A Review on Improving the Oxidative Stability of Pine Nut Oil in Extraction, Storage, and Encapsulation
Abstract
1. Introduction
2. PNO Composition and Lipid Oxidation
| Geographical Regions | Species | Characteristics of Chemical Structure FA (% of Total FA) | Product Quality | References | ||
|---|---|---|---|---|---|---|
| C18:1 Oleic Acid | All-cis-9,12–18:2 Linoleic Acid | All-cis-5,9,12–18:3 Pinolenic Acid | ||||
| Xiaoxinganling, Heilongjiang Province, Northeast China | Pinus koraiens | 25.36 | 47.67 | 14.19 | / | [10] |
| Heilongjiang Province, China | Pinuspumila | 27.47 | 45.18 | 16.94 | The total phenol content: 1.12 mg/g, AV: 2.88 mg KOH/g, PV: 5.25 mmol/kg. | [40] |
| Eumseong, Korea | Pinus koraiensis | 26.00 | 46.00 | 13.00 | / | [30] |
| The forests of eastern Afghanistan | Pinus gerardiana Wall | / | / | 19.00 | Manganese content: 8.80 mg/100 g. | [41] |
| Chilean North 31~33° latitude | Pinus pinea L. | 40.92 | 47.64 | 0.37 | A-tocopherol: 94.40 μg/kg, γ-tocopherol: 1110.50 μg/kg, total phenolic compounds: 0.27 mg GAE/g. | [7] |
| Chilean Dry Coast 34~35° latitude | Pinus pinea L. | 37.13 | 50.72 | 0.37 | A-tocopherol: 57.40 μg/kg, γ-tocopherol: 1346.90 μg/kg, total phenolic compounds: 0.39 mg GAE/g. | [7] |
| Chilean South 36~38° latitude | Pinus pinea L. | 40.28 | 48.01 | 0.39 | A-tocopherol: 25.30 μg/kg, γ-tocopherol: 756.10 μg/kg, total phenolic compounds: 0.35 mg GAE/g. | [7] |
| Carregal do Sal | Pinus pinea L. | 37.59 | 47.70 | 0.80 | / | [26] |
| North Algeria Djurdjura National Park located in Tikjda in the state of Bouira | Pinus halepensis Mill. | 24.55 | 59.25 | / | AV: 68.99 mg KOH/g, PV: 28.69 mmol/kg. | [24] |
| North Algeria the National Park of Chrea located in the state of Blida | Pinus pinea L. | 34.63 | 30.67 | / | AV: 19.63 mg KOH/g, PV: 9.95 mmol/kg. | [24] |
| North Algeria the National Park of Chrea located in the state of Blida | Pinus pinaster | 18.42 | 51.95 | / | AV: 7.26 mg KOH/g, PV: 67.42 mmol/kg. | [24] |
| North Algeria the Taza National Park located in the state of Jijel | Pinus canariensis | 17.43 | 56.75 | / | AV: 4.91 mg KOH/g, PV: 65.88 mmol/kg. | [24] |
| Algeria | Pinus halepensis Mill. | 23.95 | 57.33 | / | Contains higher levels of carotenoids, PV: 0.50 mmol/kg. | [23] |
| Turkey | Pinus halepensis Mill. | 24.62 | 57.34 | / | PV: 0.79 mmol/kg. | [23] |
| Tunis | Pinus halepensis Mill. | 6.70 | 66.60 | / | The EC50 value of PNO: 0.35 mM. | [29] |
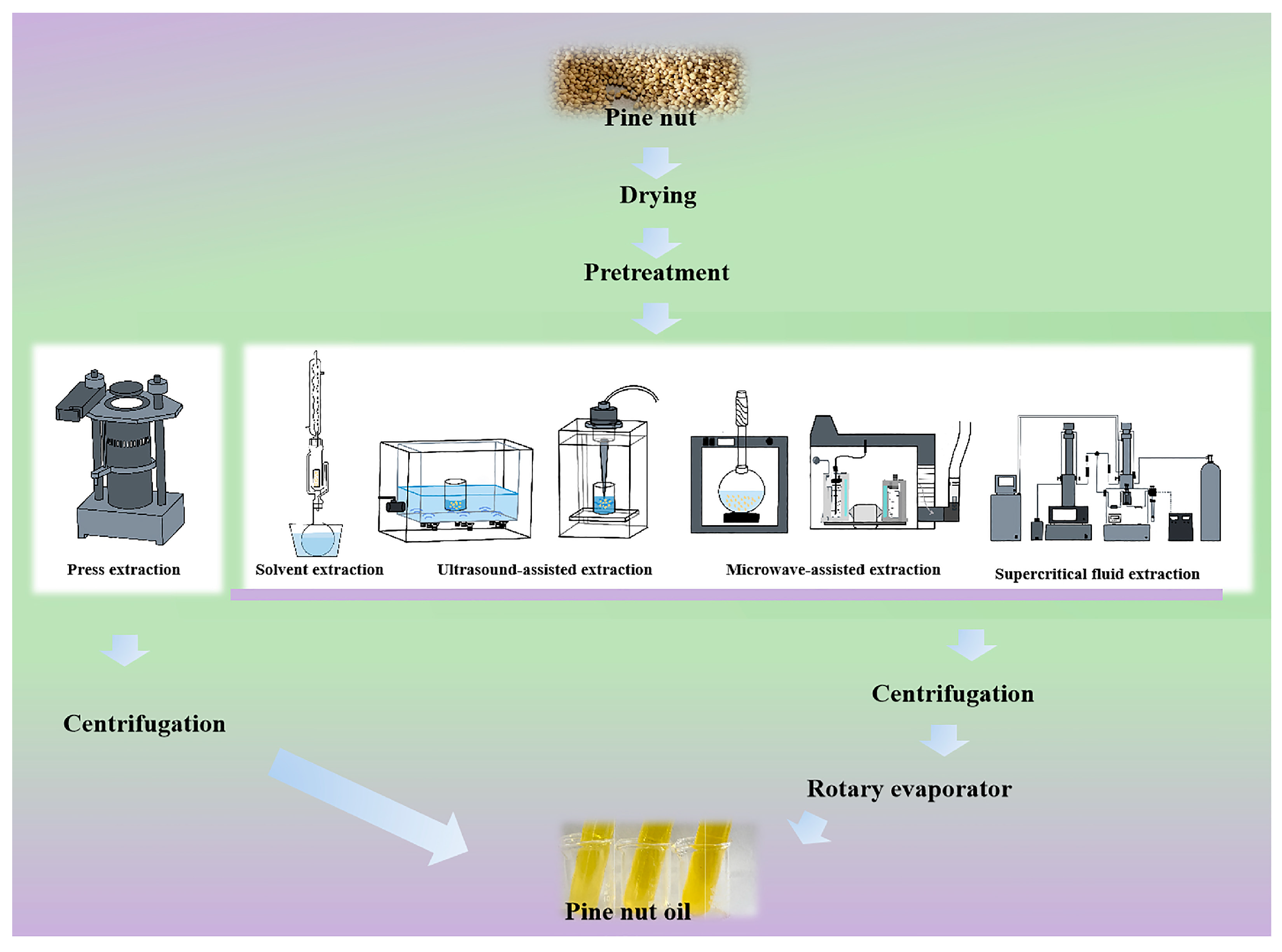
3. Processing of PNO
3.1. Pretreatment of Pine Nuts
3.2. Extraction Methodologies of PNO
| Extraction Methodologies | Reasons for Oxidation | Extraction Conditions | Oil Yield (%) | Comment | References |
|---|---|---|---|---|---|
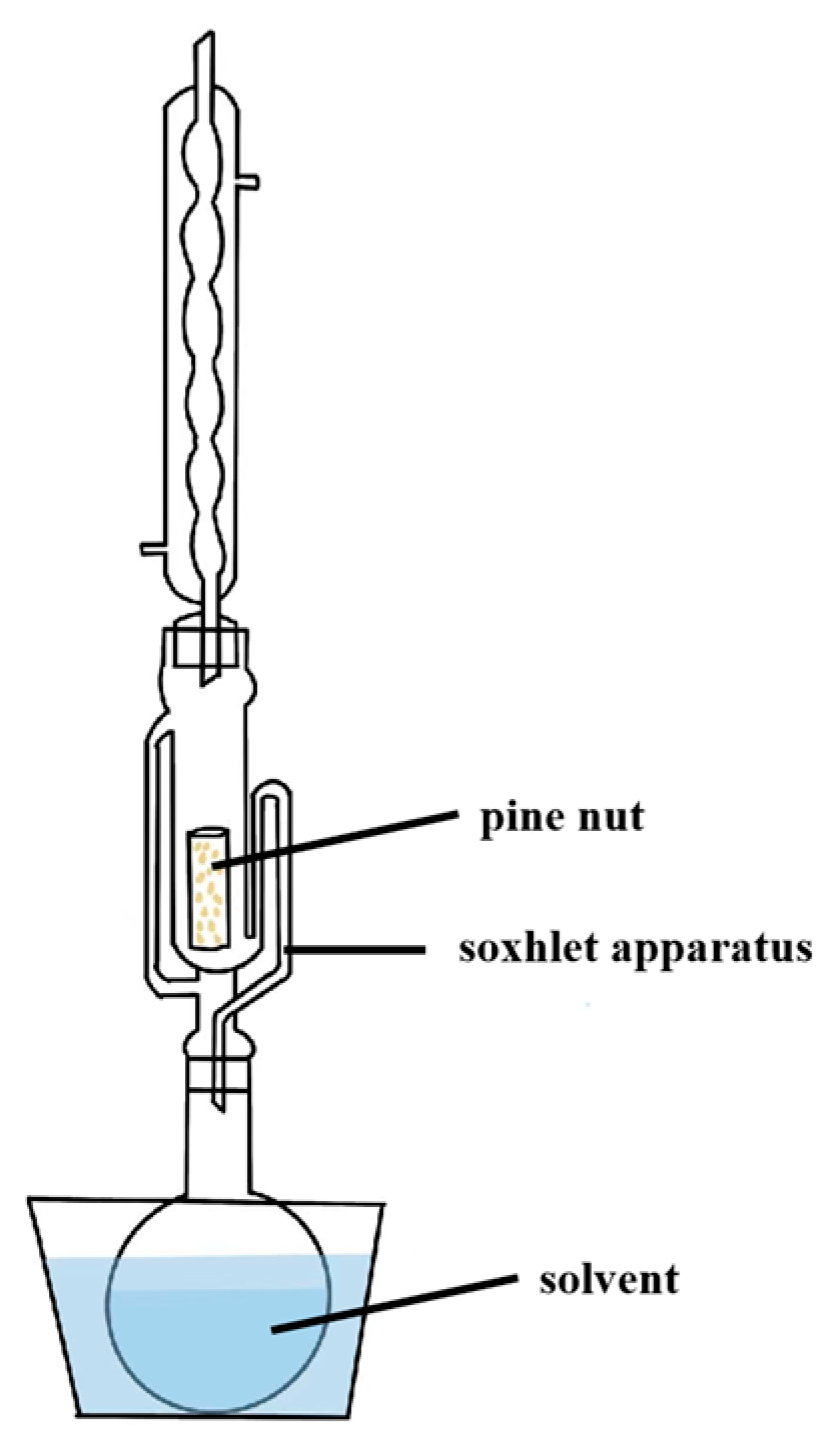
| Solvent extraction | High temperature, long time. | Extraction temperature 39.3 °C, extraction time 33.4 min, solvent/sample 10.8:1 (mL g−1). | 80.03 | PV: 0.53 mmol/kg, DPPH-HF: 8.60%, DPPH-LF: 65.07%, DPPH-oil: 73.35%, TV: 3.02. | [8] |
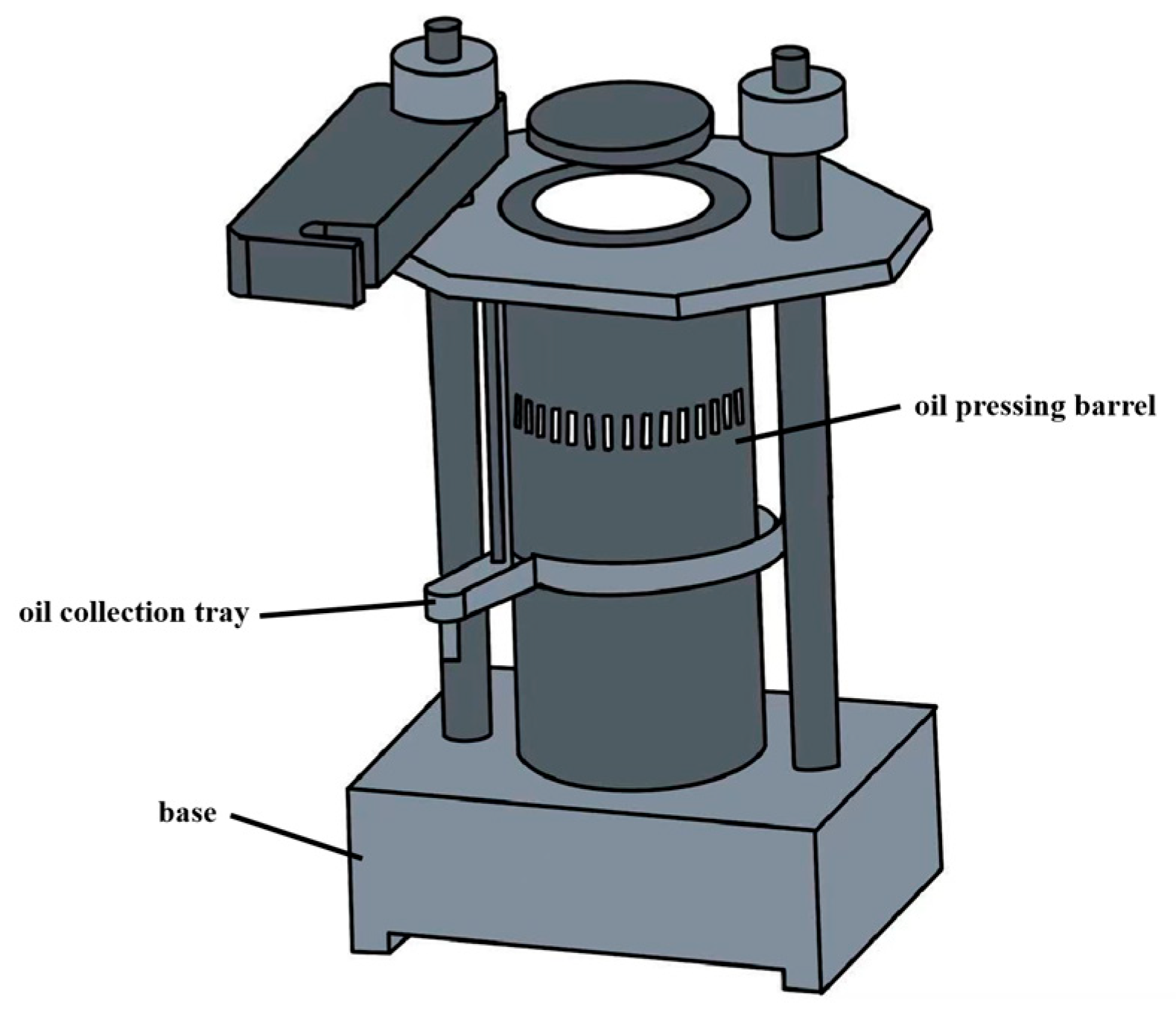
| Press extraction | High temperature of hot pressing | / | 15.50~20.67 | PV: 0.50~0.79 mmol/kg, the total amount of tocopherol: 309.42–318.04 mg/100 g, L* values: 92.24~96.04, b* values: 23.47~30.33. | [23] |
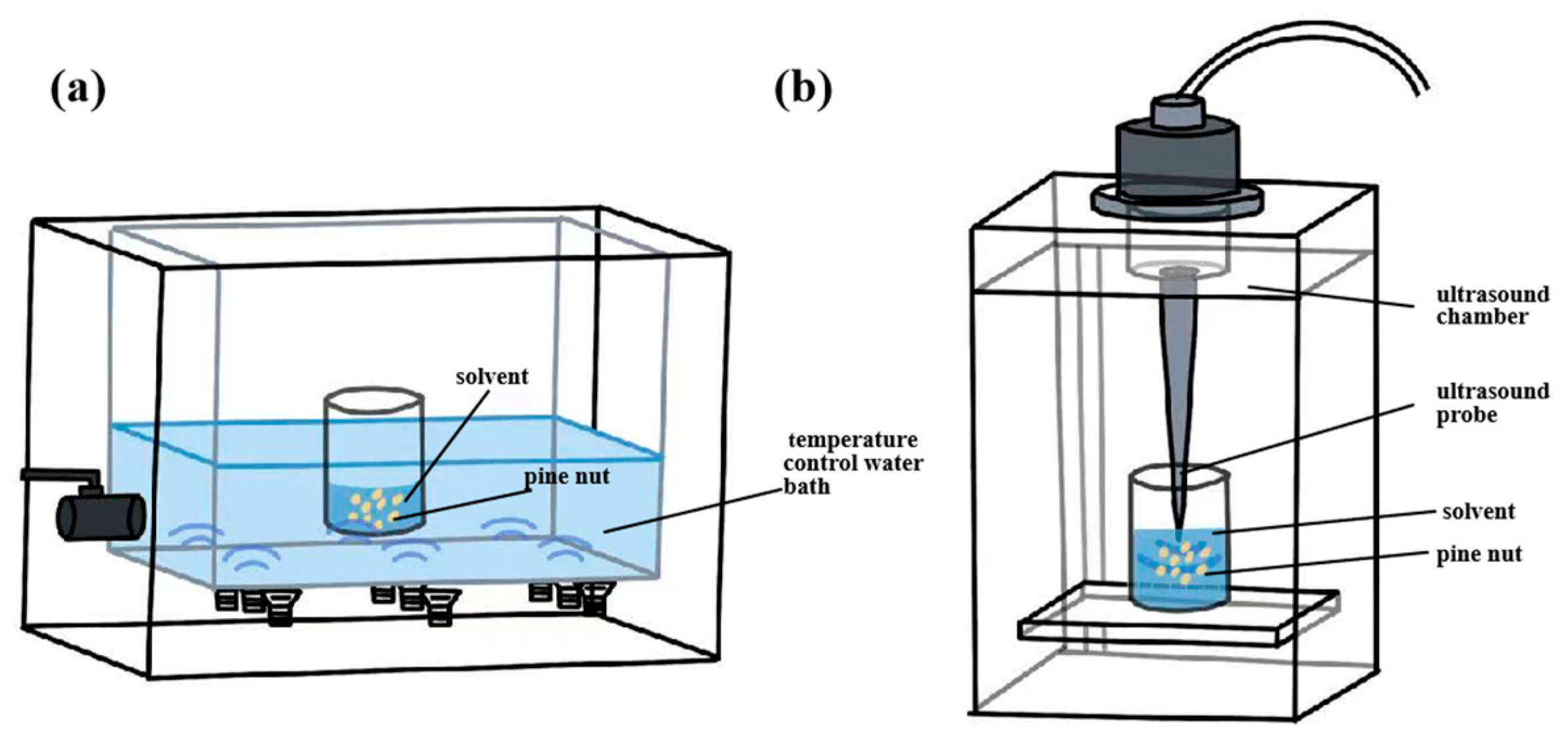
| Ultrasound-assisted extraction | Oxygen, metal probes, high temperature. | 2.5% of the enzyme solution mixture, 7.8 mL/g liquid-solid ratio, 1500 rpm stirring speed, 600 W, 50 °C, 1.7 h. | 31.89 | The total phenol content: 1.12 mg/g, PV: 5.25 mmol/kg. | [40] |
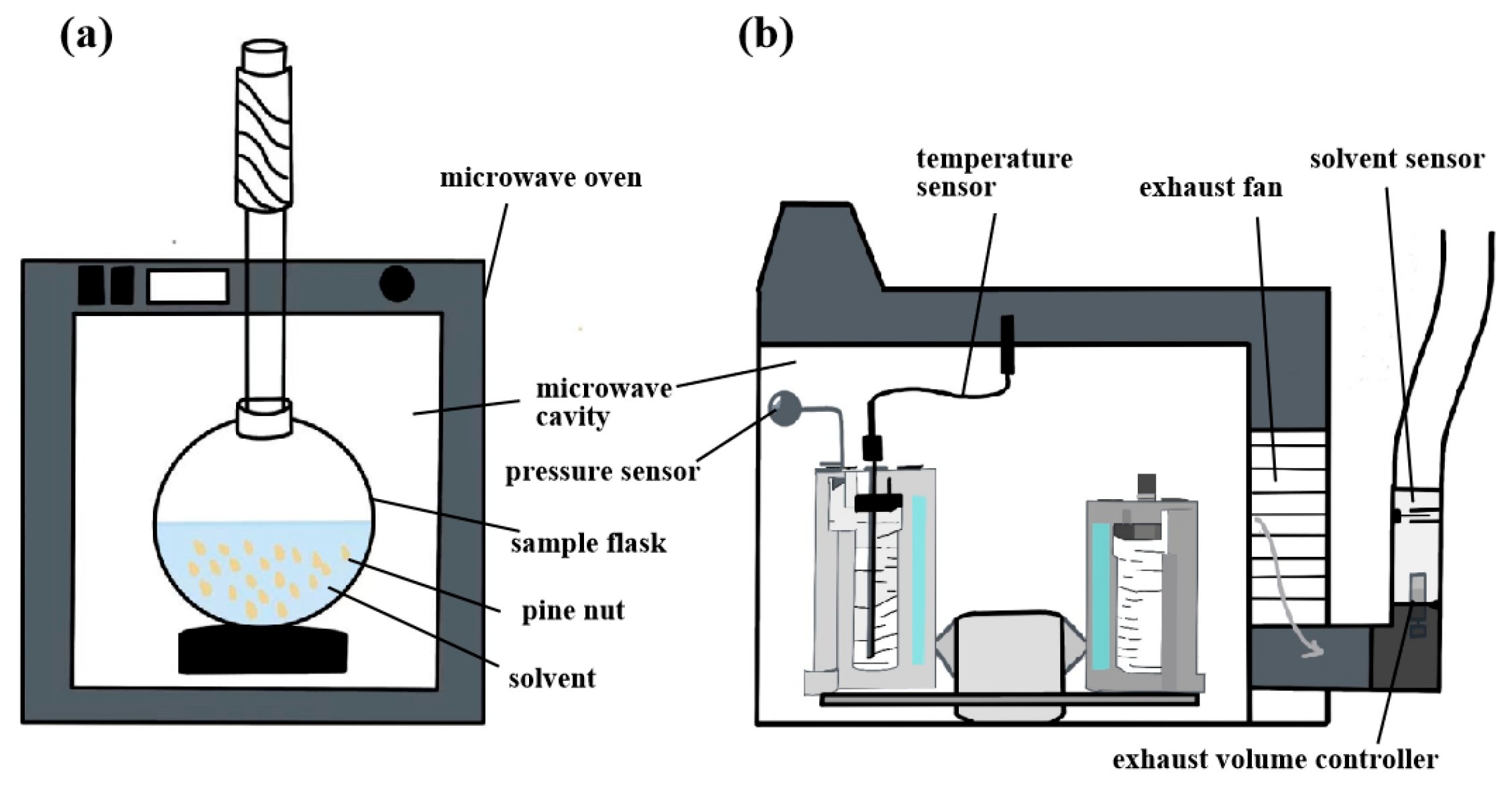
| Microwave-assisted extraction | High temperature, long time. | Microwave power 420 W, temperature 75 °C, liquid-solid ratio 7.0 mL/g and time 55 min. | 24.12 | Total phenols: 92,53 mg GAE/kg, α-tocopherol: 315.27 mg/kg, beta-carotene: 25.43 mg/kg, phytosterols: 685.68 mg/kg. | [53] |
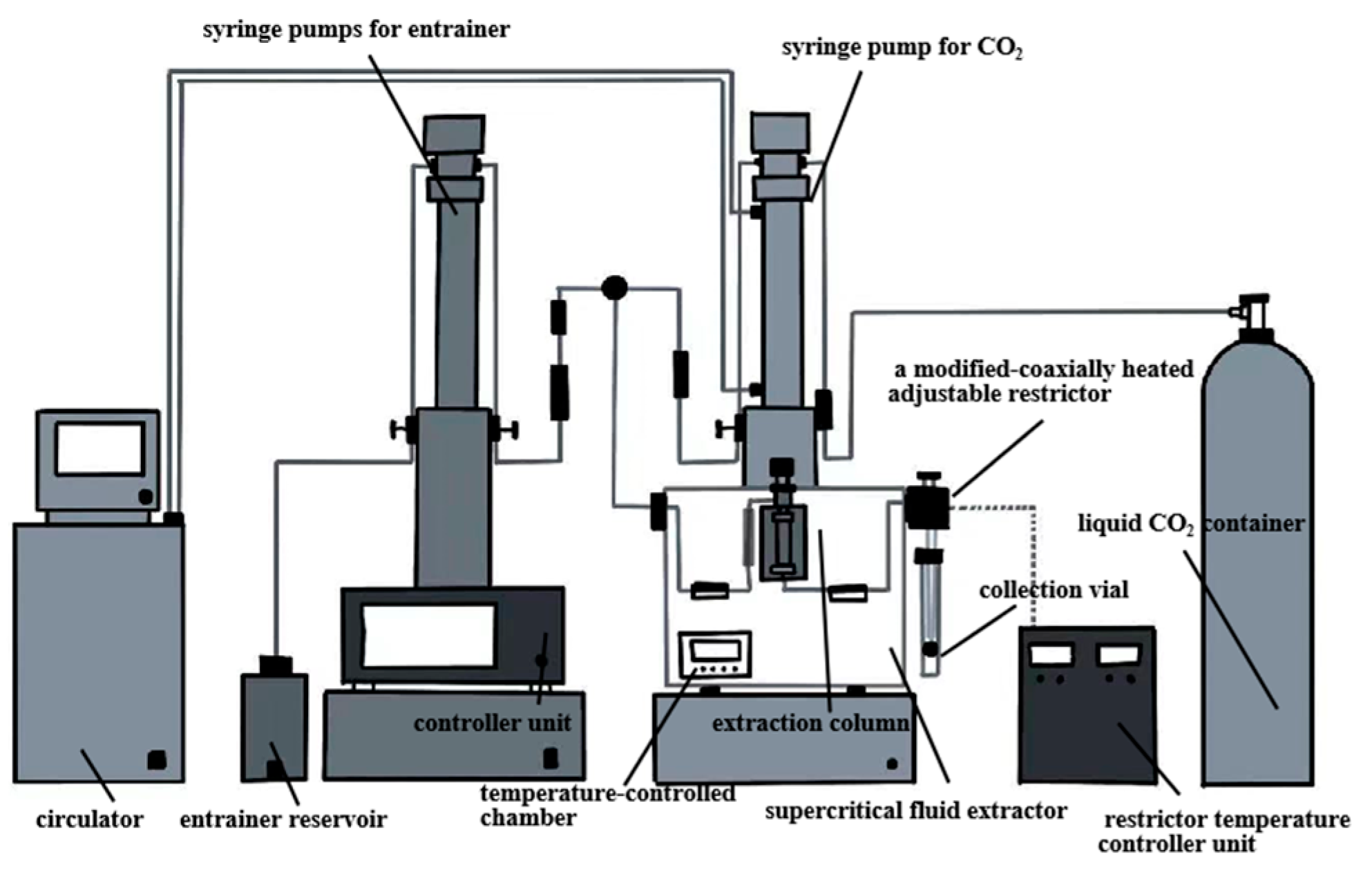
| Supercritical fluid extraction | Oxygen, high temperature. | 5760.83 PSI, 50 °C and 3.0 h. | 45.85 | More unsaturated fat, alpha-linolenic acid, total phenols and flavonoids. | [31,54] |
3.2.1. Solvent Extraction
3.2.2. Press Extraction
