The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds
Abstract
1. Background in Wound Healing
2. Diversity of Skin Microbiota in Humans
3. Bacterial Microbiome in Human Chronic Wounds
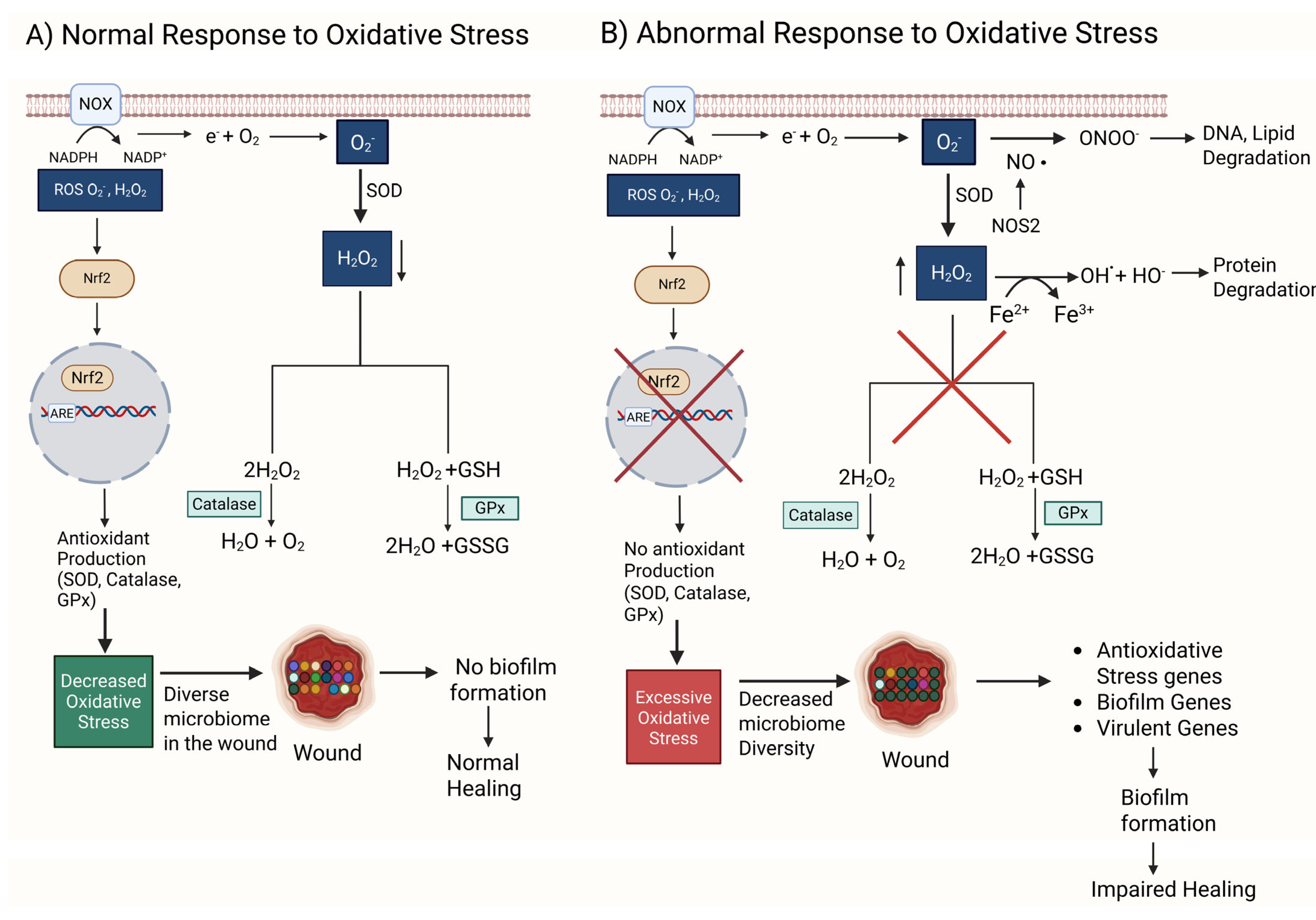
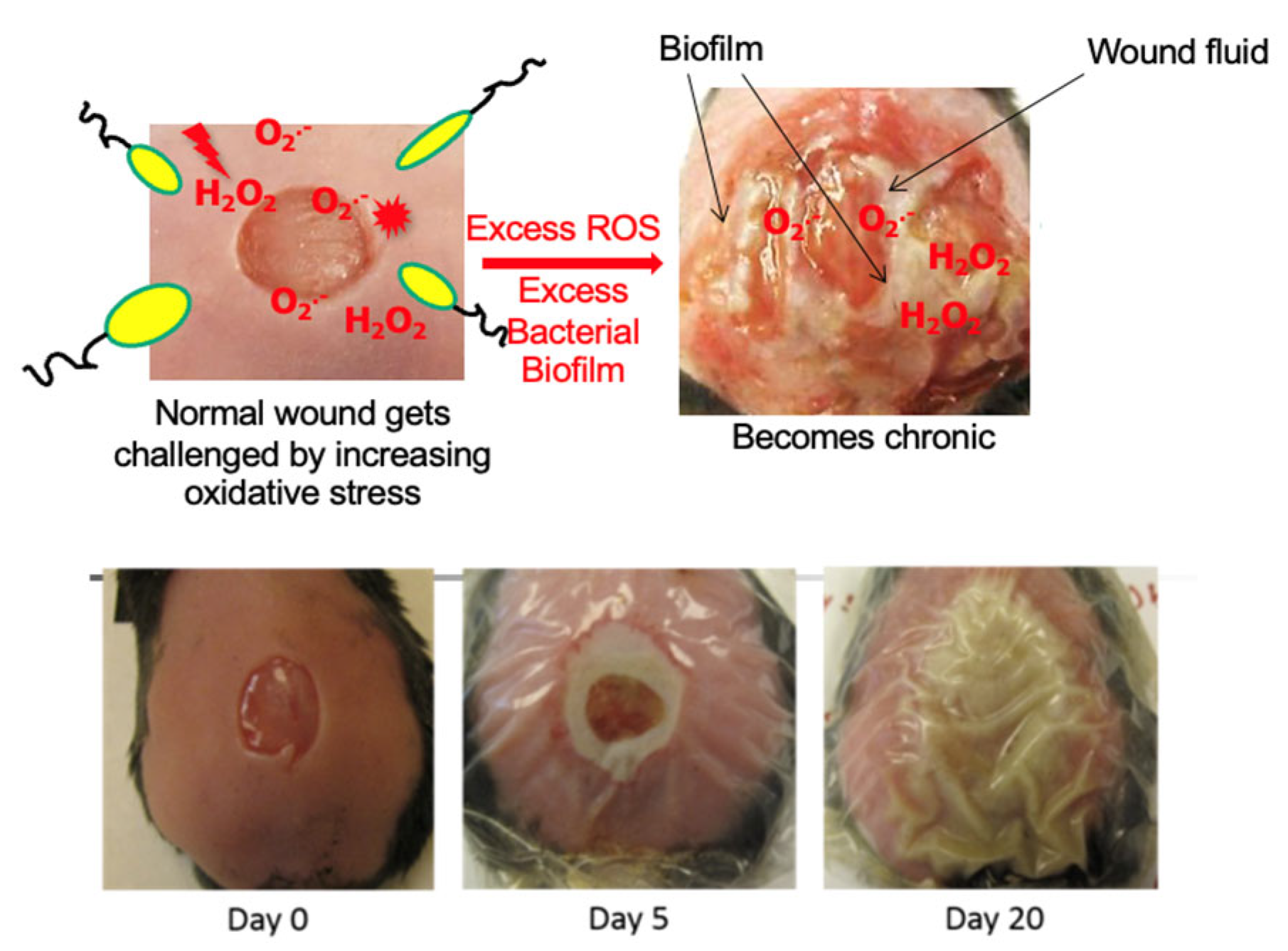
4. Oxidative Stress, Microbiome of the Skin, and Biofilm Formation
5. Importance of Treating Oxidative Stress and Biofilm to Heal Chronic Wounds
6. Challenges in Treating Human Chronic Wounds
7. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G. Wound pathophysiology, infection and therapeutic options. Ann. Med. 2002, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Martins-Green, M. Animal models for the study of acute cutaneous wound healing. Wound Repair. Regen. 2023, 31, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Martins-Green, M. Animal Models of Excessive Healing in Cutaneous Wounds. J. Dermatol. Clin. Res. 2023, 10, 1149. [Google Scholar]
- Saeed, S.; Martins-Green, M. Assessing Animal Models to Study Impaired and Chronic Wounds. Int. J. Mol. Sci. 2024, 25, 3837. [Google Scholar] [CrossRef]
- Patenall, B.L.; Carter, K.A.; Ramsey, M.R. Kick-Starting Wound Healing: A Review of Pro-Healing Drugs. Int. J. Mol. Sci. 2024, 25, 1304. [Google Scholar] [CrossRef]
- Robson, M.C.; Lea, C.E.; Dalton, J.B.; Heggers, J.P. Quantitative bacteriology and delayed wound closure. Surg. Forum 1968, 19, 501–502. [Google Scholar]
- Pierpont, Y.N.; Uberti, M.G.; Ko, F.; Robson, M.C.; Smith, C.A.; Wright, T.E.; Payne, W.G. Individualized, targeted wound treatment based on the tissue bacterial level as a biological marker. Am. J. Surg. 2011, 202, 220–224. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef]
- Kuikko, K.; Salmi, T.; Huhtala, H.; Kimpimäki, T. Characteristics of chronic ulcer patients by gender and ulcer aetiology from a multidisciplinary wound centre. Int. Wound J. 2024, 21, e70012. [Google Scholar] [CrossRef]
- Wicke, C.; Bachinger, A.; Coerper, S.; Beckert, S.; Witte, M.B.; Königsrainer, A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized Wound Care Center. Wound Repair. Regen. 2009, 17, 25–33. [Google Scholar] [CrossRef]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D.; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair. Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, C.; Calvanese, M.; Gentile, A.; Vastola, A.; Romano, P.; Ingenito, L.; Gentile, L.; Veneruso, I.; Scarano, C.; La Monica, I.; et al. Skin Microbiome Overview: How Physical Activity Influences Bacteria. Microorganisms 2025, 13, 868. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Grice, E.A. Microbial ecology of the skin in the era of metagenomics and molecular microbiology. Cold Spring Harb. Perspect. Med. 2013, 3, a015362. [Google Scholar] [CrossRef]
