Current Insight into Biological Markers of Depressive Disorder in Children and Adolescents: A Narrative Review
Abstract
1. Introduction
2. Genetic and Epigenetic Markers
3. Neurotransmitters and Their Metabolites
- Serotonin (5-hydroxytryptamin, 5-HT): Reduced levels of 5-HT and its metabolite 5-hydroxyindole acetic acid (5-HIAA) have been documented in depressed individuals [26,27]. Direct comparisons of baseline serotonin levels across age groups are limited. The observed differences in serotonin transporter (SERT) expression and selective serotonin reuptake inhibitor (SSRI) efficacy suggest that the serotonin system matures over time and potentially influences the presentation and treatment of depression. These developmental variations underscore the importance of age-specific approaches in diagnosing and treating depressive disorders.
- Noradrenalin (NA) (norepinephrine): NA affects attention, arousal, and stress responses in adults. Deficits in NA are associated with fatigue and concentration difficulties in depression. The noradrenergic system’s development in children may influence how stress and attention-related symptoms present in pediatric depression. Attention is currently being paid to studying a subset of depressed patients who might respond to new selective noradrenalin reuptake inhibitors. Understanding these variations is crucial for developing effective, age-appropriate treatments [28].
- Dopamine (DA): DA influences motivation, pleasure, and motor function. Hypoactivity is observed in DD and contributes to symptoms like anhedonia. The nucleus accumbens (NAc), also known as the ventral striatum, is a key brain region involved in reward, motivation, and decision-making. It plays a crucial role in processing both positive and negative emotions and is implicated in various neurological and psychiatric disorders, including depression. The NAc and its dopaminergic input from the ventral tegmental area (VTA) form the mesolimbic dopamine system in depression. The mesolimbic dopamine system is most commonly associated with the rewarding effects of food, sex, and drugs. Given the importance of anhedonia, reduced motivation, and reduced energy levels in most individuals with depression, it is assumed that the NAc and VTA contribute significantly to the pathophysiology and symptomatology of depression and may even be involved in its etiology. Recent studies show that manipulations of key proteins (e.g., cAMP response element-binding protein (CREB,) dynorphin, brain-derived neurotrophic factor (BDNF)) in the rodent VTA-NAc circuit produce unique behavioral phenotypes, some of which are directly related to depression. Studies of these and other proteins in the mesolimbic dopamine system have created new approaches to modeling key symptoms of depression in animals and could allow the development of antidepressant drugs with fundamentally new mechanisms of action [29].