3.2.3. Ultrasound-Assisted Extraction
3.2.4. Microwave-Assisted Extraction
3.2.5. Supercritical Fluid Extraction
3.3. Storage Conditions for PNO
| Mechanism of Damage to PNO | The Solution | References | |
|---|---|---|---|
| Light | Photo-induced oxidation accelerates the formation of pungent odor in PNO. | Darkroom storage, light-proof packaging, and the use of materials that block specific wavelengths of light (UV barrier film). | [43,79,80] |
| Oxygen | A free radical chain reaction is initiated, accelerating PNO oxidation and leading to the formation of unpleasant odors. | High-barrier packaging materials, nitrogen replacement, and vacuum sealing. | [15,83,84] |
| Temperature | When the temperature exceeded 35 °C, the stability of FA in PNO declined significantly, and the oxidation rate nearly doubled with every 10 °C increase. | Precise temperature control equipment to reduce temperature fluctuations. | [33,77,78] |
3.4. Packaging Technology for PNO
4. Technological Approaches to Improve PNO Stability
4.1. Antioxidant
| Types | Advantages | Disadvantages | References | |
|---|---|---|---|---|
| Synthetic antioxidants | Low cost, high stability, high antioxidant efficiency. | Potential toxicity, cancer risk controversy, low consumer acceptance. | [18] | |
| Natural antioxidants | Polyphenols | High safety, antioxidant capacity, and provide additional health benefits. | The extraction process is costly, the concentration requires further optimization, and it may have antagonistic effect with other components. | [77] |
| Carotenoid | Natural source, with pigment function. | High photosensitivity may promote oxidation of tocopherols. | [20,103] | |
| Tocopherol | Fat-soluble antioxidant to protect the oil phase from oxidation with high safety. | Single applications have limited effect and may fail at high concentrations. | [99,103] | |
4.2. Pickering Emulsions
4.3. Microencapsulation
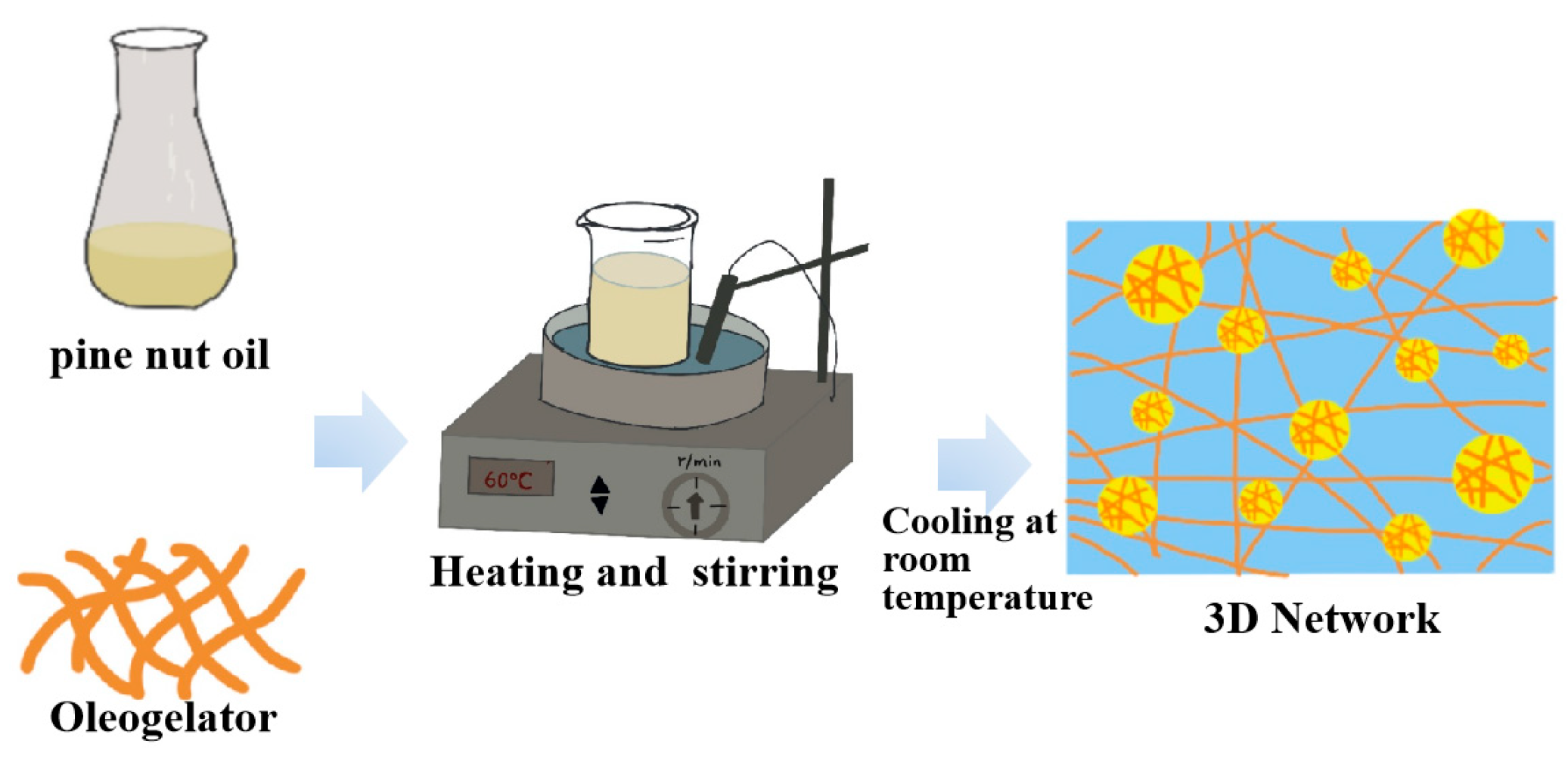
4.4. Oleogel
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNO | Pine nut oil |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasonic-assisted extraction |
| FA | Fatty acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| UFA | Unsaturated fatty acid |
| TV | Totox value |
| AV | Acid value |
| PV | Peroxide value |
| SE | Soxhlet extraction |
| BHT | Butylated hydroxytoluene |
| BHA | Butylated hydroxyanisole |
| SCF | Supercritical fluid |
| SFE | Supercritical fluid extraction |
| CA | Carnosic acid |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| GAE | Gallic acid equivalent |
References
- Godos, J.; Scazzina, F.; Castello, C.P.; Giampieri, F.; Quiles, J.L.; Urbano, M.B.; Battino, M.; Galvano, F.; Iacoviello, L.; de Gaetano, G.; et al. Underrated aspects of a true Mediterranean diet: Understanding traditional features for worldwide application of a “Planeterranean” diet. J. Transl. Med. 2024, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Li, P.W.; Ma, F.; Zhou, H.Y.; Jiang, J.; Özcan, M.M. Is the Profile of Fatty Acids, Tocopherols, and Amino Acids Suitable to Differentiate Pinus armandii Suspicious to Be Responsible for the Pine Nut Syndrome from Other Pinus Species? Chem. Biodivers. 2018, 15, e1700323. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Y.; Cao, A.L.; Aisikaer, G.; Ying, T.J. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur. J. Lipid Sci. Technol. 2013, 115, 556–563. [Google Scholar] [CrossRef]
- Lahlou, A.; Lyashenko, S.; Chileh-Chelh, T.; Belarbi, E.; Torres-García, I.; Alvarez-Corral, M.; Rodríguez-García, I.; Rincón-Cervera, M.A.; Guil-Guerrero, J.L. Fatty acid profiling in the genus Pinus in relation to its chemotaxonomy and nutritional or pharmaceutical properties. Phytochemistry 2023, 206, 113517. [Google Scholar] [CrossRef]
- An, J.Y.; Adelina, N.M.; Zhang, L.G.; Zhao, Y.H. Effect of roasting pre-treatment of two grafted pine nuts (Pinus koraiensis) on yield, color, chemical compositions, antioxidant activity, and oxidative stability of the oil. J. Food Process. Preserv. 2022, 46, e16145. [Google Scholar] [CrossRef]
- Lutz, M.; Alvarez, K.; Loewe, V. Chemical composition of pine nut (Pinus pinea L.) grown in three geographical macrozones in Chile. Cyta-J. Food 2017, 15, 284–290. [Google Scholar] [CrossRef]
