O2 | Hydrogen Peroxide |
| NO | Nitric Oxide |
| ONOO− | Peroxynitrite |
| PUFA | Polyunsaturated Fatty Acid |
| MDA | Malondialdehyde |
| 4-HNE | 4-Hydroxynonenal |
| PRDX | Peroxiredoxin |
| PRDX6 | Peroxiredoxin 6 |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| GPX | Glutathione Peroxidase |
| GST | Glutathione-S-Transferase |
| TXNRD | Thioredoxin Reductase |
| Trx | Thioredoxin |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| MSR | Methionine Sulfoxide Reductase |
| MSRA/B | Methionine Sulfoxide Reductase A/B |
| SNO | S-Nitrosylation (S-NO-modified proteins) |
| SSG | S-Glutathionylation (S-SG-modified proteins) |
| SSC0A | S-CoAlation (Coenzyme A-modified proteins) |
| SOH | Sulfenic Acid Modification |
| SO2H | Sulfinic Acid Modification |
| SO3H | Sulfonic Acid Modification |
| SSH | Sulfhydration (Persulfidation) |
| ZP | Zona Pellucida |
| EF-hand proteins | Calcium-binding proteins (named for their helix-loop-helix structure) |
| Hsp70 | Heat Shock Protein 70 |
| S6K1 | Ribosomal Protein S6 Kinase 1 |
| ODF2 | Outer Dense Fiber Protein 2 |
| GAPDHS | Glyceraldehyde-3-Phosphate Dehydrogenase, Sperm-specific |
| AKAP | A-Kinase Anchoring Protein |
| RyR | Ryanodine Receptor |
| SNAP | Soluble NSF Attachment Protein |
| iPLA2 | Calcium-Independent Phospholipase A2 |
| LPCAT | Lysophosphatidylcholine Acyltransferase |
References
- World Health Organization. Infertility. 2023. Available online: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility (accessed on 12 January 2025).
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Zheng, L.; Jiang, H.; Jiang, T. Research advances in inflammation and oxidative stress in varicocele-induced male infertility: A narrative review. Asian J. Androl. 2025, 27, 177–184. [Google Scholar] [CrossRef]
- Leisegang, K. Oxidative stress in men with obesity, metabolic syndrome and type 2 diabetes mellitus: Mechanisms and management of reproductive dysfunction. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility-Volume One; Springer: Berlin/Heidelberg, Germany, 2022; pp. 237–256. [Google Scholar]
- Zeng, J.; Yuan, L.; Chen, G.; Qi, Y.; Qie, X.; Jin, Y.; Chen, Y.; Li, H. The ferroptosis of Sertoli cells inducing blood-testis barrier damage is produced by oxidative stress in cryptorchidism. Free Radic. Biol. Med. 2025, 232, 97–106. [Google Scholar] [CrossRef]
- Ho, C.L.T.; Vaughan-Constable, D.R.; Ramsay, J.; Jayasena, C.; Tharakan, T.; Yap, T.; Whiteman, I.; Graham, N.; Minhas, S.; Homa, S.T. The relationship between genitourinary microorganisms and oxidative stress, sperm DNA fragmentation and semen parameters in infertile men. Andrologia 2021, 54, e14322. [Google Scholar] [CrossRef] [PubMed]
- Delbes, G.; Hales, B.F.; Robaire, B. Toxicants and human sperm chromatin integrity. MHR Basic Sci. Reprod. Med. 2009, 16, 14–22. [Google Scholar] [CrossRef]
- Lamirande, D.E.; O’Flaherty, C. Sperm Capacitation as an Oxidative Event. In Studies on Men’s Health and Fertility; Springer: Berlin/Heidelberg, Germany, 2012; pp. 57–94. [Google Scholar] [CrossRef]
- Maroto, M.; Torvisco, S.N.; García-Merino, C.; Fernández-González, R.; Pericuesta, E. Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules 2025, 15, 500. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanatsu-Shinohara, M.; Shinohara, T. ROS-Generating Oxidase Nox3 Regulates the Self-Renewal of Mouse Spermatogonial Stem Cells. Biol. Reprod. 2015, 92, 147. [Google Scholar] [CrossRef]
- Zubkova, E.V.; Wade, M.; Robaire, B. Changes in spermatozoal chromatin packaging and susceptibility to oxidative challenge during aging. Fertil. Steril. 2005, 84 (Suppl 2), 1191–1198. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored-PubMed. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing Capacity of Spermatozoa deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Serafini, S.; O’Flaherty, C. Redox Regulation to Modulate Phosphorylation Events in Human Spermatozoa. Antioxid. Redox Signal. 2022, 37, 437–450. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Boerke, A.; Silva, P.F.; Garcia-Gil, N.; van Gestel, R.A.; Helms, J.B.; van de Lest, C.H.; Gadella, B.M. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol. Reprod. 2011, 85, 128–136. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa†. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Tsai, C.; Harakat, A.; Gagnon, C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998, 19, 585–594. [Google Scholar] [CrossRef]
- Laatsch, B.F.; Ali, B.A.; Berthiaume, A.R.; Cunningham, G.C.; Duncan, S.M.; Hau, N.; Ho, A.; Loomis, M.A.; Lowater, H.R.; McNally, B.L. A Review of Methionine and Cysteine: Their Roles as Sinks for Reactive Oxygen and Nitrogen Species, Including Bioinformatic Analysis of Their Percent Compositions in Metabolic Proteins. Indian J. Clin. Biochem. 2024, 1–12. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Scarlata, E. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The protection of mammalian spermatozoa against oxidative stress. Reproduction 2022, 164, F67–F78. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Agarwal, A.; van der Linde, M.; du Plessis, S.S. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod. Biol. Endocrinol. 2012, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Hu, B.; Li, Y.; Cai, R.; Gao, L.; Chu, G.; Yang, G.; Pang, W. Boar seminal plasma improves sperm quality by enhancing its antioxidant capacity during liquid storage at 17 C. Zygote 2022, 30, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Zeng, Y.; Luo, L.; Wang, K. Oxidative stress-mediated protein sulfenylation in human diseases: Past, present, and future. Redox Biol. 2024, 76, 103332. [Google Scholar] [CrossRef]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 2022, 102, 1881–1906. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Wu, P.Y.; Scarlata, E.; O’Flaherty, C. Long-term adverse effects of oxidative stress on rat epididymis and spermatozoa. Antioxidants 2020, 9, 170. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- O’Flaherty, C. Iatrogenic genetic damage of spermatozoa. Genet. Damage Hum. Spermatozoa 2014, 791, 117–135. [Google Scholar]
