Abstract
Due to their negligible cytoplasm and composition of the sperm plasmalemma, spermatozoa are particularly vulnerable to lipid peroxidative damage induced by reactive oxygen species (ROS). Most ROS are referred to as free radicals because they have unpaired electrons, causing them to scavenge electrons from atoms within tissues, resulting in oxidative damage to cellular components including cell membranes, intracellular organelles, and DNA. The potential consequences of oxidative stress include impaired sperm function, DNA fragmentation, and apoptosis. Understanding the mechanisms that mediate sperm damage during cryopreservation is key to the development of improved sperm freezing media formulations and methodology to mitigate its occurrence. Historically, elucidation of those mechanisms has proven largely elusive and is complicated by the positive role that ROS also play as messengers in redox signaling and the different pathways that mediate sperm DNA damage and apoptosis. More recently, oxidative stress has been revealed as the most likely suspect in cryopreservation-induced sperm DNA damage. This narrative review was intended to provide an in-depth analysis of the mechanisms involved and offer insight into possible improvements in sperm cryopreservation.
1. Introduction
Assisted reproduction is a multidisciplinary branch of medicine requiring the coordinated efforts of specialists with different areas of expertise, including reproductive endocrinologists, anesthetists, gynecologists, urologists, andrologists, embryologists, clinical geneticists, and molecular cytogeneticists. However, precious few fertility centers benefit from the inclusion of cryobiologists among their staff, so expertise in the cryopreservation of reproductive tissues is relatively sparse. Nevertheless, cryopreservation is essential for the provision of a safe, efficient, and comprehensive clinical service for fertility patients. In this respect, it enables the donation and transport of reproductive tissues, including gametes, gonadal tissues, embryos, and stem cells. Also, it facilitates the incorporation of genetic testing of blastocysts and supports the practice of elective single embryo transfer to significantly reduce the risks of miscarriage and multiple pregnancy. Most importantly, perhaps, cryopreservation of reproductive tissues and gametes is the primary means of preserving fertility.
Human sperm cryopreservation has been an integral part of assisted reproduction since the advent of artificial insemination in the mid-20th century. Indeed, it is generally considered mandatory for semen to be quarantined in liquid nitrogen (LN2) to enable screening of prospective semen donors, ensuring they test negative for infectious diseases including Hepatitis B Virus (HBV), Hepatitis C Virus (HBC), and Human Immunodeficiency Virus (HIV) prior to use. The cryo-storage of human semen has encouraged the establishment of so-called ‘sperm banks’ around the world, which has increased access to fertility services for couples where the male is infertile and for women without a partner and those in same-sex partnerships. Precautionary semen cryopreservation may be advised in the event of diminishing sperm counts, prior to vasectomy, and where it may prove impossible to obtain a semen sample from a patient undergoing assisted reproduction with their partner, for example for individuals serving overseas in the armed forces. Furthermore, with the knowledge that sperm quality declines after the age of 35 and with the known link between paternal aging and the incidence of dominant genetic diseases in the offspring, there may be an imperative for younger men to cryopreserve their semen for future use. When faced with the necessity for gonadotoxic chemotherapy and/or radiotherapy treatment for various cancers, preservation of male fertility is entirely dependent upon prior cryopreservation of semen and testicular tissue. Similarly, potentially gonadotoxic treatment of some non-malignant diseases such as diabetes, glomerulonephritis, inflammatory bowel disease, and multiple sclerosis may also necessitate preservation of fertility [1,2,3,4]. Sperm cryopreservation is also a common ancillary to diagnostic surgical sperm retrieval (SSR) to avoid the risk of having to repeat a surgical procedure during a cycle of in vitro fertilization (IVF). Even in pre-pubertal boys, testicular tissue, testicular cell suspensions, and spermatogonial stem cells may be cryopreserved to preserve their fertility [5]. In extreme cases, testicular tissue may be cryopreserved post-mortem for potential conception by the surviving partner. Unfortunately, optimal sperm cryopreservation methodology remains elusive, and it is not uncommon for morphological, structural, and functional impairments of spermatozoa to occur due to cryopreservation [6]. Consequently, rates of cryo-survival may be low with, at best, only 50–60% of spermatozoa retaining their motility. Indeed, freezing and thawing of human semen may lead to increased generation of reactive oxygen species (ROS) and depletion of both intracellular and extracellular endogenous antioxidants, resulting in oxidative stress, increased lipid membrane peroxidation, and sperm DNA damage [7].
There has been much debate regarding the prognostic value and impact of sperm DNA quality upon laboratory and clinical outcomes, particularly following various means of assisted reproduction [8,9,10,11,12]. Of particular interest are the putative relationships between male and female age, sperm DNA damage and oocyte quality, and miscarriage [13,14]. Depending upon the quality of the oocyte’s DNA repair mechanism, the extent of single- and double-strand breaks in sperm DNA may have differential effects upon embryo viability, implantation, and ongoing pregnancy [15]. In this respect, single-stranded DNA breaks are related to oxidative stress and occur as multiple break points across the genome, leading to implantation failure. On the other hand, double-stranded breaks are more likely to be related to a lack of DNA repair during spermatogenesis and are more localized to the sperm nuclear matrix, resulting in poor embryo quality and miscarriage.
2. Reactive Oxygen Species and Oxidative Stress
One likely limitation to success in the in vitro alleviation of infertility is the oxidative stress that gametes and embryos are subjected to during various techniques performed outside of the body, including oocyte and sperm retrieval, sperm preparation, oocyte denudation, micromanipulation, embryo transfer, and cryopreservation. None of these procedures benefit from the controlled environment of an incubator where the oxygen tension is typically set much lower than that within the surrounding atmosphere. Indeed, it is a logical conclusion that increased exposure of gametes and embryos to the environment will result in cumulative oxidative stress, cryopreservation often representing the ultimate challenge to a cell following the sequential stresses of previous procedures. Under normal physiological conditions in vivo, homeostasis in the reduction–oxidation (redox) status of cells is maintained via a balance between endogenous antioxidants and oxidants. Indeed, a continuously shifting equilibrium in redox status is essential to intermediate signaling during normal cell function, but should this natural equilibrium be disrupted it can lead to cell pathology. Cells are endowed with internal defense mechanisms which can protect against oxidation including the antioxidants Vitamin C (ascorbic acid), Vitamin E (alpha-tocopherol), and Glutathione Peroxidase. Scavenging of oxygen radicals by enzymes, including Catalase, Peroxidase, and Superoxide Dismutase (SOD), is another means by which cells protect themselves from oxidative damage. Catalase and Peroxidase promote the decomposition of hydrogen peroxide (H2O2) to oxygen (O2) and water (H2O). Superoxide is converted by SOD into O2 or H2O2. Evidently, these defense mechanisms can act in concert to keep oxidative activity in check. However, if the antioxidant defense of cells becomes overwhelmed by supraphysiological levels of oxidation, then oxidative stress will ensue. Conversely, an over-response to oxidation or overuse of antioxidants can result in reductive stress, which can also render cells more vulnerable to infection [16,17]. The primary mediators of oxidative stress are a variety of molecules collectively referred to as ROS.