- Glutamate/GABA: Depression is linked to an imbalance between excitatory (glutamate) and inhibitory (GABA) neurotransmission. The paradigm shift from the monoamine hypothesis of depression to the glutamate-centered neuroplasticity hypothesis may represent a substantial advance in the hypothesis that drives research into new drugs and therapies [30].
3.1. Developmental Considerations
3.2. Summary
4. Hormonal and Endocrine Markers
4.1. Developmental Variability in Cortisol Reactivity
4.2. Summary
5. Immune and Inflammatory Markers
5.1. Developmental Variability
5.2. Summary
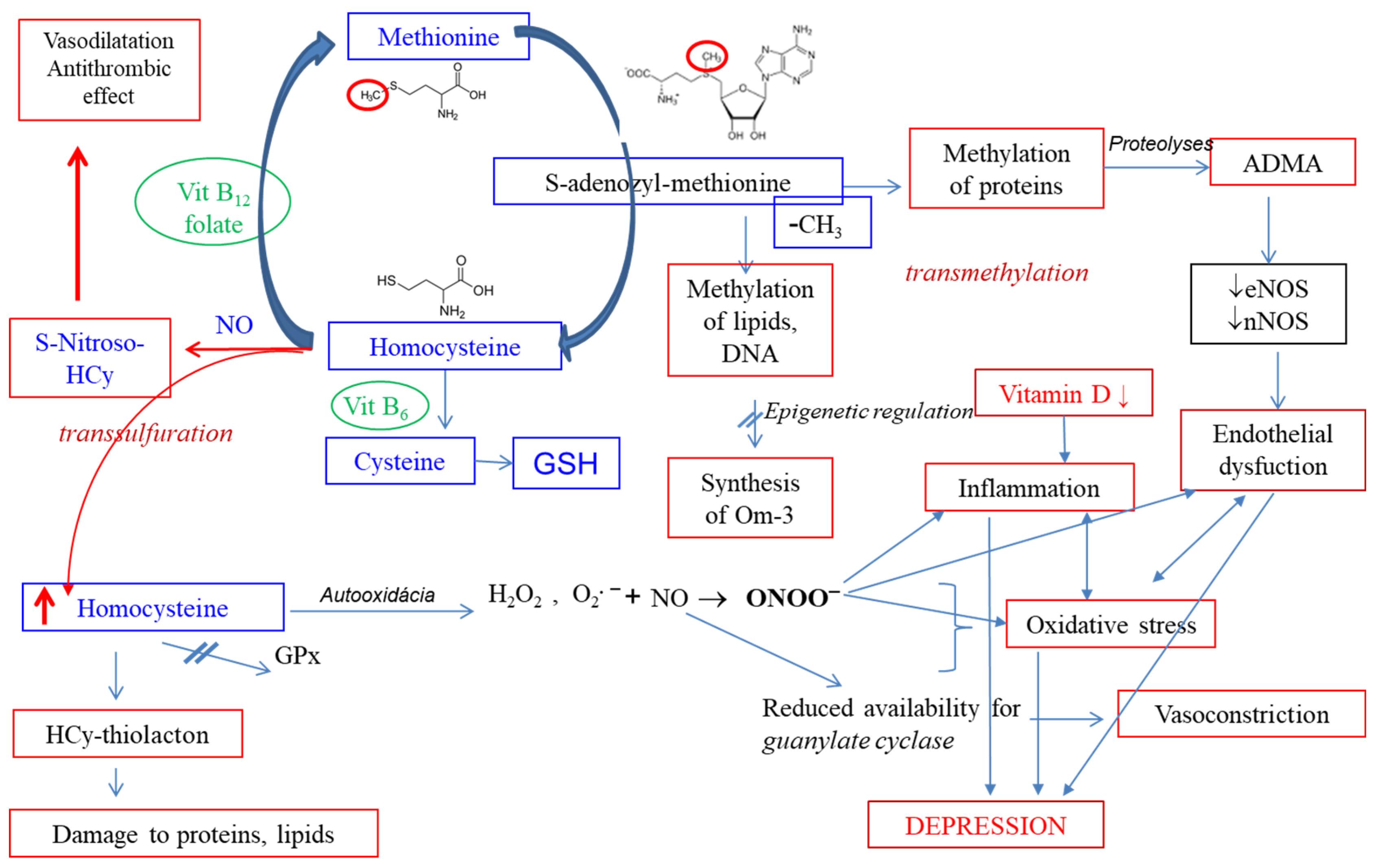
6. Vitamin D, Homocysteine, and Thromboxane in Depression
6.1. Vitamin D and Depression
6.2. Homocysteine and Depression
6.3. Thromboxane and Depression
6.4. Summary
7. Lipid Profile and Depressive Disorder
7.1. Serum Lipids and Depression
7.2. Lipoprotein Subfractions and Mental Health
7.3. Summary
8. Oxidative Stress (OS)
8.1. Biomarker Research in Paediatric Depression
8.2. Oxidative Stress in Depression: Adults vs. Youth
8.3. Mechanistic Pathways Linking Oxidative Stress to Depression
- Mitochondrial dysfunction: Impaired mitochondrial activity reduces ATP production, increases ROS, and activates inflammatory cytokines (IL-1β, IL-18), which trigger the kynurenine pathway and produce neurotoxic metabolites like quinolinic acid (QA). This activates N-methyl-D-aspartate (NMDA) receptors, increases synaptic glutamate, and exacerbates oxidative damage [105,106].
8.4. Summary
- A small number of patients (n = 60) and healthy controls (n = 20) were enrolled in the project.
- Patients with two diagnoses were included in the project: depressive disorder (n = 31) or mixed anxiety and depressive disorder (n = 29).
- An imbalance between male (n = 12) and female (n = 48) patients. This is attributed to the higher prevalence of depressive disorder in girls and the general reluctance of boys to provide biological samples. Additionally, the patient group consisted of a maximum of 58 individuals (46 females and 12 males), while the healthy control group consisted only of 20 individuals (12 females and 8 males) for ethical reasons.
- Nutritional habits of patients and controls were not monitored, preventing a complete explanation for the baseline difference in urinary tryptophan (TRP) levels between patients and controls. However, participants were instructed to follow a standard diet with an obligation to inform the responsible doctor about any deviations in eating habits.
- Furthermore, the study did not measure the levels of quinolinic acid or kynurenic acid, which would offer a more detailed understanding of the balance in TRP metabolism (neurotoxic versus neuroprotective pathways) and the involvement of TRP metabolites in the pathophysiology of depressive disorder.
- We did not determine any of the direct markers of inflammation (IL-6, IL-1, TNF, or others) in depressed children and adolescents, for technical reasons. Patients had hsCRP levels in the physiological range. We used an indirect marker, thromboxane, to indirectly monitor the inflammatory response.