- Azzi, A. Oxidative stress: What is it? Can it be measured? Where is it located? Can it be good or bad? Can it be prevented? Can it be cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef]
- Gok, B.; Gok, G.; Koc, E.; Hamidi, N.; Alijla, A.; Asil, E.; Gok, A.; Kamaci, D.; Neselioglu, S.; Ener, K. The change in Thiol–Disulphide Homeostasis levels as an oxidative stress marker after varicocelectomy: Is there a relationship with sperm parameters? Andrologia 2020, 52, e13515. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Gabriel, M.C.S.; Zini, A.; Chan, P.; O’Flaherty, C. Low Amounts and High Thiol Oxidation of Peroxiredoxins in Spermatozoa From Infertile Men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, E.; Fernandez, M.C.; O’Flaherty, C. A novel combination of γ-tocopherol-rich mixture of tocopherols and ascorbic acid restores fertility in cases of tyrosine nitration-associated male infertility in mice. Antioxidants 2020, 9, 613. [Google Scholar] [CrossRef]
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine Oxidation in Proteins: Structure, Biophysics, and Simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef]
- Roussel, X.; Boukhenouna, S.; Rahuel-Clermont, S.; Branlant, G. The rate-limiting step of sulfiredoxin is associated with the transfer of the γ-phosphate of ATP to the sulfinic acid of overoxidized typical 2-Cys peroxiredoxins. FEBS Lett. 2011, 585, 574–578. [Google Scholar] [CrossRef]
- Chang, T.-S.; Jeong, W.; Woo, H.A.; Lee, S.M.; Park, S.; Rhee, S.G. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J. Biol. Chem. 2004, 279, 50994–51001. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.A.; Chae, H.Z.; Hwang, S.C.; Yang, K.-S.; Kang, S.W.; Kim, K.; Rhee, S.G. Reversing the Inactivation of Peroxiredoxins Caused by Cysteine Sulfinic Acid Formation. Science 2003, 300, 653–656. [Google Scholar] [CrossRef]
- de Lamirande, E.; Lamothe, G. Levels of semenogelin in human spermatozoa decrease during capacitation: Involvement of reactive oxygen species and zinc. Hum. Reprod. 2010, 25, 1619–1630. [Google Scholar] [CrossRef]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 106–115. [Google Scholar] [CrossRef]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation-PubMed. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Furuya, S.; Endo, Y.; Osumi, K.; Oba, M.; Nozawa, S.; Suzuki, S. Calyculin A, protein phosphatase inhibitor, enhances capacitation of human sperm. Fertil. Steril. 1993, 59, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Machado, L.E.S. Preferential redox regulation of cysteine-based protein tyrosine phosphatases: Structural and biochemical diversity. FEBS J. 2022, 289, 5480–5504. [Google Scholar] [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 332–356. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, J.; Diaz, E.S.; Morales, P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012, 349, 765–782. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol. Hum. Reprod. 2004, 10, 355–363. [Google Scholar] [CrossRef]
- Peña, F.J.; O’Flaherty, C.; Rodríguez, J.M.O.; Cano, F.E.M.; Gaitskell-Phillips, G.; Gil, M.C.; Ferrusola, C.O. The Stallion Spermatozoa: A Valuable Model to Help Understand the Interplay Between Metabolism and Redox (De)regulation in Sperm Cells. Antioxid. Redox Signal. 2022, 37, 521–537. [Google Scholar] [CrossRef]
- Kadam, K.; Khole, V.V.; Ghosalkar, K.; Jagtap, D.; Yarramala, D.S.; Ramachandran, B. Thiol based mechanism internalises interacting partners to outer dense fibers in sperm. Free Radic. Biol. Med. 2020, 148, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Nakagata, N.; Tamura, K.; Koga, R.; Nakao, S.; Takeo, T.; Ishima, Y.; Ikeda-Imafuku, M.; Shirakado, K. Oxidation of thiol groups in membrane proteins inhibits the fertilization ability and motility of sperm by suppressing calcium influx. Biol. Reprod. 2024, 112, 563–571. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Chaney, M.O. Mechanism of GAPDH Redox Signaling by H2O2 Activation of a Two−Cysteine Switch. Int. J. Mol. Sci. 2022, 23, 4604. [Google Scholar] [CrossRef]
- Naletova, I.; Schmalhausen, E.; Tomasello, B.; Pozdyshev, D.; Attanasio, F.; Muronetz, V. The role of sperm-specific glyceraldehyde-3-phosphate dehydrogenase in the development of pathologies—From asthenozoospermia to carcinogenesis. Front. Mol. Biosci. 2023, 10, 1256963. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Liu, Y.; O’Flaherty, C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian J. Androl. 2017, 19, 73–79. [Google Scholar]
- Aboalroub, A.A.; Al Azzam, K.M. Protein S-Nitrosylation: A Chemical Modification with Ubiquitous Biological Activities. Protein J. 2024, 43, 639–655. [Google Scholar] [CrossRef]
