1. Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most important causes of morbidity, mortality, and healthcare use, affecting 400 million people worldwide [
1]. In addition, it is the third leading cause of death globally, being responsible for over 3 million deaths annually [
2]. This pulmonary disease develops slowly over many years and noticeable symptoms, such as chronic bronchitis, emphysema, and small airway obstruction, appear several years later. However, the most severe comorbidity of this illness is the development of pulmonary hypertension (PH), due in part to local hypoxia after the emphysema develops [
3]. In this context, cigarette smoke (CS) is the main risk factor that contributes to the development and progression of COPD. Nevertheless, environmental factors, such as the rising prevalence of fine particles, dust, or fumes can also play an important role, especially in non-smokers [
4].
Bronchodilators and glucocorticoids are the most widely used treatments with therapeutic effects for COPD [
5]. However, despite its profound public health impact, curative therapies for COPD or COPD-related pulmonary hypertension are not yet available. Therefore, understanding the molecular mechanisms involved in these pathologies is to develop new therapeutic approaches. In this respect, recent studies in our laboratory have shown that cigarette smoke extract (CSE) has direct effects on the pulmonary vasculature, independently of the hypoxic situation, that lead to a vascular remodeling responsible for the development of pulmonary hypertension [
6,
7]. On the other hand, studies on the airways demonstrate that CSE promotes airway hyper-responsiveness, an effect that gradually drives into an unusual narrowing of the airways due to smooth muscle contraction [
8]. In this context, bronchial smooth muscle cells play a central role in regulating airway function; therefore, it is essential to unveil the mechanisms whereby CSE treatment impairs their performance.
The accumulation of reactive oxygen species (ROS) has been closely associated with COPD pathogenesis, as evidenced by increased oxidative stress markers in exhaled breath condensates and lung tissues of COPD patients compared to control subjects [
9]. This phenomenon may be caused not only by oxidizing substances such as tobacco, but also by an excessive production of these species or a decrease in the antioxidant defenses of the cells. In this respect, other authors have identified lower levels of glutathione (GSH) in bronchoalveolar lavages (BALs) from COPD patients [
10], and our group has shown that treatment with CSE promotes not only increased oxidative stress, but also a dysregulated antioxidant response in pulmonary arterial smooth muscle cells [
7]. Indeed, the excessive ROS production triggers a redox imbalance that contributes to organelle damage and disrupted homeostasis [
11]. In particular, mitochondria are considered one of the main sources of ROS in the cell [
12], and mitochondrial ROS (mtROS) are routinely produced at several sites within the organelle. However, when a sudden or sustained increase in mtROS exceeds the natural antioxidant defense mechanisms in the cell, the resulting oxidative stress impairs cellular function and viability, ultimately contributing to the onset and progression of COPD [
13]. Therefore, elucidating the role of CSE-induced oxidative stress in airway contractile impairment is of particular interest in the context of COPD. To that end, we will first investigate a set of key proteins involved in the contractile machinery of bronchial smooth muscle cells, which may be susceptible to CSE-induced alterations.
Filamentous actin (F-actin) is one of the most representative proteins of the cell cytoskeleton. Specifically, it plays an important role in focal adhesions, alongside other proteins such as vinculin [
14]. Furthermore, actin is organized within the cytoplasm of smooth muscle cells where it interacts with myosin to form the core components of the contractile machinery. Both are involved in force generation in a cyclical mechanism that requires myosin light chain (MLC) phosphorylation. However, the contraction of smooth muscle involves many different upstream signals before the latter event occurs. For instance, calmodulin, in the presence of calcium, is known to activate myosin light chain kinase (MLCK), resulting in MLC phosphorylation and contraction [
15]. Furthermore, other upstream kinases, such as protein kinase C (PKC), can also contribute to MLCK activation through other signaling pathways [
16]. Additionally, the microtubule network also plays a critical role in intracellular dynamics; however, its regulation in smooth muscle cells, particularly in the context of respiratory pathologies such as COPD, remains poorly understood.