9. Conclusions
10. Clinical Implications and Translational Challenges
11. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegner, M.; Amatriain-Fernández, S.; Kaulitzky, A.; Murillo-Rodriguez, E.; Machado, S.; Budde, H. Systematic Review of Meta-Analyses: Exercise Effects on Depression in Children and Adolescents. Front. Psychiatry 2020, 11, 81. [Google Scholar] [CrossRef]
- Nock, M.K.; Green, J.G.; Hwang, I.; McLaughlin, K.A.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry 2013, 70, 300–310. [Google Scholar] [CrossRef]
- Fava, M. Diagnosis and Definition of Treatment-Resistant Depression. Biol. Psychiatry 2003, 53, 649–659. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Stringaris, A.; Brent, D.A.; Bloch, M.H. Annual Research Review: Defining and treating pediatric treatment-resistant depression. J. Child. Psychol. Psychiatry 2020, 61, 312–332. [Google Scholar] [CrossRef]
- Maina, G.; Adami, M.; Ascione, G.; Bondi, E.; De Berardis, D.; Delmonte, D.; Maffezzoli, S.; Martinotti, G.; Nivoli, A.; Ottavianelli, E.; et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: A Delphi panel. Ann. Gen. Psychiatry 2023, 22, 48. [Google Scholar] [CrossRef]
- Daly, M. Prevalence of Depression Among Adolescents in the U.S. from 2019 to 2019: Analysis of Trends by Sex, Race/Ethnicity, and Income. J. Adolesc. Health 2022, 70, 496–499. [Google Scholar] [CrossRef]
- Balazs, J.; Miklósi, M.; Keresztény, Á.; Apter, A.; Bobes, J.; Brunner, R.; Corcoran, P.; Cosman, D.; Haring, C.; Kahn, J.P.; et al. Prevalence of Adolescent Depression in Europe. Eur. Psychiatry 2012, 27 (Suppl. S1), 1. [Google Scholar] [CrossRef]
- Sacco, R.; Camilleri, N.; Eberhardt, J.; Umla-Runge, K.; Newbury-Birch, D. A Systematic Review and Meta-Analysis on the Prevalence of Mental Disorders among Children and Adolescents in Europe. Eur. Child Adolesc. Psychiatry 2024, 33, 2877–2894. [Google Scholar] [CrossRef]
- Arias-de la Torre, A.; Vilagut, G.; Ronaldson, A.; Serrano-Blanco, A.; Martín, V.; Peters, M.; Valderas, J.M.; Dregan, A.; Alonso, J. Prevalence and Variability of Current Depressive Disorder in 27 European Countries: A Population-Based Study. Lancet Public Health 2021, 6, e729–e738. [Google Scholar] [CrossRef]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Omega-3 Fatty Acids Modulate Symptoms of Depressive Disorder, Serum Levels of Omega-3 Fatty Acids and Omega-6/Omega-3 Ratio in Children: A Randomized, Double-Blind and Controlled Trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef]
- Katrenčíková, B.; Vaváková, M.; Waczulíková, I.; Oravec, S.; Garaiova, I.; Nagyová, Z.; Hlaváčová, N.; Ďuračková, Z.; Trebatická, J. Lipid Profile, Lipoprotein Subfractions, and Fluidity of Membranes in Children and Adolescents with Depressive Disorder: Effect of Omega-3 Fatty Acids in a Double-Blind Randomized Controlled Study. Biomolecules 2020, 10, 1427. [Google Scholar] [CrossRef]
- Paduchová, Z.; Katrenčíková, B.; Vaváková, M.; Laubertová, L.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z.; Trebatická, J. The Effect of Omega-3 Fatty Acids on Thromboxane, Brain-Derived Neurotrophic Factor, Homocysteine, and Vitamin D in Depressive Children and Adolescents: Randomized Controlled Trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in a Randomized Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Oravcová, H.; Katrenčíková, B.; Garaiová, I.; Ďuračková, Z.; Trebatická, J.; Ježová, D. Stress Hormones Cortisol and Aldosterone, and Selected Markers of Oxidative Stress in Response to Long-Term Supplementation with Omega-3 Fatty Acids in Adolescent Children with Depression. Antioxidants 2022, 11, 1546. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M., Jr.; Paduchová, Z.; Muchová, J.; Garaiová, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The Kynurenine and Serotonin Pathway, Neopterin and Biopterin in Depressed Children and Adolescents: An Impact of Omega-3 Fatty Acids, and Association with Markers Related to Depressive Disorder. A Randomized, Blinded, Prospective Study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Trans-ancestry genome-wide study of depression identifies 697 associations implicating cell types and pharmacotherapies. Cell 2025, 188, 640–652. [Google Scholar] [CrossRef]
- Xia, L.; Yao, S. The Involvement of Genes in Adolescent Depression: A Systematic Review. Front. Behav. Neurosci. 2015, 9, 329. [Google Scholar] [CrossRef]