- Lewis, S.; Donnelly, E.; Sterling, E.; Kennedy, M.; Thompson, W.; Chakravarthy, U. Nitric oxide synthase and nitrite production in human spermatozoa: Evidence that endogenous nitric oxide is beneficial to sperm motility. Mol. Hum. Reprod. 1996, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Lamothe, G.; Villemure, M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Costamagna, C.; Moffa, F.; Aldieri, E.; Ochetti, S.; Bosia, A.; Massobrio, M.; Lindblom, B.; Ghigo, D. Signaling Pathway of Nitric Oxide-Induced Acrosome Reaction in Human Spermatozoa1. Biol. Reprod. 2001, 64, 1708–1712. [Google Scholar] [CrossRef]
- Hezavehei, M.; Kouchesfahani, H.M.; Shahverdi, A.; Sharafi, M.; Salekdeh, G.H.; Eftekhari-Yazdi, P. Preconditioning of sperm with sublethal nitrosative stress: A novel approach to improve frozen–thawed sperm function. Reprod. Biomed. Online 2019, 38, 413–425. [Google Scholar] [CrossRef]
- Hezavehei, M.; Mirzaei, M.; Sharafi, M.; Wu, Y.; Gupta, V.; Fitzhenry, M.; Kouchesfahani, H.M.; Eftekhari-Yazdi, P.; Baharvand, H.; Dalman, A.; et al. Proteomics study reveals the molecular mechanisms underlying cryotolerance induced by mild sublethal stress in human sperm. Cell Tissue Res. 2021, 387, 143–157. [Google Scholar] [CrossRef]
- Algieri, C.; Blanco-Prieto, O.; Llavanera, M.; Yeste, M.; Spinaci, M.; Mari, G.; Bucci, D.; Nesci, S. Effects of cryopreservation on the mitochondrial bioenergetics of bovine sperm. Reprod. Domest. Anim. 2022, 58, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Staicu, F.-D.; Martínez-Soto, J.C.; Canovas, S.; Matás, C. Nitric oxide-targeted protein phosphorylation during human sperm capacitation. Sci. Rep. 2021, 11, 20979. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aguirregomezcorta, J.; Soriano-Úbeda, C.; Matás, C. Involvement of nitric oxide during in vitro oocyte maturation, sperm capacitation and in vitro fertilization in pig. Res. Vet. Sci. 2021, 134, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Janta, A.; Majewska, A.; Ciereszko, A.; Mostek, A.; Janta, A.; Majewska, A.; Ciereszko, A. Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins. Int. J. Mol. Sci. 2021, 22, 7903. [Google Scholar] [CrossRef]
- Loggia, D.; O’Flaherty, C. Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation. Antioxidants 2024, 13, 885. [Google Scholar] [CrossRef]
- Lee, G.Y.; Lee, J.-Y.; Ko, Y.-G.; Jin, D.; Lee, S.Y.; Kim, B.; Kim, S.W. Nitric oxide enhances sperm viability and fertility in rooster semen. J. Anim. Reprod. Biotechnol. 2025, 40, 2–11. [Google Scholar] [CrossRef]
- Fadl, A.M.; El-Shahat, K.H.; Hashem, M.F. Effect of pentoxifylline on the testicular hemodynamic, volume, testosterone, nitric oxide levels and semen quality in Ossimi rams during non breeding-season. Theriogenology 2023, 209, 126–133. [Google Scholar] [CrossRef]
- Banihani, S.A.; Khaled, H.J. Caffeine increased progressive motility of human spermatozoa in normozoospermic and asthenozoospermic semen samples and enhanced activity of seminal creatine kinase. Andrologia 2021, 53, e14052. [Google Scholar] [CrossRef]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- Bumanlag, E.; Scarlata, E.; O’Flaherty, C. Peroxiredoxin 6 Peroxidase and Ca2+-Independent Phospholipase A2 Activities Are Essential to Support Male-Mouse Fertility. Antioxidants 2022, 11, 226. [Google Scholar] [CrossRef]
- Serrano, R.; Garrido, N.; Céspedes, J.A.; González-Fernández, L.; García-Marín, L.J.; Bragado, M.J. Molecular mechanisms involved in the impairment of boar sperm motility by peroxynitrite-induced nitrosative stress. Int. J. Mol. Sci. 2020, 21, 1208. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, A.; Macanovic, B.; Garalejic, E.; Korac, A.; Otasevic, V.; Korac, B. Level of NO/nitrite and 3-nitrotyrosine in seminal plasma of infertile men: Correlation with sperm number, motility and morphology. Chem.-Biol. Interact. 2018, 291, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Treulen, F.; Boguen, R.; Sánchez, R.; Villegas, J.V. Nitrosative stress by peroxynitrite impairs ATP production in human spermatozoa. Andrologia 2016, 49, e12615. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Boguen, R.; Treulen, F.; Sánchez, R.; Villegas, J.V. Peroxynitrite-mediated nitrosative stress decreases motility and mitochondrial membrane potential in human spermatozoa. Mol. Hum. Reprod. 2014, 21, 237–243. [Google Scholar] [CrossRef]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric oxide, 8-hydroxydeoxyguanosine, and total antioxidant capacity in human seminal plasma of infertile men and their relationship with sperm parameters. Clin. Exp. Reprod. Med. 2020, 47, 54–60. [Google Scholar] [CrossRef]
- Uribe, P.; Cabrillana, M.E.; Fornés, M.W.; Treulen, F.; Boguen, R.; Isachenko, V.; Isachenko, E.; Sánchez, R.; Villegas, J.V. Nitrosative stress in human spermatozoa causes cell death characterized by induction of mitochondrial permeability transition-driven necrosis. Asian J. Androl. 2018, 20, 600–607. [Google Scholar]
- Uribe, P.; Barra, J.; Painen, K.; Zambrano, F.; Schulz, M.; Moya, C.; Isachenko, V.; Isachenko, E.; Mallmann, P.; Sánchez, R. FeTPPS, a Peroxynitrite Decomposition Catalyst, Ameliorates Nitrosative Stress in Human Spermatozoa. Antioxidants 2023, 12, 1272. [Google Scholar] [CrossRef]
- Alnasser, S.M. The role of glutathione S-transferases in human disease pathogenesis and their current inhibitors. Genes Dis. 2025, 12, 101482. [Google Scholar] [CrossRef]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef]