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The Wound Microbiome: Modern Approaches to Examining the Role of Microorganisms in Impaired Chronic Wound Healing. Adv. Wound Care 2014, 3, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Roume, H.; Heintz-Buschart, A.; Muller, E.E.L.; May, P.; Satagopam, V.P.; Laczny, C.C.; Narayanasamy, S.; Lebrun, L.A.; Hoopmann, M.R.; Schupp, J.M.; et al. Comparative integrated omics: Identification of key functionalities in microbial community-wide metabolic networks. NPJ Biofilms Microbiomes 2015, 1, 15007. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Loesche, M.; Hodkinson Brendan, P.; Heilmann, K.; Ruthel, G.; Gardner Sue, E.; Grice Elizabeth, A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. MBio 2016, 7, e01058-16. [Google Scholar] [CrossRef]
- Pang, M.; Zhu, M.; Lei, X.; Chen, C.; Yao, Z.; Cheng, B. Changes in Foot Skin Microbiome of Patients with Diabetes Mellitus Using High-Throughput 16S rRNA Gene Sequencing: A Case Control Study from a Single Center. Med. Sci. Monit. 2020, 26, e921440. [Google Scholar] [CrossRef]
- Navas-Molina, J.A.; Peralta-Sánchez, J.M.; González, A.; McMurdie, P.J.; Vázquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzym. 2013, 531, 371–444. [Google Scholar] [CrossRef]
- Tsilimigras, M.C.; Fodor, A.A. Compositional data analysis of the microbiome: Fundamentals, tools, and challenges. Ann. Epidemiol. 2016, 26, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Simmering, R. The Microbiota of the Human Skin. Adv. Exp. Med. Biol. 2016, 902, 61–81. [Google Scholar] [CrossRef]
- Egert, M.; Simmering, R.; Riedel, C.U. The Association of the Skin Microbiota With Health, Immunity, and Disease. Clin. Pharmacol. Ther. 2017, 102, 62–69. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.-h.; Pei, Z.; Blaser, M.J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef]
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Crompton, R.A.; Thomason, H.A.; Campbell, L.; Singh, G.; McBain, A.J.; Cruickshank, S.M.; Hardman, M.J. Cutaneous Nod2 Expression Regulates the Skin Microbiome and Wound Healing in a Murine Model. J. Investig. Dermatol. 2017, 137, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Miller, L.S. Host–pathogen interactions between the skin and Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahamed, A.; Chen, K.; Lebig, E.G.; Petros, B.; Saeed, S.; Martins-Green, M. Chapter 21—Skin microbiota and its role in health and disease with an emphasis on wound healing and chronic wound development. In Microbiome, Immunity, Digestive Health and Nutrition; Bagchi, D., Downs, B.W., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 297–311. [Google Scholar] [CrossRef]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Morgan, S.J.; Lippman, S.I.; Bautista, G.E.; Harrison, J.J.; Harding, C.L.; Gallagher, L.A.; Cheng, A.-C.; Siehnel, R.; Ravishankar, S.; Usui, M.L.; et al. Bacterial fitness in chronic wounds appears to be mediated by the capacity for high-density growth, not virulence or biofilm functions. PLoS Pathog. 2019, 15, e1007511. [Google Scholar] [CrossRef]