- Hankin, B.L.; Young, J.F.; Abela, J.R.; Smolen, A.; Jenness, J.L.; Gulley, L.D.; Technow, J.R.; Gottlieb, A.B.; Cohen, J.R.; Oppenheimer, C.W. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. J. Abnorm. Psychol. 2015, 124, 803–816. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Culverhouse, R.C.; Saccone, N.L.; Horton, A.C.; Ma, Y.; Anstey, K.J.; Banaschewski, T.; Burmeister, M.; Etain, B.; Fisher, H.L.; Goldman, N.; et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 2018, 23, 133–142. [Google Scholar] [CrossRef]
- Avecilla, V.; Avecilla, A. Inhibitor of DNA-Binding/Differentiation Proteins and Environmental Toxicants: Genomic Impact on the Onset of Depressive Dysfunction. Med. Sci. 2019, 7, 7. [Google Scholar] [CrossRef]
- Shim, I.H.; Yi, J.M.; Ha, S.H.; Kwon, K.A.; Bae, D.S. Glucocorticoid Receptor Gene (NR3C1) Expression in the Pathogenesis of Depression in Cancer. Alpha Psychiatry 2022, 23, 294–297. [Google Scholar] [CrossRef]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef]
- Shadrina, M.; Bondarenko, E.A.; Slominsky, P.A. Genetic Factors in Major Depression Disease. Front. Psychiatry 2018, 9, 609. [Google Scholar] [CrossRef]
- Ceylan, D.; Karacicek, B.; Tufekci, K.U.; Aksahin, I.C.; Senol, S.H.; Genc, S. Mitochondrial DNA oxidation, methylation, and copy number alterations in major and bipolar depression. Front. Psychiatry 2023, 14, 1304660. [Google Scholar] [CrossRef]
- Jayamohananan, H.; Manoj Kumar, M.K. 5-HIAA as a Potential Biological Marker for Neurological and Psychiatric Disorders. Adv. Pharm. Bull. 2019, 9, 374–381. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin. Chem. 1994, 40, 288–295. [Google Scholar] [CrossRef]
- Charney, D.S. Monoamine dysfunction and the pathophysiology and treatment of depression. J. Clin. Psychiatry 1998, 59 (Suppl. S14), 11–14. [Google Scholar]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a Glutamate Hypothesis of Depression: An Emerging Frontier of Neuropsychopharmacology for Mood Disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Bowman, M.A.; Daws, L.C. Targeting Serotonin Transporters in the Treatment of Juvenile and Adolescent Depression. Front. Neurosci. 2019, 13, 434875. [Google Scholar] [CrossRef]
- Wang, L.; Yang, P.; Yang, C.; Yang, D.; Wu, X.; Cao, T.; Zeng, C.; Chen, Q.; Zhang, S.; Zhu, Z.; et al. Disturbance of Neurotransmitter Metabolism in Drug-Naïve, First-Episode Major Depressive Disorder: A Comparative Study on Adult and Adolescent Cohorts. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1283–1296. [Google Scholar] [CrossRef]
- Gabbay, V.; Klein, R.G.; Katz, Y.; Mendoza, S.; Guttman, L.E.; Alonso, C.M.; Babb, J.S.; Hirsch, G.S.; Liebes, L. The Possible Role of the Kynurenine Pathway in Adolescent Depression with Melancholic Features. J. Child Psychol. Psychiatry 2010, 51, 935–943. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Cernackova, A.; Durackova, Z.; Trebaticka, J.; Mravec, B. Neuroinflammation and Depressive Disorder: The Role of the Hypothalamus. J. Clin. Neurosci. 2020, 75, 5–10. [Google Scholar] [CrossRef]
- Hage, M.P.; Azar, S.T. The Link between Thyroid Function and Depression. J. Thyroid Res. 2012, 2012, 590648. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Gunnar, M.; Quevedo, K. The Neurobiology of Stress and Development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic–Pituitary–Adrenal Axis Dysregulation in Depressed Children and Adolescents: A Meta-Analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef]
- Humphreys, K.L.; Moore, S.R.; Davis, E.G.; MacIsaac, J.L.; Lin, D.T.S.; Kobor, M.S.; Gotlib, I.H. DNA Methylation of HPA-Axis Genes and the Onset of Major Depressive Disorder in Adolescent Girls: A Prospective Analysis. Transl. Psychiatry 2019, 9, 245. [Google Scholar] [CrossRef]
- Gardini, E.S.; Schaub, S.; Neuhauser, A.; Ramseier, E.; Villiger, A.; Ehlert, U.; Lanfranchi, A.; Turecki, G. Methylation of the Glucocorticoid Receptor Promoter in Children: Links with Parents as Teachers, Early Life Stress, and Behavior Problems. Dev. Psychopathol. 2022, 34, 810–822. [Google Scholar] [CrossRef]
- Wang, Q.; Shelton, R.C.; Dwivedi, Y. Interaction between Early-Life Stress and FKBP5 Gene Variants in Major Depressive Disorder and Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2018, 225, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.; Melhem, N.; Ferrell, R.; Emslie, G.; Wagner, K.D.; Ryan, N.; Vitiello, B.; Birmaher, B.; Mayes, T.; Zelazny, J.; et al. Association of FKBP5 Polymorphisms with Suicidal Events in the Treatment of Resistant Depression in Adolescents (TORDIA) Study. Am. J. Psychiatry 2010, 167, 190–197. [Google Scholar] [CrossRef]
