Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Assays
2.1.1. Human Microglial Cell Culture
2.1.2. Cell Treatment
2.1.3. ELISA
2.1.4. Real-Time PCR
2.1.5. Western Blot
2.1.6. Flow Cytometry Analysis
2.2. In Vivo Assays
2.2.1. Preparation of Aβ Oligomers
2.2.2. Animal Model and Experimental Design
2.2.3. Behavioral Tests
2.2.4. Sample Collection
2.2.5. Biochemical Analysis
2.2.6. Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
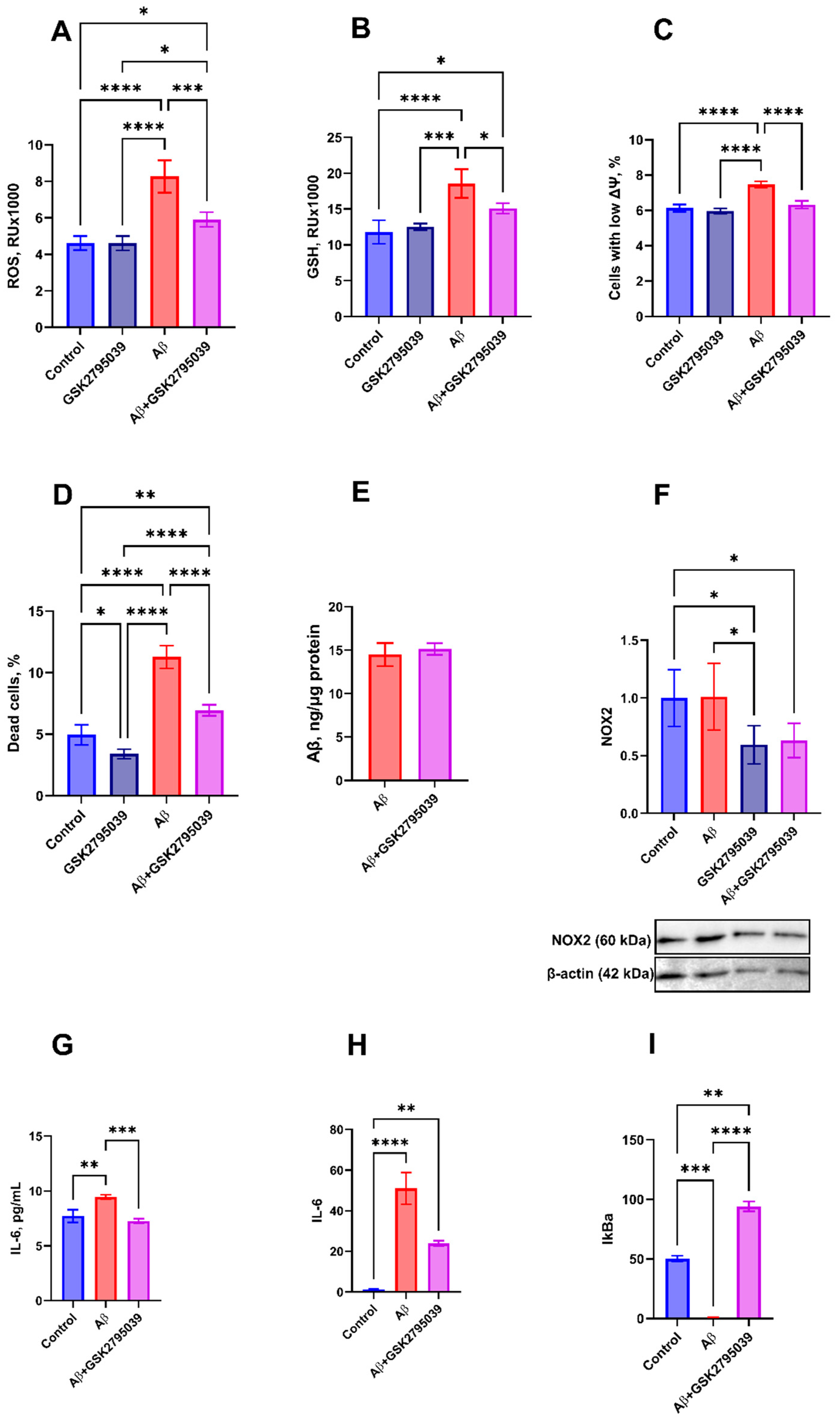
3.1. GSK2795039 Inhibits Oxidative Stress and Inflammatory Response to Aβ in Microglial Cells
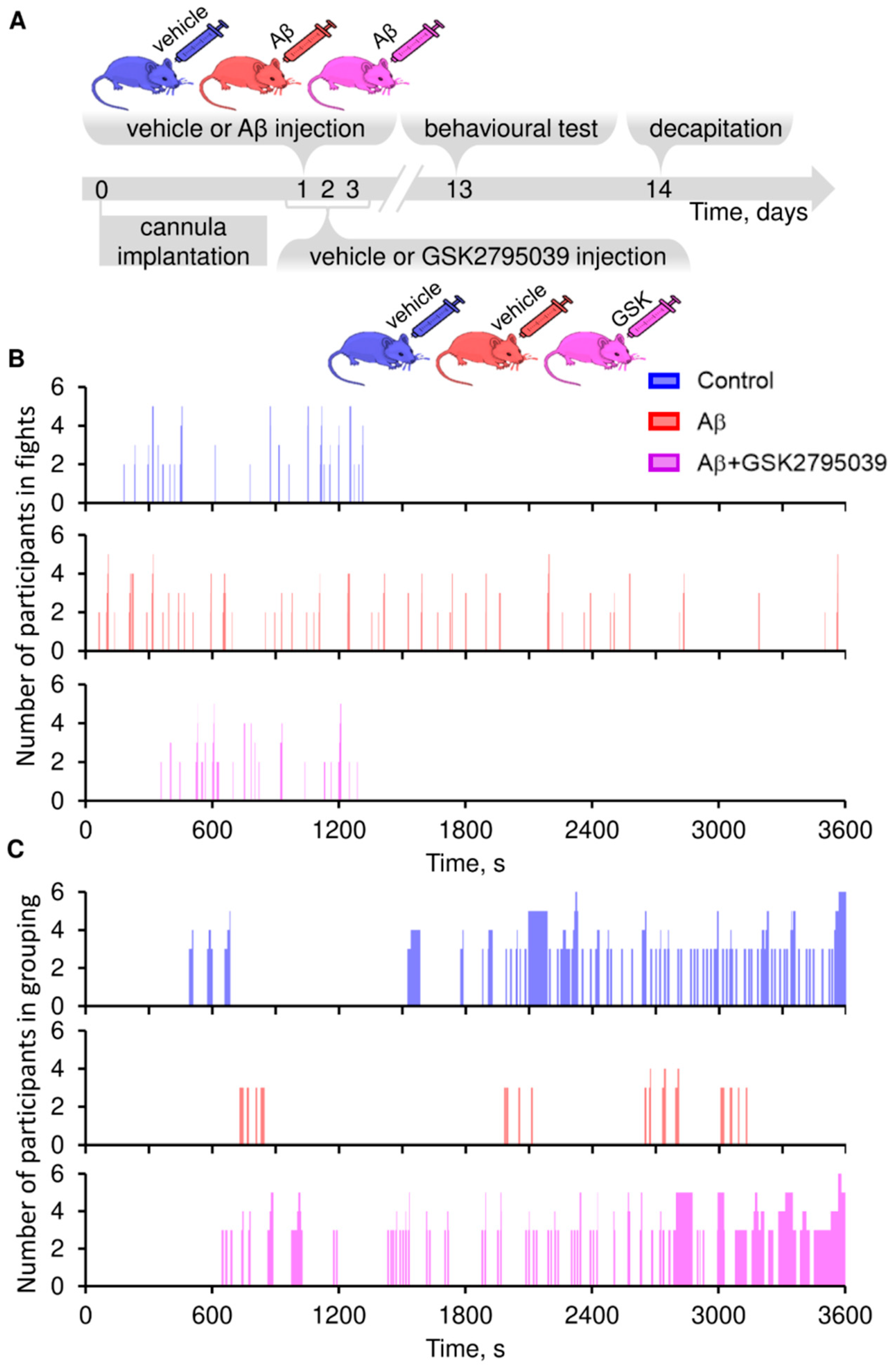
3.2. GSK2795039 Prevents Aβ–Induced Behavioral Abnormalities in Mice
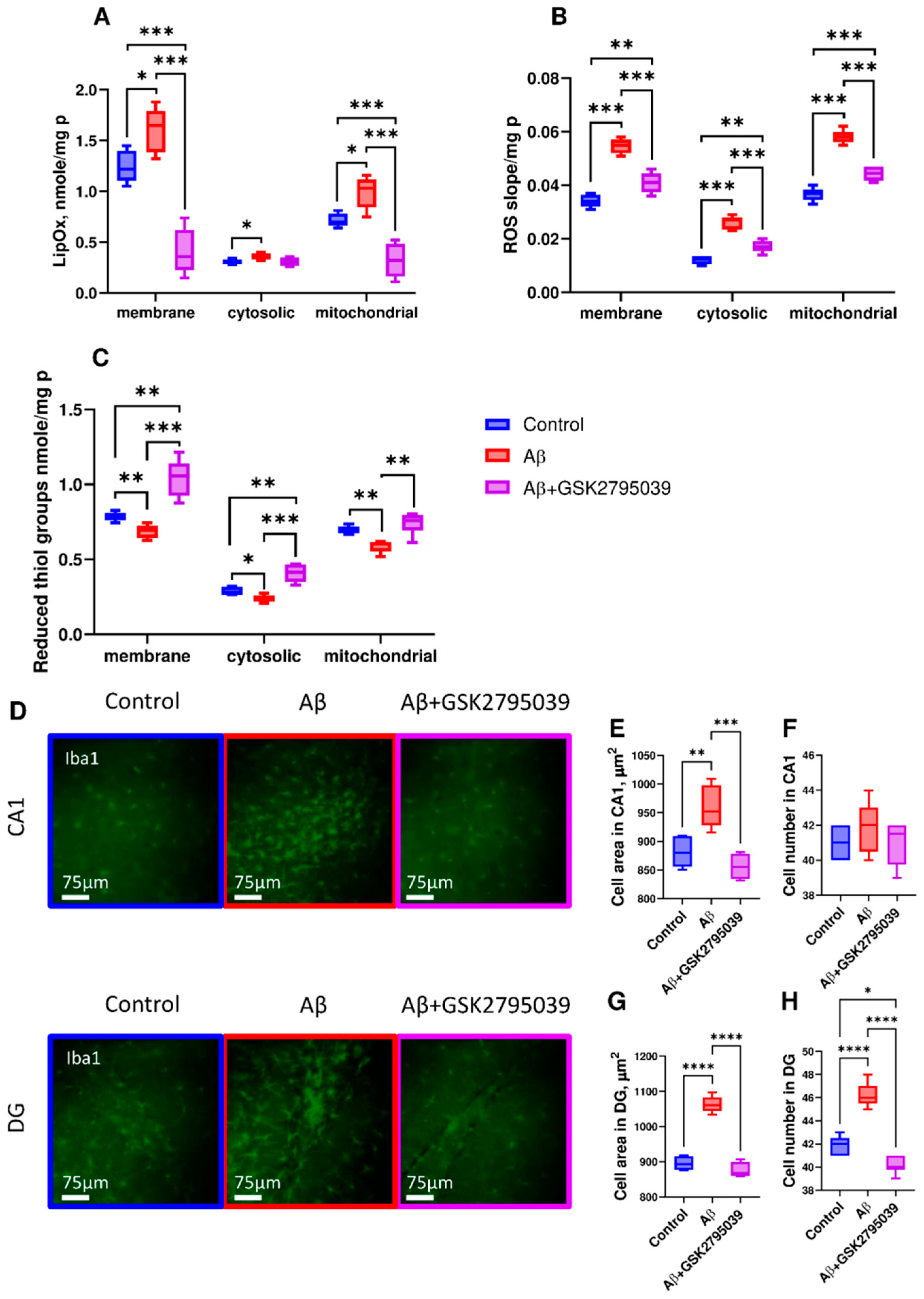
3.3. GSK2795039 Reduces Oxidative Stress and Microglial Activation in the Brain Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glabe, C.G. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging 2006, 27, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, Y.; Tabuena, D.R.; Zilberter, M. NOX-induced oxidative stress is a primary trigger of major neurodegenerative disorders. Prog. Neurobiol. 2023, 231, 102539. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Landreth, G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflamm. 2006, 3, 30. [Google Scholar] [CrossRef][Green Version]
- Mander, P.K.; Jekabsone, A.; Brown, G.C. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006, 176, 1046–1052. [Google Scholar] [CrossRef]
- Gjoneska, E.; Pfenning, A.R.; Mathys, H.; Quon, G.; Kundaje, A.; Tsai, L.-H.; Kellis, M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 2015, 518, 365–369. [Google Scholar] [CrossRef]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Gao, T.; Xiao, H.; Betarbet, R.; Duong, D.M.; Webster, J.A.; Hales, C.M.; Lah, J.J.; et al. Quantitative proteomics of acutely-isolated mouse microglia identifies novel immune Alzheimer’s disease-related proteins. Mol. Neurodegener. 2018, 13, 34. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Althafar, Z.M. Targeting Microglia in Alzheimer’s Disease: From Molecular Mechanisms to Potential Therapeutic Targets for Small Molecules. Molecules 2022, 27, 4124. [Google Scholar] [CrossRef]