- Kemerli-Kalbaran, T.; Ozdemir, M. Multi-response optimization of oil extraction from pine nut (Pinus pinea L.) by response surface methodology: Extraction efficiency, physicochemical properties and antioxidant activity. LWT-Food Sci. Technol. 2019, 103, 34–43. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Choy, E. The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders. Int. J. Mol. Sci. 2023, 24, 1171. [Google Scholar] [CrossRef]
- Wei, G.; Rong, K.; Yang, K.X.; Bao, Z.Y.; Zhang, X.T.; Zhang, Z.; Gong, Y.N.; Wang, J.F. Effects of active molecules of Korean pine seed on rodent health and implications for forest regeneration. J. For. Res. 2022, 33, 1045–1060. [Google Scholar] [CrossRef]
- Wang, J.R.; Wang, X.M.; Wang, W.Q.; Zhang, L.G.; Zhao, Y.H. Functionalization of pine kernel protein by pH-shifting combined with ultrasound treatments: Further improvement with increasing acidity. Int. J. Biol. Macromol. 2023, 248, 125884. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, K.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Edible oil to powder technologies: Concepts and advances. Food Biosci. 2023, 53, 102567. [Google Scholar] [CrossRef]
- Kouamé, K.; Bora, A.F.M.; Li, X.D.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Hoffmann, J.F.; Galli, V.; Zambiazi, R.C. Composition and impact of pre- and post-harvest treatments/factors in pecan nuts quality. Trends Food Sci. Technol. 2023, 131, 46–60. [Google Scholar] [CrossRef]
- Ogundipe, S.O.; Usack, J.G.; Pegg, R.B.; Suh, J.H. Thermal and Non-thermal Processing on the Physical and Chemical Properties of Tree Nuts: A Review. Food Bioprocess. Technol. 2024, 17, 1727–1751. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, A.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Odeh, D.; Kraljic, K.; Skukan, A.B.; Skevin, D. Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants 2021, 10, 785. [Google Scholar] [CrossRef]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef]
- Wang, L.; Lu, S.M.; Deng, Y.P.; Wu, W.W.; Wang, L.; Liu, Y.J.; Zu, Y.G.; Zhao, X.H. Pickering emulsions stabilized by luteolin micro-nano particles to improve the oxidative stability of pine nut oil. J. Sci. Food Agric. 2021, 101, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Z.Y.; Quan, W.; Xue, C.Y.; Qu, T.; Wang, T.; Chen, Q.M.; Wang, Z.J.; Zeng, M.M.; Qin, F.; et al. Pine pollen: A review of its chemical composition, health effects, processing, and food applications. Trends Food Sci. Technol. 2023, 138, 599–614. [Google Scholar] [CrossRef]
- Atmane, S.A.; Özbek, Z.A.; Ergönül, P.G.; Khettal, B. Valorization of Pinus halepensis Mill. seed oil: Physicochemical characteristics, bioactive compounds, and antioxidant activity as affected by location and extraction method. J. Food Process. Preserv. 2021, 45, e15548. [Google Scholar] [CrossRef]
- Kadri, N.; Khettal, B.; Aid, Y.; Kherfellah, S.; Sobhi, W.; Barragan-Montero, V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chem. 2015, 188, 184–192. [Google Scholar] [CrossRef]
- Nasri, N.; Khaldi, A.; Hammami, M.; Triki, S. Fatty acid composition of two Tunisian pine seed oils. Biotechnol. Prog. 2005, 21, 998–1001. [Google Scholar] [CrossRef]
- Evaristo, I.; Batista, D.; Correia, I.; Correia, P.; Costa, R. Chemical profiling of Portuguese Pinus pinea L. nuts. J. Sci. Food Agric. 2010, 90, 1041–1049. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Andrews, R.; Zhou, Y.; Farhat, M.; Elmajee, M.; Rundle, S.; Choy, E. Pinolenic acid exhibits anti-inflammatory and anti-atherogenic effects in peripheral blood-derived monocytes from patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 8807. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Andrews, R.; Zhou, Y.; Burston, J.; Choy, E. Anti-inflammatory and immunoregulatory effects of pinolenic acid in rheumatoid arthritis. Rheumatology 2022, 61, 992–1004. [Google Scholar] [CrossRef]
- Khammassi, M.; Amato, G.; Caputo, L.; Nazzaro, F.; Fratianni, F.; Kouki, H.; Amri, I.; Hamrouni, L.; De Feo, V. Fatty Acid Profiles and Biological Activities of the Vegetable Oils of Argania spinosa, Pinus halepensis and Pistacia atlantica Grown in Tunisia: A Preliminary Study. Molecules 2024, 29, 160. [Google Scholar] [CrossRef]