- Hiles, S.A.; Baker, A.L.; de Malmanche, T.; Attia, J. A Meta-Analysis of Differences in IL-6 and IL-10 between People with and without Depression: Exploring the Causes of Heterogeneity. Brain Behav. Immun. 2012, 26, 1180–1188. [Google Scholar] [CrossRef]
- D’Acunto, G.; Nageye, F.; Zhang, J.; Masi, G.; Cortese, S. Inflammatory Cytokines in Children and Adolescents with Depressive Disorders: A Systematic Review and Meta-Analysis. J. Child Adolesc. Psychopharmacol. 2019, 29, 362–369. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reimek, E.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Zonca, V.; Marizzoni, M.; Saleri, S.; Zajkowska, Z.; Manfro, P.H.; Souza, L.; Viduani, A.; Sforzini, L.; Swartz, J.R.; Fisher, H.L.; et al. Inflammation and Immune System Pathways as Biological Signatures of Adolescent Depression–The IDEA-RiSCo Study. Transl. Psychiatry 2024, 14, 230. [Google Scholar] [CrossRef]
- Schumacher, A.; Muha, J.; Campisi, S.C.; Bradley-Ridout, G.; Lee, A.C.; Korczak, D.J. The Relationship Between Neurobiological Function and Inflammation in Depressed Children and Adolescents: A Scoping Review. Neuropsychobiology 2024, 83, 61. [Google Scholar] [CrossRef]
- Ho, T.C.; Kulla, A.; Teresi, G.I.; Sisk, L.M.; Rosenberg-Hasson, Y.; Maecker, H.T.; Gotlib, L.H. Inflammatory Cytokines and Callosal White Matter Microstructure in Adolescents. Brain Behav. Immun. 2022, 100, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Teresi, G.I.; Segarra, J.R.; Ojha, A.; Walker, J.C.; Gu, M.; Spielman, D.M.; Sacchet, M.D.; Jiang, F.; Rosenberg-Hasson, Y.; et al. Higher Levels of Pro-inflammatory Cytokines Are Associated with Higher Levels of Glutamate in the Anterior Cingulate Cortex in Depressed Adolescents. Front. Psychiatry 2021, 12, 642976. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, P.; Sagar, R.; Mehta, M.; Sharma, S.; Subramanium, A.; Shamshi, F.; Sengupta, U.; Pandey, R.M.; Mukhopadhyay, A.K. Serum Cytokines and Anxiety in Adolescent Depression Patients: Gender Effect. Psychiatry Res. 2015, 229, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ely, B.A.; Simkovic, S.J.; Tao, A.; Wolchok, R.; Alonso, C.M.; Gabbay, V. Correlates of C-Reactive Protein with Neural Reward Circuitry in Adolescents with Psychiatric Symptoms. Brain Behav. Immun. Health 2020, 9, 100153. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tang, J.; Xu, G.; Chen, X.; Fang, K.; He, F.; Zheng, Y. Investigating the Relationship Between Inflammatory Cytokines and Adolescent Depression: A Comparative Analysis. Front. Psychiatry 2025, 16, 1524015. [Google Scholar] [CrossRef]
- Mills, N.T.; Scott, J.G.; Wray, N.R.; Cohen-Woods, S.; Baune, B.T. Research Review: The Role of Cytokines in Depression in Adolescents: A Systematic Review. J. Child Psychol. Psychiatry 2013, 54, 816–835. [Google Scholar] [CrossRef]
- Murphy, P.K.; Wagner, C.L. Vitamin D and Mood Disorders Among Women: An Integrative Review. J. Midwifery Womens Health 2008, 53, 440–446. [Google Scholar] [CrossRef]
- Folstein, M.; Liu, T.; Peter, I.; Buell, J.; Arsenault, L.; Scott, T.; Qiu, W.W. The Homocysteine Hypothesis of Depression. Am. J. Psychiatry 2007, 164, 861–867. [Google Scholar] [CrossRef]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and Its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.J.; McGrath, J.J. Vitamin D, Effects on Brain Development, Adult Brain Function, and the Links Between Low Levels of Vitamin D and Neuropsychiatric Disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative Stress, Inflammation and Treatment Response in Major Depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Grudet, C.; Wolkowitz, O.M.; Mellon, S.H.; Malm, J.; Reus, V.I.; Brundin, L.; Nier, B.M.; Dhabhar, F.S.; Hough, C.M.; Westrin, Å.; et al. Vitamin D and Inflammation in Major Depressive Disorder. J. Affect. Disord. 2020, 267, 33–41. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of Vitamin D Supplementation on Depression and Some Involved Neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef]
- Libuda, L.; Timmesfeld, N.; Antel, J.; Hirtz, R.; Bauer, J.; Führer, D.; Zwanziger, D.; Öztürk, D.; Langenbach, G.; Hahn, D.; et al. Effect of Vitamin D Deficiency on Depressive Symptoms in Child and Adolescent Psychiatric Patients: Results of a Randomized Controlled Trial. Eur. J. Nutr. 2020, 59, 3415–3424. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, H. The Influence of Vitamin D on the Severity of Depressive Symptoms in Children and Adolescents: A Systematic Review. 2024 Awards for Excellence in Student Research and Creative Activity-Documents, 9. 2024. Available online: https://thekeep.eiu.edu/lib_awards_2024_docs/9 (accessed on 5 June 2025).