- St-Pierre, M.K.; VanderZwaag, J.; Loewen, S.; Tremblay, M.È. All roads lead to heterogeneity: The complex involvement of astrocytes and microglia in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2022, 16, 932572. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, X.; So, K.F.; Jiang, W.; Chiu, K. Targeting Microglia in Alzheimer’s Disease: Pathogenesis and Potential Therapeutic Strategies. Biomolecules 2024, 14, 833. [Google Scholar] [CrossRef]
- Verdier, Y.; Zarándi, M.; Penke, B. Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: Binding sites and implications for Alzheimer’s disease. J. Pept. Sci. 2004, 10, 229–248. [Google Scholar] [CrossRef]
- Ahn, E.H.; Park, J.B. Molecular Mechanisms of Alzheimer’s Disease Induced by Amyloid-β and Tau Phosphorylation Along with RhoA Activity: Perspective of RhoA/Rho-Associated Protein Kinase Inhibitors for Neuronal Therapy. Cells 2025, 14, 89. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Sun, Z.D.; Hu, J.X.; Wu, J.R.; Zhou, B.; Huang, Y.P. Toxicities of amyloid-beta and tau protein are reciprocally enhanced in the Drosophila model. Neural Regen. Res. 2022, 17, 2286–2292. [Google Scholar] [CrossRef]
- Begum, R.; Thota, S.; Abdulkadir, A.; Kaur, G.; Bagam, P.; Batra, S. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell. Mol. Immunol. 2022, 19, 660–686. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Esteras, N.; Kundel, F.; Amodeo, G.F.; Pavlov, E.V.; Klenerman, D.; Abramov, A.Y. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. 2021, 288, 127–141. [Google Scholar] [CrossRef]
- Keeney, M.T.; Hoffman, E.K.; Farmer, K.; Bodle, C.R.; Fazzari, M.; Zharikov, A.; Castro, S.L.; Hu, X.; Mortimer, A.; Kofler, J.K.; et al. NADPH oxidase 2 activity in Parkinson’s disease. Neurobiol. Dis. 2022, 170, 105754. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.; Popova, I.; Ivanov, A.; Jang, S.-S.; Yoon, S.Y.; Osypov, A.; Huang, Y.; Zilberter, Y.; Zilberter, M. Aβ initiates brain hypometabolism, network dysfunction and behavioral abnormalities via NOX2-induced oxidative stress in mice. Commun. Biol. 2021, 4, 1054. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Waghela, B.N.; Vaidya, F.U.; Agrawal, Y.; Santra, M.K.; Mishra, V.; Pathak, C. Molecular insights of NADPH oxidases and its pathological consequences. Cell Biochem. Funct. 2021, 39, 218–234. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, L.; Hong, J.S.; Zhang, D.; Zhao, J.; Wang, Q. Nicotinamide Adenine Dinucleotide Phosphate Oxidase and Neurodegenerative Diseases: Mechanisms and Therapy. Antioxid. Redox Signal. 2020, 33, 374–393. [Google Scholar] [CrossRef]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef]
