Exo70 Protects Against Memory and Synaptic Impairments Following Mild Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Animals
2.3. Lentiviral Transduction in HEK293 Cells
2.4. Intrahippocampal Lentiviral Injection
2.5. Immunohistochemistry
2.6. Western Blot Analysis
2.7. Mild Traumatic Brain Injury (mTBI) Procedure
2.8. Behavioral Tests
2.9. Electrophysiology
2.10. Image and Statistical Analysis
3. Results
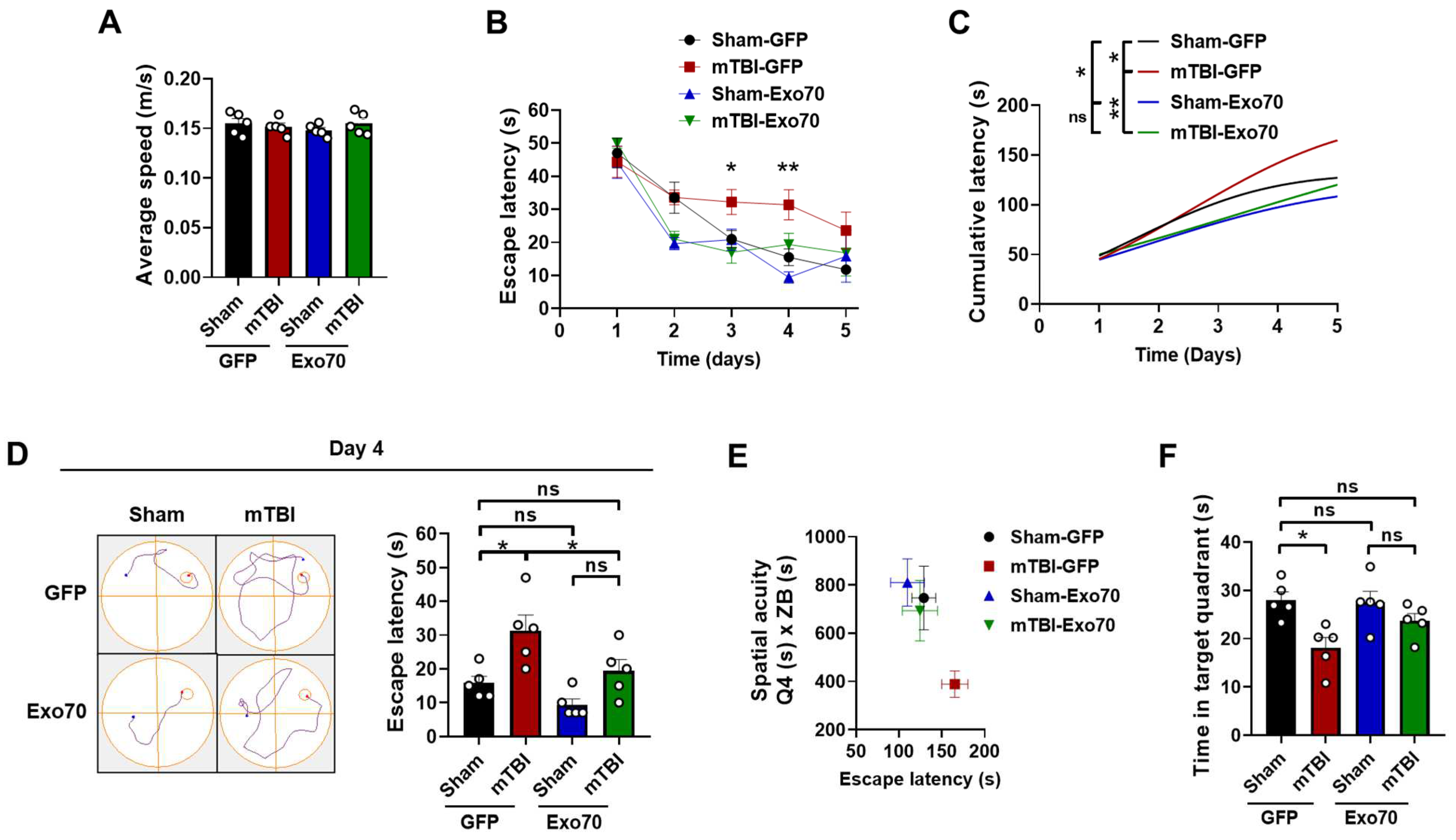
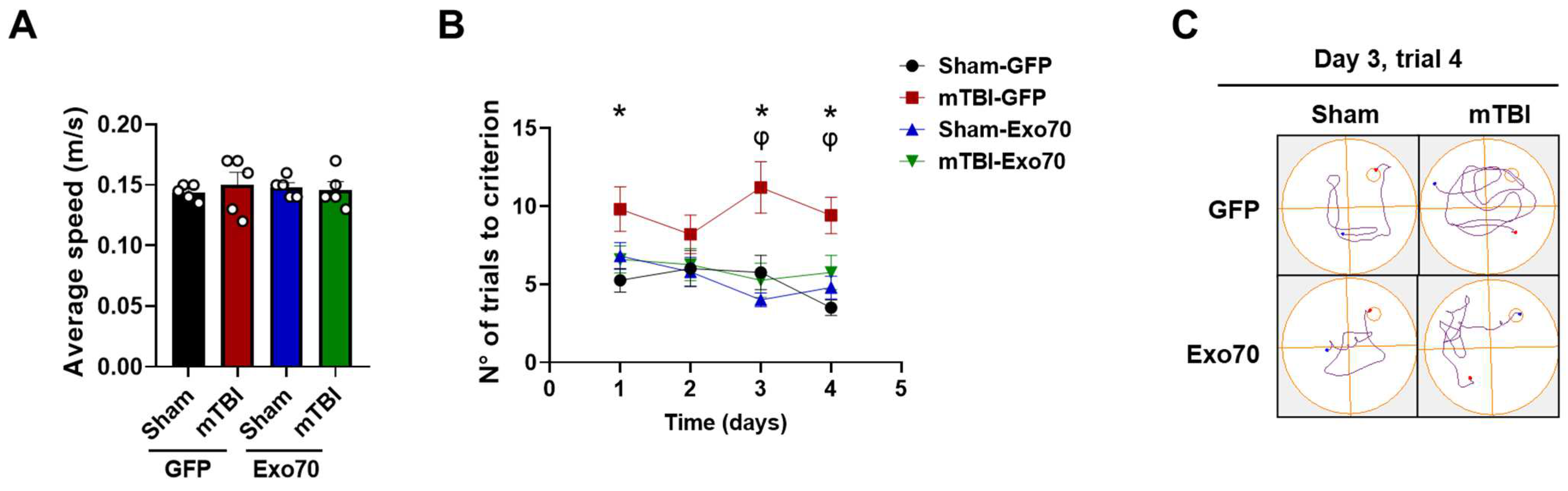
3.1. Evaluation of Exo70 Overexpression in Learning and Memory
3.2. Synaptic Transmission Evaluation
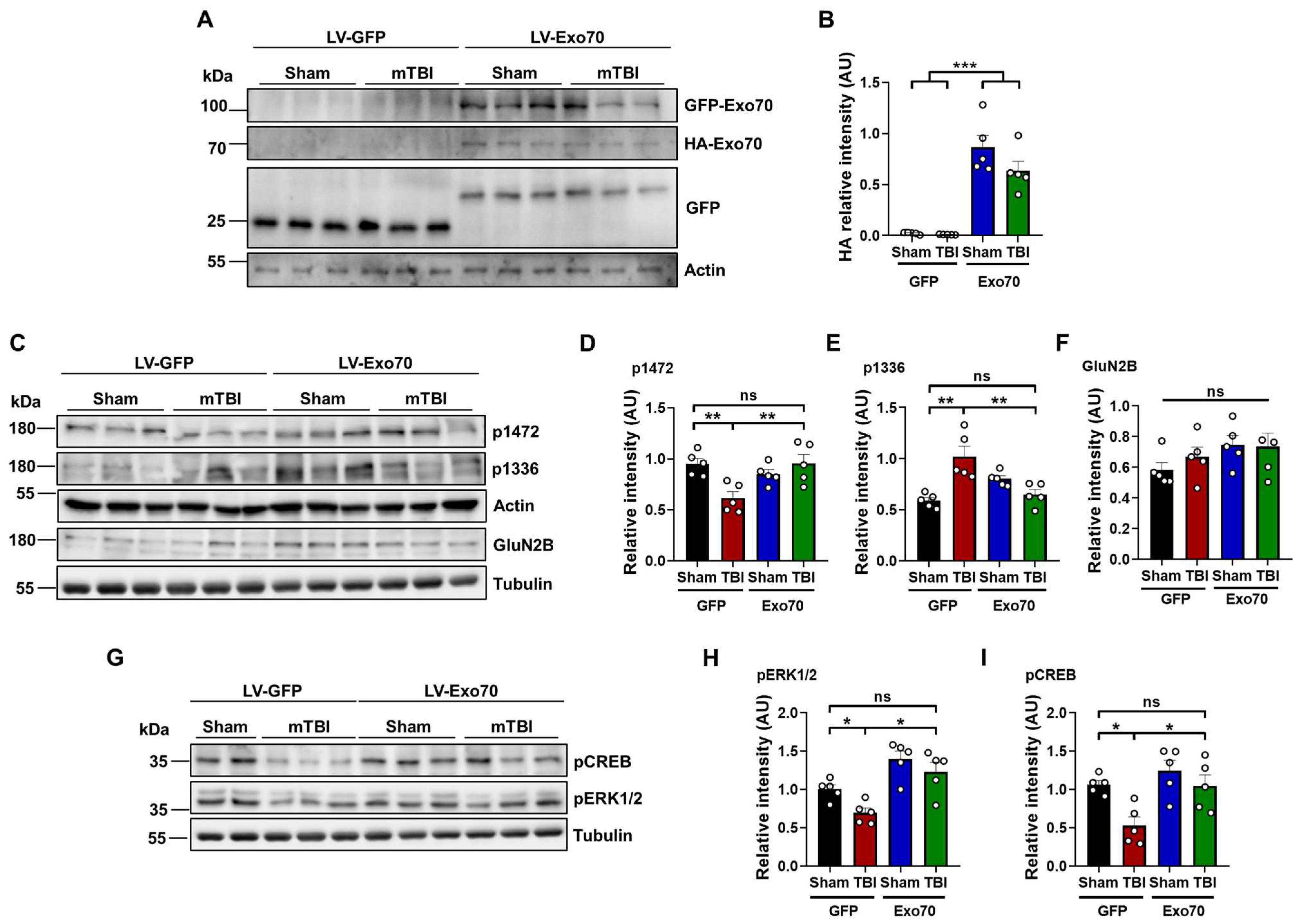
3.3. NMDAR Synaptic Availability
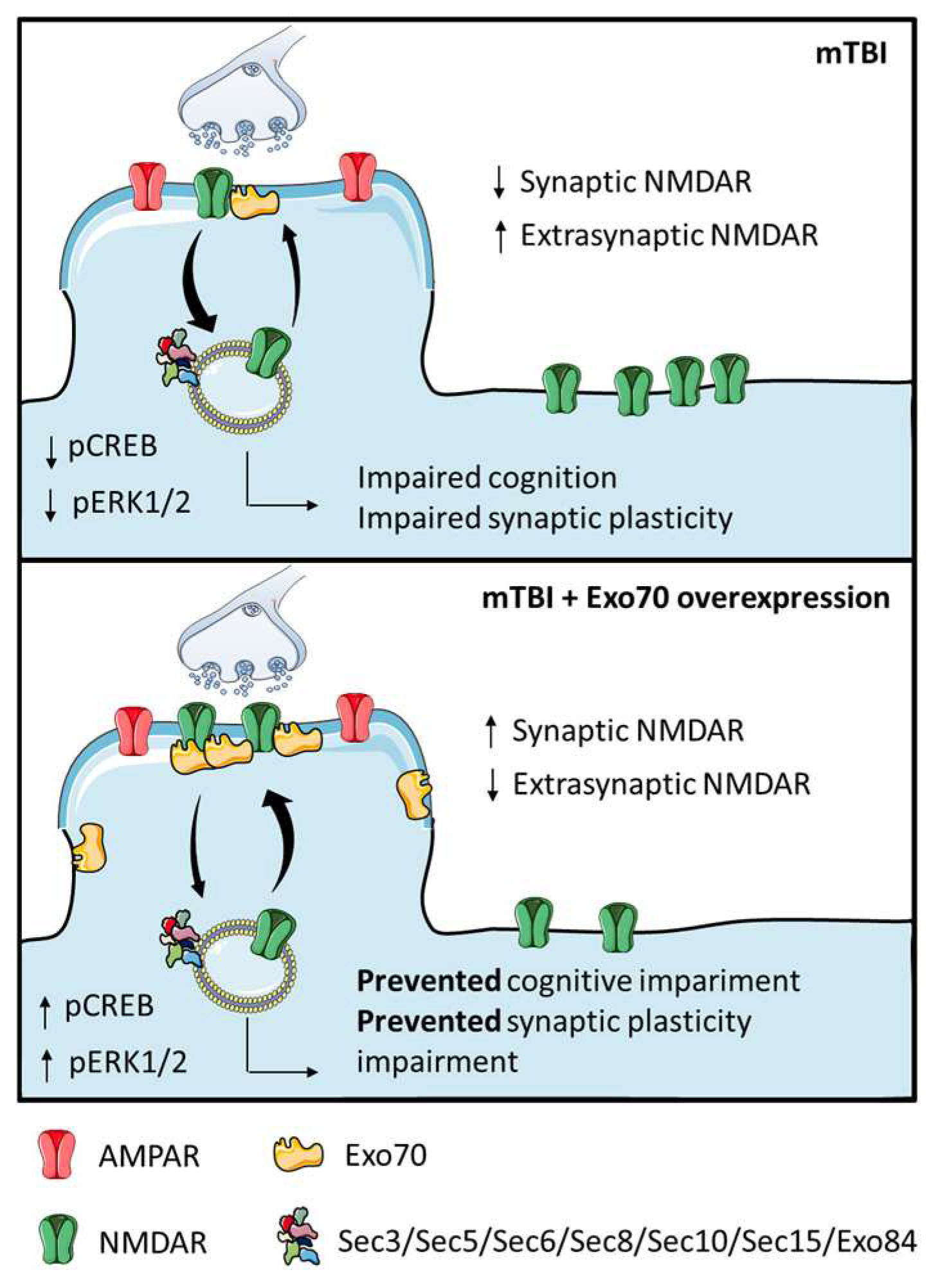
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I.; The Demographics and Clinical Assessment Working Group of the International; Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury; Psychological Health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Sabella, S.A.; Andrzejewski, J.H.; Wallgren, A. Financial hardship after traumatic brain injury: A brief scale for family caregivers. Brain Inj. 2018, 32, 926–932. [Google Scholar] [CrossRef]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Aubry, M.; Cantu, B.; Dvorak, J.; Echemendia, R.; Engebretsen, L.; Johnston, K.; Kutcher, J.; Raftery, M.; et al. Consensus statement on Concussion in Sport—The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2013, 14, e1–e13. [Google Scholar] [CrossRef]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Primers 2016, 2, 16084. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A. Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13000. [Google Scholar] [CrossRef]
- Davis, C.K.; Vemuganti, R. Antioxidant therapies in traumatic brain injury. Neurochem. Int 2022, 152, 105255. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Patel, A.B.; Kioutchoukova, I.P.; Diaz, M.J.; Lucke-Wold, B. Mechanisms of Mitochondrial Oxidative Stress in Brain Injury: From Pathophysiology to Therapeutics. Oxygen 2023, 3, 163–178. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Mattison, H.A.; Cerpa, W. Role of NMDA Receptor-Mediated Glutamatergic Signaling in Chronic and Acute Neuropathologies. Neural Plast. 2016, 2016, 2701526. [Google Scholar] [CrossRef]