- Trebatická, J.; Dukát, A.; Ďuračková, Z.; Muchová, J. Cardiovascular Diseases, Depression Disorders and Potential Effects of Omega-3 Fatty Acids. Physiol. Res. 2017, 66, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, F.E.; Schram, M.T.; Schalkwijk, C.G.; Stehouwer, C.D.; Henry, R.M.; Dagnelie, P.C.; Schaper, N.C.; Van der Kallen, C.J.; Koster, A.; Sep, S.J.; et al. Associations of Low Grade Inflammation and Endothelial Dysfunction with Depression–The Maastricht Study. Brain Behav. Immun. 2016, 56, 390–396. [Google Scholar] [CrossRef]
- Nakao Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folates, Vitamin B12, Homocysteine, and the MTHFR 677C→T Polymorphism in Anxiety and Depression: The Hordaland Homocysteine Study. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef]
- Gilbody, S.; Lightfoot, T.; Sheldon, T. Is Low Folate a Risk Factor for Depression? A Meta-Analysis and Exploration of Heterogeneity. J. Epidemiol. Community Health 2007, 61, 631–637. [Google Scholar] [CrossRef]
- Kamath, A.; Rao, G.; Upadhya, S. Vitamin B12 and folate status in depression in children and adolescents: A case-control study. Indian J. Psychiatry 2010, 52, 122–125. [Google Scholar] [CrossRef]
- Almeida, O.P.; McCaul, K.; Hankey, G.J.; Norman, P.; Jamrozik, K.; Flicker, L. Homocysteine and depression in later life. Arch. Gen. Psychiatry 2008, 65, 1286–1294. [Google Scholar] [CrossRef]
- Lieb, J.; Karmali, R.; Horrobin, D. Elevated levels of prostaglandin e2 and thromboxane B2 in depression. Prostaglandins Leukot. Med. 1983, 10, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Fimognari, F.L.; Infantino, V.; Monteleone, G.; Fimognari, G.B.; Falletti, D.; Marigliano, V. High Plasma Concentrations of Cortisol and Thromboxane B2 in Patients with Depression. Am. J. Med. Sci. 1994, 307, 228–232. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. Importance of maintaining a low omega-6/omega-3 ratio for reducing platelet aggregation, coagulation and thrombosis. Open Heart 2019, 6, e001011. [Google Scholar] [CrossRef] [PubMed]
- Sinopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef]
- Husted, K.S.; Bouzinova, E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Parekh, A.; Smeeth, D.; Milner, Y.; Thuret, S. The Role of Lipid Biomarkers in Major Depression. Healthcare 2017, 5, 5. [Google Scholar] [CrossRef]
- Tedders, S.H.; Fokong, K.D.; McKenzie, L.E.; Wesely, C.; Yu, L.; Zhang, J. Low cholesterol is associated with depression among US household population. J. Affect. Disord. 2011, 135, 115–121. [Google Scholar] [CrossRef]
- Nakao, M.; Yano, E. Relationship between major depression and high serum cholesterol in Japanese men. Tohoku J. Exp. Med. 2004, 204, 273–287. [Google Scholar] [CrossRef]
- Cheon, S.Y. Impaired Cholesterol Metabolism, Neurons, and Neuropsychiatric Disorders. Exp. Neurobiol. 2023, 32, 57. [Google Scholar] [CrossRef]
- Persons, J.E.; Fiedorowicz, J.G. Depression and serum low-density lipoprotein: A systematic review and meta-analysis. J. Affect. Disord. 2016, 206, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Khalfan, A.F.; Campisi, S.C.; Lo, R.F.; McCrindle, B.W.; Korczak, D.J. The association between adolescent depression and dyslipidemia. J. Affect. Disord. 2023, 338, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.; Muniz, N. Differences in HDL subfraction distribution in normolipidemic versus dyslipidemic individuals (pdf format). Presented at AACC,57th Annual Meeting, Orlando, FL, USA, 24–28 July 2005. [Google Scholar]
- Oravec, S.; Gruber, K.; Dostal, E.; Mikl, J. Hyper-betalipoproteinemia LDL 1,2: A newly identified nonatherogenic hypercholes terolemia in a group of hypercholesterolemic subjects. Neuro Endocrinol. Lett. 2011, 32, 322–327. [Google Scholar]