In addition to the above-mentioned alterations, other species such as hydroxyl radical and hydroperoxyl [
17] can trigger the peroxidation of polyunsaturated fatty acids in biological tissues. Indeed, these products have been shown to display higher levels in breath condensate and in the lungs of stable COPD patients. Moreover, they also correlate negatively with the lung function marker forced expiratory volume in one second (FEV1), suggesting that lipid peroxidation plays an important role in the decline of lung function [
18]. Furthermore, it has been shown that this phenomenon also regulates podocyte migration and cytoskeleton structure [
19]. Therefore, it is plausible that this phenomenon might also occur within the airway smooth muscle and contribute to the CSE-negative effects in this compartment, such as the dysregulated contractile response.
This study aims to delve deeper into the molecular mechanisms underlying the development of airway hyper-responsiveness induced by cigarette smoke. Our findings demonstrate that cigarette smoke exposure promotes oxidative stress, increases intracellular calcium levels, and disrupts the contractile machinery, collectively contributing to functional impairment of the bronchial smooth muscle layer. Furthermore, we show that several pharmacological agents, particularly antioxidants, effectively mitigate these CSE-induced alterations in the airways.
2. Materials and Methods
Cell culture. Primary bronchial smooth muscle cells (hBSMCs) were obtained from ScienCell Research Laboratories (#3400), and cultured following the manufacturer’s recommendations in smooth muscle cell medium (ScienCell, #1101, Carlsbad, CA, USA) with 2% fetal bovine serum (ScienCell, #0010), 1× smooth muscle cell growth supplement (ScienCell, #1152), and penicillin/streptomycin solution (ScienCell, #0503). Cells were grown to 90% maximum confluence, maintained in incubators at 37 °C and 5% CO2 in a humidified atmosphere, and used for a maximum of eight passages. In the indicated experiments, the cells were pretreated with 25 nM of the mitochondrial antioxidant mitoTEMPO (Sigma, SML0737, St. Louis, CA, USA) for 24 h or with 500 μM N-acetylcysteine (NAC) (Sigma, A7250) for 2 h before being co-treated with 15% CSE. The rest of the treatments were as follows: before CSE treatment, cells were pretreated with 50 μM 2-Aminoethoxydiphenyl borate (2-APB) (Merck Millipore, 100065, Darmstadt, Germany) for 2 h or 5 μM cytochalasin B (Merk, C6762) for 2 h. Afterwards, the cell medium was washed out and replaced with fresh medium containing 15% CSE. When treated with CSE, cells were maintained in a different incubator to avoid the exposure of non-treated cells to volatile substances from the CSE.
Cigarette smoke extract preparation. CSE was prepared from commercial Kentucky 3R4F cigarettes (University of Kentucky Lexington, KY, 9.4 mg tar and 0.73 mg nicotine per cigarette). The smoke from the complete burning of five cigarettes was bubbled into 150 mL of phosphate-buffered saline using the VACUSAFE aspiration system (INTEGRA Biosciences AG, Zizers, Switzerland). The CSE solution was then filtered, aliquoted, and kept frozen at −80 °C until use. To ensure a similar preparation amongst different batches, the CSE concentration of the initial solution was measured spectrophotometrically at a 320 nm wavelength. This solution was considered to be 100% CSE, being then diluted to obtain the desired concentration for each experiment.
Western blot analysis. Cells were grown to 90% confluence on culture plates in the presence or absence of CSE for different times and lysates were prepared in non-reducing 2X Laemmli buffer, boiled at 95 °C for 10 min in the presence of 20 mM Dithiothreitol (DTT), electrophoretically separated by either an 8% (high MW proteins) or a 15% (low MW proteins) SDS-PAGE gel and transferred onto 0.45 μm nitrocellulose membranes (GE Healthcare Life Sciences, 10600003, Marlborough, MA, USA). Total protein bands were reversibly stained with Fast Green FCF (Sigma, F7252) and imaged on Amersham ImageQuant 800 (GE Healthcare Life Sciences) for total protein quantification. Transferred proteins were incubated overnight at 4 °C with specific primary antibodies (
Table 1). Horseradish peroxidase conjugated secondary antibodies (
Table 2) were added for 1 h at room temperature and protein signal was then visualized using Immobilon Forte (MerkMillipore, WBLUF0500,) on an Amersham ImageQuant 800. The intensity of specific protein bands was quantified by densitometry using ImageJ 1.51 software (U.S. National Institutes of Health, Bethesda, Maryland, USA) and normalized to the intensity of Fast Green FCF staining from each gel line.