- Geng, L.; Fan, L.M.; Liu, F.; Smith, C.; Li, J.-M. Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Chéret, C.; Gervais, A.; Lelli, A.; Colin, C.; Amar, L.; Ravassard, P.; Mallet, J.; Cumano, A.; Krause, K.H.; Mallat, M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J. Neurosci. 2008, 28, 12039–12051. [Google Scholar] [CrossRef]
- Dohi, K.; Ohtaki, H.; Nakamachi, T.; Yofu, S.; Satoh, K.; Miyamoto, K.; Song, D.; Tsunawaki, S.; Shioda, S.; Aruga, T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflamm. 2010, 7, 41. [Google Scholar] [CrossRef]
- Mason, H.; Rai, G.; Kozyr, A.; De Jonge, N.; Gliniewicz, E.; Berg, L.J.; Wald, G.; Dorrier, C.; Henderson, M.J.; Zakharov, A.; et al. Development of an improved and specific inhibitor of NADPH oxidase 2 to treat traumatic brain injury. Redox Biol. 2023, 60, 102611. [Google Scholar] [CrossRef]
- Juric, M.; Rawat, V.; Amaradhi, R.; Zielonka, J.; Ganesh, T. Novel NADPH Oxidase-2 Inhibitors as Potential Anti-Inflammatory and Neuroprotective Agents. Antioxidants 2023, 12, 1660. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, S.; Magi, S.; Preziuso, A.; Serfilippi, T.; Cerqueni, G.; Orciani, M.; Amoroso, S.; Lariccia, V. The Hidden Notes of Redox Balance in Neurodegenerative Diseases. Antioxidants 2022, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Nocella, C.; D’amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G.; et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. [Google Scholar] [CrossRef]
- Hirano, K.; Chen, W.S.; Chueng, A.L.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G.; et al. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Padilha, E.C.; Shah, P.; Rai, G.; Xu, X. NOX2 inhibitor GSK2795039 metabolite identification towards drug optimization. J. Pharm. Biomed. Anal. 2021, 201, 114102. [Google Scholar] [CrossRef]
- Diebold, B.A.; Smith, S.M.; Li, Y.; Lambeth, J.D. NOX2 As a Target for Drug Development: Indications, Possible Complications, and Progress. Antioxid. Redox Signal. 2015, 23, 375–405. [Google Scholar] [CrossRef] [PubMed]