Molecules known as ROS are highly reactive chemicals derived from diatomic O2, H2O and H2O2. Essentially, they are intrinsically unstable molecules containing oxygen and include products of aerobic metabolism such as H2O2, the hydroxyl radical (.OH), and the superoxide anion (O2−). Except for H2O2, most ROS have unpaired electrons and, therefore, are referred to as free radicals. Hence, by definition, free radicals tend to readily react with other molecules. Indeed, free radicals will readily scavenge electrons from other atoms present within cells, which can result in oxidative damage to various cellular components such as lipids, proteins, and DNA, as well as intracellular organelles including mitochondria. Intracellular, extracellular, and environmental triggers and sources of ROS may adversely impact cell function and viability. Endogenous sources of ROS include mitochondrial Oxidoreductase, membrane-bound enzyme complexes such as Nicotinamide Adenine Dinucleotide Phosphate Oxidase (NADPH oxidase), and enzyme activities such as Xanthine Oxidase (XO). Production of the O2− free radical is catalyzed by NADPH oxidase via its transfer of an electron to oxygen. The activity of XO generates various ROS including H2O2 and superoxide ions. Exogenous triggers of ROS include environmental variables such as ambient oxygen tension, humidity, and light and the presence of metal ions including copper and iron. Also, increased levels of ROS have been associated with individual-specific factors including a sedentary lifestyle, diet and obesity, stress, consumption of alcohol, and smoking and recreational drug use [18]. Alarmingly, increased exposure to environmental pollution, including endocrine-disrupting chemicals, microplastics, and radiofrequency electromagnetic radiation, has also been identified as a potential trigger of ROS-induced oxidative stress [19]. Unsurprisingly perhaps, increased levels of ROS have been observed in several reproductive pathologies including endometriosis, intrauterine growth retardation, miscarriage, polycystic ovary syndrome, and preeclampsia [17].
A normal byproduct of cellular aerobic metabolism, the generation of ROS in spermatozoa is primarily mediated by electron leakage from the mitochondrial electron transport chain, cytosolic L-amino acid oxidases, or from the NADPH oxidase system, situated within the sperm plasmalemma [20]. However, it is the redox balance within spermatozoa which is relevant because at moderate levels ROS are necessary for the stability and protection of sperm DNA and normal sperm functions including capacitation, hyperactivation, the acrosome reaction, and fertilization of the oocyte [21]. Indeed, reductive stress and oxidative stress can both be detrimental to sperm function, so the correct balance must be maintained [22]. Under normal physiology, ROS are usually neutralized by the cell’s endogenous antioxidant system but can damage lipids, proteins, and nucleic acids if allowed to reach critical levels. Indeed, a vicious cycle of oxidative DNA damage can arise, in which ROS-induced lipid peroxidation releases lipid aldehydes which can bind to proteins in the mitochondrial electron transport chain, leading to further production of ROS [23,24]. With respect to sperm preparation, ambient temperature, incubation at 37 °C, and excessive centrifugation are additional potential triggers of ROS generation [25].
3. The Impact of Reactive Oxygen Species upon Spermatozoa
Due to their limited cytosol, high levels of polyunsaturated fatty acids in their cell membranes; lack of endogenous, cytoplasmic, antioxidant enzymes; and DNA repair mechanisms, spermatozoa are particularly vulnerable to peroxidative damage by ROS. Therefore, sperm structural and functional integrity may be impaired by oxidative stress, especially during cryopreservation. Toxic byproducts of lipid peroxidation, 4-Hydroxynonenal (4-HNE), Malondialdehyde (MDA), and Acrolein, disrupt the sperm plasmalemma and mitochondrial proteins of the electron transport chain [26]. Lipid peroxidation caused by oxidative stress results in reduced sperm plasmalemma fluidity and integrity, which can impair sperm motility, capacitation, the acrosome reaction, and fertilization [20,27]. Metabolic disruption of the mitochondrial electron transport chain can adversely impact sperm quality via increased production of mitochondrial ROS [28]. Mitochondrial membrane damage can also adversely impact sperm motility [29]. Furthermore, ROS can damage sperm chromatin and DNA and, thereby, negatively impact male fertility [30]. The oxidative stress of ROS upon sperm DNA integrity is characterized by the generation of the DNA base adduct 8-Hydroxy-2′-Deoxyguanosine (8OHDG), the first enzyme in the base excision repair (BER) pathway, 8-Oxoguanine DNA Glycosylase 1 (OGG1) being the sperm’s only DNA repair mechanism [24]. Cryoinjury to sperm may be mediated by various mechanisms, including accidental cell death, due to extracellular and/or intracellular ice formation, intrinsic or extrinsic programmed cell death or apoptosis, and non-apoptotic regulated cell death including ferroptosis and necroptosis, as well as oxidative stress. These pathways of cryo-induced sperm DNA fragmentation overlap and interact to some extent, oxidative stress being able to trigger one or more forms of regulated cell death, and vice versa [16]. Accidental cell death during cryopreservation is discussed further in the Section 4 below.
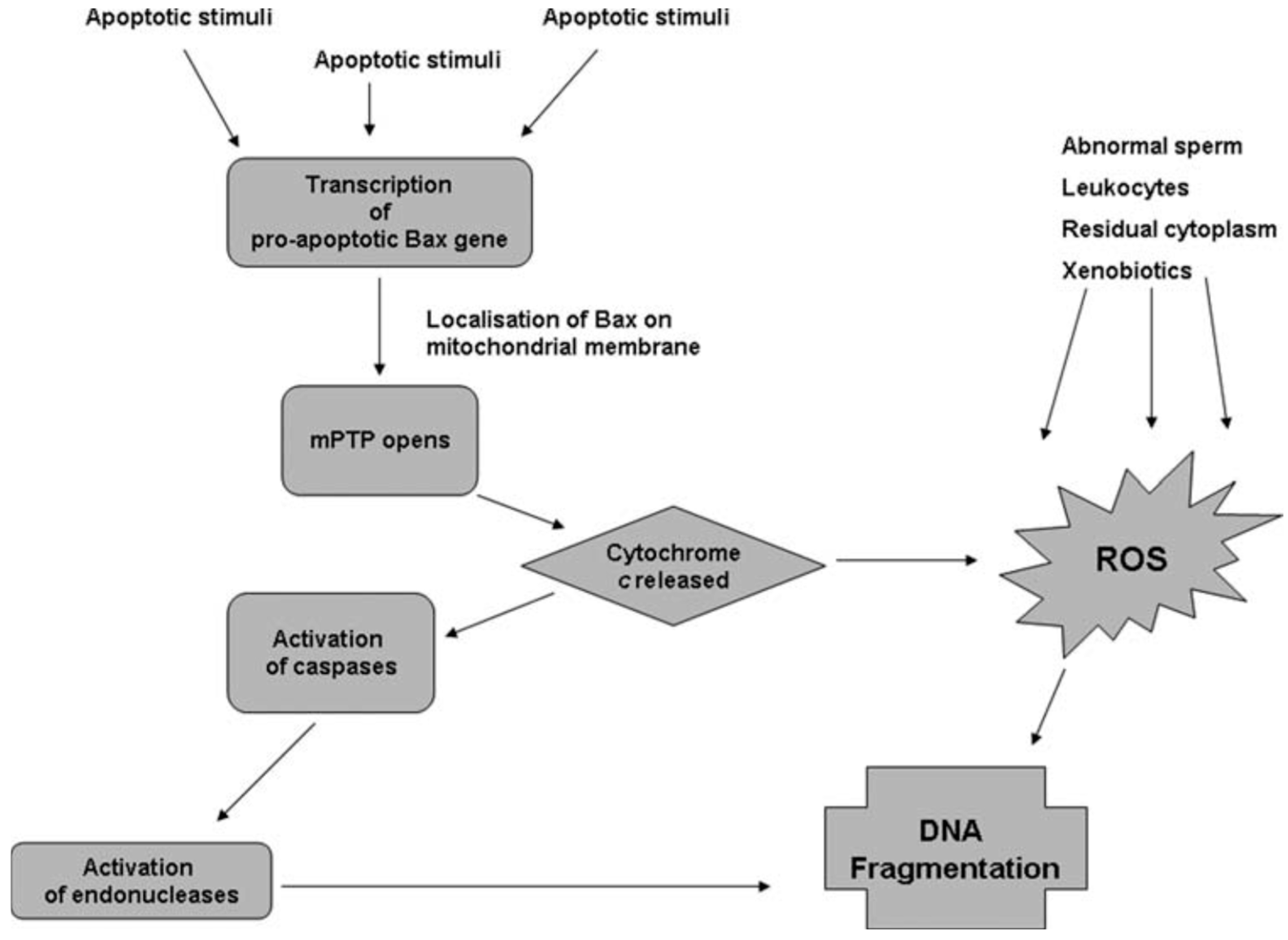
Apoptosis is regulated by products of lipid peroxidation which can activate the Nuclear Factor Kappa B, Mitogen-Activated Protein Kinase, and Protein Kinase C signaling pathways. Sperm cryodamage may be characterized by various markers of apoptosis including activation of caspases, externalization of phosphatidylserine, and a reduction in mitochondrial membrane potential [31]. Interestingly, though apoptosis is a normal feature of spermatogenesis, incomplete or abortive apoptosis occurs in response to the induction of sperm DNA fragmentation by endonucleases and may be responsible for the appearance of DNA-damaged spermatozoa within the ejaculate [32]. Caspases, which are specific aspartic acid-directed cysteine proteases, are known to play a role in the stress-induced apoptotic cascade and eventual cell death [33]. Indeed, correlations have been drawn between caspase activity, apoptotic markers, and sperm parameters within ejaculated human semen [34,35]. Activation of caspases has been observed in human spermatozoa following cryopreservation, and the presence of glycerol in the sperm freezing medium may exacerbate this phenomenon [36,37]. The differential relationships between oxidative stress and apoptosis with sperm DNA damage can be illustrated by the concurrent pathways believed to be involved in sperm DNA fragmentation (Figure 1). In the apoptosis pathway, apoptotic stimuli trigger the transcription of pro-apoptotic genes, including Bax, which encodes the mitochondrial membrane’s pro-apoptotic Bax protein [38]. Bax can open the mitochondrial permeability transition pore, resulting in mitochondrial swelling and release of Cytochrome C [39]. Once Cytochrome C enters the cytoplasm, it activates caspases which activate specific endonucleases, causing sperm DNA fragmentation [38]. As shown in Figure 1, Cytochrome C can also cause DNA fragmentation via increased production of ROS and oxidative DNA damage [40]. Hence, in the ejaculate at least, ROS generated by abnormal spermatozoa and non-germ cells may compound any DNA fragmentation caused by the apoptotic cascade. On the other hand, sperm DNA fragmentation appears to occur independently of caspase activation in cryopreserved semen, presumably within non-apoptotic spermatozoa [41].