- Oravec, S.; Dostal, E.; Dukát, A.; Gavorník, P.; Kučera, M.; Gruber, K. HDL subfractions analysis: A new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuroendocrinol. Lett. 2011, 32, 502–509. [Google Scholar]
- Žitňanová, I.; Oravec, S.; Janubová, M.; Koňariková, K.; Dvořaková, M.; Laubertová, L.; Králová, M.; Šimko, M.; Muchová, J. Gender differences in LDL and HDL subfractions in atherogenic and nonatherogenic phenotypes. Clin. Biochem. 2020, 79, 9–13. [Google Scholar] [CrossRef]
- Muchová, J.; Andrezálová, L.; Oravec, S.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z. High density lipoprotein subfractions and paraoxonase 1 in children. Acta Biochim. Pol. 2016, 63, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S.M.; Ruusunen, A.; Niskanen, L.; Tolmunen, T.; Voutilainen, S.; Viinamäki, H.; Kaplan, G.A.; Kauhanen, J. Elevated depressive symptoms and compositional changes in LDL particles in middle-aged men. Eur. J. Epidemiol. 2010, 25, 403. [Google Scholar] [CrossRef]
- Yazıcı, T.; Uçucu, S. Lipoprotein subfractions in patients with depression: The lipoprint system. Ann. Clin. Anal. Med. 2022, 13, 470–474. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Ďuračková, Z. Free Radicals and Antioxidants for Non-Experts. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–38. [Google Scholar] [CrossRef]
- Vaváková, M.; Duračková, Z.; Trebatická, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef]
- Maes, M.; Chang, Y.S.; Galecki, P.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the neurodegenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2014, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Martin, A.; King, R.A.; Charney, D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol. Psychiatry. 2001, 49, 980–1001. [Google Scholar] [CrossRef]
- Zwolińska, W.; Dmitrzak-Węglarz, M.; Słopień, A. Biomarkers in Child and Adolescent Depression. Child. Psychiatry Hum. Dev. 2023, 54, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ait Tayeb, A.E.; Poinsignon, V.; Chappell, K.; Bouligand, J.; Becquemont, L.; Verstuyft, C. Major Depressive Disorder and Oxidative Stress: A Review of Peripheral and Genetic Biomarkers According to Clinical Characteristics and Disease Stages. Antioxidants 2023, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2014, 51, 164–175. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. Int. J. Transl. Med. 2024, 4, 176–196. [Google Scholar] [CrossRef]
- Miniksar, Y.D.; Göçmen, A.Y. Childhood Depression and Oxidative Stress. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 1–9. [Google Scholar] [CrossRef]
- Maes, M.; Landucci Bonifacio, K.; Morelli, N.R.; Vargas, H.O.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol. Neurobiol. 2019, 56, 141–156. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Samaryn, E.; Maciejczyk, M.; Zalewska, A.; Waszkiewicz, N. Urine 3-Nitrotyrosine and Serum HDL as Potential Biomarkers of Depression. J. Clin. Med. 2023, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15, 17590914231181037. [Google Scholar] [CrossRef] [PubMed]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell. Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.A.D.P.; de Athaide, M.M.; Rahman, A.U.; de Mattos Barbosa, M.G.; Jardim, M.M.; Moraes, M.O.; Pinheiro, R.O. Kynurenines in the Pathogenesis of Peripheral Neuropathy During Leprosy and COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 815738. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachere, N.E.; Bar-Or, A. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the Glutamatergic System to Develop Novel, Improved Therapeutics for Mood Disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting Glutamate Signalling in Depression: Progress and Prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF Against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Catena-Dell’Osso, M.; Rotella, F.; Dell’Osso, A.; Fagiolini, A.; Marazziti, D. Inflammation, Serotonin and Major Depression. Curr. Drug Targets 2013, 14, 571–577. [Google Scholar] [CrossRef]