- Tipton, C.D.; Mathew, M.E.; Wolcott, R.A.; Wolcott, R.D.; Kingston, T.; Phillips, C.D. Temporal dynamics of relative abundances and bacterial succession in chronic wound communities. Wound Repair. Regen. 2017, 25, 673–679. [Google Scholar] [CrossRef]
- Dhall, S.; Do, D.; Garcia, M.; Wijesinghe, D.S.; Brandon, A.; Kim, J.; Sanchez, A.; Lyubovitsky, J.; Gallagher, S.; Nothnagel, E.A.; et al. A Novel Model of Chronic Wounds: Importance of Redox Imbalance and Biofilm-Forming Bacteria for Establishment of Chronicity. PLoS ONE 2014, 9, e109848. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Tolker-Nielsen, T. Biofilm Development. Microbiol. Spectr. 2015, 3, 51–66. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Vivas, A.C. Lower-extremity ulcers: Diagnosis and management. Br. J. Dermatol. 2015, 173, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, K.E.; Jordan, C.Y.; Crichton, E.; Barnes, J.E.; Harkin, G.E.; Hall, L.M.L.; Jones, J.D. A retrospective analysis of the microbiology of diabetic foot infections at a Scottish tertiary hospital. BMC Infect. Dis. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair. Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Gontcharova, V.; Youn, E.; Sun, Y.; Wolcott, R.D.; Dowd, S.E. A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol. J. 2010, 4, 8–19. [Google Scholar] [CrossRef]
- Dowd, S.E.; Sun, Y.; Secor, P.R.; Rhoads, D.D.; Wolcott, B.M.; James, G.A.; Wolcott, R.D. Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008, 8, 43. [Google Scholar] [CrossRef]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef]
- Price, L.B.; Liu, C.M.; Melendez, J.H.; Frankel, Y.M.; Engelthaler, D.; Aziz, M.; Bowers, J.; Rattray, R.; Ravel, J.; Kingsley, C.; et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: Impact of diabetes and antibiotics on chronic wound microbiota. PLoS ONE 2009, 4, e6462. [Google Scholar] [CrossRef]
- Wu, Y.K.; Cheng, N.C.; Cheng, C.M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Bacterial chatter in chronic wound infections. Wound Repair. Regen. 2021, 29, 106–116. [Google Scholar] [CrossRef]