- Chung, M.Y.; Woo, H.; Kim, J.; Kong, D.; Choi, H.D.; Choi, I.W.; Kim, I.H.; Noh, S.K.; Kim, B.H. Pinolenic Acid in Structured Triacylglycerols Exhibits Superior Intestinal Lymphatic Absorption As Compared to Pinolenic Acid in Natural Pine Nut Oil. J. Agric. Food Chem. 2017, 65, 1543–1549. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhang, Y.; Wang, Z.Y.; Zu, Y.G. In vivo antioxidant activity of Pinus koraiensis nut oil obtained by optimised supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Huang, W.C.; Shen, H.J.; Chen, R.Y.; Chang, H.; Ho, Y.S.; Tsai, P.J.; Chuang, L.T. Investigation of Modulatory Effect of Pinolenic Acid (PNA) on Inflammatory Responses in Human THP-1 Macrophage-Like Cell and Mouse Models. Inflammation 2020, 43, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Ahad, T.; Gull, A. Effect of synthetic antioxidants, packaging materials and storage periods on quality characteristics of walnut kernels. Biomass Convers. Biorefinery 2023, 14, 28729–28739. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable oils oxidation: Mechanisms, consequences and protective strategies. Food Rev. Int. 2023, 39, 4180–4197. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.; Kang, M.C.; Chun, Y.G.; Kim, B.K.; Song, K.M. Intestinal epithelial barrier protective effect of nut oils (walnut, almond, pistachio, and pine nut) on TNF-α/IFN-γ induced damage in Caco-2 cell monolayers. J. Funct. Foods 2023, 111, 105887. [Google Scholar] [CrossRef]
- Nejatian, M.; Yazdi, A.P.G.; Fattahi, R.; Saberian, H.; Bazsefidpar, N.; Assadpour, E.; Jafari, S.M. Improving the storage and oxidative stability of essential fatty acids by different encapsulation methods; a review. Int. J. Biol. Macromol. 2024, 260, 129548. [Google Scholar] [CrossRef]
- Yildiz, A.Y.; Karaca, H. The protective role of shell, packaging technique and storage temperature in lipid oxidation in walnuts of different varieties. Postharvest Biol. Technol. 2024, 210, 112747. [Google Scholar] [CrossRef]
- Teh, S.S.; Lau, H.L.N. Phytonutrient content and oil quality of selected edible oils upon twelve months storage. J. Am. Oil Chem. Soc. 2023, 100, 651–661. [Google Scholar] [CrossRef]
- Shojaee, A.; Rastegar, S.; Tajeddin, B.; Sayyad-Amin, P. Quality Preservation of Walnut Kernels: Effect of Storage Temperature and Vacuum Packaging. Erwerbs-Obstbau 2023, 65, 2407–2418. [Google Scholar] [CrossRef]
- Chen, F.L.; Zhang, Q.; Gu, H.Y.; Yang, L. An approach for extraction of kernel oil from Pinus pumila using homogenate-circulating ultrasound in combination with an aqueous enzymatic process and evaluation of its antioxidant activity. J. Chromatogr. A 2016, 1471, 68–79. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Vali, M.; Haghighi-Zade, M.H.; Siahpoosh, A.; Malihi, R. The Effect of Chilgoza Pine Nut (Pinus gerardiana Wall.) on Blood Glucose and Oxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes.-Targets Ther. 2020, 13, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, H. Effect of different packaging materials and storage on lipid characteristics, oxidative stability and antioxidant properties of hazelnut. J. Food Meas. Charact. 2023, 18, 647–663. [Google Scholar] [CrossRef]
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT-Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.L.; Lu, X.Z.; Sun, H.; Wang, F.J. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res. Int. 2021, 150, 110791. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Cai, L.Y.; Cao, A.L.; Luo, Z.S.; Mao, L.C.; Ying, T.J. Ultrastructure characteristics and quality changes of low-moisture Chilgoza pine nut (Pinus gerardiana) during the near-freezing-temperature storage. Cyta-J. Food 2017, 15, 466–473. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.L.; Su, E.R.; Chen, Y.Z.; Cao, F.L. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Emadi, A.; Jayedi, A.; Mirmohammadkhani, M.; Abdolshahi, A. Aflatoxin reduction in nuts by roasting, irradiation and fumigation: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5056–5066. [Google Scholar] [CrossRef]