- Babaei, P. NMDA and AMPA receptors dysregulation in Alzheimer’s disease. Eur. J. Pharmacol. 2021, 908, 174310. [Google Scholar] [CrossRef]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef]
- Mira, R.G.; Lira, M.; Quintanilla, R.A.; Cerpa, W. Alcohol consumption during adolescence alters the hippocampal response to traumatic brain injury. Biochem. Biophys. Res. Commun. 2020, 528, 514–519. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Lin, Y.; Tan, T.; Yang, Z.; Dayao, C.; Liu, L.; Jiang, R.; Zhang, J. Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res. 2011, 1382, 165–172. [Google Scholar] [CrossRef]
- Miller, L.P.; Lyeth, B.G.; Jenkins, L.W.; Oleniak, L.; Panchision, D.; Hamm, R.J.; Phillips, L.L.; Dixon, C.E.; Clifton, G.L.; Hayes, R.L. Excitatory amino acid receptor subtype binding following traumatic brain injury. Brain Res. 1990, 526, 103–107. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Nicosia, N.; Giovenzana, M.; Misztak, P.; Mingardi, J.; Musazzi, L. Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders. Int. J. Mol. Sci. 2024, 25, 6521. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP61 Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. [Google Scholar] [CrossRef]
- Park, Y.; Luo, T.; Zhang, F.; Liu, C.; Bramlett, H.M.; Dietrich, W.D.; Hu, B. Downregulation of Src-kinase and glutamate-receptor phosphorylation after traumatic brain injury. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1642–1649. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Mira, R.G.; Rovegno, M.; Minniti, A.N.; Cerpa, W. Age-related NMDA signaling alterations in SOD2 deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2010–2020. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sacher, M.; Barrowman, J.; Ferro-Novick, S.; Novick, P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000, 10, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Heider, M.R.; Munson, M. Exorcising the exocyst complex. Traffic 2012, 13, 898–907. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Transport-vesicle targeting: Tethers before SNAREs. Nat. Cell Biol. 1999, 1, E17–E22. [Google Scholar] [CrossRef]
- Whyte, J.R.; Munro, S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002, 115 Pt 13, 2627–2637. [Google Scholar] [CrossRef]
- Lira, M.; Mira, R.G.; Carvajal, F.J.; Zamorano, P.; Inestrosa, N.C.; Cerpa, W. Glutamatergic Receptor Trafficking and Delivery: Role of the Exocyst Complex. Cells 2020, 9, 2402. [Google Scholar] [CrossRef] [PubMed]
- Martin-Urdiroz, M.; Deeks, M.J.; Horton, C.G.; Dawe, H.R.; Jourdain, I. The Exocyst Complex in Health and Disease. Front. Cell Dev. Biol. 2016, 4, 24. [Google Scholar] [CrossRef]
- Hazuka, C.D.; Foletti, D.L.; Hsu, S.C.; Kee, Y.; Hopf, F.W.; Scheller, R.H. The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 1324–1334. [Google Scholar] [CrossRef]
- Peng, Y.; Lee, J.; Rowland, K.; Wen, Y.; Hua, H.; Carlson, N.; Lavania, S.; Parrish, J.Z.; Kim, M.D. Regulation of dendrite growth and maintenance by exocytosis. J. Cell Sci. 2015, 128, 4279–4292. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, S.; Grassi, D.; Bernis, M.E.; Sosa, L.; Bisbal, M.; Gastaldi, L.; Jausoro, I.; Caceres, A.; Pfenninger, K.H.; Quiroga, S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13292–13301. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Backos, D.S.; Rupasinghe, C.N.; Spaller, M.R.; Esteban, J.A. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006, 25, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Sans, N.; Prybylowski, K.; Petralia, R.S.; Chang, K.; Wang, Y.X.; Racca, C.; Vicini, S.; Wenthold, R.J. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 2003, 5, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Guo, W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev. Cell 2012, 22, 967–978. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, M.Q.; van de Ven, W.; Zhang, Z.M.; Wu, B.; Young, M.C.; Synek, L.; Borchardt, D.; Harrison, R.; Pan, S.; et al. Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc. Natl. Acad. Sci. USA 2016, 113, E41–E50. [Google Scholar] [CrossRef]
- Lira, M.; Arancibia, D.; Orrego, P.R.; Montenegro-Venegas, C.; Cruz, Y.; Garcia, J.; Leal-Ortiz, S.; Godoy, J.A.; Gundelfinger, E.D.; Inestrosa, N.C.; et al. The Exocyst Component Exo70 Modulates Dendrite Arbor Formation, Synapse Density, and Spine Maturation in Primary Hippocampal Neurons. Mol. Neurobiol. 2019, 56, 4620–4638. [Google Scholar] [CrossRef]
- Borsani, G.; Piovani, G.; Zoppi, N.; Bertini, V.; Bini, R.; Notarangelo, L.; Barlati, S. Cytogenetic and molecular characterization of a de-novo t(2p;7p) translocation involving TNS3 and EXOC6B genes in a boy with a complex syndromic phenotype. Eur. J. Med. Genet. 2008, 51, 292–302. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Nishida-Fukuda, H.; Li, Y.; McDonald, W.H.; Gradinaru, C.C.; Macara, I.G. Exocyst dynamics during vesicle tethering and fusion. Nat. Commun. 2018, 9, 5140. [Google Scholar] [CrossRef]
- Boyd, C.; Hughes, T.; Pypaert, M.; Novick, P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004, 167, 889–901. [Google Scholar] [CrossRef]
- Hamburger, Z.A.; Hamburger, A.E.; West, A.P., Jr.; Weis, W.I. Crystal structure of the S.cerevisiae exocyst component Exo70p. J. Mol. Biol. 2006, 356, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Fruhmesser, A.; Blake, J.; Haberlandt, E.; Baying, B.; Raeder, B.; Runz, H.; Spreiz, A.; Fauth, C.; Benes, V.; Utermann, G.; et al. Disruption of EXOC6B in a patient with developmental delay, epilepsy, and a de novo balanced t(2;8) translocation. Eur. J. Hum. Genet. EJHG 2013, 21, 1177–1180. [Google Scholar] [CrossRef]