Cell hypertrophy assessment. Cells were grown in 35 mm plates in the presence of CSE for 48 h. Afterwards, they were detached with 0.05% trypsin–EDTA (Gibco, 25300-054, NY, USA) and analyzed by flow cytometry. Median forward scattering of light from Ar 488 nm laser was quantified on a FACS Canto™ II cytometer (BD Biosciences, NJ, USA), excluding cell debris and doublets from the analysis.
Proliferation assay. Cells were stained with 5 μM fluorescent probe carboxyfluorescein succinimidyl ester (CFSE, CellTraceTM C34554) from Thermo Fisher Scientific (MA, USA) in PBS for 20 min at 37 °C. Afterwards, complete medium was added for another 5 min to stop the staining process and the cells were allowed to attach on 24-well plates before CSE challenge. The cells were detached with 0.05% trypsin–EDTA (Gibco, 25300-054), and median CFSE fluorescence intensity was measured on a FACS CantoTM II cytometer (BD Biosciences), illuminating with an Ar 488 nm laser, right before CSE exposure (day 0 condition) or 72 h later. The results were expressed as the lost CFSE fluorescence with respect to day 0 condition and normalized to CSE-untreated cells’ fluorescence levels.
Cell senescence assay. Cell senescence was measured with the Invitrogen™ CellEvent™ Senescence Green Flow Cytometry Assay Kit (C10840, Thermo Fisher Sci. MA, USA). Cells were treated with or without 15% CSE for 72 h. After this time, the cells were trypsinized and washed twice with 1× PBS. Then, the cells were fixed in 4% paraformaldehyde for 10 min at room temperature, and stained with the CellEvent™ Senescence Green Probe diluted 1/1000 in CellEvent™ Senescence Buffer for 2 h at 37 °C. the cells were washed in 1× PBS with 1% BSA, resuspended in 1× PBS, and analyzed by flow cytometry.
Quantification of calcium, cytosolic, and mitochondrial superoxide levels. hBSMCs were grown in 35 mm plates and exposed to CSE and/or different drugs. After that, cytosolic and mitochondrial superoxide levels, as well as intracellular calcium levels, were analyzed with fluorescent probes with 10 μM dihydroethidium (DHE) (Invitrogen, D11347), 5 μM MitoSOXTM Red (Thermo Fisher Scientific, M36008), and 1 μM Fluo-4 AM (ThermoFisher Scientific, F14201), respectively. Afterwards, the cells were trypsinized and quantified by flow cytometry. Events were collected on a FACS Canto II flow cytometer. To ensure accuracy and consistency in the flow-cytometry-based quantification of these parameters, the cells were initially selected based on their forward scatter (FSC) and side scatter (SSC) parameters to exclude debris and non-cellular events. Subsequently, doublets were excluded using FSC-H vs. FSC-A analysis, ensuring that only single cells were analyzed. Finally, viable cells were identified by excluding cell labelled positive with ghost dye (Cytekbio, 13-0865-T100, CA, USA), thereby allowing the analysis to focus exclusively on live, single hBSMCs. All collected data were analyzed by GraphPad Prism 9.5.1.