- Teneva, O.; Petkova, Z.; Toshev, N.; Solakov, N.; Loginovska, K.; Platov, Y. Effect of roasting on the chemical and lipid composition of pine nuts in two regions in Russia. Heliyon 2024, 10, e34576. [Google Scholar] [CrossRef]
- Sen, D.; Gokmen, V. Kinetic modeling of Maillard and caramelization reactions in sucrose-rich and low moisture foods applied for roasted nuts and seeds. Food Chem. 2022, 395, 133583. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.K.; Zheng, L.; Mao, J.H.; Zhao, C.W.; Huang, J.H.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. Effects of the variety and oil extraction method on the quality, fatty acid composition and antioxidant capacity of Torreya grandis kernel oils. LWT-Food Sci. Technol. 2018, 91, 398–405. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, K.; Liu, Y.T.; Liu, A.P.; Zhang, Q.; Han, G.Q.; Liu, S.L.; Yang, Y.; Zhu, Y.D.; Zhu, D.F. Optimization of microwave-assisted extraction of oil from tiger nut (Cyperus esculentus L.) and its quality evaluation. Ind. Crops Prod. 2018, 115, 290–297. [Google Scholar] [CrossRef]
- Hao, L.Y.; Lv, C.Y.; Cui, X.N.; Yi, F.P.; Su, C. Study on biological activity of perilla seed oil extracted by supercritical carbon dioxide. LWT-Food Sci. Technol. 2021, 146, 111457. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green. Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils-A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Satriana, S.; Supardan, M.D.; Arpi, N.; Mustapha, W.A.W. Development of Methods Used in the Extraction of Avocado Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800210. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.Y.; Zeng, Y.; Liu, G. Application and Perspectives of Supercritical Fluid Technology in the Nutraceutical Industry. Adv. Sustain. Syst. 2022, 6, 2200055. [Google Scholar] [CrossRef]
- Szydlowska-Czerniak, A.; Momot, M.; Stawicka, B.; Rabiej-Koziol, D. Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils. Antioxidants 2022, 11, 1556. [Google Scholar] [CrossRef]
- Sookwong, P.; Yuenyong, J.; Bennett, C. Bioactive Constituents in Cold-Pressed Plant Oils: Their Structure, Bioactivity and Chromatographic Analysis. J. Oleo Sci. 2024, 73, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Laskos, K.; Pisulewska, E.; Waligórski, P.; Janowiak, F.; Janeczko, A.; Sadura, I.; Polaszczyk, S.; Czyczylo-Mysza, I.M. Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils. Antioxidants 2021, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to increase the oxidative stability of cold pressed oils. LWT-Food Sci. Technol. 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Sicaire, A.G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochemistry 2016, 31, 319–329. [Google Scholar] [CrossRef]
- Shen, L.P.; Pang, S.X.; Zhong, M.M.; Sun, Y.F.; Qayum, A.; Liu, Y.X.; Rashid, A.; Xu, B.G.; Liang, Q.F.; Ma, H.L.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochemistry 2023, 101, 106646. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.J.; Zhao, S.N.; Yang, X.L.; Xu, W.D.; Guo, M.M.; Xu, E.B.; Ding, T.; Ye, X.Q.; Liu, D.H. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies-A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Rani, H.; Sharma, S.; Bala, M. Technologies for extraction of oil from oilseeds and other plant sources in retrospect and prospects: A review. J. Food Process Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Chen, F.C.; Xu, B.; Cui, W.Y.; Wang, Y.F.; Wan, F.C.; Cheng, A.W. Effects of microwave treatment on vegetable oil quality & biological activities. Trends Food Sci. Technol. 2024, 153, 104748. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Lopez-Cervantes, J.; Yousefabad, S.H.A.; Hosseini, S.F.; Karimi, M.; Motazedian, A.; Asadifard, S. Novel edible oil sources: Microwave heating and chemical properties. Food Res. Int. 2017, 92, 147–153. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trac-Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Salgin, U.; Salgin, S. Effect of main process parameters on extraction of pine kernel lipid using supercritical green solvents: Solubility models and lipid profiles. J. Supercrit. Fluids 2013, 73, 18–27. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Taheri, S.E.H.; Bazargan, M.; Vosough, P.R.; Sadeghian, A. A comprehensive insight into peanut: Chemical structure of compositions, oxidation process, and storage conditions. J. Food Compos. Anal. 2024, 125, 105770. [Google Scholar] [CrossRef]