- Ercolani, L.; Scirè, A.; Galeazzi, R.; Massaccesi, L.; Cianfruglia, L.; Amici, A.; Piva, F.; Urbanelli, L.; Emiliani, C.; Principato, G. A possible S-glutathionylation of specific proteins by glyoxalase II: An in vitro and in silico study. Cell Biochem. Funct. 2016, 34, 620–627. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Sharov, V.S.; Klotz, L.; Briviba, K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations: A new function for selenoproteins as peroxynitrite reductase. J. Biol. Chem. 1997, 272, 27812–27817. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, X.; Jiang, C. S-glutathionylation of ion channels: Insights into the regulation of channel functions, thiol modification crosstalk, and mechanosensing. Antioxid. Redox Signal. 2014, 20, 937–951. [Google Scholar] [CrossRef]
- Kukulage, D.S.K.; Samarasinghe, K.T.G.; Matarage Don, N.N.J.; Shivamadhu, M.C.; Shishikura, K.; Schiff, W.; Mashhadi Ramezani, F.; Padmavathi, R.; Matthews, M.L.; Ahn, Y.-H. Protein phosphatase PP2Cα S-glutathionylation regulates cell migration. J. Biol. Chem. 2024, 300, 107784. [Google Scholar] [CrossRef]
- Rashdan, N.A.; Shrestha, B.; Pattillo, C.B. S-glutathionylation, friend or foe in cardiovascular health and disease. Redox Biol. 2020, 37, 101693. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Turanov, A.A.; Mariotti, M.; Hwang, Y.J.; Wang, H.; Lee, S.-G.; Paulo, A.J.; Yim, H.S.; Gygi, P.S.; Chung, J.-J.; et al. Selenoprotein TXNRD3 supports male fertility via the redox regulation of spermatogenesis. J. Biol. Chem. 2022, 298, 102183. [Google Scholar] [CrossRef]
- Fafula, R.V.; Paranyak, N.M.; Besedina, A.S.; Vorobets, D.Z.; Iefremova, U.P.; Onufrovych, O.K.; Vorobets, Z.D. Biological Significance of Glutathione S-Transferases in Human Sperm Cells. J. Hum. Reprod. Sci. 2019, 12, 24–28. [Google Scholar] [CrossRef]
- Petit, F.; Serres, C.; Bourgeon, F.; Pineau, C.; Auer, J. Identification of sperm head proteins involved in zona pellucida binding. Hum. Reprod. 2013, 28, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, S.; Zhang, C.; Liu, H.; Liu, M.; Zhang, G.-H.; Duan, G.; Chen, S.; Ren, J. The moderation effect of GSTM1/GSTT1 gene polymorphisms on the association of sperm mitochondrial DNA copy number and sperm mobility. Sci. Rep. 2024, 14, 24790. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 61. [Google Scholar] [CrossRef]
- Garriga, F.; Llavanera, M.; Viñolas-Vergés, E.; Recuero, S.; Tamargo, C.; Delgado-Bermúdez, A.; Yeste, M. Glutathione S-transferase Mu 3 is associated to in vivo fertility, but not sperm quality, in bovine. Animal 2022, 16, 100609. [Google Scholar] [CrossRef]
- Schneider, M.; Förster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumüller, C.; Deutsch, M.J.; Walch, A.; Hrabé de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. Faseb. J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Pipolo, S.; Puglisi, R.; Mularoni, V.; Esposito, V.; Fuso, A.; Lucarelli, M.; Fiorenza, M.T.; Mangia, F.; Boitani, C. Involvement of sperm acetylated histones and the nuclear isoform of Glutathione peroxidase 4 in fertilization. J. Cell. Physiol. 2017, 233, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Mostek-Majewska, A.; Majewska, A.; Janta, A.; Ciereszko, A. New insights into posttranslational modifications of proteins during bull sperm capacitation. Cell Commun. Signal. 2023, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Mostek-Majewska, A.; Janta, A.; Majewska, A.; Ciereszko, A.; Mostek-Majewska, A.; Janta, A.; Majewska, A.; Ciereszko, A. Effect of 2-Cys Peroxiredoxins Inhibition on Redox Modifications of Bull Sperm Proteins. Int. J. Mol. Sci. 2021, 22, 12888. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Frontiers | Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Garriga, F.; Maside, C.; Padilla, L.; Recuero, S.; Rodríguez-Gil, J.E.; Yeste, M.; Garriga, F.; Maside, C.; Padilla, L.; Recuero, S.; et al. Heat shock protein 70 kDa (HSP70) is involved in the maintenance of pig sperm function throughout liquid storage at 17 °C. Sci. Rep. 2024, 14, 13383. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Gong, W.; Liu, Z.; Wu, H.; Hu, W.; Chen, X.; Wang, L.; Wu, S.; Chen, C.; et al. S-Glutathionylation of human inducible Hsp70 reveals a regulatory mechanism involving the C-terminal α-helical lid. J. Biol. Chem. 2020, 295, 8302–8324. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, M.V.; Kleimenov, S.Y.; Samygina, V.R.; Muronetz, V.I.; Schmalhausen, E.V. S-nitrosylation and S-glutathionylation of GAPDH: Similarities, differences, and relationships. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2023, 1867, 130418. [Google Scholar] [CrossRef]
- Makarova, N.; Romanov, Y.A.; Dolgushina, N.; Parker, M.; Krasnyi, A. Comparative analysis of the expression of glutathione peroxidase and glutathione reductase genes in human sperm after cryopreservation. Bull. Exp. Biol. Med. 2018, 165, 166–170. [Google Scholar] [CrossRef]
- Zou, J.; Wei, L.; Li, D.; Zhang, Y.; Wang, G.; Zhang, L.; Cao, P.; Yang, S.; Li, G. Effect of glutathione on sperm quality in Guanzhong dairy goat sperm during cryopreservation. Front. Vet. Sci. 2021, 8, 771440. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z.; Khodaei-Motlagh, M. Effects of reduced glutathione on the quality of rooster sperm during cryopreservation. Theriogenology 2019, 128, 149–155. [Google Scholar] [CrossRef]
- Gangwar, C.; Saxena, A.; Patel, A.; Singh, S.; Yadav, S.; Kumar, R.; Singh, V. Effect of reduced glutathione supplementation on cryopreservation induced sperm cryoinjuries in Murrah bull semen. Anim. Reprod. Sci. 2018, 192, 171–178. [Google Scholar] [CrossRef]