Quantification of lipid peroxidation. hBSMCs were grown on 35 mm plates and exposed to CSE and/or different drugs. Afterwards, C11-BODIPY 581/591 staining was conducted. Briefly, a 1.5 mM solution of C11-BODIPY was prepared in DMSO and then diluted in Opti-MEM to achieve a final concentration of 1 μM. The cells were then incubated in the working solution for 30 min at 37 °C, trypsinized, and quantified by flow cytometry. The degree of lipid peroxidation was quantified by calculating the fluorescence intensity ratio between 510 nm (the emission peak of its oxidized form) and 590 nm (the emission peak of its reduced form). Events were collected on a FACS Canto II flow cytometer. To ensure accuracy and consistency in the flow cytometry-based quantification of these parameters, the cells were initially selected based on their forward scatter (FSC) and side scatter (SSC) parameters to exclude debris and non-cellular events. Subsequently, doublets were excluded using FSC-H vs. FSC-A analysis, ensuring that only single cells were analyzed. Finally, viable cells were identified by excluding ghost dye (Cytekbio, 13-0865-T100)-positive events, thereby allowing the analysis to focus exclusively on live, single hBSMCs. All collected data were analyzed by GraphPad Prism 9.5.1.
Immunofluorescence. hBSMCs were seeded onto fibronectin (5 μg/mL)-coated 13 mm glass coverslips and then incubated under the desired experimental conditions. The cells were then fixed with 4% paraformaldehyde in PBS for 20 min at 4 °C and permeabilized with 0.3% saponin in 0.2% BSA/PBS. Next, they were labeled with fluorescent primary antibodies (anti-P-MLC and anti-vinculin) followed by Alexa Fluor 488- and 568-labeled secondary antibodies, respectively, and phalloidin-conjugated Alexa 647. A DAPI probe was used to stain the cell nuclei. Cells mounted in coverslips with Faramount Aqueous Medium (Agilent, S3025, CA, USA) were imaged using the THUNDER Imager 3D fluorescence microscope (Leica Microsystems, Wetzlar, GE) and processed with LASX software version 5.1.0. Ten randomly selected images were collected for each sample.
Cytoskeleton recovery assay. The actin cytoskeleton was destabilized by incubation for 2 h with 5 μM cytochalasin B (cytB) (Sigma-Aldrich, C6762-1MG, St. Louis, CA, USA) followed by a 1 h recovery period, during which cells were cultured with or without 15% CSE exposure. NAC pretreatment was carried out for 2 h before adding cytB. Then, NAC was withdrawn during destabilization with cytB and reintroduced during the recovery period. Finally, the cells were labeled with Phalloidin Alexa FluorTM 568 (Invitrogen, A12380) at 1:200, trypsinized, and analyzed by flow cytometry. To calculate the percentage of repolymerized F-actin, the MFI of cells with cytoskeletons destabilized by cytB was normalized to the MFI of cells subjected to the same conditions without cytB.
Bronchial reactivity analysis. Bronchial segments from 8-week-old C57BL/6J female mice were carefully dissected free of surrounding tissue and cut into rings (1.8–2 mm length). The bronchial rings were then incubated for 24 h in DMEM (Sigma-Aldrich, D5796) medium containing a vehicle (Control) and CSE with or without mitoTEMPO (25 nM). Thereafter, the rings were mounted in a wire myograph (610 M, Danish Myo Technology, Hinnerup, Denmark) with Krebs physiological solution, maintained at 37 °C, and bubbled with a mixture of 21% O2 and 5% CO2. Contractility was recorded by an isometric force transducer and a displacement device coupled with a digitalization and data acquisition system (PowerLab, ADInstruments, Dunedin, NZ). The rings were stretched to give an equivalent transmural pressure of 20 mmHg. After equilibration, bronchial rings were first stimulated with KCl (80 mM). Then, the preparations were washed three times and allowed to recover before a new stimulation. After that, concentration–response curves for the response to serotonin (5-HT, 30 nM–10 µM) or acetylcholine (ACh 1 nM–10 µM) were created.