- Potapov, K.V.; Platonov, D.N.; Belyy, A.Y.; Novikov, M.A.; Tomilov, Y.V.; Anashkina, A.A.; Mukhina, K.A.; Kechko, O.I.; Solyev, P.N.; Novikov, R.A.; et al. Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo[2,3-b]pyridine-Based Inhibitors of NADPH Oxidase 2. Int. J. Mol. Sci. 2025, 26, 3647. [Google Scholar] [CrossRef]
- Akhter, R.; Shao, Y.; Formica, S.; Khrestian, M.; Bekris, L.M. TREM2 Alters the Phagocytic, Apoptotic and Inflammatory Response to Aβ42 in HMC3 Cells. Mol. Immunol. 2021, 131, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Varshavskaya, K.B.; Petrushanko, I.Y.; Mitkevich, V.A.; Barykin, E.P.; Makarov, A.A. Post-translational modifications of beta-amyloid alter its transport in the blood-brain barrier in vitro model. Front. Mol. Neurosci. 2024, 17, 1362581. [Google Scholar] [CrossRef] [PubMed]
- Wander, C.M.; Li, Y.D.; Bao, H.; Asrican, B.; Luo, Y.J.; Sullivan, H.A.; Chao, T.H.; Zhang, W.T.; Chéry, S.L.; Tart, D.S.; et al. Compensatory Remodeling of a Septo-Hippocampal GABAergic Network in the Triple Transgenic Alzheimer’s Mouse Model. J. Transl. Med. 2023, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Power, S.K.; Venkatesan, S.; Qu, S.; McLaurin, J.; Lambe, E.K. Enhanced Prefrontal Nicotinic Signaling as Evidence of Active Compensation in Alzheimer’s Disease Models. Transl. Neurodegener. 2024, 13, 58. [Google Scholar] [CrossRef]
- Luo, F.; Rustay, N.R.; Ebert, U.; Hradil, V.P.; Cole, T.B.; Llano, D.A.; Mudd, S.R.; Zhang, Y.; Fox, G.B.; Day, M. Characterization of 7- and 19-Month-Old Tg2576 Mice Using Multimodal In Vivo Imaging: Limitations as a Translatable Model of Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 933–944. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Tang, X.; Walter, E.; Wohleb, E.; Fan, Y.; Wang, C. ATG5 (Autophagy Related 5) in Microglia Controls Hippocampal Neurogenesis in Alzheimer Disease. Autophagy 2024, 20, 847–862. [Google Scholar] [CrossRef]
- Lindenau, J.; Noack, H.; Asayama, K.; Wolf, G. Enhanced Cellular Glutathione Peroxidase Immunoreactivity in Activated Astrocytes and in Microglia During Excitotoxin Induced Neurodegeneration. Glia 1998, 24, 252–256. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Gutterer, J.M.; Kussmaul, L.; Hamprecht, B.; Dringen, R. Microglial Cells in Culture Express a Prominent Glutathione System for the Defense Against Reactive Oxygen Species. Dev. Neurosci. 2000, 22, 384–392. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [PubMed]
