Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Reagents and Antibodies
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Cell Morphology Analysis
2.6. Colony Formation Assay
2.7. Cell Migration and Invasion Assay
2.8. ROS Assay
2.9. Cell Apoptosis Analysis
2.10. Western Blot Analysis
2.11. Immunofluorescence (IF) Staining
2.12. Cell-Derived Xenograft (CDX) Mouse Tumor Model
2.13. IHC Analysis
2.14. Statistical Analysis
3. Results
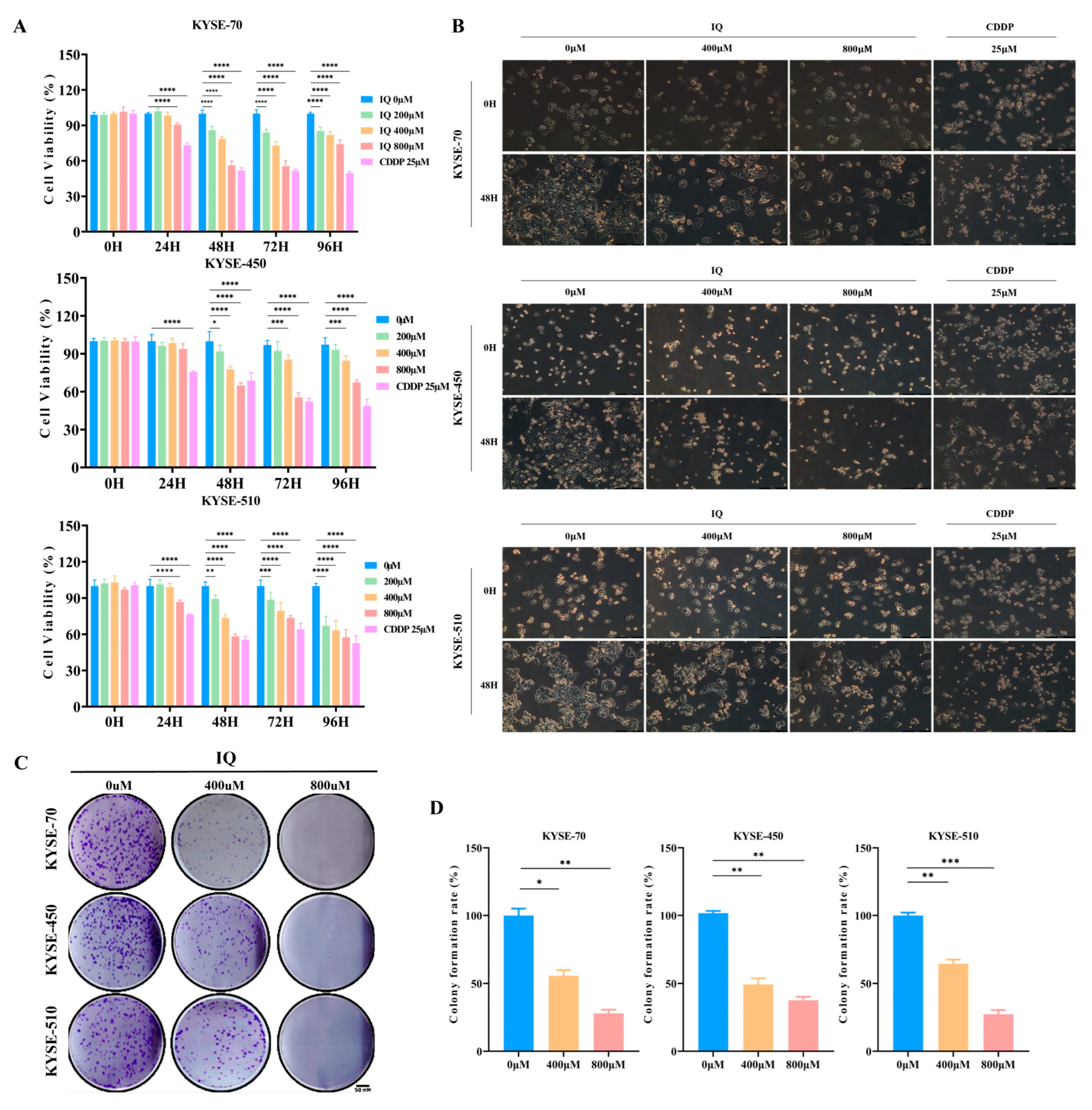
3.1. IQ Inhibits ESCC Cell Growth
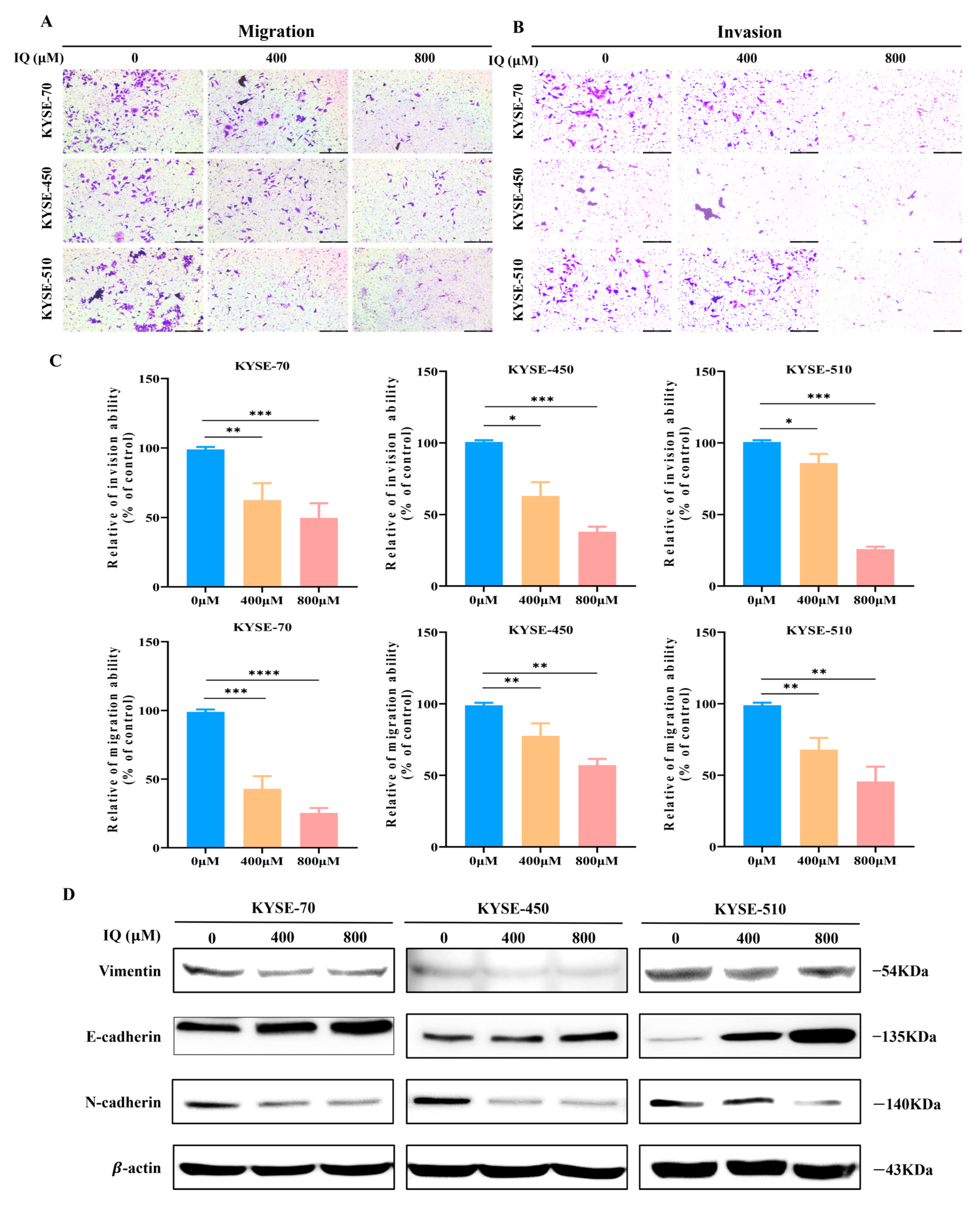
3.2. IQ Suppresses the Migration and Invasion of ESCC Cells
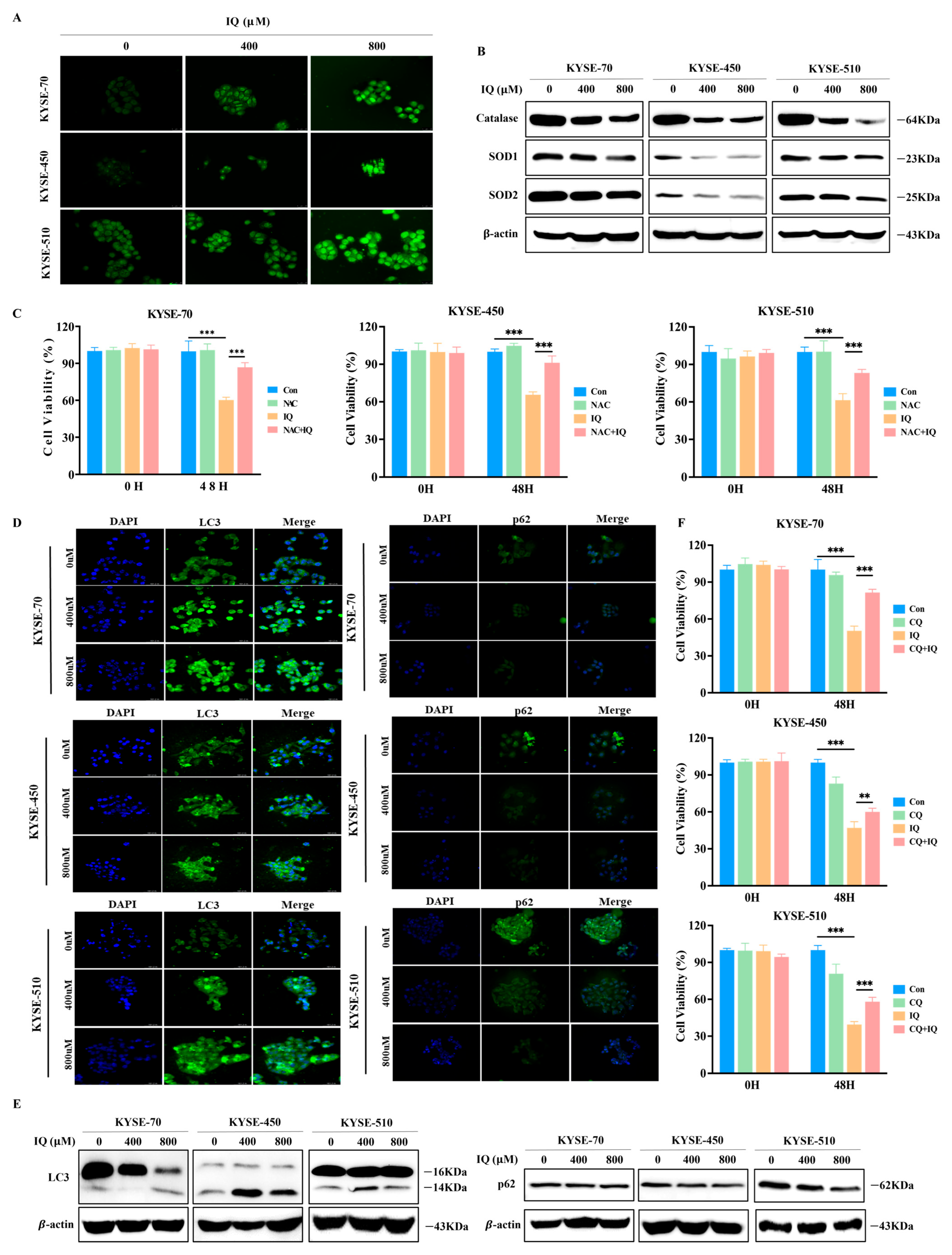
3.3. IQ Induces ROS Generation and Promotes Autophagy in ESCC Cells
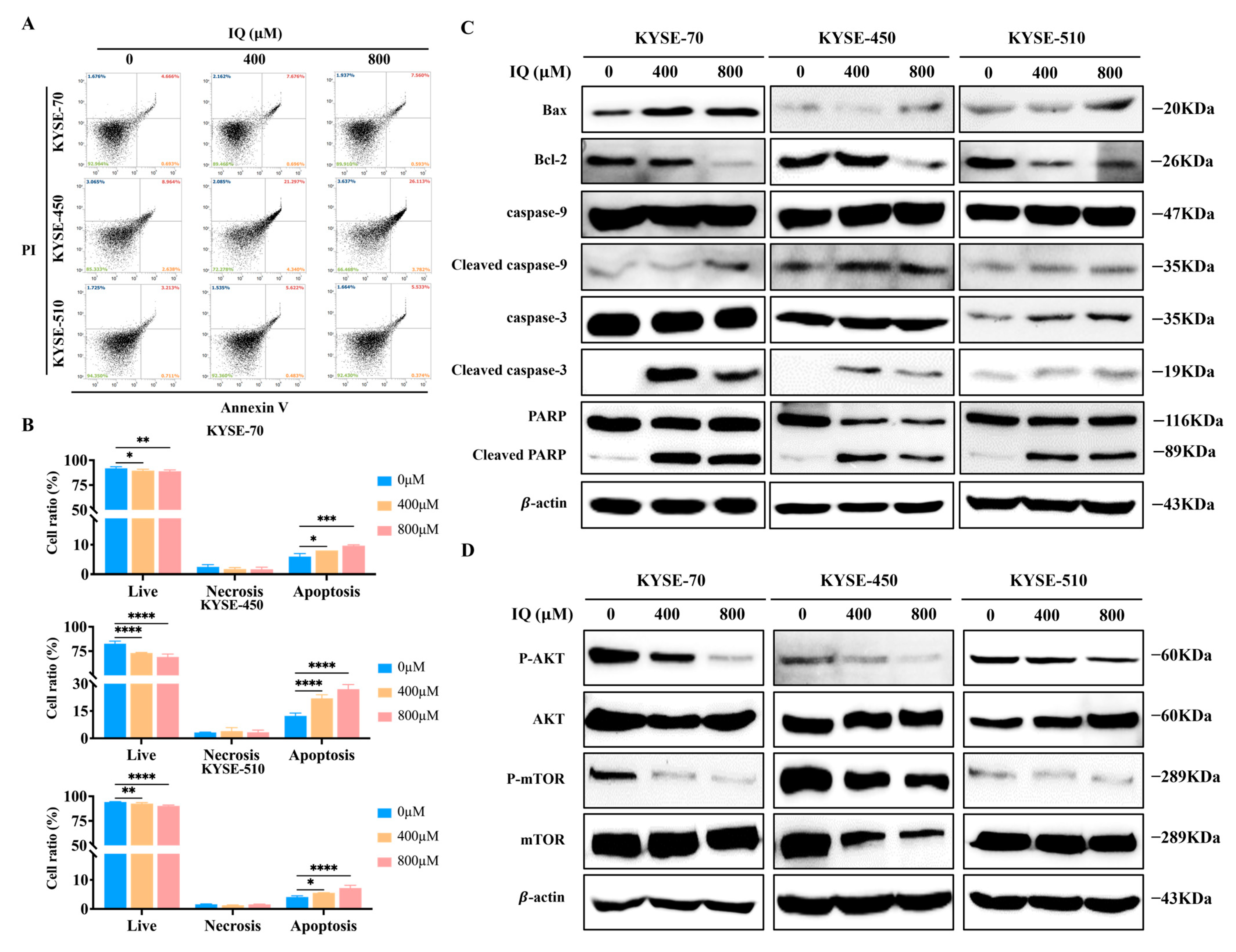
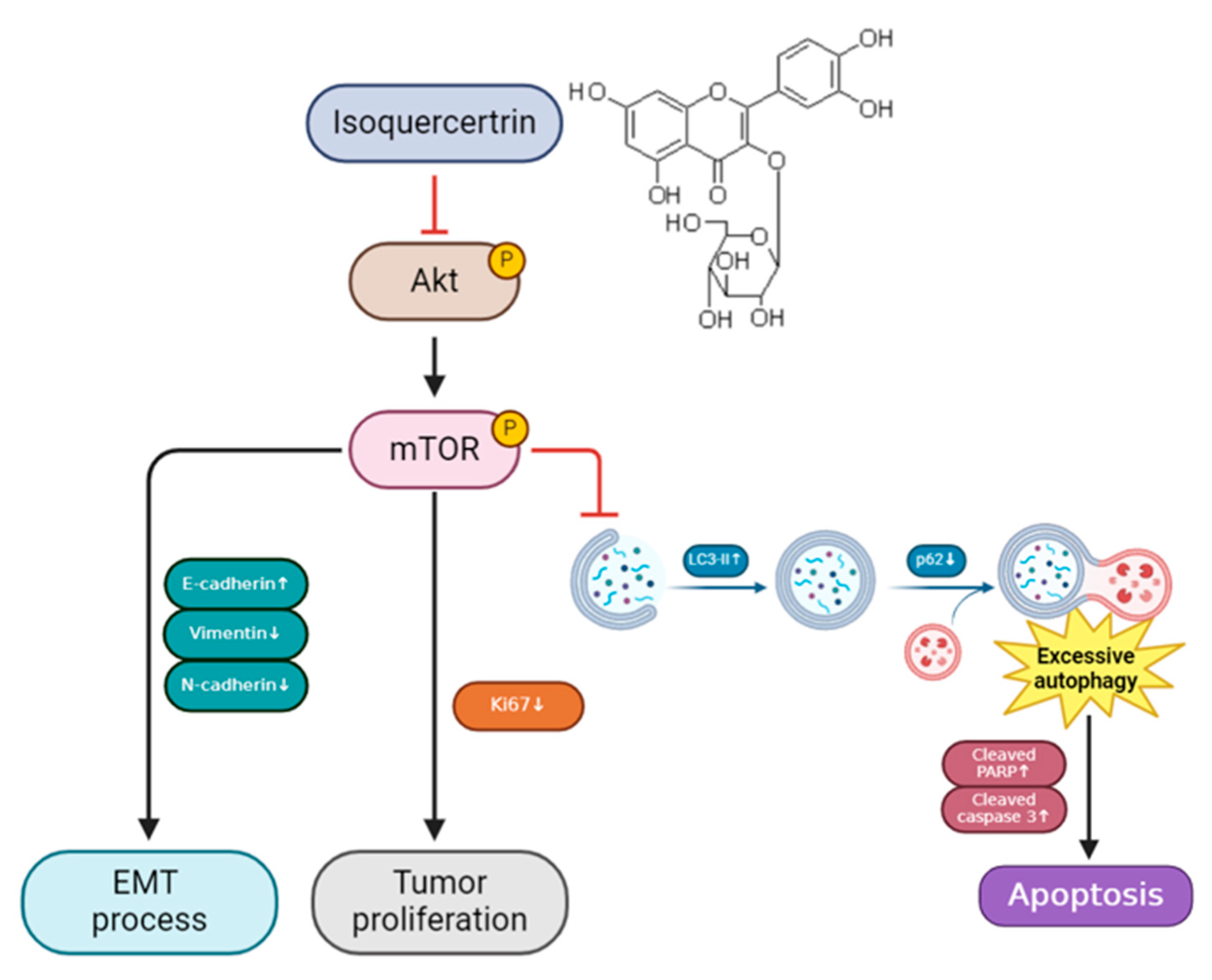
3.4. IQ Induces Apoptosis in ESCC Cells by Modulating the AKT/mTOR Signaling Pathway
3.5. IQ Suppresses the Growth of ESCC Tumors In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Esophageal cancer: Epidemiology, pathogenesis and prevention. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 517–526. [CrossRef] [PubMed]