Statistics. Data are presented as the mean ± SEM. Two-sided unpaired Student’s t-tests were used to compare two experimental groups, and one-way or two-way ANOVAs followed by Sidak’s post hoc test were used when comparing three or more groups in accordance with the conditions of normality and homoscedasticity. In case the assumptions of normality and homoscedasticity were not accomplished, the Wilcoxon test was used to compare two groups and the non-parametrical Kruskal–Wallis test was used to compare three or more groups, followed by a Wilcoxon post hoc test to compare between groups. No statistically significant differences were considered to be present when p values were larger than 0.05. Statistical and graphical analyses were performed with GraphPad Prism 9.5.1 software (San Diego, CA, USA).
4. Discussion
COPD is a complex respiratory pathology characterized by chronic bronchitis, emphysema, small airway obstruction, and persistent respiratory symptoms. It is well known that cigarette smoke, which is the main risk factor of this illness, is key to promoting airway hyper-responsiveness. However, the mechanisms involved in CSE-mediated effects on airway smooth muscle are poorly understood. Here, we have delved into the molecular mechanisms responsible for the generation of ROS and the deregulation of calcium levels in bronchial smooth muscle cells following exposure to tobacco smoke, and how these pathways contribute to the smoke-induced dysregulation in airway hyperreactivity.
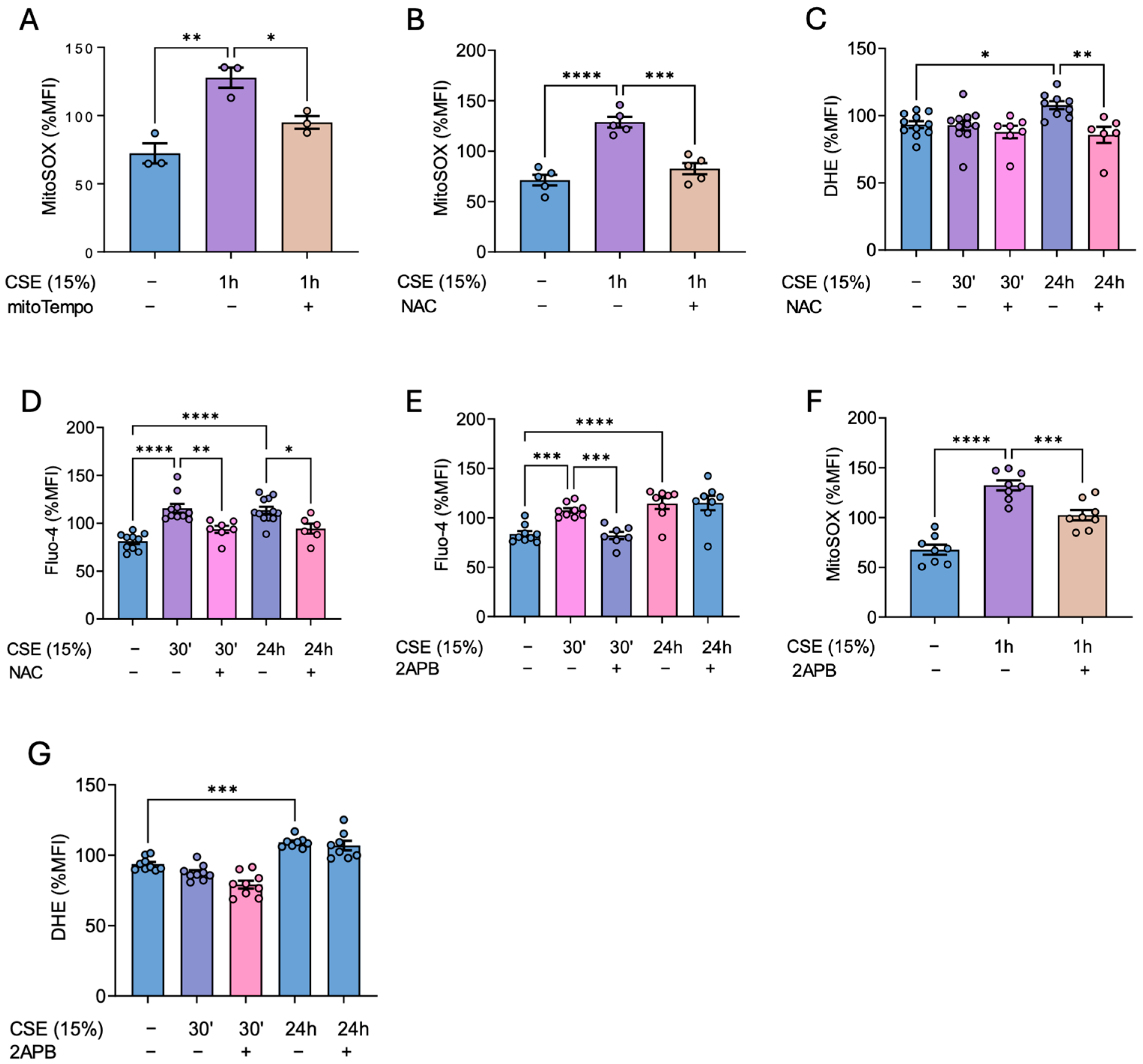
We documented two different peaks of calcium occurring at 30′ and 24 h after CSE exposure. This suggests that, despite calcium signaling occurring rapidly after the initial stimuli, this signal could be extended for longer periods of time due factors such as the continuous presence of CSE in the cell culture or the activation of “long-lasting” calcium channels. This could explain the increase in calcium observed after 24 h of CSE exposure. Furthermore, this second wave of calcium may be amplified by the increase in ROS levels at later times. This is supported by previous results reporting a feedback regulatory mechanism between calcium and ROS [