- Wen, J.; Lopes, F.; Soares, G.; Farrell, S.A.; Nelson, C.; Qiao, Y.; Martell, S.; Badukke, C.; Bessa, C.; Ylstra, B.; et al. Phenotypic and functional consequences of haploinsufficiency of genes from exocyst and retinoic acid pathway due to a recurrent microdeletion of 2p13.2. Orphanet J. Rare Dis. 2013, 8, 100. [Google Scholar] [CrossRef]
- Shalata, A.; Lauhasurayotin, S.; Leibovitz, Z.; Li, H.; Hebert, D.; Dhanraj, S.; Hadid, Y.; Mahroum, M.; Bajar, J.; Egenburg, S.; et al. Biallelic mutations in EXOC3L2 cause a novel syndrome that affects the brain, kidney and blood. J. Med. Genet. 2019, 56, 340–346. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Ahmed, S.M.; Collins, F.; Cowley, M.; Vetro, A.; Dale, R.C.; Hock, D.H.; de Caestecker, C.; Menezes, M.; Massey, S.; et al. Mutations in the exocyst component EXOC2 cause severe defects in human brain development. J. Exp. Med. 2020, 217, e20192040. [Google Scholar] [CrossRef]
- Miller, J.E.; Shivakumar, M.K.; Lee, Y.; Han, S.; Horgousluoglu, E.; Risacher, S.L.; Saykin, A.J.; Nho, K.; Kim, D.; Alzheimer’s Disease Neuroimaging Initiative. Rare variants in the splicing regulatory elements of EXOC3L4 are associated with brain glucose metabolism in Alzheimer’s disease. BMC Med. Genom. 2018, 11 (Suppl. S3), 76. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.; Zamorano, P.; Cerpa, W. Exo70 intracellular redistribution after repeated mild traumatic brain injury. Biol. Res. 2021, 54, 5. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.P.; Chen, X.F.; Xu, X.S.; Zhang, N.; Li, M.; Li, J.N.; Zhang, L.; Zhang, D.; Zhang, X.; Mao, R.R.; et al. A Temporal Activity of CA1 Neurons Underlying Short-Term Memory for Social Recognition Altered in PTEN Mouse Models of Autism Spectrum Disorder. Front. Cell. Neurosci. 2021, 15, 699315. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kang, M.; Kim, Y.S.; Kim, D.H.; Nam, D.W.; Song, E.J.; Mook-Jung, I.; Moon, M. Intrahippocampal injection of a lentiviral vector expressing neurogranin enhances cognitive function in 5XFAD mice. Exp. Mol. Med. 2018, 50, e461. [Google Scholar] [CrossRef]