- Grey, J.E.; Harding, K.G.; Enoch, S. Venous and arterial leg ulcers. BMJ 2006, 332, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Browse, N.L.; Burnand, K.G. The cause of venous ulceration. Lancet 1982, 2, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Moosa, H.H.; Nemeth, A.J.; Alstadt, S.P.; Eaglstein, W.H. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch. Dermatol. 1987, 123, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Eaglstein, W.H. The “trap” hypothesis of venous ulceration. Lancet 1993, 341, 1006–1008. [Google Scholar] [CrossRef]
- Abbade, L.P.; Lastória, S.; Rollo Hde, A. Venous ulcer: Clinical characteristics and risk factors. Int. J. Dermatol. 2011, 50, 405–411. [Google Scholar] [CrossRef]
- Vlajinac, H.; Marinkovic, J.; Maksimovic, M.; Radak, D. Factors related to venous ulceration: A cross-sectional study. Angiology 2014, 65, 824–830. [Google Scholar] [CrossRef]
- Shibata, K.; Ogai, K.; Ogura, K.; Urai, T.; Aoki, M.; Arisandi, D.; Takahashi, N.; Okamoto, S.; Sanada, H.; Sugama, J. Skin Physiology and its Microbiome as Factors Associated with the Recurrence of Pressure Injuries. Biol. Res. Nurs. 2021, 23, 75–81. [Google Scholar] [CrossRef]
- de Wert, L.A.; Rensen, S.S.; Soons, Z.; Poeze, M.; Bouvy, N.D.; Penders, J. The cutaneous microbiome in hospitalized patients with pressure ulcers. Sci. Rep. 2020, 10, 5963. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Morrissey, K.; Tripet, B.P.; Van Leuven, J.T.; Han, A.; Lazarus, G.S.; Zenilman, J.M.; Stewart, P.S.; James, G.A.; Copié, V. Biochemical Association of Metabolic Profile and Microbiome in Chronic Pressure Ulcer Wounds. PLoS ONE 2015, 10, e0126735. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.E.; Halliwell, B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem. Biophys. Res. Commun. 2012, 423, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Ruegger, P.R.; Lebig, E.G.; VanSchalkwyk, S.; Jeske, D.R.; Hsiao, A.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation. Front. Cell Infect. Microbiol. 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress and Skin Microbiome are Critical for Initiation and Development of Chronic Wounds in Diabetic Mice. Sci. Rep. 2019, 9, 19318. [Google Scholar] [CrossRef]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef]
- Kim, J.H.; Martins-Green, M. Protocol to Create Chronic Wounds in Diabetic Mice. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Kim, J.H.; Dong, J.; Le, B.H.; Lonergan, Z.R.; Gu, W.; Girke, T.; Zhang, W.; Newman, D.K.; Martins-Green, M. Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity. Antioxidants 2024, 13, 655. [Google Scholar] [CrossRef]