- Mitkevich, V.A.; Petrushanko, I.Y.; Yegorov, Y.E.; Simonenko, O.V.; Vishnyakova, K.S.; Kulikova, A.A.; Tsvetkov, P.O.; Makarov, A.A.; Kozin, S.A. Isomerization of Asp7 Leads to Increased Toxic Effect of Amyloid-β42 on Human Neuronal Cells. Cell Death Dis. 2013, 4, e939. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Tu, D.; Velagapudi, R.; Gao, Y.; Hong, J.S.; Zhou, H.; Gao, H.M. Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration. Free Radic. Biol. Med. 2023, 200, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Lin, L.; Ren, J.; He, R.; Sun, K. Inhibition of NADPH oxidase 2 (NOX2) reverses cognitive deficits by modulating excitability and excitatory transmission in the hippocampus after traumatic brain injury. Biochem. Biophys. Res. Commun. 2022, 617, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Luo, L. An Effective NADPH Oxidase 2 Inhibitor Provides Neuroprotection and Improves Functional Outcomes in Animal Model of Traumatic Brain Injury. Neurochem. Res. 2020, 45, 1097–1106. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Li, G.G.; Yu, H.H.; Bai, S.; Guo, G.Y.; Guo, W.L.; Ma, Y.; Wang, J.H.; Liu, N.; et al. P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage. Neural Regen. Res. 2021, 16, 1582–1591. [Google Scholar] [CrossRef]
- Peng, L.; Ji, Y.; Li, Y.; You, Y.; Zhou, Y. PRDX6-iPLA2 aggravates neuroinflammation after ischemic stroke via regulating astrocytes-induced M1 microglia. Cell Commun. Signal. 2024, 22, 76. [Google Scholar] [CrossRef]
- Teixeira-Santos, L.; Veríssimo, E.; Martins, S.; Sousa, T.; Albino-Teixeira, A.; Pinho, D. Effects of NADPH Oxidase Isoform-2 (NOX2) Inhibition on Behavioral Responses and Neuroinflammation in a Mouse Model of Neuropathic Pain. Biomedicines 2023, 11, 416. [Google Scholar] [CrossRef]
- Okada, N.; Nakamura, S.; Shimazawa, M. 3-Nitropropionic Acid Enhances Ferroptotic Cell Death via NOX2-Mediated ROS Generation in STHdhQ111 Striatal Cells Carrying Mutant Huntingtin. Biol. Pharm. Bull. 2023, 46, 177–186. [Google Scholar] [CrossRef]