- Cerpa, W.; Hancke, J.L.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Marin, P.P.; Inestrosa, N.C. The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer’s mouse model. Curr. Alzheimer Res. 2010, 7, 126–133. [Google Scholar] [CrossRef]
- Kilbourne, M.; Kuehn, R.; Tosun, C.; Caridi, J.; Keledjian, K.; Bochicchio, G.; Scalea, T.; Gerzanich, V.; Simard, J.M. Novel model of frontal impact closed head injury in the rat. J. Neurotrauma 2009, 26, 2233–2243. [Google Scholar] [CrossRef]
- Risling, T.E.; Caulkett, N.A.; Florence, D. Open-drop anesthesia for small laboratory animals. Can. Vet. J. La Rev. Vet. Can. 2012, 53, 299–302. [Google Scholar]
- Minerbi, A.; Kahana, R.; Goldfeld, L.; Kaufman, M.; Marom, S.; Ziv, N.E. Long-term relationships between synaptic tenacity, synaptic remodeling, and network activity. PLoS Biol. 2009, 7, e1000136. [Google Scholar] [CrossRef]
- Barbash, S.; Hanin, G.; Soreq, H. Stereotactic injection of microRNA-expressing lentiviruses to the mouse hippocampus ca1 region and assessment of the behavioral outcome. J. Vis. Exp. 2013, 76, e50170. [Google Scholar] [CrossRef]
- Kanninen, K.; Heikkinen, R.; Malm, T.; Rolova, T.; Kuhmonen, S.; Leinonen, H.; Yla-Herttuala, S.; Tanila, H.; Levonen, A.L.; Koistinaho, M.; et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16505–16510. [Google Scholar] [CrossRef]
- Farook, J.M.; Shields, J.; Tawfik, A.; Markand, S.; Sen, T.; Smith, S.B.; Brann, D.; Dhandapani, K.M.; Sen, N. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013, 4, e754. [Google Scholar] [CrossRef]
- Marschner, L.; Schreurs, A.; Lechat, B.; Mogensen, J.; Roebroek, A.; Ahmed, T.; Balschun, D. Single mild traumatic brain injury results in transiently impaired spatial long-term memory and altered search strategies. Behav. Brain Res. 2019, 365, 222–230. [Google Scholar] [CrossRef]