- Zhuang, Y.; Dong, J.; He, X.M.; Wang, J.P.; Li, C.M.; Dong, L.; Zhang, Y.; Zhou, X.F.; Wang, H.X.; Yi, Y.; et al. Impact of Heating Temperature and Fatty Acid Type on the Formation of Lipid Oxidation Products During Thermal Processing. Front. Nutr. 2022, 9, 913297. [Google Scholar] [CrossRef]
- Breschi, C.; Guerrini, L.; Zanoni, B.; Masella, P.; Lunetti, L.; Parenti, A. Simulation of Transport under Different Temperature Conditions: Effects on Extra Virgin Olive Oil Quality. Eur. J. Lipid Sci. Technol. 2022, 124, 2100242. [Google Scholar] [CrossRef]
- Lazarou, K.; Tsagkaris, A.S.; Drakopoulou, S.; Kyriakopoulos, A.M.; Martakos, I.; Pentogenis, M.; Glyniadaki, M.; Kritikou, E.; Koupa, A.; Kostakis, M.; et al. Long-term stability of extra virgin olive oil: Effects of filtration and refrigeration storage on the Kolovi variety. J. Sci. Food Agric. 2024, 104, 9673–9683. [Google Scholar] [CrossRef]
- Ahmed, I.; Chatha, S.A.S.; Iftikhar, N.; Farooq, M.F.; Zulfiqar, H.; Ali, S.; Hussain, S.M.; Alshehri, M.A.; Al-Ghanim, K.A.; Hussain, A.I. Nutritional quality of selected commercially available seed oils and effect of storage conditions on their oxidative stability. PLoS ONE 2024, 19, e0308117. [Google Scholar] [CrossRef]
- Sattar, A.; Jan, M.; Ahmad, A.; Hussain, A.; Khan, I. Light-induced oxidation of nut oils. Nahrung 1989, 33, 213–215. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Nosrati, P. Scavenging effect of pasipay (Passiflora incarnate L.) on singlet oxygen generation and fatty acid photooxygenation. Food Sci. Nutr. 2018, 6, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Huvaere, K.; Skibsted, L.H. Flavonoids protecting food and beverages against light. J. Sci. Food Agric. 2015, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Manzini, R.; Accorsi, R.; Piana, F.; Regattieri, A. Accelerated life testing for packaging decisions in the edible oils distribution. Food Packag. Shelf Life 2017, 12, 114–127. [Google Scholar] [CrossRef]
- Thewes, F.R.; Both, V.; Thewes, F.R.; Brackmann, A.; Schultz, E.E.; Berghetti, M.R.P.; Soldateli, F.J.; Wendt, L.M.; Führ, A.; Wagner, R.; et al. Interaction of oxygen and moisture content on ‘Barton’ and ‘Jackson’ pecan storage. Postharvest Biol. Technol. 2021, 179, 111584. [Google Scholar] [CrossRef]
- Veneziani, G.; García-González, D.L.; Esposto, S.; Nucciarelli, D.; Taticchi, A.; Boudebouz, A.; Servili, M. Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils. Foods 2023, 12, 612. [Google Scholar] [CrossRef]
- Marcinkowski, D.; Bochniak, M.; Werenska, M.; Czwartkowski, K. The Influence of Storage Conditions of Cold-Pressed Rapeseed Oil on Its Quality Parameters. Appl. Sci. 2023, 13, 1746. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef]
- Huyan, Z.Y.; Ding, S.X.; Mao, X.H.; Wu, C.E.; Yu, X.Z. Effects of packaging materials on oxidative product formation in vegetable oils: Hydroperoxides and volatiles. Food Packag. Shelf Life 2019, 21, 100328. [Google Scholar] [CrossRef]
- Wang, H.; Zu, G.; Yang, L.; Zu, Y.G.; Wang, H.; Zhang, Z.H.; Zhang, Y.; Zhang, L.; Wang, H.Z. Effects of Heat and Ultraviolet Radiation on the Oxidative Stability of Pine Nut Oil Supplemented with Carnosic Acid. J. Agric. Food Chem. 2011, 59, 13018–13025. [Google Scholar] [CrossRef]
- Dhibi, M.; Issaoui, M.; Brahmi, F.; Mechri, B.; Mnari, A.; Cheraif, I.; Skhiri, F.; Gazzah, N.; Hammami, M. Nutritional quality of fresh and heated Aleppo pine (Pinus halepensis Mill.) seed oil: trans-fatty acid isomers profiles and antioxidant properties. J. Food Sci. Technol. 2014, 51, 1442–1452. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.H.; Chai, Y.Y. Preparation of microcapsule antioxidative wall materials of pine nut oil by the Maillard reaction. J. Sci. Food Agric. 2019, 99, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, G.Q.; Bogojevic, O.; Pedersen, J.N.; Guo, Z. Edible oleogels as solid fat alternatives: Composition and oleogelation mechanism implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2077–2104. [Google Scholar] [CrossRef] [PubMed]