- Firdous, S.M.; Khan, S.A.; Maity, A. Oxidative stress-mediated neuroinflammation in Alzheimer’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8189–8209. [Google Scholar] [CrossRef] [PubMed]
- Baligács, N.; Albertini, G.; Borrie, S.C.; Serneels, L.; Pridans, C.; Balusu, S.; De Strooper, B. Homeostatic microglia initially seed and activated microglia later reshape amyloid plaques in Alzheimer’s Disease. Nat. Commun. 2024, 15, 10634. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.; da Cruz, E.; Silva, O.A.B.; Henriques, A.G. Impact of Cytokines and Chemokines on Alzheimer’s Disease Neuropathological Hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Chen, C.; Lu, J.; Peng, W.; Mak, M.S.; Yang, Y.; Zhu, Z.; Wang, S.; Hou, J.; Zhou, X.; Xin, W.; et al. Acrolein, an endogenous aldehyde induces Alzheimer’s disease-like pathologies in mice: A new sporadic AD animal model. Pharmacol. Res. 2022, 175, 106003. [Google Scholar] [CrossRef]
- Righi, D.; Manco, C.; Pardini, M.; Stufano, A.; Schino, V.; Pelagotti, V.; Massa, F.; De Stefano, N.; Plantone, D. Investigating interleukin-8 in Alzheimer’s disease: A comprehensive review. J. Alzheimers Dis. 2025, 103, 38–55. [Google Scholar] [CrossRef]
- Ju Hwang, C.; Choi, D.Y.; Park, M.H.; Hong, J.T. NF-κB as a Key Mediator of Brain Inflammation in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 3–10. [Google Scholar] [CrossRef]
- Codocedo, J.F.; Mera-Reina, C.; Bor-Chian Lin, P.; Fallen, P.B.; Puntambekar, S.S.; Casali, B.T.; Jury-Garfe, N.; Martinez, P.; Lasagna-Reeves, C.A.; Landreth, G.E. Therapeutic targeting of immunometabolism reveals a critical reliance on hexokinase 2 dosage for microglial activation and Alzheimer’s progression. Cell Rep. 2024, 43, 114488. [Google Scholar] [CrossRef]
- Aalten, P.; Verhey, F.R.; Boziki, M.; Bullock, R.; Byrne, E.J.; Camus, V.; Caputo, M.; Collins, D.; De Deyn, P.P.; Elina, K.; et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: Part I. Dement. Geriatr. Cogn. Disord. 2007, 24, 457–463. [Google Scholar] [CrossRef]