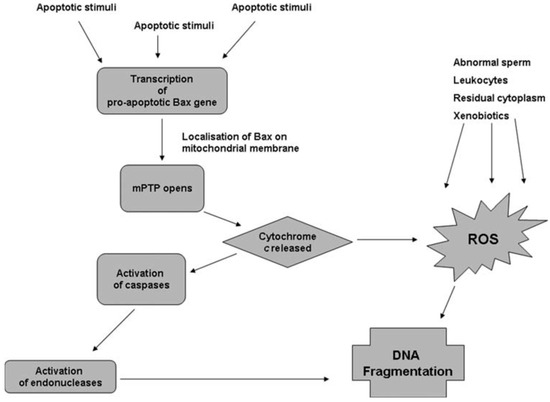
Figure 1.
Pathways proposed to lead to sperm DNA fragmentation [41].
It is well understood that ROS may be a cause of oxidative stress to spermatozoa, which provides an alternative mechanism for possible induction of sperm DNA damage by cryopreservation [25,42]. Indeed, the superoxide anion, which is capable of spontaneously or enzymatically undergoing a redox reaction to yield H2O2, is well-known to be generated by human spermatozoa [43,44]. In this respect, H2O2 is considered especially cytotoxic as it can cross the cell membrane and has high activity as an oxidant. Furthermore, the extremely noxious hydroxyl radical, which can attack almost every cellular component, is a product of superoxide and H2O2 [45]. Under normal circumstances, spermatozoa are protected from oxidative stress by the presence of enzymatic and non-enzymatic antioxidants within the seminal plasma [21]. These include oxygen radical scavengers such as Catalase and Peroxidase, which promote the breakdown of H2O2 into O2 and H2O—and SOD. However, particularly following removal of seminal plasma during sperm preparation, generation of ROS during sperm cryopreservation may overwhelm endogenous antioxidants, resulting in oxidative stress and DNA damage [46]. Indeed, the generation of ROS during sperm cryopreservation has been observed in several species, suggesting a relationship between oxidative stress and cryoinjury to sperm DNA [47,48,49,50]. The oxidative stress experienced by spermatozoa is compounded by cryopreservation since absorption of their cytoplasm reduces their reserves of enzymatic and ROS-scavenging antioxidants, and metabolic recovery during freezing and thawing causes a surge in their endogenous levels of ROS. Interestingly, semen cryopreservation may be especially detrimental to the spermatozoa of male factor infertility patients [51,52]. Furthermore, any spermatozoa with already compromised chromatin structure and DNA integrity may prove particularly susceptible to the oxidative stress of cryopreservation [24,53]. Lipid peroxidation of the sperm plasmalemma by ROS and the resulting oxidative stress is also intricately linked to other pathways of regulated cell death. One of these pathways, termed ferroptosis, is characterized by a lethal level of widespread, iron-dependent, lipid peroxidation [54]. Cell protection against ferroptosis is achieved by scavenging and reduction in lipid peroxides, particularly by Coenzyme Q10 (CoQ10), which is abundant throughout the cell membrane [55]. Another pathway like apoptosis is necroptosis, which is characterized by morphological features typical of cell necrosis and has been associated with increased exposure of the cell to H2O2 and inactivation of Caspase 8 [56]. Interestingly, Necrostatin-1 which inhibits activation of Receptor Interacting Protein 1 (RIP1), a key mediator of necroptosis, can significantly improve the cryopreservation of spermatogonial stem cells [57].
4. Sperm Cryopreservation and Sperm Cryoinjury
Basic research in cryobiology has led to the development of various methods for the cryopreservation of semen, spermatozoa, and testicular tissue, including dry ice pelleting, LN2 vapor freezing, rate-controlled slow freezing, and ultra-rapid cooling or vitrification. These techniques mostly rely upon the incorporation of various permeating and non-permeating cryoprotective agents (CPAs) to avoid or minimize sperm cryoinjury during both cooling/freezing and warming/thawing procedures. In this respect, the primary objective is to preserve sperm viability, membrane function, and motility. The main approaches to sperm cryopreservation are slow freezing, rapid freezing, and vitrification. Slow or controlled-rate freezing employs either passive manual exposure to LN2 vapor or a computer-controlled device to control a pre-programmed decrease in temperature over an extended period. Rapid freezing is typically achieved via direct contact between sperm straws and LN2 vapor for 8–10 min, followed by direct immersion in LN2 to rapidly reduce the temperature to −196 °C. Like rapid freezing, vitrification also relies upon direct immersion into LN2. Unlike slow freezing and rapid freezing, vitrification is better suited to cryopreservation of small numbers of spermatozoa, such as those retrieved from the epididymis and testis via SSR.
With slow freezing, spermatozoa may suffer extensive chemical and physical damage, most likely due to intracellular ice crystallization. Primarily, this is because of the water to ice transition phase that occurs between −15 °C and −60 °C, which spermatozoa must survive during both freezing and thawing [58]. Prior to that, during the cooling phase, cells and tissues lose their osmotic equilibrium with the freezing medium which freezes at a higher temperature (−5 °C) than the cells, which have a lower freezing point due to the salts within their cytoplasm. Cells become supercooled between −5 °C and −10 °C, and extracellular ice formation increases, resulting in an increased extracellular solute concentration. This forces cells to osmotically equilibrate with the extracellular environment by rapidly losing water, leading to severe dehydration and shrinkage, which increases the risk of osmotic shock. Cells may then be subjected to additional physical damage due to the ‘packing effect’ of increasing cell–ice and cell–cell contacts that occur between −10 °C and −15 °C, following the expansion of the extracellular ice. By this stage, further osmotic equilibrium is achieved via the assembly of trans-membrane ice crystal–hydrogen bonds, resulting in intracellular freezing. Furthermore, during thawing spermatozoa may again be subjected to osmotic shock due to excessive cellular swelling [59]. Indeed, it has been suggested that sperm cryoinjury occurs predominantly during thawing due to loss of antioxidant defense activity and structural damage to the cytoskeleton [60,61]. Hence, the major threats to sperm cryo-survival are osmotic shock and intracellular ice formation.