- Yang, S.; Xu, W.; Hua, Y.; Ruan, Q.; Meng, Z. Incorporation of reduced glutathione to the extender improves frozen-thawed sperm function and fertility potential in mandarin fish (Siniperca chuatsi). Aquac. Rep. 2025, 40, 102590. [Google Scholar] [CrossRef]
- Abdullah, F.; Khan Nor-Ashikin, M.N.; Agarwal, R.; Kamsani, Y.S.; Abd Malek, M.; Bakar, N.S.; Mohammad Kamal, A.A.; Sarbandi, M.S.; Abdul Rahman, N.S.; Musa, N.H. Glutathione (GSH) improves sperm quality and testicular morphology in streptozotocin-induced diabetic mice. Asian J. Androl. 2021, 23, 281–287. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Zhang, W.; Wang, B.; Ma, Y.; Su, L.; Dai, Y.; Liu, G. Glutathione and selenium nanoparticles have a synergistic protective effect during cryopreservation of bull semen. Front. Vet. Sci. 2023, 10, 1093274. [Google Scholar] [CrossRef]
- Masoudi, R.; Sharafi, M.; Pourazadi, L.; Dadashpour Davachi, N.; Asadzadeh, N.; Esmaeilkhanian, S.; Dirandeh, E. Supplementation of chilling storage medium with glutathione protects rooster sperm quality. Cryobiology 2020, 92, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Hatami, M.; Esmaeilkhanian, S.; Zarei, F.; Sharafi, M.; Hatefi, A. Preservation of rooster post-thawed sperm epigenetic modifications, fertility potential and other quality parameters in different extenders using reduced glutathione. Theriogenology 2024, 215, 24–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Y.; Zeng, W. Cysteine improves boar sperm quality via glutathione biosynthesis during the liquid storage. Anim. Biosci. 2022, 35, 166–176. [Google Scholar] [CrossRef]
- Njoroge, W.E.; Zhu, Z.; Umehara, T.; Yamanaka, T.; Zeng, W.; Okazaki, T.; Shimada, M. Synthesis of functional enzymes involved in glutathione production during linear motility in boar sperm. Free Radic. Biol. Med. 2025, 228, 126–136. [Google Scholar] [CrossRef]
- Al-Mutary, M.G. Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review. J. King Saud Univ.-Sci. 2021, 33, 101226. [Google Scholar] [CrossRef]
- Ortega-Ferrusola, C.; Martin Muñoz, P.; Ortiz-Rodriguez, J.M.; Anel-López, L.; Balao da Silva, C.; Álvarez, M.; de Paz, P.; Tapia, J.A.; Anel, L.; Silva- Rodríguez, A.; et al. Depletion of thiols leads to redox deregulation, production of 4-hydroxinonenal and sperm senescence: A possible role for GSH regulation in spermatozoa†. Biol. Reprod. 2019, 100, 1090–1107. [Google Scholar] [CrossRef]
- Erbayram, Z.F.; Menevse, E.; Dursunoglu, D. Semen testis expressed protein 101 and spermatid-specific thioredoxin reductase 3 levels may be biomarkers in infertile male. Turk. J. Biochem. 2021, 46, 581–586. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Pan, W.; Xu, Z.; Yang, H.; Luo, Q.; Ye, X.; Cheng, X. Effects of L-carnitine combined with pancreatic kininogenase on thioredoxin 2, thioredoxin reductase 1, and sperm quality in patients with oligoasthenospermia. Transl. Androl. Urol. 2021, 10, 3515–3523. [Google Scholar] [CrossRef]
- Moradi, M.-N.; Karimi, J.; Khodadadi, I.; Amiri, I.; Karami, M.; Saidijam, M.; Vatannejad, A.; Tavilani, H. Evaluation of the p53 and Thioredoxin reductase in sperm from asthenozoospermic males in comparison to normozoospermic males. Free Radic. Biol. Med. 2018, 116, 123–128. [Google Scholar] [CrossRef]
- Otašević, V.; Stančić, A.; Korać, A.; Janković, A.; Korać, B. Reactive Oxygen, Nitrogen, and Sulfur Species in Human Male Fertility. A Crossroad of Cellular Signaling and Pathology. Biofactors 2019, 46, 206–219. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Absence of peroxiredoxin 6 amplifies the effect of oxidant stress on mobility and SCSA/CMA3 defined chromatin quality and impairs fertilizing ability of mouse spermatozoa. Biol. Reprod. 2016, 94, 68, 1–10. [Google Scholar] [CrossRef]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef]
- Leonardi, R.; Zhang, Y.M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Prog. Lipid Res. 2005, 44, 125. [Google Scholar] [CrossRef]
- Martinez, L.D.; Tsuchiya, Y.; Gout, I. Coenzyme A biosynthetic machinery in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, L.F.; Sibon, C.M.O.; Jackowski, S.; Gout, I. Coenzyme A and its derivatives: Renaissance of a textbook classic. Biochem. Soc. Trans. 2014, 42, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, R.; Erasmus, E. Xenobiotic/medium chain fatty acid: CoA ligase–a critical review on its role in fatty acid metabolism and the detoxification of benzoic acid and aspirin. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1169–1179. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Peak-Chew, S.Y.; Newell, C.; Miller-Aidoo, S.; Mangal, S.; Zhyvoloup, A.; Bakovic’, J.; Malanchuk, O.; Pereira, G.C.; Kotiadis, V.; et al. Protein CoAlation: A redox-regulated protein modification by coenzyme A in mammalian cells. Biochem. J. 2017, 474, 2489–2508. [Google Scholar] [CrossRef]
- Tossounian, M.-A.; Baczynska, M.; Dalton, W.; Peak-Chew, S.Y.; Undzenas, K.; Korza, G.; Filonenko, V.; Skehel, M.; Setlow, P.; Gout, I.; et al. Bacillus subtilis YtpP and Thioredoxin A Are New Players in the Coenzyme-A-Mediated Defense Mechanism against Cellular Stress. Antioxidants 2023, 12, 938. [Google Scholar] [CrossRef]
- Malanchuk, O.; Bdzhola, A.; Palchevskyi, S.; Bdzhola, V.; Chai, P.; Pardo, O.E.; Seckl, M.J.; Banerjee, A.; Peak-Chew, S.Y.; Skehel, M.; et al. Investigating the Regulation of Ribosomal Protein S6 Kinase 1 by CoAlation. Int. J. Mol. Sci. 2024, 25, 8747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nelson, O.D.; Price, I.R.; Zhu, C.; Lu, X.; Fernandez, I.R.; Weiss, R.S.; Lin, H. Long-chain fatty acyl coenzyme A inhibits NME1/2 and regulates cancer metastasis. Proc. Natl. Acad. Sci. USA 2022, 119, e2117013119. [Google Scholar] [CrossRef]