21]. In parallel, antioxidant enzymes such as SOD1, SOD2, SOD3, and catalase were decreased upon CSE treatment, indicating that the rise in oxidative stress is not only due to the direct effects of tobacco smoke, but also to impaired antioxidant defenses in bronchial smooth muscle cells. These findings are in line with previous results from our group regarding pulmonary artery smooth muscle cells [
7]. Notably, N-acetylcysteine not only prevented the increase in ROS observed 24 h post CSE exposure, but also blocked the calcium peaks to both 30 min and 24 h after CSE. This result supports the existence of a feedback loop between calcium and ROS and suggests that the early calcium influx in the cells may contribute to the late ROS surge. Additionally, CSE treatment increased mitochondrial superoxide levels, which was also prevented by NAC, further highlighting the effectiveness of this antioxidant in our cells. On the other hand, the reduction in antioxidant defenses and the resulting accumulation of hydrogen peroxide (H₂O₂), due to elevated cytosolic superoxide, likely accounts for the observed increase in lipid peroxidation after 24 h of CSE exposure. Consistent with these findings, NAC effectively prevented this rise in lipid peroxidation, including the peak levels detected at 48 h.
IP
3 receptors, which mediate calcium release from intracellular stores, are mainly located in the endoplasmic reticulum [
22]. In this regard, 2-APB, an antagonist of these receptors, was shown to block the early CSE-driven increase in intracellular calcium as well as the subsequent increase in mitochondrial superoxide. Thus, these results demonstrate that, in response to CSE, an initial cytosolic calcium surge originating from the ER triggers a subsequent overproduction of mitochondrial ROS. This phenomenon may be attributed to mitochondrial calcium uptake through calcium-permeable channels within this organelle, such as voltage-dependent anion channels (VDACs) located in the mitochondrial outer membrane [
23]. This situation will, in turn, lead to a mitochondrial calcium overload responsible for the generation of mitochondrial oxidative stress [
24].