- Kapasi, A.; Yu, L.; Leurgans, S.E.; Agrawal, S.; Boyle, P.A.; Bennett, D.A.; Schneider, J.A. Association between hippocampal microglia, AD and LATE-NC, and cognitive decline in older adults. Alzheimers Dement. 2024, 20, 3193–3202. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemèle, E.J.; Fulton, D.J.R.; Fukai, T. Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Dalle-Donne, I. Redox proteomics. Antioxid. Redox Signal. 2012, 17, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Peralta, D.; Bronowska, A.K.; Morgan, B.; Dóka, É.; Van Laer, K.; Nagy, P.; Gräter, F.; Dick, T.P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015, 11, 156–163. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.-K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Tang, Y.; Liow, J.-S.; Zhang, Z.; Li, J.; Long, T.; Li, Y.; Tang, B.; Hu, S. The Evaluation of Dynamic FDG-PET for Detecting Epileptic Foci and Analyzing Reduced Glucose Phosphorylation in Refractory Epilepsy. Front. Neurosci. 2018, 12, 993. [Google Scholar] [CrossRef]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and Signaling: Cell Logistics Shape the Eukaryotic Cell Plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Sequence | NCBI Reference Sequence |
|---|---|---|
| IL-6-F | 5′-GGAGCCCAGCTATGAACTCC-3′ | NM_000600.5 |
| IL-6-R | 5′-GGTCAGGGGTGGTTATTGCA-3′ | |
| IkBa-F | 5′-GTGGGGCTGATGTCAACAGA-3′ | NM_020529.3 |
| IkBa-R | 5′-GGTCAGTCACTCGAAGCACA-3′ | |
| GAPDH-F | 5′-TGCACCACCAACTGCTTAC-3′ | NM_001256799.3 |
| GAPDH-R | 5′-GGCATGGACTGTGGTCATGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhina, K.A.; Kechko, O.I.; Osypov, A.A.; Petrushanko, I.Y.; Makarov, A.A.; Mitkevich, V.A.; Popova, I.Y. Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice. Antioxidants 2025, 14, 663. https://doi.org/10.3390/antiox14060663
Mukhina KA, Kechko OI, Osypov AA, Petrushanko IY, Makarov AA, Mitkevich VA, Popova IY. Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice. Antioxidants. 2025; 14(6):663. https://doi.org/10.3390/antiox14060663
Chicago/Turabian StyleMukhina, Kristina A., Olga I. Kechko, Alexander A. Osypov, Irina Yu. Petrushanko, Alexander A. Makarov, Vladimir A. Mitkevich, and Irina Yu. Popova. 2025. "Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice" Antioxidants 14, no. 6: 663. https://doi.org/10.3390/antiox14060663
APA StyleMukhina, K. A., Kechko, O. I., Osypov, A. A., Petrushanko, I. Y., Makarov, A. A., Mitkevich, V. A., & Popova, I. Y. (2025). Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice. Antioxidants, 14(6), 663. https://doi.org/10.3390/antiox14060663