The damage that cells and tissues might otherwise suffer during cryopreservation may be modulated to some extent by CPAs, which are organic solutes that modulate the phase transition between water and ice [62]. Primarily, CPAs prevent excessive cell shrinkage and inhibit intracellular and extracellular ice formation during freezing but also help preserve cell membranes and promote vitrification. By virtue of their molecular size and ability to diffuse through cell membranes, CPAs occupy either the intracellular or extracellular space, cell membranes generally only being permeable to smaller molecules such as dimethyl sulfoxide (DMSO), glycerol, and 1,2-propanediol, also known as propylene glycol. Serendipitously in 1949, glycerol, ethylene glycol, and propylene glycol were the first CPAs discovered to improve human sperm cryo-survival following vitrification, and they remain in common usage [63]. Ethylene glycol, glycerol, and 1,2-propanediol are kosmotropic, meaning that they possess anti-freeze properties, forming strong hydrogen bonds with water, thereby competing with the hydrogen bonds connecting water molecules. Hence, they effectively disrupt the potential for intracellular ice formation, specific alignment of water molecules being necessary for crystalline ice formation. Unfortunately, the ability of cell-permeable CPAs to prevent intracellular ice formation depends upon a slow cooling rate which can be detrimental to sperm viability per se. Used to reduce ice formation in diverse applications, DMSO is an aqueous, hydrogen bonding, polar organosulfur compound, with very high permeability through the cell membrane. Cell-permeable CPAs have various mechanisms of action; they reduce the temperature at which cells freeze and raise the temperature at which they vitrify, and some increase cell membrane permeability, which facilitates cell dehydration. Some cell-permeable CPAs prevent membrane damage by inhibition of adjacent cell membrane fusion via interaction with phospholipids within the lipid bilayer. Cell-permeable CPAs also protect intracellular organelles. These cell-permeable cryoprotectants all have a molecular mass below 100 g.mol−1. In contrast the disaccharides, sucrose and trehalose, have a molecular mass of 342.3 g.mol−1 and 342.296 g.mol−1, respectively and, therefore, are unable to diffuse across the cell membrane.
Non-permeating CPAs influence the osmolality of the extracellular environment, thereby modulating osmosis during cellular dehydration and rehydration. Generally, at the same molar concentrations, non-permeating CPAs are less toxic to cells than permeating CPAs and reduce the concentration of permeating CPAs required for vitrification. During cooling, non-permeating CPAs increase cellular solute concentration via dehydration and increase carrier solution viscosity, thereby promoting colligative freezing point depression and vitrification, respectively. Non-permeating CPAs are also believed to adsorb to the outer cell membrane, thereby protecting the cell from extracellular crystal lattice ice formation. Furthermore, non-permeating disaccharides lower the lipid membrane’s phase transition temperatures via non-specific osmotic and volumetric effects, thereby helping maintain cell membrane integrity.
The intrinsic response of spermatozoa to cryopreservation is likely to vary depending upon whether they are within seminal plasma, in cell suspension, or embedded within other cell types present within the testis. Uniformly rapid cooling and warming rates are evidently more difficult to achieve within complex tissues. Intuitively, it may seem preferable to cryopreserve the entire ejaculate since the viability and cryo-stability of mitochondrial and sperm cell membranes is supported by lipoproteins within the seminal plasma [64]. Furthermore, it has been suggested that removal of seminal plasma brings about premature capacitation, sperm membrane instability, and a reduction in the viable lifespan of spermatozoa [65]. Seminal plasma proteins also possess antioxidant properties [66,67]. This concept has been supported by several studies reporting that freezing prior to preparation of semen results in significantly increased viable, progressively motile sperm counts [67,68]. However, this practice has been questioned recently, with density gradient centrifugation (DGC) preparation of semen prior to rate-controlled slow freezing having been shown to yield a highly significant increase in the total number of viable, progressively motile spermatozoa following thawing [69]. Indeed, this is a debatable issue, with other studies also supporting the pre-preparation approach to sperm freezing [70,71,72,73,74]. A possible explanation for these observations is that removal of seminal plasma increases sperm membrane fluidity, thereby increasing permeability to CPAs to maximize cryoprotection [75]. Hence, the relationship between semen preparation and sperm cryopreservation appears to be a complex one, with optimization of approach being complicated by the need to balance access of CPAs with maintenance of the sperm membrane’s architectural integrity.
Ultra-rapid liquid solidification or vitrification of spermatozoa may mitigate the risk of intracellular ice formation to some extent, plunging into the LN2 avoiding phase transition damage [76]. Vitrification may also be achieved in the absence of potentially toxic cell-permeable CPAs [77]. Indeed, successful vitrification may be achieved using only non-permeating compounds such as human serum albumin and sucrose by virtue of their antioxidant and osmotic qualities, respectively. This method has been used successfully for even large volumes of semen in the absence of any CPAs [78]. With respect to sperm DNA integrity, vitrification in the absence of CPAs compares favorably against cryopreservation using a wide range of commercially available sperm freezing media [53]. Indeed, there appears to be no detrimental impact of cryoprotectant-free vitrification upon sperm DNA fragmentation and hyaluronan binding when compared with rapid freezing and freezing using cell-permeable CPAs [79]. Interestingly, even vitrification in the absence of both sucrose and cell-permeable CPAs has been reported to yield higher sperm viability and lower cryodamage to sperm chromatin and sperm DNA [80,81]. However, the benefit of cryoprotectant-free vitrification of human semen is not a universal finding, with some authors recommending vitrification of spermatozoa following separation from seminal plasma [82].
During cryopreservation, spermatozoa are subjected to oxidative stress via consumption of antioxidants due to sperm cytosol absorption and disruption of the extracellular environment, which destabilizes sperm metabolism and increases the levels of ROS. Unfortunately, there has been little evolution in the composition of human sperm freezing media for decades, and there is clearly a need for greater understanding of the mechanisms involved in sperm DNA damage during cryopreservation. Further research in this area will, hopefully, lead to improvements in cryomedia and cryopreservation techniques.
5. Antioxidants and Their Mitigation of Sperm Cryodamage
Oral antioxidant therapy for men suspected of being exposed to oxidative stress has proven to be of limited value to their semen quality and fertility [83]. Unfortunately, this area of research has been largely undermined by a general failure to effectively diagnose those suffering from oxidative stress and, therefore, identify patient groups most likely to benefit from administration of oral antioxidants [84]. Furthermore, it has been pointed out that little value in the administration of antioxidants can be expected in the absence of a deficit in endogenous antioxidant activity [84]. Conversely, there can be little doubt that spermatozoa will be subjected to oxidative stress during various procedures conducted in vitro. Indeed, a range of antioxidants such as Catalase, CoQ10, Ethylenediaminetetraacetic Acid (EDTA), Melatonin, and vitamins have been shown to improve sperm parameters during various in vitro processes [85,86]. Interestingly, a multi-antioxidant approach may better address the diversity of the redox systems known to operate within spermatozoa, a synergistic effect of various antioxidants during cryopreservation having been demonstrated in the animal model [87,88,89,90]. Therefore, it is likely that a similar approach might be beneficial to the cryopreservation of human semen and spermatozoa.