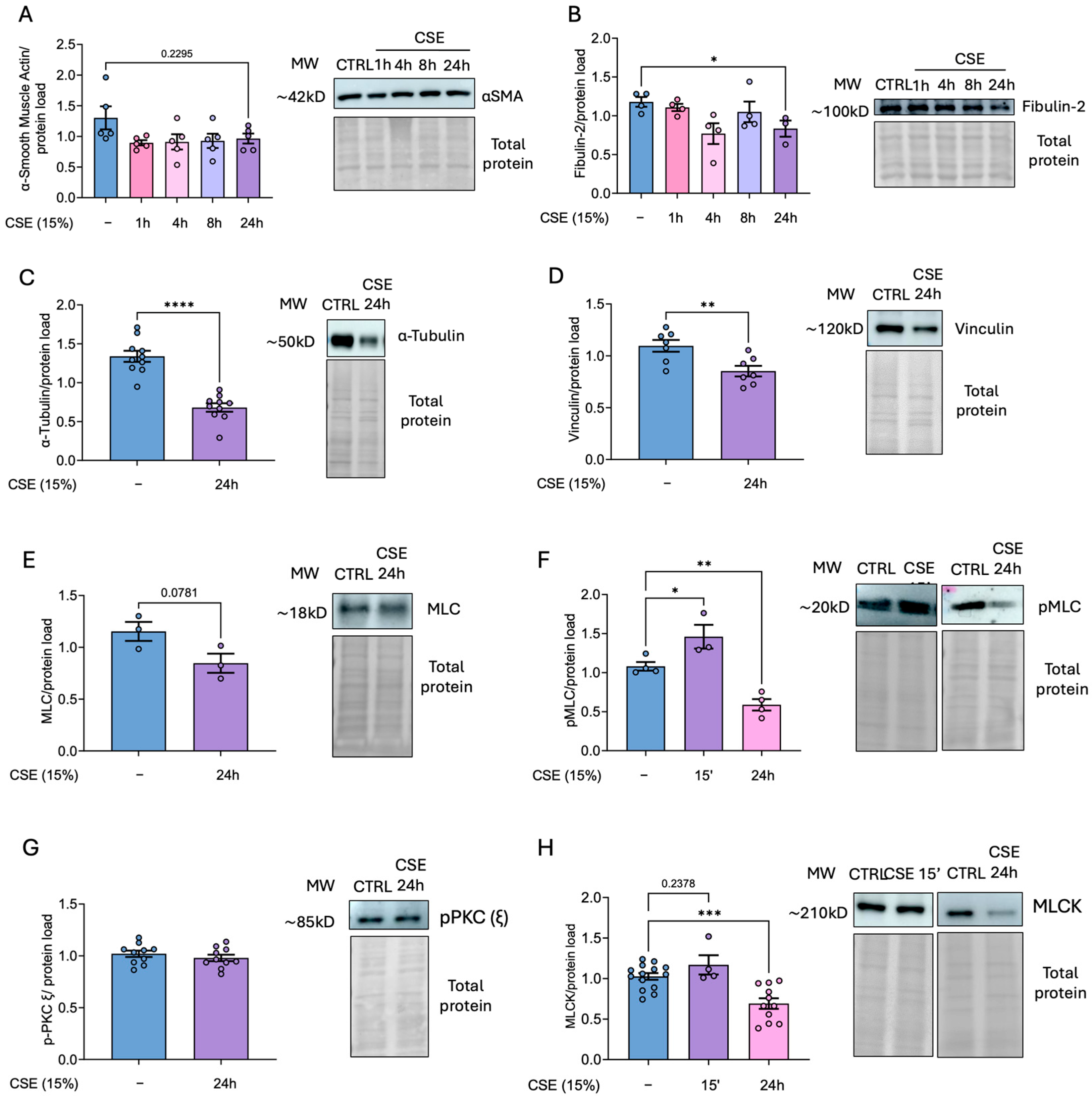
CSE-mediated oxidative stress and calcium alterations in hBSMCs may be potentially linked to the impaired cytoskeleton function and organization observed in our cells, as some of these effects were prevented with antioxidants. In this context, previous studies have shown that nicotine [
25], tobacco smoke exposure [
26], and particularly elevated ROS levels [
27,
28] significantly impact cytoskeletal architecture, focal adhesion dynamics, and the organization of actin and tubulin networks. We also detected altered actin dynamics and the decreased phosphorylation of myosin light chain (MLC), both critical markers of impaired contractile function. These alterations appear to be closely linked to CSE-induced oxidative stress, as they were mitigated by treatment with the antioxidant N-acetylcysteine (NAC). Supporting this, previous research suggests that MLCK activity is modulated by the cellular redox state, with elevated oxidative conditions potentially inhibiting its function, thus reducing MLC phosphorylation [
28]. This mechanism may underlie the diminished reactivity of CSE-challenged bronchi observed in our study.