- Puscas, A.; Muresan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef]
- Park, C.; Maleky, F. A Critical Review of the Last 10 Years of Oleogels in Food. Front. Sustain. Food Syst. 2020, 4, 139. [Google Scholar] [CrossRef]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sorensen, A.D.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and oxidative stability in emulsions. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1864–1901. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Mansour, H.M.M.; El-Sohaimy, S.A.; Zeitoun, A.M.; Abdo, E.M. Effect of Natural Antioxidants from Fruit Leaves on the Oxidative Stability of Soybean Oil during Accelerated Storage. Antioxidants 2022, 11, 1691. [Google Scholar] [CrossRef]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Pitino, R.; De Marchi, M.; Manuelian, C.L.; Johnson, M.; Simoni, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Yield, Quality, and Oxidative Status of Poultry Products: A Review of the Literature of the Last 20 Years. Antioxidants 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.A.; Spotti, M.L.; Vassallo, M.; Spotti, M.J.; Carrara, C.R.; Fioramonti, S.A. The prooxidant effect of natural antioxidants combination when co-encapsulated to chia oil-based nutraceutical edible powders: More is not always better. Eur. J. Lipid Sci. Technol. 2023, 126, 2300156. [Google Scholar] [CrossRef]
- Bordón, M.G.; Bodoira, R.M.; González, A.; Piloni, R.; Ribotta, P.D.; Martínez, M.L. Spray-Drying, Oil Blending, and the Addition of Antioxidants Enhance the Storage Stability at Room Temperature of Omega-3-Rich Microcapsules Based on Chia Oil. Eur. J. Lipid Sci. Technol. 2022, 124, 2100181. [Google Scholar] [CrossRef]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering emulsions: Interfacial tension, colloidal layer morphology, and trapped-particle motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Schröder, A.; Sprakel, J.; Boerkamp, W.; Schroën, K.; Berton-Carabin, C.C. Can we prevent lipid oxidation in emulsions by using fat-based Pickering particles? Food Res. Int. 2019, 120, 352–363. [Google Scholar] [CrossRef]
- Abdullah; Weiss, J.; Ahmad, T.; Zhang, C.; Zhang, H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Technol. 2020, 106, 91–103. [Google Scholar] [CrossRef]
- da Silva, L.C.; Castelo, R.M.; Cheng, H.N.; Biswas, A.; Furtado, R.F.; Alves, C.R. Methods of Microencapsulation of Vegetable Oil: Principles, Stability and Applications-A Minireview. Food Technol. Biotechnol. 2022, 60, 308–320. [Google Scholar] [CrossRef]
- de Oliveira, T.S.; Freitas-Silva, O.; Kluczkovski, A.M.; Campelo, P.H. Potential use of vegetable proteins to reduce Brazil nut oil oxidation in microparticle systems. Food Res. Int. 2020, 137, 109526. [Google Scholar] [CrossRef]
- Rossi, Y.E.; Vanden Braber, N.L.; Vergara, L.I.D.; Montenegro, M.A. Bioactive Ingredients Obtained from Agro-industrial Byproducts: Recent Advances and Innovation in Micro- and Nanoencapsulation. J. Agric. Food Chem. 2021, 69, 15066–15075. [Google Scholar] [CrossRef]
- Huang, J.; Feng, X.; Zhang, S.; Wang, L.Z.; Yue, J.J.; Chu, L.L. Preparation and characterization of astaxanthin-loaded microcapsules and its application in effervescent tablets. J. Sci. Food Agric. 2023, 103, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen, I.; Mert, B. Recent developments of oleogel utilizations in bakery products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2460–2479. [Google Scholar] [CrossRef] [PubMed]
- Ursachi, C.S.; Perta-Crisan, S.; Tolan, I.; Chambre, D.R.; Chereji, B.D.; Condrat, D.; Munteanu, F.D. Development and Characterization of Ethylcellulose Oleogels Based on Pumpkin Seed Oil and Rapeseed Oil. Gels 2024, 10, 384. [Google Scholar] [CrossRef]