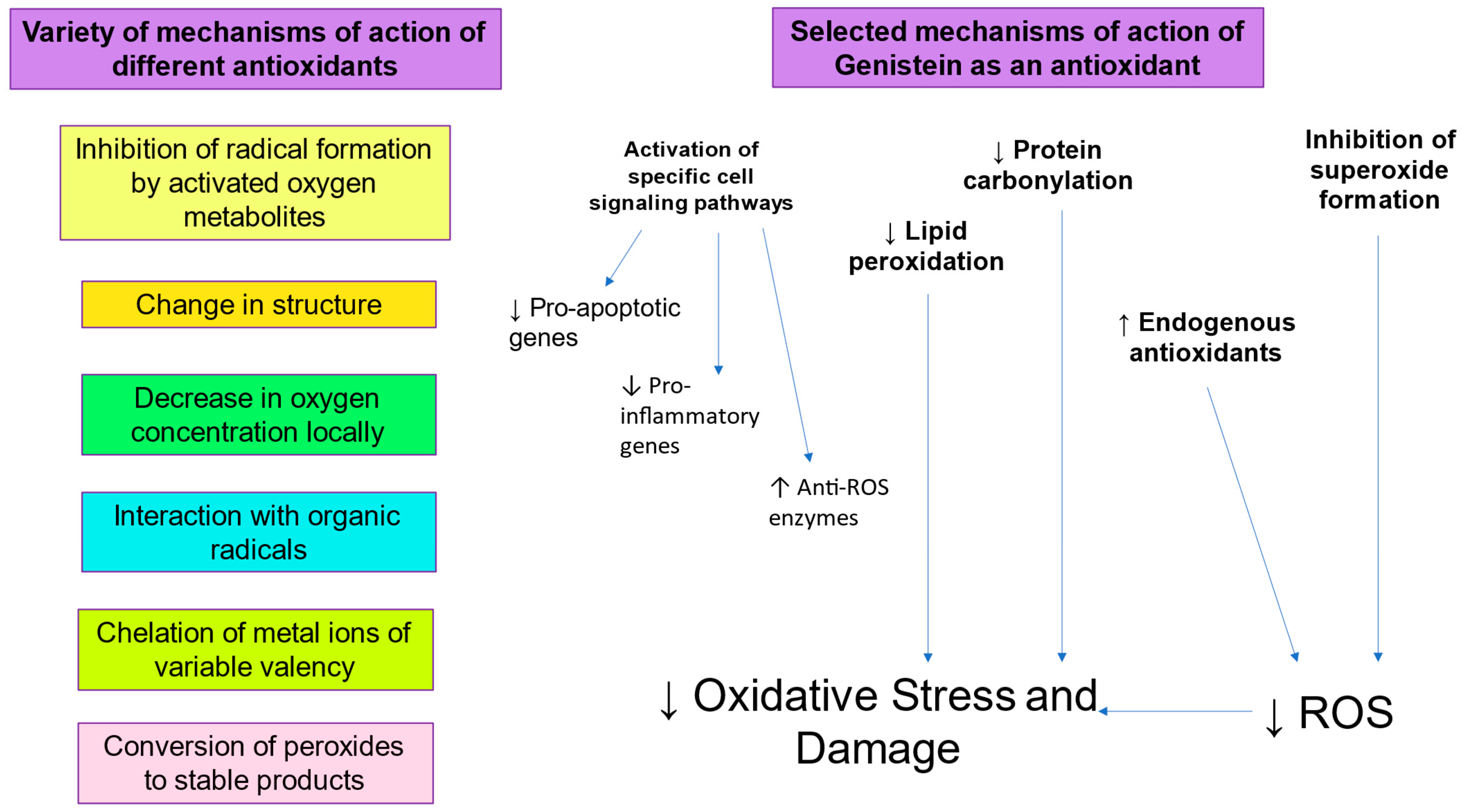
There is clearly an imperative for the development and clinical application of more effective cryomedia and cryopreservation protocols [91]. In this respect, the inclusion of antioxidants such as Genistein to prevent sperm DNA damage due to oxidative stress during cryopreservation would be a logical strategy (Figure 2). Genistein, a phyto-estrogen derived from soyabeans, has been found to protect against oxidative stress, lipid peroxidation, and resulting sperm DNA fragmentation [41]. Genistein appears to have antioxidant activity since it can protect cells from oxidative stress, lipid peroxidation, and DNA fragmentation [92,93]. Its mechanism of action includes inhibition of enzymes capable of generating ROS, such as XO, inhibition of lipid peroxidation by scavenging of peroxyl radicals, and enhancement of the activity of antioxidants endogenous to spermatozoa, such as Glutathione Peroxidase, Glutathione Reductase, and SOD [94,95]. Interestingly, Genistein also displays anti-mutagenic activity and can inhibit ROS-induced DNA fragmentation [96,97]. Indeed, Genistein has a significant protective effect against oxidative DNA damage and sperm DNA fragmentation, suggesting that increased oxidative stress generates sperm DNA damage during cryopreservation [41]. Sperm vitality and post-thaw motility is also improved by the presence of Genistein, suggesting a link between oxidative stress and sperm function.
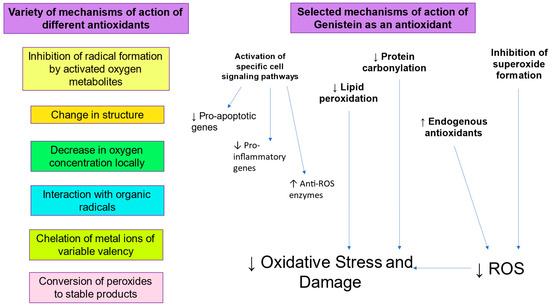
Figure 2.
Role of antioxidants in mitigating oxidative stress.
Various other enzymatic and non-enzymatic antioxidants have been shown to be beneficial to sperm function and DNA integrity [98]. Hypotaurine and the powerful antioxidant, Glutathione, have been shown to protect against H2O2-induced sperm DNA damage [99]. Interestingly, Hypotaurine also has a protective effect against apoptosis, but only when sperm are separated by DGC prior to freezing [100]. The antioxidants, Vitamin C and Vitamin E, also protect against sperm DNA damage during sperm preparation [101,102]. Other studies of Vitamin C and Vitamin E have reported a reduction in cryopreservation-induced ROS production and increase in post-thaw motility, respectively [103]. Edaravone is a low-molecular-weight antioxidant which scavenges both lipid- and water-soluble peroxyl radicals by donating an electron to them [104]. Other novel antioxidants merit further research. For example, the nucleophilic thiol, Penicillamine, has been shown to protect against oxidative stress in several studies involving human spermatozoa. In one of these studies, Penicillamine was able to protect against oxidative stress induced by Thiostrepton-mediated inhibition of the antioxidant enzymes 2-Cys Peroxiredoxins [105]. A mechanistic study of Penicillamine demonstrated that its protective effects against oxidative stress were mediated by a stabilization of 4-HNE generation and a decline in 8OHDG formation, affording protection of thiol groups within spermatozoa [106]. In another study, Penicillamine was shown to protect against Ionomycin and H2O2-induced oxidative stress in normozoospermic donor semen samples [107]. When added to the sperm incubation medium containing Ionomycin or H2O2, Penicillamine was able to reduce levels of ROS, resulting in improved preservation of mitochondrial membrane potential, ATP levels, and sperm motility. The amino acids, Cysteine and Glutamine, can also serve as effective antioxidants, decreasing intracellular ROS and sperm DNA damage, resulting in a significant improvement in sperm plasmalemma integrity, mitochondrial membrane potential, and progressive sperm motility [108]. Another naturally occurring antioxidant, Astaxanthin, has been found to decrease the levels of ROS and increase human sperm motility and DNA integrity following both slow freezing and vitrification [109,110,111].
6. Discussion
The cryopreservation of semen, spermatozoa, and testicular tissue is clearly not a stress-free process, which is hardly surprising given that the various procedures are performed under atmospheric conditions, with oxygen tensions of 20–21%. So, by definition, it is likely that spermatozoa will be subjected to oxidative stress. Though correlations between sperm DNA fragmentation and apoptosis have been reported previously, the evidence is not that strong and, besides, there is a paucity of evidence for an increase in sperm DNA fragmentation mediated by a caspase cascade during cryopreservation [112,113]. Though increased sperm DNA damage following cryopreservation has been extensively reported previously [7,20,23,24,114], there are relatively few studies demonstrating an association between oxidative base damage and sperm DNA fragmentation [41,112]. Supporting a link between cryo-induced oxidative DNA damage and sperm DNA fragmentation, there have also been reports of increased ROS production during sperm cryopreservation [47,48]. Since oxidative stress, sperm DNA fragmentation, and apoptosis occur within spermatozoa regardless of whether semen samples have been separated by DGC before or after cryopreservation, it appears that cryoinjury is suffered by both good- and poor-quality spermatozoa [113]. Notably, there appears to be a commonality between the mechanisms mediating the generation and exacerbation of oxidative DNA damage and sperm DNA fragmentation during cryopreservation [114].