- Yu, B.Y.K.; Tossounian, M.-A.; Hristov, S.D.; Lawrence, R.; Arora, P.; Tsuchiya, Y.; Peak-Chew, S.Y.; Filonenko, V.; Oxenford, S.; Angell, R.; et al. Regulation of metastasis suppressor NME1 by a key metabolic cofactor coenzyme A. Redox Biol. 2021, 44, 101978. [Google Scholar] [CrossRef]
- Tossounian, M.-A.; Baczynska, M.; Dalton, W.; Newell, C.; Ma, Y.; Das, S.; Semelak, A.J.; Estrin, A.D.; Filonenko, V.; Trujillo, M.; et al. Profiling the Site of Protein CoAlation and Coenzyme A Stabilization Interactions. Antioxidants 2022, 11, 1362. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Byrne, D.P.; Burgess, S.G.; Bormann, J.; Baković, J.; Huang, Y.; Zhyvoloup, A.; Yu, B.Y.K.; Peak-Chew, S.; Tran, T. Covalent Aurora A regulation by the metabolic integrator coenzyme A. Redox Biol. 2020, 28, 101318. [Google Scholar] [CrossRef]
- Filonenko, V.; Gout, I. Discovery and functional characterisation of protein CoAlation and the antioxidant function of coenzyme A. BBA Adv. 2023, 3, 100075. [Google Scholar] [CrossRef]
- Zhyvoloup, A.; Yu, B.Y.K.; Baković, J.; Davis-Lunn, M.; Tossounian, M.-A.; Thomas, N.; Tsuchiya, Y.; Peak-Chew, S.Y.; Wigneshweraraj, S.; Filonenko, V.; et al. Analysis of disulphide bond linkage between CoA and protein cysteine thiols during sporulation and in spores of Bacillus species. FEMS Microbiol. Lett. 2020, 367, fnaa174. [Google Scholar] [CrossRef] [PubMed]
- Aloum, L.; Brimson, C.A.; Zhyvoloup, A.; Baines, R.; Baković, J.; Filonenko, V.; Thompson, C.R.L.; Gout, I. Coenzyme A and protein CoAlation levels are regulated in response to oxidative stress and during morphogenesis in Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 2019, 511, 294–299. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Zhyvoloup, A.; Baković, J.; Thomas, N.; Yu, K.Y.B.; Das, S.; Orengo, C.; Newell, C.; Ward, J.; Saladino, G.; et al. Protein CoAlation and antioxidant function of coenzyme A in prokaryotic cells. Biochem. J. 2018, 475, 1909–1937. [Google Scholar] [CrossRef] [PubMed]
- Baković, J.; Yu, B.Y.K.; Silva, D.; Baczynska, M.; Peak-Chew, S.Y.; Switzer, A.; Burchell, L.; Wigneshweraraj, S.; Vandanashree, M.; Gopal, B. Redox regulation of the quorum-sensing transcription factor AgrA by coenzyme A. Antioxidants 2021, 10, 841. [Google Scholar] [CrossRef]
- Baković, J.; Yu, B.Y.K.; Silva, D.; Chew, S.P.; Kim, S.; Ahn, S.-H.; Palmer, L.; Aloum, L.; Stanzani, G.; Malanchuk, O.; et al. A key metabolic integrator, coenzyme A, modulates the activity of peroxiredoxin 5 via covalent modification. Mol. Cell. Biochem. 2019, 461, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Petrone, O.; Serafini, S.; Yu, B.Y.K.; Filonenko, V.; Gout, I.; O’Flaherty, C. Changes of the Protein CoAlation Pattern in Response to Oxidative Stress and Capacitation in Human Spermatozoa. Int. J. Mol. Sci. 2023, 24, 12526. [Google Scholar] [CrossRef]
- Smyth, P.S.; Nixon, B.; Skerrett-Byrne, A.D.; Burke, D.N.; Bromfield, G.E. Building an Understanding of Proteostasis in Reproductive Cells: The Impact of Reactive Carbonyl Species on Protein Fate. Antioxid. Redox Signal. 2024, 41, 296–321. [Google Scholar] [CrossRef]
- Akagawa, M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radic. Res. 2021, 55, 307–320. [Google Scholar] [CrossRef]
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm protein carbonylation. Andrologia 2019, 51, e13233. [Google Scholar] [CrossRef]
- Mohanty, G.; Swain, N.; Goswami, C.; Kar, S.; Samanta, L. Histone retention, protein carbonylation, and lipid peroxidation in spermatozoa: Possible role in recurrent pregnancy loss. Syst. Biol. Reprod. Med. 2016, 62, 201–212. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, M.A.; Ladouce, R.; Friguet, B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J. Proteom. 2013, 92, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Westfalewicz, B.; Słowińska, M.; Dietrich, M.A.; Judycka, S.; Ciereszko, A. Differences in sperm protein abundance and carbonylation level in bull ejaculates of low and high quality. PLoS ONE 2018, 13, e0206150. [Google Scholar] [CrossRef]
- Shi, H.; Li, Q.-Y.; Li, H.; Wang, H.-Y.; Fan, C.-X.; Dong, Q.-Y.; Pan, B.-C.; Ji, Z.-L.; Li, J.-Y. ROS-induced oxidative stress is a major contributor to sperm cryoinjury. Hum. Reprod. 2023, 39, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Słowińska, M.; Judycka, S.; Karol, H.; Ciereszko, A.; Dietrich, M.A. Identification of oxidatively modified proteins due to cryopreservation of carp semen1. J. Anim. Sci. 2018, 96, 1453–1465. [Google Scholar] [CrossRef]
- Al Smadi, M.A.; Hammadeh, M.E.; Batiha, O.; Al Sharu, E.; Altalib, M.M.; Jahmani, M.Y.; Mahdy, A.; Amor, H. Elevated seminal protein carbonyl concentration is correlated with asthenozoospermia and affects adversely the laboratory intracytoplasmic sperm injection (ICSI) outcomes. Andrologia 2021, 53, e14232. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z.; Wang, B.; Wang, Y.; Zhang, J.; et al. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Soria-Tiedemann, M.; Michel, G.; Urban, I.; Aldrovandi, M.; O’Donnell, V.B.; Stehling, S.; Kuhn, H.; Borchert, A. Unbalanced Expression of Glutathione Peroxidase 4 and Arachidonate 15-Lipoxygenase Affects Acrosome Reaction and In Vitro Fertilization. Int. J. Mol. Sci. 2022, 23, 9907. [Google Scholar] [CrossRef]
- Mavi, G.K.; Dubey, P.P.; Cheema, R.S. Association of antioxidant defense system with semen attributes vis a vis fertility in exotic and indigenous chicken breeds. Theriogenology 2020, 144, 158–163. [Google Scholar] [CrossRef]