However, these findings are somewhat unexpected in this cellular compartment, as previous studies have reported that CSE exposure leads to increased airway hyperreactivity, primarily driven by enhanced contraction of airway smooth muscle cells [
8]. This could be explained by the fact that phosphorylation is a rapid post-translational modification that occurs within minutes in response to specific stimuli. In fact, the analysis of MLC phosphorylation shortly after CSE exposure demonstrated an early increase in pMLC levels, accompanied by a slight but concurrent rise in MLCK, the kinase responsible for MLC phosphorylation. These findings, along with the quick increase in cytosolic calcium, suggest that hBSMCs exhibit an enhanced but short-lived contractile response immediately following CSE exposure. Previous findings from the literature indicate that while chronic exposure to cigarette smoke generally enhances airway contractility, short-term or acute exposure can transiently reduce airway tone, particularly through nicotine-mediated pathways [
29,
30,
31]. A plausible explanation for the reduced pMLC levels observed after 24 h is that CSE may impair global protein synthesis in hBSMCs, which could also account for the decreased levels of other cytoskeleton proteins. In addition, oxidative stress may contribute to protein degradation via oxidative modifications [
32]. Notably, treatment with the antioxidant NAC prevented the CSE-mediated loss of cytoskeleton recovery ability and preserved the organization and/or the levels of key cytoskeletal proteins including vinculin, pMLC, and F-actin. These findings indicate that the dysregulation of ROS and calcium homeostasis underlies the observed alterations in cytoskeletal integrity and contractile machinery. Additionally, increased cell senescence in response to CSE point to a broader cellular stress response that compromises both the structural integrity and proliferative capacity of hBSMCs. However, to further characterize this phenotype, future analyses of specific senescence markers such as p53 and p21 would be valuable.
Along with the disrupted expression and organization of crucial cytoskeleton proteins, we also confirmed that CSE reduced bronchial contraction in response to KCl (used as a positive control), 5-HT, and ACh. In this regard, mitoTEMPO alleviated the loss of contractility in response to ACh, and, more importantly, it significantly prevented the CSE-mediated decrease in contractility with KCl and 5-HT. Therefore, these results further reinforce the importance of the early CSE-mediated increase in calcium and ROS in the contractile machinery dysfunction of hBSMCs.
Nevertheless, this study has also certain limitations that should be acknowledged. First, 2-APB may exert additional effects on other calcium channels, such as members of the TRPC family. In this regard, we also performed experiments using verapamil, a calcium channel blocker that primarily inhibits L-type calcium channels on the plasma membrane. Unlike the effects observed with 2-APB on calcium and ROS levels, verapamil treatment did not produce significant changes regarding both of them (
Supplementary Figure S1), therefore reinforcing the specificity of 2-APB towards IP3R. On the other hand, our functional experiments were conducted using bronchial rings from female mice only, precluding the assessment of potential sex-based differences in CSE responses or antioxidant efficacy, which requires further research. In addition, although the combination of in vitro and ex vivo models provides valuable mechanistic insight, the extrapolation of these findings to the in vivo context is limited. Long-term in vivo studies using chronic cigarette smoke exposure models would be essential to validate the physiological relevance of the observed alterations.
In conclusion, our results demonstrate that CSE exposure disrupts cytoskeleton organization and function in hBSMCs. This disruption is accompanied by increased levels of superoxide, intracellular calcium, and lipid peroxidation, factors that likely contribute to the detrimental effects of CSE on the contractile apparatus. This hypothesis is further supported by our observation that both antioxidants and calcium-modulating agents effectively mitigate these alterations. Therefore, the use of these drugs, in combination with current therapies, could be a promising therapeutic approach for the management of COPD.