Given that there are at least two distinct putative mechanisms of cryoinjury to sperm DNA, we designed a study to possibly differentiate between them using semen samples from 60 patients tested for sperm DNA fragmentation, oxidative DNA damage, and apoptosis, before and after cryopreservation [41]. Aliquots of each liquefied semen sample, with or without prior DGC preparation, were frozen by rate-controlled, slow freezing in either the absence (n = 20 controls) or presence of the soyabean-derived, estrogenic antioxidant Genistein (n = 20) or the cell-permeable, pan-caspase irreversible inhibitor, Z-VAD(OMe)-FMK (n = 20). Prior to freezing, a highly significant positive correlation was found between oxidative DNA damage and sperm DNA fragmentation in semen samples before (p < 0.001) and after (p < 0.01) DGC, a similar positive correlation (p < 0.001) between sperm DNA fragmentation and the percentage of caspase positive cells only being observed in the DGC separated fraction. Cryopreservation in the absence of Genistein and Z-VAD(OMe)-FMK resulted in a significant increase in oxidative DNA damage, sperm DNA fragmentation, and apoptosis, regardless of whether the semen samples were separated by DGC. Genistein addition prior to freezing was found to have a significant protective effect following thawing against oxidative DNA damage and sperm DNA fragmentation, resulting in a significant increase in sperm motility and vitality. In contrast, Z-VAD(OMe)-FMK addition prior to freezing was unable to provide any protective effect against oxidative DNA damage and sperm DNA fragmentation following thawing. These results suggest that the damage to sperm DNA induced during cryopreservation is predominantly mediated by oxidative stress rather than apoptosis, offering some potential opportunity to improve sperm cryopreservation media.
Since our study, there have been reports from other groups supporting our findings. To differentiate between the impact of sperm cryopreservation on single- and double-stranded DNA fragmentation, an analysis of the fresh and frozen semen samples from 30 males was conducted using alkaline and neutral Comet assays, respectively [115]. A 10% increase in single-stranded DNA fragmentation was observed following cryopreservation, regardless of male fertility status, whereas there was no significant increase in double-stranded DNA fragmentation. These results led the authors to conclude that oxidative stress is the major effector of sperm DNA damage since single-stranded sperm DNA fragmentation has been previously correlated with oxidative damage [116,117]. Another study compared the levels of apoptosis, oxidative stress, and sperm DNA damage in 60 infertility patients and 20 normozoospermic sperm donors prior to and following slow freezing of their semen samples, using an Annexin V-FITC apoptosis kit, BIODIPY/8OH-DG, and TUNEL staining, respectively [118]. The sperm donors were found to have significantly lower levels of apoptosis, oxidative stress, and DNA damage than those undergoing fertility treatment, and BIODIPY/8OH-DG levels were significantly higher than Annexin V levels post-thawing, suggesting the greater relevance of oxidative stress during cryopreservation.
7. Future Directions
The relative benefit of antioxidants during cryopreservation may be species-specific, tissue specific, and/or depend to some extent upon the freezing method employed. For example, the universal antioxidant, α-Lipoic Acid, which acts as a cofactor for mitochondrial enzyme activity and is capable of regenerating other antioxidants such as Glutathione, has been shown to protect against cryo-induced sperm DNA damage and improve sperm motility and acrosome integrity in boar spermatozoa [119]. On the other hand, with simple sucrose vitrification of semen samples from normozoospermic sperm donors, triple antioxidant (Acetyl-L-Carnitine, N-Acetyl-L-Cysteine, and α-Lipoic Acid) supplementation of the vitrification medium did not decrease extracellular levels of ROS and was found to have only a moderate, non-significant, protective effect against sperm DNA fragmentation [120]. This conundrum serves to illustrate the challenge in developing improved methods for sperm cryopreservation in the human and other mammalian species, given the confounding variables of sperm source, sperm preparation, sperm freezing medium, and cryopreservation technique. Furthermore, it may be unrealistic to expect antioxidants that act by increasing the activity of endogenous, enzymatic, antioxidant pathways within the limited sperm cytosol to be as effective as those that scavenge ROS. To complicate matters further, there are other groups of molecules related to ROS that may also be involved in cryoinjury to spermatozoa including reactive halogen (bromine and chlorine) species, reactive nitrogen species, and reactive sulfur species. Also, there are additional, oxidative stress-derived, regulated, cell death pathways which merit further research such as cuproptosis, where excessive accumulation of copper within the cell can cause metabolic disturbances. There exists precious little published data exploring these alternative pathways during sperm cryopreservation.
8. Conclusions
Spermatozoa are particularly vulnerable to oxidative stress by virtue of the high cellular content of polyunsaturated fatty acids within the sperm plasmalemma and limited reserves of antioxidants within their residual cytoplasm following spermiogenesis. Furthermore, timely genomic responses to environmental stress, such as the maintenance of ATP synthesis and redox homeostasis, are rendered impossible by the highly condensed chromatin within the sperm nucleus. Semen, spermatozoa, and testicular tissue are exposed to oxidative stress during cryopreservation, cryoinjury of sperm DNA predominantly occurring via the ROS pathway rather than via the apoptotic cascade. Therefore, vitrification and the addition of antioxidants to sperm cryomedia may mitigate the increased sperm DNA fragmentation observed following freezing and thawing.
Author Contributions
Conceptualization, S.D.F. and L.K.T.; writing—original draft preparation, S.D.F.; writing—review and editing, S.D.F. and L.K.T.; visualization, S.D.F. and L.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they do not have any commercial or associative interests that represent a conflict of interest in connection with the work submitted. S.D.F. is also an employee of CooperSurgical Fertility Solutions, but the company had no knowledge of, or role in the design, execution, interpretation, or writing of this review article.