- Xu, X.; Cowan, M.; Beraldo, F.; Schranz, A.; McCunn, P.; Geremia, N.; Brown, Z.; Patel, M.; Nygard, K.L.; Khazaee, R.; et al. Repetitive mild traumatic brain injury in mice triggers a slowly developing cascade of long-term and persistent behavioral deficits and pathological changes. Acta Neuropathol. Commun. 2021, 9, 60. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef]
- Xu, F.; Plummer, M.R.; Len, G.W.; Nakazawa, T.; Yamamoto, T.; Black, I.B.; Wu, K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006, 1121, 22–34. [Google Scholar] [CrossRef]
- Goebel-Goody, S.M.; Davies, K.D.; Alvestad Linger, R.M.; Freund, R.K.; Browning, M.D. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 2009, 158, 1446–1459. [Google Scholar] [CrossRef]
- Wu, B.; Guo, W. The Exocyst at a Glance. J. Cell Sci. 2015, 128, 2957–2964. [Google Scholar] [CrossRef]
- Lee, S.L.T.; Lew, D.; Wickenheisser, V.; Markus, E.J. Interdependence between dorsal and ventral hippocampus during spatial navigation. Brain Behav. 2019, 9, e01410. [Google Scholar] [CrossRef]
- Titus, D.J.; Wilson, N.M.; Freund, J.E.; Carballosa, M.M.; Sikah, K.E.; Furones, C.; Dietrich, W.D.; Gurney, M.E.; Atkins, C.M. Chronic Cognitive Dysfunction after Traumatic Brain Injury Is Improved with a Phosphodiesterase 4B Inhibitor. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 7095–7108. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, M.; Gao, Y.; Cao, Y.; Bao, J.G.; Lin, Y.Y.; Wang, Y.; Luo, Q.Z.; Jiang, J.Y.; Zhong, C.L. Acute histopathological responses and long-term behavioral outcomes in mice with graded controlled cortical impact injury. Neural Regen. Res. 2019, 14, 997–1003. [Google Scholar] [CrossRef]
- Aungst, S.L.; Kabadi, S.V.; Thompson, S.M.; Stoica, B.A.; Faden, A.I. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 1223–1232. [Google Scholar] [CrossRef]
- Schwarzbach, E.; Bonislawski, D.P.; Xiong, G.; Cohen, A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 2006, 16, 541–550. [Google Scholar] [CrossRef]