- Blouin, T.; Saini, N. Aldehyde-induced DNA-protein crosslinks- DNA damage, repair and mutagenesis. Front. Oncol. 2024, 14, 1478373. [Google Scholar] [CrossRef] [PubMed]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2017, 50, e12926. [Google Scholar] [CrossRef]
- Trevizan, T.J.; Carreira, T.J.; Carvalho, R.I.; Kipper, H.B.; Nagata, B.W.; Perri, V.H.S.; Oliveira, F.E.M.; Pierucci, C.J.; Koivisto, D.B.M. Does lipid peroxidation and oxidative DNA damage differ in cryopreserved semen samples from young, adult and aged Nellore bulls? Anim. Reprod. Sci. 2018, 195, 8–15. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Torres-Garrido, M.; Ribas-Maynou, J.; Llavanera, M.; Barranco, I.; Yeste, M.; Miró, J.; Catalán, J.; Yánez-Ortiz, I.; et al. Impact of Seminal Plasma Antioxidants on DNA Fragmentation and Lipid Peroxidation of Frozen–Thawed Horse Sperm. Antioxidants 2024, 13, 322. [Google Scholar] [CrossRef]
- Restrepo, G.; Varela, E.; Duque, E.J.; Gómez, E.J.; Rojas, M. Freezing, Vitrification, and Freeze-Drying of Equine Spermatozoa: Impact on Mitochondrial Membrane Potential, Lipid Peroxidation, and DNA Integrity. J. Equine Vet. Sci. 2019, 72, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, B.; Nasiri-Foomani, N.; Saedi, A.; Hasani-Baferani, A.; Samadi, F. Effect of selenium nanoparticles-supplemented INRA96 extender on Turkmen stallion sperm quality and lipid peroxidation during storage at 5 °C. J. Equine Vet. Sci. 2024, 136, 105073. [Google Scholar] [CrossRef]
- Abd El-Hamid, I.S. Improving chilled and frozen buck sperm characteristics by adding melatonin and L-carnitine to the preservation medium. Reprod. Domest. Anim. 2024, 59, e14504. [Google Scholar] [CrossRef] [PubMed]
- Zuha, S.; Rakha, B.A.; Akhter, S.; Ansari, M.S.; Waseem, K. Effect of reduced glutathione on quality, lipid peroxidation and antioxidant potential of frozen-thawed ring-necked pheasant semen. Reprod. Domest. Anim. 2024, 59, e14535. [Google Scholar] [CrossRef]
- Naderi Noreini, S.; Malmir, M.; Ghafarizadeh, A.; Faraji, T.; Bayat, R. Protective effect of L-carnitine on apoptosis, DNA fragmentation, membrane integrity and Lipid peroxidation of spermatozoa in the asthenoteratospermic men. Andrologia 2020, 53, e13932. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Rico de Souza, A. Hydrogen Peroxide Modifies Human Sperm Peroxiredoxins in a Dose-Dependent Manner1. Biol. Reprod. 2010, 84, 238–247. [Google Scholar] [CrossRef]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Malone Rubright, S.L.; Pearce, L.L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zeng, W.T.; Lee, D.Y. H(2)S- and Redox-State-Mediated PTP1B S-Sulfhydration in Insulin Signaling. Int. J. Mol. Sci. 2023, 24, 2898. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef] [PubMed]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H(2)S biosynthesis and catabolism: New insights from molecular studies. Cell Mol. Life Sci. 2017, 74, 1391–1412. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Q.; Zhang, Y.; Zheng, F.; Xue, T.; Ge, X.; Ma, R.; Li, X.; Wu, R.; Liang, K.; et al. Omega-3 polyunsaturated fatty acids alleviate hydrogen sulfide-induced blood-testis barrier disruption in the testes of adult mice. Reprod. Toxicol. 2020, 98, 233–241. [Google Scholar] [CrossRef]
- Song, Y.; Mao, C.; Zhong, Q.; Zhang, R.; Jiang, D.; Sun, X. Role of hydrogen sulfide in the male reproductive system. Front. Endocrinol. 2024, 15, 1377090. [Google Scholar] [CrossRef]
- Pintus, E.; Jovičić, M.; Kadlec, M.; Ros-Santaella, J.L. Divergent effect of fast-and slow-releasing H2S donors on boar spermatozoa under oxidative stress. Sci. Rep. 2020, 10, 6508. [Google Scholar] [CrossRef]
- Řimnáčová, H.; Moravec, J.; Štiavnická, M.; Havránková, J.; Monsef, L.; Hošek, P.; Prokešová, Š.; Žalmanová, T.; Fenclová, T.; Petr, J.; et al. Evidence of endogenously produced hydrogen sulfide (H2S) and persulfidation in male reproduction. Sci. Rep. 2022, 12, 11426. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, M.; Pintus, E.; Ros-Santaella, J.L. The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress. Animals 2022, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Mokhlis, H.A.; Rashed, M.H.; Saleh, I.G.; Eldeib, M.G.; El-Husseiny, A.A.; Khidr, E.G.; Gomaa, M.H.; Gad, H.S.; Aglan, A.; Mokhlis, H.A.; et al. Hydrogen sulfide alleviates acrylamide-induced testicular toxicity in male rats. Toxicol. Environ. Health Sci. 2022, 15, 41–51. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafarpour, F.; Vash, N.T.; Hajian, M.; Nasr-Esfahani, M.H.; Mohammadi, A.; Jafarpour, F.; Vash, N.T.; Hajian, M.; Nasr-Esfahani, M.H. Supplementation of sperm cryopreservation media with H2S donors enhances sperm quality, reduces oxidative stress, and improves in vitro fertilization outcomes. Sci. Rep. 2024, 14, 12400. [Google Scholar] [CrossRef]
- Azarbarz, N.; Seifabadi, S.Z.; Moaiedi, Z.M.; Mansouri, E. Assessment of the effect of sodium hydrogen sulfide (hydrogen sulfide donor) on cisplatin-induced testicular toxicity in rats. Environ. Sci. Pollut. Res. 2020, 27, 8119–8128. [Google Scholar] [CrossRef]
- Salehiyeh, S.; Faiz, A.F.; Manzourolhojeh, M.; Bagheri, A.M.; Lorian, K.; Salehiyeh, S.; Faiz, A.F.; Manzourolhojeh, M.; Bagheri, A.M.; Lorian, K. The functions of hydrogen sulfide on the urogenital system of both males and females: From inception to the present. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 6391–6415. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.-D.; Liu, X.-Q.; Zhang, P.-F.; Hao, Y.-N.; Li, L.; Chen, L.; Shen, W.; Tang, X.-F.; Min, L.-J. Hydrogen sulfide and/or ammonia reduces spermatozoa motility through AMPK/AKT related pathways. Sci. Rep. 2016, 6, 37884. [Google Scholar] [CrossRef] [PubMed]