References
- Ranganathan, P.; Mahran, A.M.; Hallak, J.; Agarwal, A. Sperm cryopreservation for men with nonmalignant, systemic diseases: A descriptive study. J. Androl. 2002, 23, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, R.; Miller, S.D.; Meyers, K.E.; Ginsberg, J.P. Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr. Nephrol. 2015, 30, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Scarpa, M.; Pathak, S.; Burra, P.; Sturniolo, G.C.; Russo, F.P.; Murugesan, R.; D’Incá, R. Inflammatory Bowel Disease Therapies Adversely Affect Fertility in Men- A Systematic Review and Meta-analysis. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, P.; Rovei, V.; Masera, S.; Vercellino, M.; Massobrio, M.; Mutani, R.; Revelli, A. Fertility in patients with multiple sclerosis: Current knowledge and future perspectives. Neurol. Sci. 2006, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Onofre, J.; Baert, Y.; Faes, K.; Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum. Reprod. Update 2016, 22, 744–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nijs, M.; Ombelet, W. Cryopreservation of human sperm. Hum. Fertil. 2001, 4, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Askari, A.S.; Georg, T.; Rosenbaum, P.; Schmidt, W. Effect of freeze-thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int. J. Androl. 1999, 22, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.H.; Morshedi, M.; Taylor, S.; Oehninger, S. Sperm DNA quality predicts intrauterine insemination outcome: A prospective cohort study. Hum. Reprod. 2002, 17, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod. 2003, 18, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Gardner, D.K.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004, 82, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.A.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Lornage, J.; Mazoyer, C.; Lejeune, H.; Salle, B.; François Guerin, J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil. Steril. 2007, 87, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Coomarasamy, A.; Frew, L.; Hutton, R.; Kirkman-Brown, J.; Lawlor, M.; Lewis, S.; Partanen, R.; Payne-Dwyer, A.; Román-Montañana, C.; et al. Sperm selection with hyaluronic acid improved live birth outcomes among older couples and was connected to sperm DNA quality, potentially affecting all treatment outcomes. Hum. Reprod. 2022, 37, 1106–1125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribas-Maynou, J.; Benet, J. Single and Double Strand Sperm DNA Damage: Different Reproductive Effects on Male Fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Henkel, R.; Finelli, R.; Robert, K.A.; Iovine, C.; Baskaran, S. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic. Biol. Med. 2020, 152, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Adel, M.; Fleming, S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J. Pers. Med. 2024, 14, 198. [Google Scholar] [CrossRef]
- Sciorio, R.; Greco, P.F.; Greco, E.; Tramontano, L.; Fathy, E.M.; Fleming, S. Potential Effects of Environmental Toxicants on Sperm Quality and Potential Risk for Fertility in Humans. Front. Endocrinol. 2025, in press. [Google Scholar]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, D.K.; Atreja, S.K. Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation. Reprod. Domest. Anim. 2015, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.M.; Forell, F.; Oliveira, A.T.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.L.; Taylor, S.L.; Morshedi, M.; Schuffner, A.; Duran, E.H.; Beebe, S.; Oehninger, S. Caspase activity and apoptotic markers in ejaculated human sperm. Mol. Hum. Reprod. 2002, 8, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Gallego, M.A.; Defossez, A.; Formstecher, P.; Marchetti, P. Staining of human sperm with fluorochrome-labeled inhibitor of caspases to detect activated caspases: Correlation with apoptosis and sperm parameters. Hum. Reprod. 2004, 19, 1127–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zini, A.; de Lamirande, E.; Gagnon, C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993, 16, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wündrich, K.; Paasch, U.; Leicht, M.; Glander, H.J. Activation of caspases in human spermatozoa during cryopreservation—An immunoblot study. Cell Tissue Bank. 2006, 7, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Weng, S.L.; Fox, P.; Duran, E.H.; Morshedi, M.S.; Oehninger, S.; Beebe, S.J. Somatic cell apoptosis markers and pathways in human ejaculated sperm: Potential utility as indicators of sperm quality. Mol. Hum. Reprod. 2004, 10, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Soto, V.; Zazueta, C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int. J. Biochem. Cell Biol. 2007, 39, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Holmes, E.H.; Greene, T.G.; Liu, P.K. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000, 14, 955–967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Hargreave, T.B.; Irvine, D.S.; Wu, F.C. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J. Androl. 1989, 10, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Wingate, J.K.; Koppers, A.J.; McLaughlin, E.A.; Aitken, R.J. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- van Overveld, F.W.; Haenen, G.R.; Rhemrev, J.; Vermeiden, J.P.; Bast, A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem. Biol. Interact. 2000, 127, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, F.; Rossi, T.; Sabatini, L.; Pulcinelli, F.M.; Rapone, S.; Dondero, F.; Gazzaniga, P.P. Human sperm cryopreservation and reactive oxygen species (ROS) production. Acta Eur. Fertil. 1995, 26, 145–148. [Google Scholar] [PubMed]
- Wang, A.W.; Zhang, H.; Ikemoto, I.; Anderson, D.J.; Loughlin, K.R. Reactive oxygen species generation by seminal cells during cryopreservation. Urology 1997, 49, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Baumber, J.; Ball, B.A.; Linfor, J.J.; Meyers, S.A. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J. Androl. 2003, 24, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Peris, S.I.; Bilodeau, J.F.; Dufour, M.; Bailey, J.L. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol. Reprod. Dev. 2007, 74, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.T.; Steele, E.K.; McClure, N.; Lewis, S.E. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum. Reprod. 2001, 16, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- de Paula, T.S.; Bertolla, R.P.; Spaine, D.M.; Cunha, M.A.; Schor, N.; Cedenho, A.P. Effect of cryopreservation on sperm apoptotic deoxyribonucleic acid fragmentation in patients with oligozoospermia. Fertil. Steril. 2006, 86, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Fleming, S.D.; Schulke, L.; Barone, K.; Zieschang, J.A.; Clark, A.M. The DNA integrity of cryopreserved spermatozoa separated for use in assisted reproductive technology is unaffected by the type of cryoprotectant used but is related to the DNA integrity of the fresh separated preparation. Fertil. Steril. 2009, 92, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hai, E.; Li, B.; Zhang, J.; Zhang, J. Sperm freezing damage: The role of regulated cell death. Cell Death Discov. 2024, 10, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, S.E.; Ahn, J.S.; Kim, Y.H.; Oh, H.J.; Kim, B.J.; Ryu, B.Y. Necrostatin-1 improves the cryopreservation efficiency of murine spermatogonial stem cells via suppression of necroptosis and apoptosis. Theriogenology 2020, 158, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.; Paynter, S. Fundamentals of cryobiology in reproductive medicine. Reprod. Biomed. Online 2004, 9, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Y.; Liu, J.; Liu, C.; McGann, L.E.; Watson, P.F.; Kleinhans, F.W.; Mazur, P.; Critser, E.S.; Critser, J.K. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 1995, 10, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Storey, B.T. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J. Androl. 1992, 13, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Storey, B.T. Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: Glycerol and other polyols as sole cryoprotectant. J. Androl. 1993, 14, 199–209. [Google Scholar] [PubMed]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef] [PubMed]
- Jobim, M.I.; Oberst, E.R.; Salbego, C.G.; Souza, D.O.; Wald, V.B.; Tramontina, F.; Mattos, R.C. Two-dimensional polyacrylamide gel electrophoresis of bovine seminal plasma proteins and their relation with semen freezability. Theriogenology 2004, 61, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Grizard, G.; Chevalier, V.; Griveau, J.F.; Le Lannou, D.; Boucher, D. Influence of seminal plasma on cryopreservation of human spermatozoa in a biological material-free medium: Study of normal and low-quality semen. Int. J. Androl. 1999, 22, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Cryopreservation of human semen and prepared sperm: Effects on motility parameters and DNA integrity. Fertil. Steril. 2001, 76, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Saritha, K.R.; Bongso, A. Comparative evaluation of fresh and washed human sperm cryopreserved in vapor and liquid phases of liquid nitrogen. J. Androl. 2001, 22, 857–862. [Google Scholar] [PubMed]