- Zhang, Z.; Leng, Z.; Fang, K.; Sun, M.; Li, Z.; Kang, L.; Chen, T.; Xu, M. Global research trend of esophageal squamous cell carcinoma from 2012 to 2022: A bibliometric analysis. Front. Oncol. 2022, 12, 977935. [Google Scholar] [CrossRef]
- Niu, C.; Liu, Y.; Wang, J.; Liu, Y.; Zhang, S.; Zhang, Y.; Zhang, L.; Zhao, D.; Liu, F.; Chao, L.; et al. Risk factors for esophageal squamous cell carcinoma and its histological precursor lesions in China: A multicenter cross-sectional study. BMC Cancer 2021, 21, 1034. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Fan, J.H.; Qiao, Y.L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol. Med. 2017, 14, 33–41. [Google Scholar] [CrossRef]
- Allan, J.M.; Travis, L.B. Mechanisms of therapy-related carcinogenesis. Nat. Rev. Cancer 2005, 5, 943–955. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Kim, E.; Yi, J.; Huang, H.; Kim, H.; Raza, M.A.; Park, S.; Jang, S.; Kim, K.; et al. Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 8507. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Z.; Kim, E.; Huang, K.; Kim, C.Y.; Kim, H.; Park, K.; Kwon, W.S.; Lee, S.I.; Kim, Y.G.; et al. Parishin A Inhibits Oral Squamous Cell Carcinoma via the AKT/mTOR Signaling Pathway. Pharmaceuticals 2024, 17, 1277. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Orfali, G.; Duarte, A.C.; Bonadio, V.; Martinez, N.P.; de Araújo, M.E.; Priviero, F.B.; Carvalho, P.O.; Priolli, D.G. Review of anticancer mechanisms of isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.S.; Kim, J.H.; Lizardo, R.C.M.; Min, H.J.; Cho, H.D.; Hong, S.M.; Seo, K.I. The Flavonol Isoquercitrin Promotes Mitochondrial-Dependent Apoptosis in SK-Mel-2 Melanoma Cell via the PI3K/AKT/mTOR Pathway. Nutrients 2020, 12, 3683. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Chang, K.C.; Liu, P.F.; Chang, C.H.; Lin, Y.C.; Chen, Y.J.; Shu, C.W. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci. 2022, 12, 1. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Hänggi, K.; Ruffell, B. Cell death, therapeutics, and the immune response in cancer. Trends Cancer 2023, 9, 381–396. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Hu, W.; Jiang, B.; Zhou, X.; Zhang, M.; Song, M. Curcumin Induces Autophagy-mediated Ferroptosis by Targeting the PI3K/AKT/mTOR Signaling Pathway in Gastric Cancer. Turk. J. Gastroenterol. 2024, 35, 625–633. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Tang, Q.; Huang, L.; Zhan, Z.; Li, Y.; Wang, G.; Dai, X.; Zhang, Y. A Novel Aniline Derivative from Peganum harmala L. Promoted Apoptosis via Activating PI3K/AKT/mTOR-Mediated Autophagy in Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2023, 24, 12626. [Google Scholar] [CrossRef]
- Conti, V.; Polcaro, G.; De Bellis, E.; Donnarumma, D.; De Rosa, F.; Stefanelli, B.; Corbi, G.; Sabbatino, F.; Filippelli, A. Natural Health Products for Anti-Cancer Treatment: Evidence and Controversy. J. Pers. Med. 2024, 14, 685. [Google Scholar] [CrossRef]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The Role of Natural Products and Their Multitargeted Approach to Treat Solid Cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef]
- Roleira, F.M.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.E.; Marlein, C.; Walker, H.F.; Frame, F.M.; Mann, V.M.; Simms, M.S.; Davies, B.R.; Collins, A.T.; Maitland, N.J. Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 2017, 8, 56698–56713. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Ariosa, A.R.; Lahiri, V.; Lei, Y.; Yang, Y.; Yin, Z.; Zhang, Z.; Klionsky, D.J. A perspective on the role of autophagy in cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166262. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Levine, B.; Yuan, J. Autophagy in cell death: An innocent convict? J. Clin. Investig. 2005, 115, 2679–2688. [Google Scholar] [CrossRef]
- Wu, W.Y.; Cui, Y.K.; Hong, Y.X.; Li, Y.D.; Wu, Y.; Li, G.; Li, G.R.; Wang, Y. Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J. Cell Mol. Med. 2020, 24, 12141–12153. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR Pathways in Cancer and Autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Du, R.; Liu, W.; Huang, G.; Dong, Z.; Li, X. PI3K/Akt/mTOR Signaling Pathway: Role in Esophageal Squamous Cell Carcinoma, Regulatory Mechanisms and Opportunities for Targeted Therapy. Front. Oncol. 2022, 12, 852383. [Google Scholar] [CrossRef]
- Huang, R.; Dai, Q.; Yang, R.; Duan, Y.; Zhao, Q.; Haybaeck, J.; Yang, Z. A Review: PI3K/AKT/mTOR Signaling Pathway and Its Regulated Eukaryotic Translation Initiation Factors May Be a Potential Therapeutic Target in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 817916. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Wang, H.; Li, Z. Isoquercitrin Induces Endoplasmic Reticulum Stress and Immunogenic Cell Death in Gastric Cancer Cells. Biochem. Genet. 2023, 61, 1128–1142. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Huang, K.; Huang, H.; Kim, E.; Kim, H.; Kim, C.Y.; Kim, D.J.; Lee, S.I.; Kim, S.; Kim, D.Y.; et al. Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway. Antioxidants 2025, 14, 694. https://doi.org/10.3390/antiox14060694
Liu Z, Huang K, Huang H, Kim E, Kim H, Kim CY, Kim DJ, Lee SI, Kim S, Kim DY, et al. Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway. Antioxidants. 2025; 14(6):694. https://doi.org/10.3390/antiox14060694
Chicago/Turabian StyleLiu, Zhibin, Ke Huang, Hai Huang, Eungyung Kim, Hyeonjin Kim, Chae Yeon Kim, Dong Joon Kim, Sang In Lee, Sangsik Kim, Do Yoon Kim, and et al. 2025. "Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway" Antioxidants 14, no. 6: 694. https://doi.org/10.3390/antiox14060694
APA StyleLiu, Z., Huang, K., Huang, H., Kim, E., Kim, H., Kim, C. Y., Kim, D. J., Lee, S. I., Kim, S., Kim, D. Y., Liu, K., Ryoo, Z. Y., Lee, M.-H., Ma, L., & Kim, M. O. (2025). Isoquercitrin Suppresses Esophageal Squamous Cell Carcinoma (ESCC) by Inducing Excessive Autophagy and Promoting Apoptosis via the AKT/mTOR Signaling Pathway. Antioxidants, 14(6), 694. https://doi.org/10.3390/antiox14060694