- Teodoro, R.O.; Pekkurnaz, G.; Nasser, A.; Higashi-Kovtun, M.E.; Balakireva, M.; McLachlan, I.G.; Camonis, J.; Schwarz, T.L. Ral mediates activity-dependent growth of postsynaptic membranes via recruitment of the exocyst. EMBO J. 2013, 32, 2039–2055. [Google Scholar] [CrossRef]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef]
- Hanson, G.T.; Aggeler, R.; Oglesbee, D.; Cannon, M.; Capaldi, R.A.; Tsien, R.Y.; Remington, S.J. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 13044–13053. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Ilavazhagan, G.; Rossignol, J.; Dunbar, G.L. Molecular regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases. Neurosci. Biobehav. Rev. 2015, 59, 208–237. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Remodeling dendritic spines for treatment of traumatic brain injury. Neural Regen. Res. 2019, 14, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Jamjoom, A.A.B.; Rhodes, J.; Andrews, P.J.D.; Grant, S.G.N. The synapse in traumatic brain injury. Brain 2021, 144, 18–31. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira, M.; Abarca, J.; Mira, R.G.; Zamorano, P.; Cerpa, W. Exo70 Protects Against Memory and Synaptic Impairments Following Mild Traumatic Brain Injury. Antioxidants 2025, 14, 640. https://doi.org/10.3390/antiox14060640
Lira M, Abarca J, Mira RG, Zamorano P, Cerpa W. Exo70 Protects Against Memory and Synaptic Impairments Following Mild Traumatic Brain Injury. Antioxidants. 2025; 14(6):640. https://doi.org/10.3390/antiox14060640
Chicago/Turabian StyleLira, Matías, Jorge Abarca, Rodrigo G. Mira, Pedro Zamorano, and Waldo Cerpa. 2025. "Exo70 Protects Against Memory and Synaptic Impairments Following Mild Traumatic Brain Injury" Antioxidants 14, no. 6: 640. https://doi.org/10.3390/antiox14060640
APA StyleLira, M., Abarca, J., Mira, R. G., Zamorano, P., & Cerpa, W. (2025). Exo70 Protects Against Memory and Synaptic Impairments Following Mild Traumatic Brain Injury. Antioxidants, 14(6), 640. https://doi.org/10.3390/antiox14060640