- Shuai, X.X.; McClements, D.J.; Dai, T.T.; Geng, Q.; Wei, C.B.; Wang, W.L.; Chen, J.; Zhang, M.; Du, L.Q. Effect of different oleogelators on physicochemical properties, oxidative stability and astaxanthin delivery of macadamia oil-based oleogels. Food Res. Int. 2024, 196, 115131. [Google Scholar] [CrossRef]
- Pehlivanoglu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Sabet, S.; Pinto, T.C.; Kirjoranta, S.J.; Garcia, A.K.; Valoppi, F. Clustering of oleogel production methods reveals pitfalls and advantages for sustainable, upscalable, and oxidative stable oleogels. J. Food Eng. 2023, 357, 111659. [Google Scholar] [CrossRef]
- Malvano, F.; Laudisio, M.; Albanese, D.; D’Amore, M.; Marra, F. Olive Oil-Based Oleogel as Fat Replacer in a Sponge Cake: A Comparative Study and Optimization. Foods 2022, 11, 2643. [Google Scholar] [CrossRef]
- Feng, Z.L.; He, D.X.; Zhang, L.T.; Li, Q.; Xue, C.F.; Yi, X.Z.; Liao, L.; Pei, Z.S.; Shen, X.R. Preparation of myofibrillar protein oleogels by emulsion template method: Application of fat substitute for sponge cakes. LWT-Food Sci. Technol. 2025, 216, 117350. [Google Scholar] [CrossRef]
- Chen, J.Y.; Shi, W.J.; Liu, Y.Z.; Wang, Z.Y.; Wang, J.L.; Yang, Y.; Lu, S.L.; Dong, J.; Wang, J.Y.; Wang, Q.L. Effectiveness of wax-bovine bone protein-grapeseed oil composite oleogels as a margarine substitute in cookies: Characteristics of fat substitutes and baking properties. Int. J. Biol. Macromol. 2025, 306, 141649. [Google Scholar] [CrossRef]
| Methodologies | Mechanism of Action | Advantages | Disadvantages | Application in PNO | References |
|---|---|---|---|---|---|
| Antioxidant | Scavenging free radical to inhibit oxidation reaction | Reduce the rate of oxidation and provide health benefits. | The use of antioxidants in combination with PNO may pose a risk of reduced oxidative stability. | PNO supplemented with 0.2 mg/g carnosic acid showed a favorable oxidative effect. | [89,90] |
| Pickering emulsions | Solid particles form a mechanical barrier at the oil–water interface, which physically isolates the oil and thereby slows its oxidation | Increased interfacial protein content, enhanced rheological properties, and improved protection during digestion. | With potential toxicity and allergy, small-molecule prooxidants can still penetrate the granular layer. | Luteolin micro/nanoparticles can serve as stabilizers. They not only maintain the structural integrity of emulsion droplets but also enhance the oxidative stability of PNO emulsions. | [21] |
| Microencapsulation | Forms a protective layer to store biologically active substances | Converts liquids to solids, enriches food range improves oxidative stability and shelf life. | Toxicity and biological activity of micro- and nanoparticles. | PNO microcapsules were prepared using gelatin– gum arabic–maltodextrin (2:2:1, w/w) as the Maillard reaction-based wall material. | [74,91] |
| Oleogel | Physical isolation reduces the amount of oil molecules in the environment | Low-saturated, trans-fat-free health product, efficient carrier of bioactives. | Processing technology requirements are high; there are food compatibility problems. | \ | [92,93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, Z.; Wang, Y.; Mu, Z.; Lv, X.; Wang, Z.; Dong, A.; Fan, Z.; Zhang, H. A Review on Improving the Oxidative Stability of Pine Nut Oil in Extraction, Storage, and Encapsulation. Antioxidants 2025, 14, 716. https://doi.org/10.3390/antiox14060716
Zhu J, Li Z, Wang Y, Mu Z, Lv X, Wang Z, Dong A, Fan Z, Zhang H. A Review on Improving the Oxidative Stability of Pine Nut Oil in Extraction, Storage, and Encapsulation. Antioxidants. 2025; 14(6):716. https://doi.org/10.3390/antiox14060716
Chicago/Turabian StyleZhu, Jingwen, Zhenzhou Li, Yisen Wang, Zhexuan Mu, Xiaohong Lv, Zhenyu Wang, Aijun Dong, Ziluan Fan, and Hua Zhang. 2025. "A Review on Improving the Oxidative Stability of Pine Nut Oil in Extraction, Storage, and Encapsulation" Antioxidants 14, no. 6: 716. https://doi.org/10.3390/antiox14060716
APA StyleZhu, J., Li, Z., Wang, Y., Mu, Z., Lv, X., Wang, Z., Dong, A., Fan, Z., & Zhang, H. (2025). A Review on Improving the Oxidative Stability of Pine Nut Oil in Extraction, Storage, and Encapsulation. Antioxidants, 14(6), 716. https://doi.org/10.3390/antiox14060716