- Aledo, J.C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 2019, 28, 1785–1796. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Yurina, L.; Vasilyeva, A. Functional Role of Methionine Oxidation in Proteins: Arguments for and against. Biol. Bull. Rev. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Lim, J.M.; Kim, G.; Levine, R.L. Methionine in proteins: It’s not just for protein initiation anymore. Neurochem. Res. 2018, 44, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.S.; Ferrington, D.A.; Squier, T.C.; Schöneich, C. Diastereoselective reduction of protein-bound methionine sulfoxide by methionine sulfoxide reductase. FEBS Lett. 1999, 455, 247–250. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.D.; Shi, Y.; Wang, F.; Wu, P.F.; Chen, J.G. Methionine oxidation of actin cytoskeleton attenuates traumatic memory retention via reactivating dendritic spine morphogenesis. Redox Biol. 2024, 77, 103391. [Google Scholar] [CrossRef]
- Kaya, A.; Lee, B.C.; Gladyshev, V.N. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid. Redox Signal. 2015, 23, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Frémont, S.; Hammich, H.; Bai, J.; Wioland, H.; Klinkert, K.; Rocancourt, M.; Kikuti, C.; Stroebel, D.; Romet-Lemonne, G.; Pylypenko, O.; et al. Oxidation of F-actin controls the terminal steps of cytokinesis. Nat. Commun. 2017, 8, 14528. [Google Scholar] [CrossRef]
- Lim, J.C.; Kim, G.; Levine, R.L. Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A. Free Radic. Biol. Med. 2013, 61, 257–264. [Google Scholar] [CrossRef]
- Veredas, F.J.; Cantón, F.R.; Aledo, J.C. Methionine residues around phosphorylation sites are preferentially oxidized in vivo under stress conditions. Sci. Rep. 2017, 7, 40403. [Google Scholar] [CrossRef]
- Oien, D.B.; Moskovitz, J. Substrates of the Methionine Sulfoxide Reductase System and Their Physiological Relevance. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 80, pp. 93–133. [Google Scholar]
- Indhu, M.S.; Ramamoorthy, M.; Pandey, S.; Mathesh, K.; Mahawar, M.; Sarkar, M.; Ghosh, S.K.; Taru Sharma, G.; Bhure, S.K. Improved quality and fertilizability of cryopreserved buffalo spermatozoa with the supplementation of methionine sulfoxide reductase A. Andrology 2021, 9, 1943–1957. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.-J. Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 2019, 177, 1480–1494.e1419. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Miao, Y.; Peng, J.; Wei, H. Effects of Methionine Restriction from Different Sources on Sperm Quality in Aging Mice. Nutrients 2023, 15, 4782. [Google Scholar] [CrossRef] [PubMed]

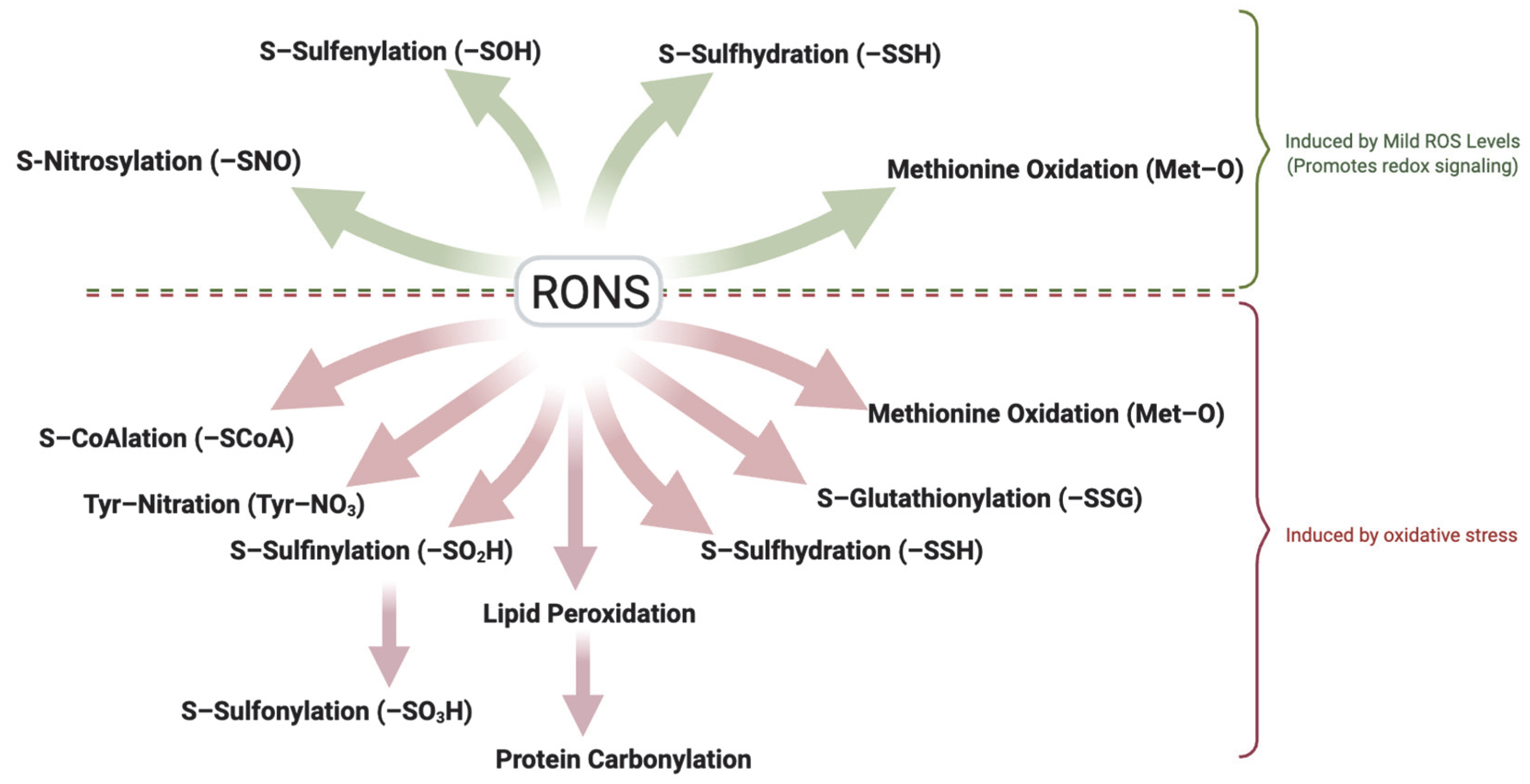
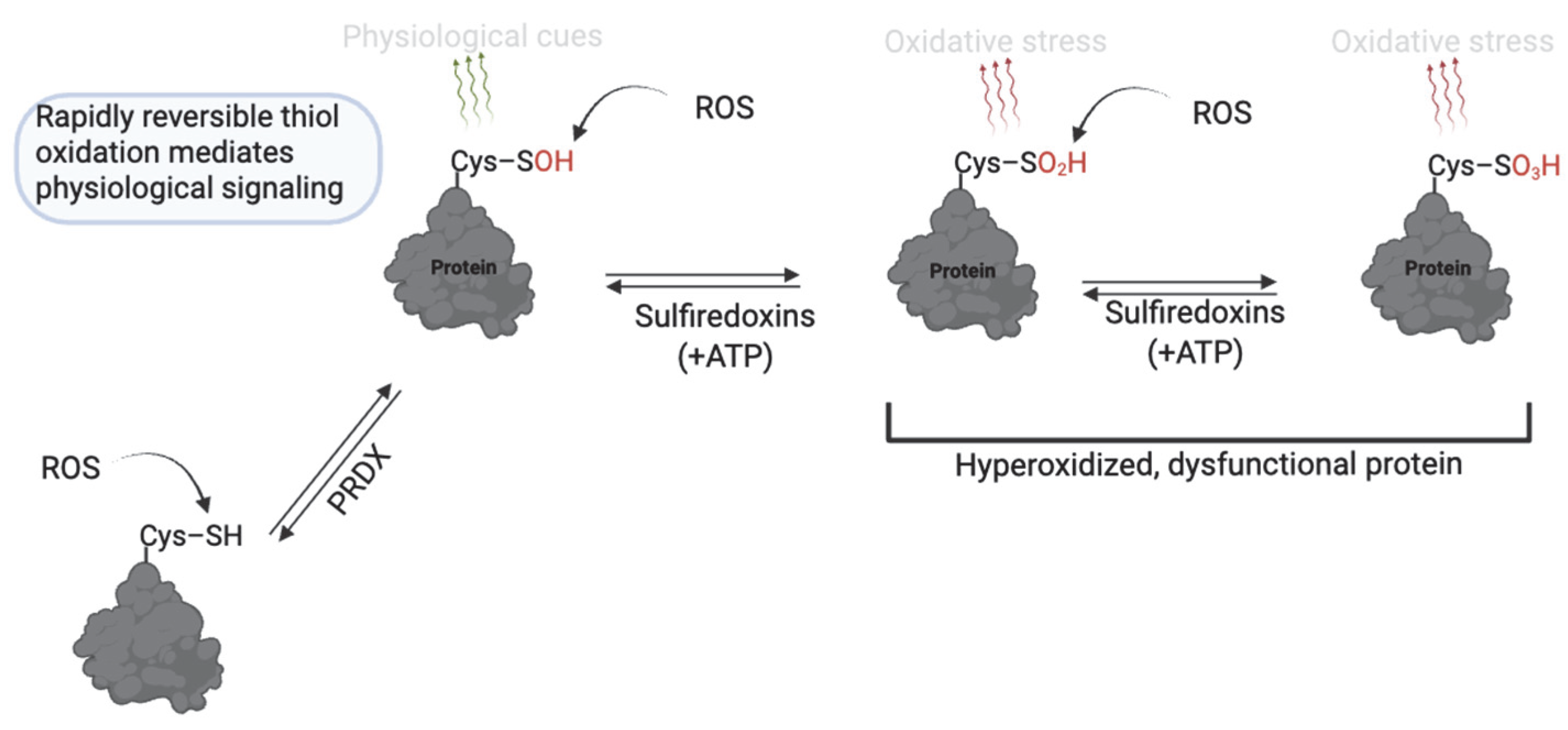
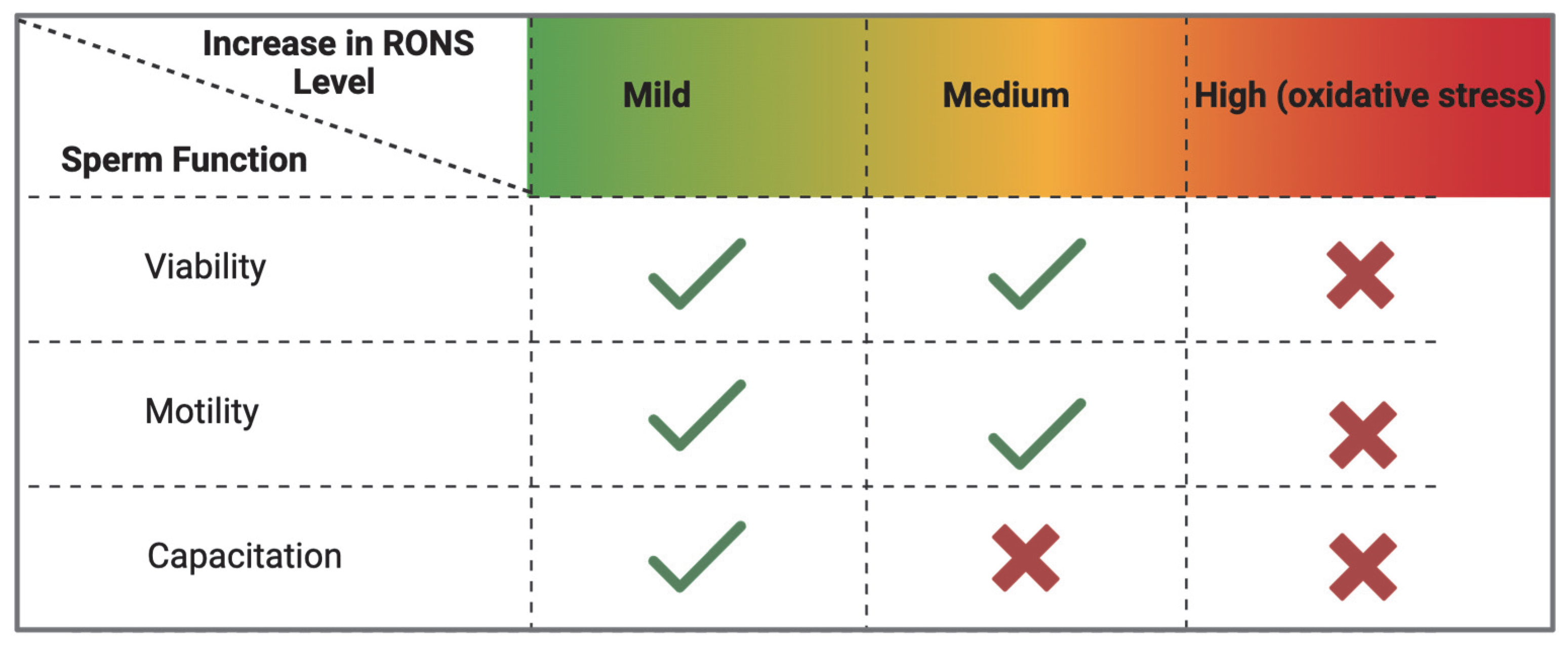
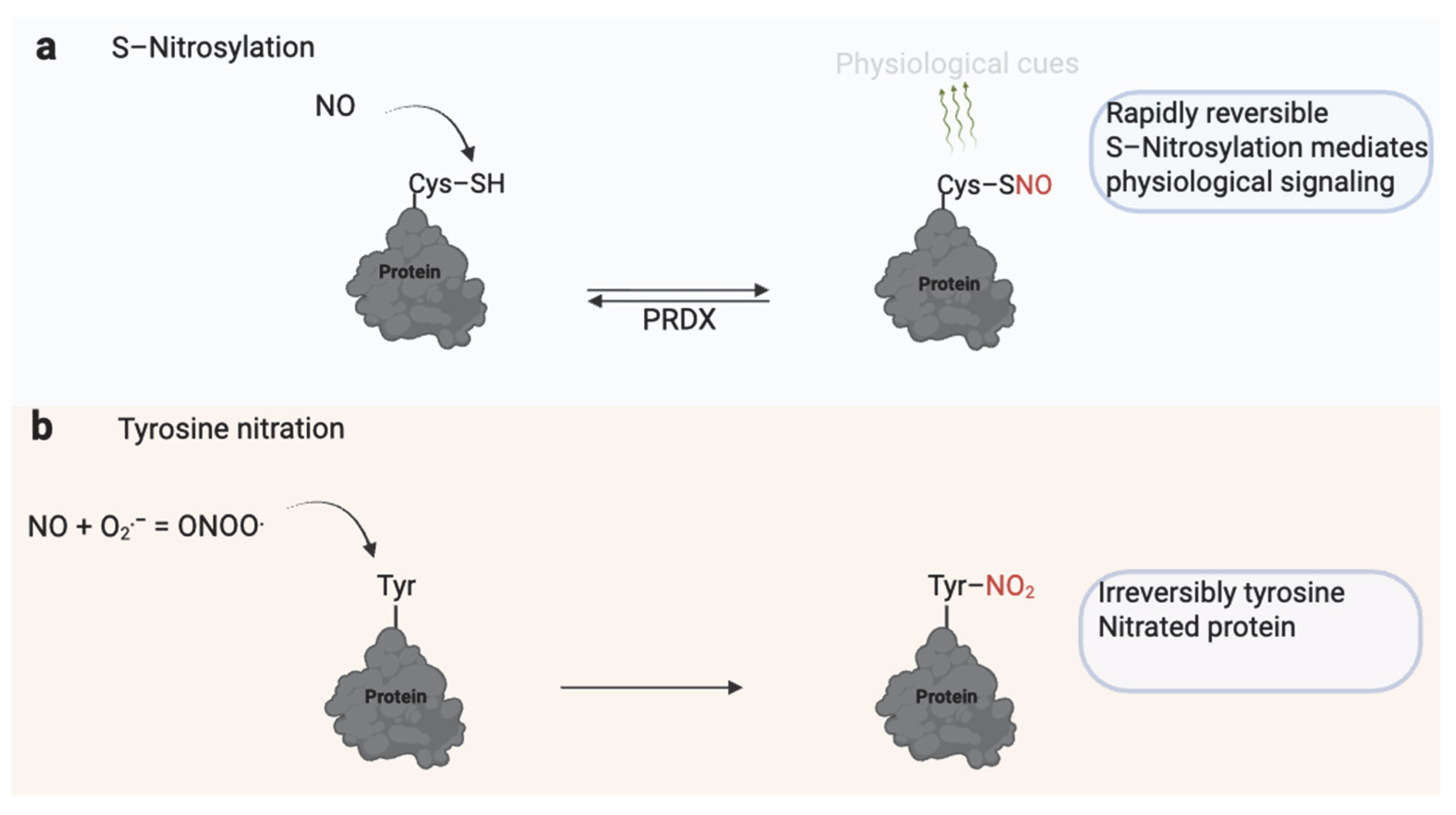
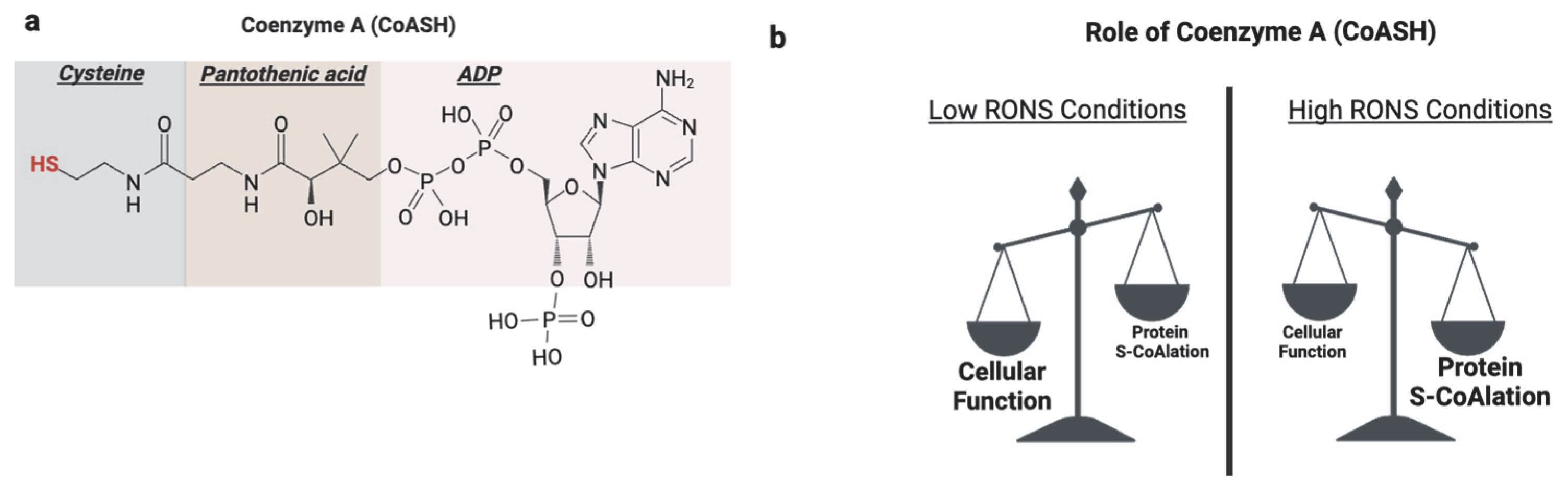
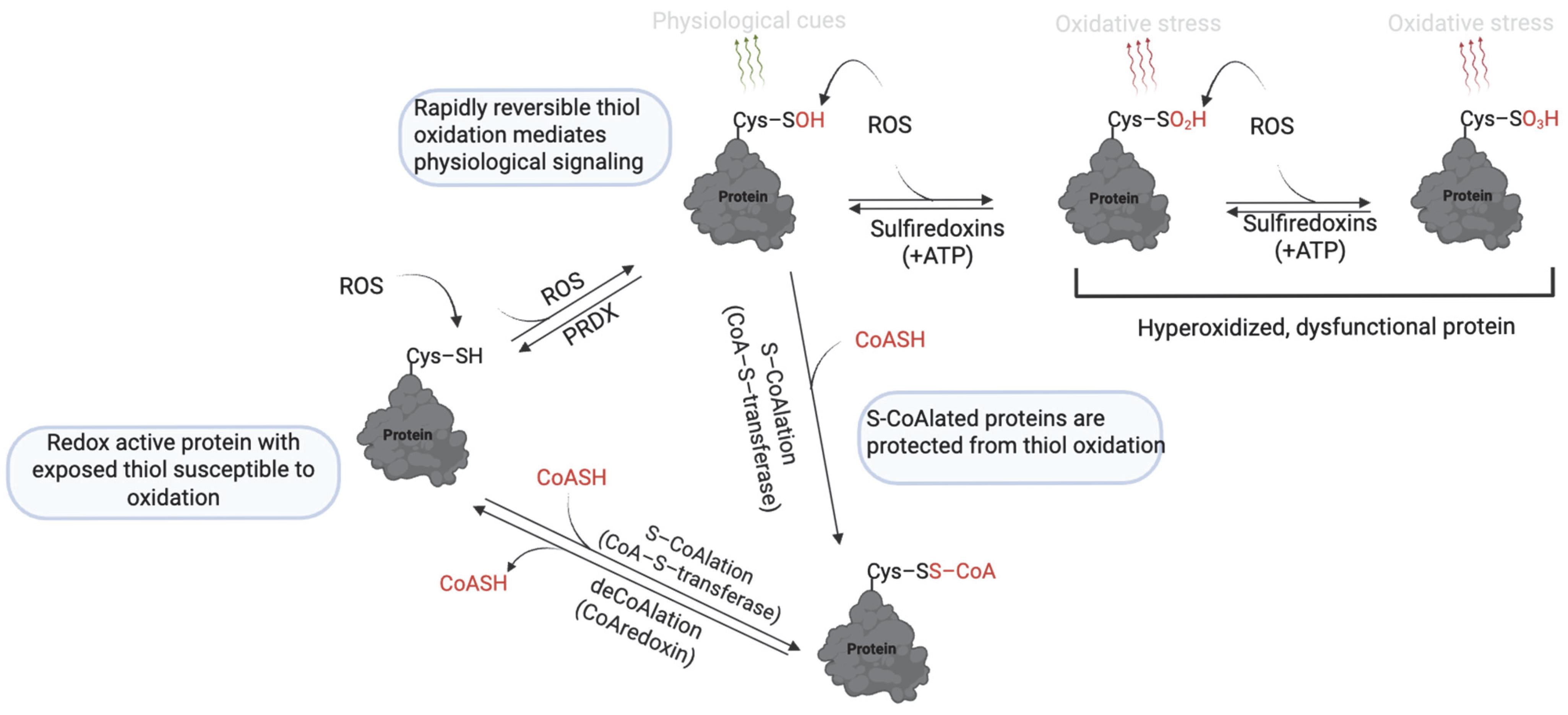
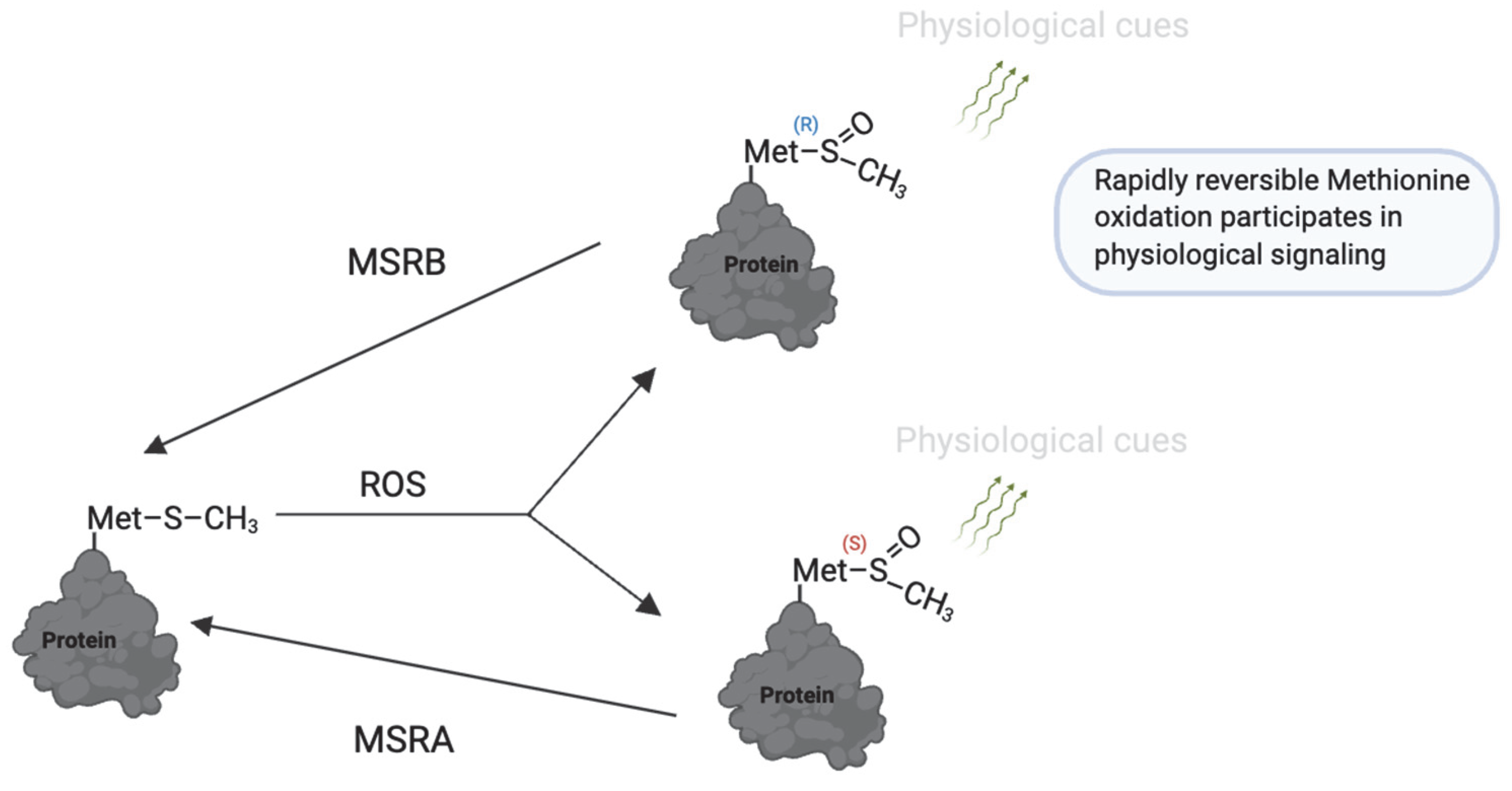
| Modification | Effect | Species | Reference |
|---|---|---|---|
| S-Nitrosylation | Enhances capacitation | Human, Boar | [64,66] |
| Enhances cryosurvival | Human, Rooster | [60,61,67] | |
| Enhances motility | Human, Ram | [68,69] | |
| Tyrosine Nitration | Impairs motility | Boar, Human | [72,74,75] |
| Impairs acrosome reaction | Human | [35,76] | |
| Promotes mitochondrial permeability transition (MPT)-driven apoptosis | Human | [77] |
| Effect of S-CoAlation | Model | Reference |
|---|---|---|
| Antioxidant function | Mammalian cells, Bacteria, Amoeba | [127,135,136] |
| CoAlation induced by carbon starvation, sporulation and oxidative stress | Bacillus megaterium, Bacillus subtilis | [135] |
| Reversible inactivation of metabolic enzymes (e.g., GAPDH) under oxidative stress | Staphylococcus aureus | [137] |
| Reversible inactivation of antioxidant enzymes (e.g., Peroxiredoxin 5) | Mammalian Cardiomyocytes | [139] |
| Modulates signaling pathways by reversible inhibition of kinases (e.g., S6K1, Aurora kinase A) | Mammalian cells (HEK293) | [129,133] |
| Inhibition of catalytic activity through ADP moiety interaction (e.g., NME1/NME2) | Mammalian cells (HEK293)/Recombinant proteins from E. coli (BL21 DE3) | [130,131] |
| Increases in human spermatozoa exposed to oxidative stress, suggesting antioxidant function | Human spermatozoa | [140] |
| Decreases during capacitation, possibly relieving inhibition of phosphorylation pathways | Human spermatozoa | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onochie, C.; Evi, K.; O’Flaherty, C. Role of Redox-Induced Protein Modifications in Spermatozoa in Health and Disease. Antioxidants 2025, 14, 720. https://doi.org/10.3390/antiox14060720
Onochie C, Evi K, O’Flaherty C. Role of Redox-Induced Protein Modifications in Spermatozoa in Health and Disease. Antioxidants. 2025; 14(6):720. https://doi.org/10.3390/antiox14060720
Chicago/Turabian StyleOnochie, Chika, Keturah Evi, and Cristian O’Flaherty. 2025. "Role of Redox-Induced Protein Modifications in Spermatozoa in Health and Disease" Antioxidants 14, no. 6: 720. https://doi.org/10.3390/antiox14060720
APA StyleOnochie, C., Evi, K., & O’Flaherty, C. (2025). Role of Redox-Induced Protein Modifications in Spermatozoa in Health and Disease. Antioxidants, 14(6), 720. https://doi.org/10.3390/antiox14060720