- Malynn, S.; Campos-Torres, A.; Moynagh, P.; Haase, J. The Pro-Inflammatory Cytokine TNF-α Regulates the Activity and Expression of the Serotonin Transporter (SERT) in Astrocytes. Neurochem. Res. 2013, 38, 694–704. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Sadalla, S.; Milani, S. The Relationship between the Serotonin Metabolism, Gut-Microbiota and the Gut-Brain Axis. Curr. Drug Metab. 2019, 20, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic Modulation of the Microbiota-Gut-Brain Axis and Behaviour in Zebrafish. Sci. Rep. 2016, 6, 30046. [Google Scholar] [CrossRef] [PubMed]
- Freimer, D.; Yang, T.T.; Ho, T.C.; Tymofiyeva, O.; Leung, C. The Gut Microbiota, HPA Axis, and Brain in Adolescent-Onset Depression: Probiotics as a Novel Treatment. Brain Behav. Immun. Health 2022, 26, 100541. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, S.P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone Mediates Stress-Related Increased Intestinal Permeability in a Region-Specific Manner. Neurogastroenterol. Motil. 2013, 25, e127–e139. [Google Scholar] [CrossRef]

 —symbol of inhibition.
—symbol of inhibition.
 —symbol of inhibition.
—symbol of inhibition.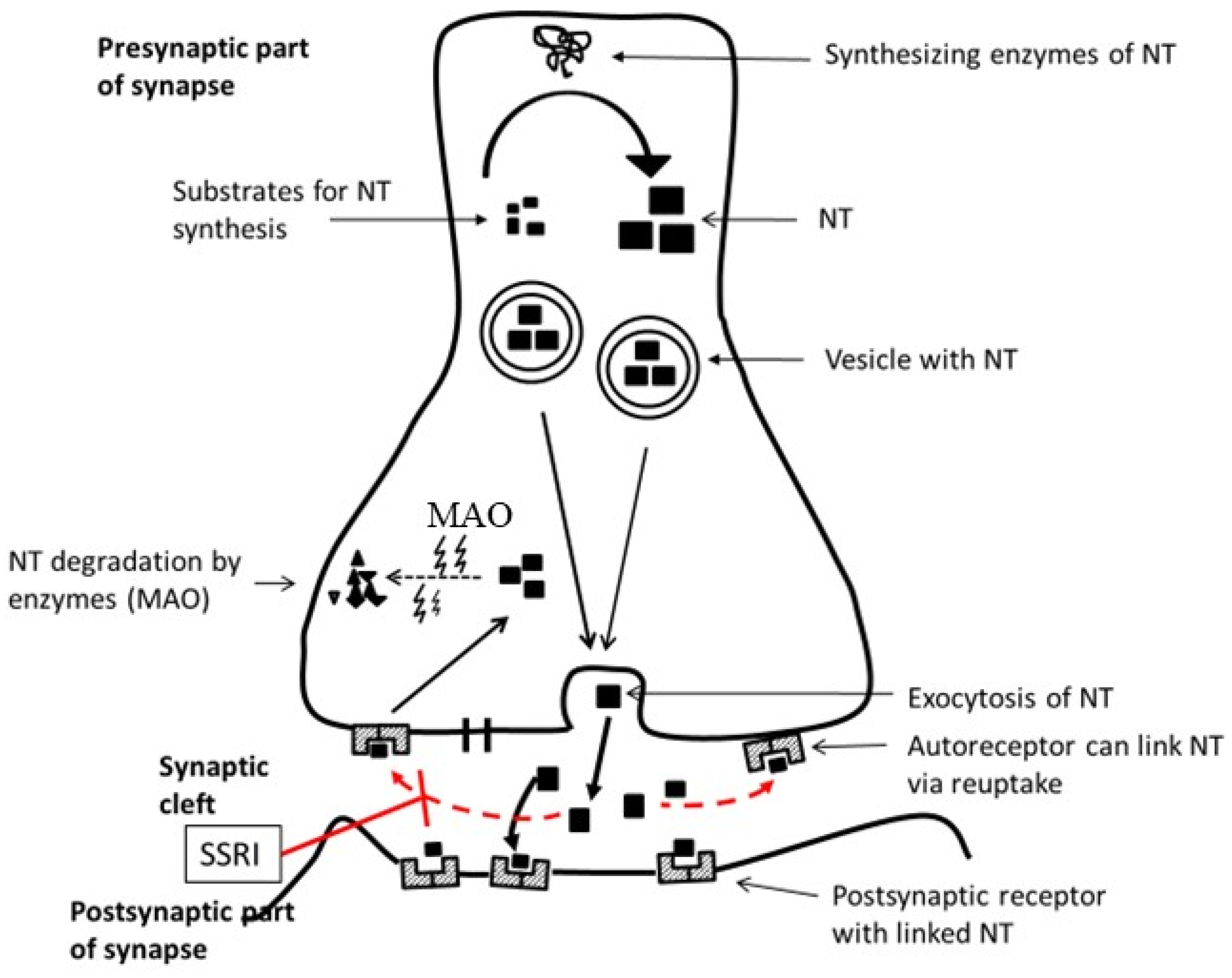

 —symbol of inhibition.
—symbol of inhibition.
 —symbol of inhibition.
—symbol of inhibition.
| Neurotransmitter | Role in Depression | Adult Presentation | Pediatric Considerations |
|---|---|---|---|
| Serotonin | Mood, sleep, appetite regulation | Low mood, sleep disturbances | Mood swings, appetite changes |
| Dopamine | Motivation, pleasure, motor function | Anhedonia, psychomotor slowing | Irritability, behavioural issues |
| Noradrenaline | Attention, arousal, stress response | Fatigue, concentration issues | Attention deficits, heightened stress responses |
 | Mitochondria dysfunction | OS → ROS ↑ → ATP ↓ AP1 ↑ → pro-IC ↑ → Ca2+ ↑ → nNOS ↑ → Mtch dysfunction |  |
| Neuro- inflammation | OS → Mtch damage ROS ↑ → NF-κB ↑ → IDO/TDO ↑ → QA ↑ → → NMDA ↑ → GLU excitotoxicity → Ca2+ ↑ | ||
| GLU excitotoxicity | OS → GLT1 ↑ OS → GLS ↑ OS → QA ↑ | ||
| BDNT/TrkB dysfunction | OS ↑ → CREB ↓ → BDNF ↓ → NF-κB ↑ | ||
| Serotonin deficiency | Induction of inflammation → disruption of MGB OS → IDO/TDO ↑ → KP ↑ → 5-HT ↓ OS → TNF-α ↑ → IL-1β ↑ → SERT ↓ | ||
| MGB axis | OS → Homeostasis of neurotransmitters ↓ through BDNF/TrkB OS → 5-HT ↓ | ||
| HPA axis dysregulation | OS → GR ↑ → negative feedback CRH/ACTH → restoration of HPA homeostasis OS → GR → NF-κB → Inflammation OS → GR agonist → IL-6/TNFα ↓ ROS ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebatická, J.; Vatrál, M.; Katrenčíková, B.; Muchová, J.; Ďuračková, Z. Current Insight into Biological Markers of Depressive Disorder in Children and Adolescents: A Narrative Review. Antioxidants 2025, 14, 699. https://doi.org/10.3390/antiox14060699
Trebatická J, Vatrál M, Katrenčíková B, Muchová J, Ďuračková Z. Current Insight into Biological Markers of Depressive Disorder in Children and Adolescents: A Narrative Review. Antioxidants. 2025; 14(6):699. https://doi.org/10.3390/antiox14060699
Chicago/Turabian StyleTrebatická, Jana, Martin Vatrál, Barbora Katrenčíková, Jana Muchová, and Zdeňka Ďuračková. 2025. "Current Insight into Biological Markers of Depressive Disorder in Children and Adolescents: A Narrative Review" Antioxidants 14, no. 6: 699. https://doi.org/10.3390/antiox14060699
APA StyleTrebatická, J., Vatrál, M., Katrenčíková, B., Muchová, J., & Ďuračková, Z. (2025). Current Insight into Biological Markers of Depressive Disorder in Children and Adolescents: A Narrative Review. Antioxidants, 14(6), 699. https://doi.org/10.3390/antiox14060699