- Pletts, M.W.; Burrell, R.E. Clinically relevant evaluation of the antimicrobial and anti-inflammatory properties of nanocrystalline and nanomolecular silver. Wound Repair. Regen. 2025, 33, e13249. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Yadav, R. Manuka honey: A promising wound dressing material for the chronic nonhealing discharging wounds: A retrospective study. Natl. J. Maxillofac. Surg. 2021, 12, 233–237. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Kim, J.H.; Spero, M.; Lebig, E.G.; Lonergan, Z.R.; Trindade, I.B.; Newman, D.K.; Martins-Green, M. Targeting Anaerobic Respiration in Pseudomonas aeruginosa with Chlorate Improves Healing of Chronic Wounds. Adv. Wound Care 2024, 13, 53–69. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.; Wu, J.; Ahamed, A.I.; Wang, Y.; Martins-Green, M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J. Diabetes Res. 2020, 2020, 9589507. [Google Scholar] [CrossRef]
- Eming, S.A.; Tomic-Canic, M. Updates in wound healing: Mechanisms and translation. Exp. Dermatol. 2017, 26, 97–98. [Google Scholar] [CrossRef]
- Pastar, I.; Balukoff, N.C.; Marjanovic, J.; Chen, V.Y.; Stone, R.C.; Tomic-Canic, M. Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harb. Perspect. Biol. 2023, 15, a041243. [Google Scholar] [CrossRef]
- Elliot, S.; Wikramanayake, T.C.; Jozic, I.; Tomic-Canic, M. A Modeling Conundrum: Murine Models for Cutaneous Wound Healing. J. Investig. Dermatol. 2018, 138, 736–740. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Wu, C.; Xiong, Z.F.; Fang, X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review). Mol. Med. Rep. 2015, 12, 2411–2416. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]

| Skin Sites and Physiology | Alpha Diversity | Beta Diversity | Microbial Composition |
|---|---|---|---|
| Dry (hypothenar palm, volar forearm) | High | High interpersonal variation | Actinobacteria (Propionibacterium 13% and Corynebacterium 15%) Firmicutes, Proteobacteria (41%) and Bacteroidetes (14%) |
| Moist (Nare, antecubital fossa, inguinal crease, popliteal fossa) | Low | Low | Colonized predominantly by Firmicutes like Staphylococcus (21%), Corynebacterium spp. (28%), and Proteobacteria (26%) |
| Sebaceous (check, glabella, external auditory canal, occiput, back) | Lower | lower | Colonized predominantly by Propionibacterium spp. (46%) and Staphylococcus (16%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Green, M.; Kim, J.; Aziz, K. The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds. Antioxidants 2025, 14, 682. https://doi.org/10.3390/antiox14060682
Martins-Green M, Kim J, Aziz K. The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds. Antioxidants. 2025; 14(6):682. https://doi.org/10.3390/antiox14060682
Chicago/Turabian StyleMartins-Green, Manuela, Jane Kim, and Klara Aziz. 2025. "The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds" Antioxidants 14, no. 6: 682. https://doi.org/10.3390/antiox14060682
APA StyleMartins-Green, M., Kim, J., & Aziz, K. (2025). The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds. Antioxidants, 14(6), 682. https://doi.org/10.3390/antiox14060682