- Palomar Rios, A.; Gascón, A.; Martínez, J.V.; Balasch, S.; Molina Botella, I. Sperm preparation after freezing improves motile sperm count, motility, and viability in frozen-thawed sperm compared with sperm preparation before freezing-thawing process. J. Assist. Reprod. Genet. 2018, 35, 237–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Androni, D.A.; Dodds, S.; Tomlinson, M.; Maalouf, W.E. Is pre-freeze sperm preparation more advantageous than post-freeze? Reprod. Fertil. 2021, 2, 17–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Sánchez, F.; Cooper, T.G.; Yeung, C.H.; Nieschlag, E. Improvement in quality of cryopreserved human spermatozoa by swim-up before freezing. Int. J. Androl. 1994, 17, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Sharma, R.K.; Thomas, A.J., Jr.; Agarwal, A. Improvement in motion characteristics and acrosome status in cryopreserved human spermatozoa by swim-up processing before freezing. Hum. Reprod. 2000, 15, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Counsel, M.; Bellinge, R.; Burton, P. Vitality of oligozoospermic semen samples is improved by both swim-up and density gradient centrifugation before cryopreservation. J. Assist. Reprod. Genet. 2004, 21, 137–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petyim, S.; Neungton, C.; Thanaboonyawat, I.; Laokirkkiat, P.; Choavaratana, R. Sperm preparation before freezing improves sperm motility and reduces apoptosis in post-freezing-thawing sperm compared with post-thawing sperm preparation. J. Assist. Reprod. Genet. 2014, 31, 1673–1680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brugnon, F.; Ouchchane, L.; Pons-Rejraji, H.; Artonne, C.; Farigoule, M.; Janny, L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.J. Rapidly cooled human sperm: No evidence of intracellular ice formation. Hum. Reprod. 2006, 21, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: From past practical difficulties to present success. Reprod. Biomed. Online 2003, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, M.; du Plessis, S.S.; Huyser, C. Large volume cryoprotectant-free vitrification: An alternative to conventional cryopreservation for human spermatozoa. Andrologia 2015, 47, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Agha-Rahimi, A.; Khalili, M.A.; Nabi, A.; Ashourzadeh, S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: Effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod. Biomed. Online 2014, 28, 352–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.; Li, L.; Qian, Y.; Xu, C.; Zhu, Y.; Huang, H.; Jin, F.; Ye, Y. Small-volume vitrification for human spermatozoa in the absence of cryoprotectants by using Cryotop. Andrologia 2015, 47, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Agha-Rahimi, A.; Khalili, M.A.; Nottola, S.A.; Miglietta, S.; Moradi, A. Cryoprotectant-free vitrification of human spermatozoa in new artificial seminal fluid. Andrology 2016, 4, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.A.; Adib, M.; Halvaei, I.; Nabi, A. Vitrification of neat semen alters sperm parameters and DNA integrity. Urol. J. 2014, 11, 1465–1470. [Google Scholar] [PubMed]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 5, CD007411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Gharagozloo, P. The assessment of oxidative stress in human semen: Chaos and confusion in pursuit of diagnostic precision. Reprod. Biomed. Online 2025, 50, 104488. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.J.; Kim, J.H.; Ryu, C.S.; Lee, J.Y.; Park, J.S.; Chung, D.Y.; Choi, S.Y.; Kim, M.H.; Chun, E.K.; Roh, S.I. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum. Reprod. 2008, 23, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giaretta, E.; Estrada, E.; Bucci, D.; Spinaci, M.; Rodríguez-Gil, J.E.; Yeste, M. Combining reduced glutathione and ascorbic acid has supplementary beneficial effects on boar sperm cryotolerance. Theriogenology 2015, 83, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Feng, T.; Dai, G.; Wang, Y.; Zhu, H.; Hu, J. Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation. Cryobiology 2018, 84, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tanhaei Vash, N.; Nadri, P.; Karimi, A. Synergistic effects of myo-inositol and melatonin on cryopreservation of goat spermatozoa. Reprod. Domest. Anim. 2022, 57, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rao, Y.; Du, C.; Wang, A.; Wu, Y.; Lin, R.; Xiao, T.; Lin, W. Sodium alginate and bovine serum albumin co-combined improved the cryopreservation quality of boar sperm through the PI3K-AKT pathway. Reprod. Fertil. Dev. 2025, 37, RD24127. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129749. [Google Scholar] [CrossRef] [PubMed]
- Bennetts, L.E.; De Iuliis, G.N.; Nixon, B.; Kime, M.; Zelski, K.; McVicar, C.M.; Lewis, S.E.; Aitken, R.J. Impact of estrogenic compounds on DNA integrity in human spermatozoa: Evidence for cross-linking and redox cycling activities. Mutat. Res. 2008, 641, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Qiu, S.F.; Shen, J.; Sun, L.N.; Wang, J.Y.; Bruce, I.C.; Xia, Q. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci. Lett. 2008, 438, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bowen, R.; Cai, Q.; Barnes, S.; Wang, Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc. Soc. Exp. Biol. Med. 1995, 208, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Gebicki, J.; Grynkiewicz, G. Radical scavenging properties of genistein. Free Radic. Biol. Med. 2003, 35, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Sakano, K.; Nakamura Si Kosaka, H. Antimutagenic activity of isoflavones from soybean seeds (Glycine max merrill). J. Agric. Food Chem. 1999, 47, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Win, W.; Cao, Z.; Peng, X.; Trush, M.A.; Li, Y. Different effects of genistein and resveratrol on oxidative DNA damage in vitro. Mutat. Res. 2002, 513, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility--a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Glutathione and hypotaurine in vitro: Effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis 2000, 15, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Lewis, S.E.; McKelvey-Martin, V.J.; Thompson, W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum. Reprod. 1998, 13, 1240–1247, Erratum in Hum. Reprod. 1998, 13, 3284. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999, 14, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Lin, Q.L.; Liu, R.J.; Xie, W.Y.; Xiao, W.F. Reducing oxidative DNA damage by adding antioxidants in human semen samples undergoing cryopreservation procedure. Zhonghua Yi Xue Za Zhi 2007, 87, 3174–3177. (In Chinese) [Google Scholar] [PubMed]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Nazarian, H.; Mofarahe, Z.S.; Raee, P.; Moradi, A.; Khavari, Z.; Novin, M.G. The Effect of Edaravone Supplementation Prior to Cryopreservation on Sperm Parameters, DNA Integrity, Apoptosis, Lipid Peroxidation, and Mitochondrial Membrane Potential in Infertile Men with Asthenoteratozoospermia. Reprod. Sci. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Meriño, J.; Bravo, A.; Zambrano, F.; Schulz, M.; Villegas, J.V.; Sánchez, R. Antioxidant effects of penicillamine against in vitro-induced oxidative stress in human spermatozoa. Andrologia 2020, 52, e13553. [Google Scholar] [CrossRef] [PubMed]
- Koohestanidehaghi, Y.; Torkamanpari, M.; Shirmohamadi, Z.; Lorian, K.; Vatankhah, M. The effect of cysteine and glutamine on human sperm functional parameters during vitrification. Andrologia 2021, 53, e13870. [Google Scholar] [CrossRef] [PubMed]
- Dede, G.; Saylan, A. The effect of astaxanthin on human sperm parameters after cryopreservation. Can. Urol. Assoc. J. 2022, 16, E552–E557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghantabpour, T.; Nashtaei, M.S.; Nekoonam, S.; Rezaei, H.; Amidi, F. The Effect of Astaxanthin on Motility, Viability, Reactive Oxygen Species, Apoptosis, and Lipid Peroxidation of Human Spermatozoa During the Freezing-Thawing Process. Biopreserv. Biobank. 2022, 20, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Thanintranon, S.; Saeng-Anan, U.; Vutyavanich, T.; Piromlertamorn, W.; Somsak, P.; Sanmee, U. Effects of astaxanthin supplementation during vitrification and liquid nitrogen vapor freezing on motility, morphology, survival, reactive oxygen species (ROS), and DNA fragmentation of post-cryopreserved human sperm. JBRA Assist. Reprod. 2024, 28, 611–617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Fleming, S.D.; Barone, K.; Zieschang, J.A.; Clark, A.M. The effect of repeated freezing and thawing on human sperm DNA fragmentation. Fertil. Steril. 2010, 93, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Fernández-Encinas, A.; García-Peiró, A.; Prada, E.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Human semen cryopreservation: A sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology 2014, 2, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Enciso, M.; Sarasa, J.; Agarwal, A.; Fernández, J.L.; Gosálvez, J. A two-tailed Comet assay for assessing DNA damage in spermatozoa. Reprod. Biomed. Online 2009, 18, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; García-Peiró, A.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Alkaline and neutral Comet assay profiles of sperm DNA damage in clinical groups. Hum. Reprod. 2012, 27, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Uriondo, H.; Nodar, F.; Alvarez Sedó, C. Cryopreservation Promotes Sperm DNA Damage Through Oxidative Stress [38N]. Obstet. Gynecol. 2018, 131, 162S. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, Z.L.; Li, C.J.; Hu, X.C.; Li, Q.W. Effect of alpha-lipoic acid on boar spermatozoa quality during freezing-thawing. Zygote 2016, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Juanpanich, T.; Suttirojpattana, T.; Parnpai, R.; Vutyavanich, T. The relationship between reactive oxygen species, DNA fragmentation, and sperm parameters in human sperm using simplified sucrose vitrification with or without triple antioxidant supplementation. Clin. Exp. Reprod. Med. 2022, 49, 117–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).