Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Materials and Regents
2.2. Experimental Design
2.3. Body Size Assay and Locomotory Behavior
2.3.1. Body Size Assay
2.3.2. Locomotory Behavior
2.3.3. Foraging Behavior and Chemotaxis Assay
2.4. ADHP, DHE, and NDA Fluorescent Staining of C. elegans
2.5. Levels of Endogenous ROS and Accumulation of Lipofuscin in Nematodes
2.6. Morphological Changes of Neurotransmitter Systems
2.7. Neurotransmitter Levels
2.8. DAF-16::GFP Localization and SOD 3::GFP and GST-4::GFP Quantification
2.9. qRT–PCR Analysis
2.10. Statistical Analysis
3. Results
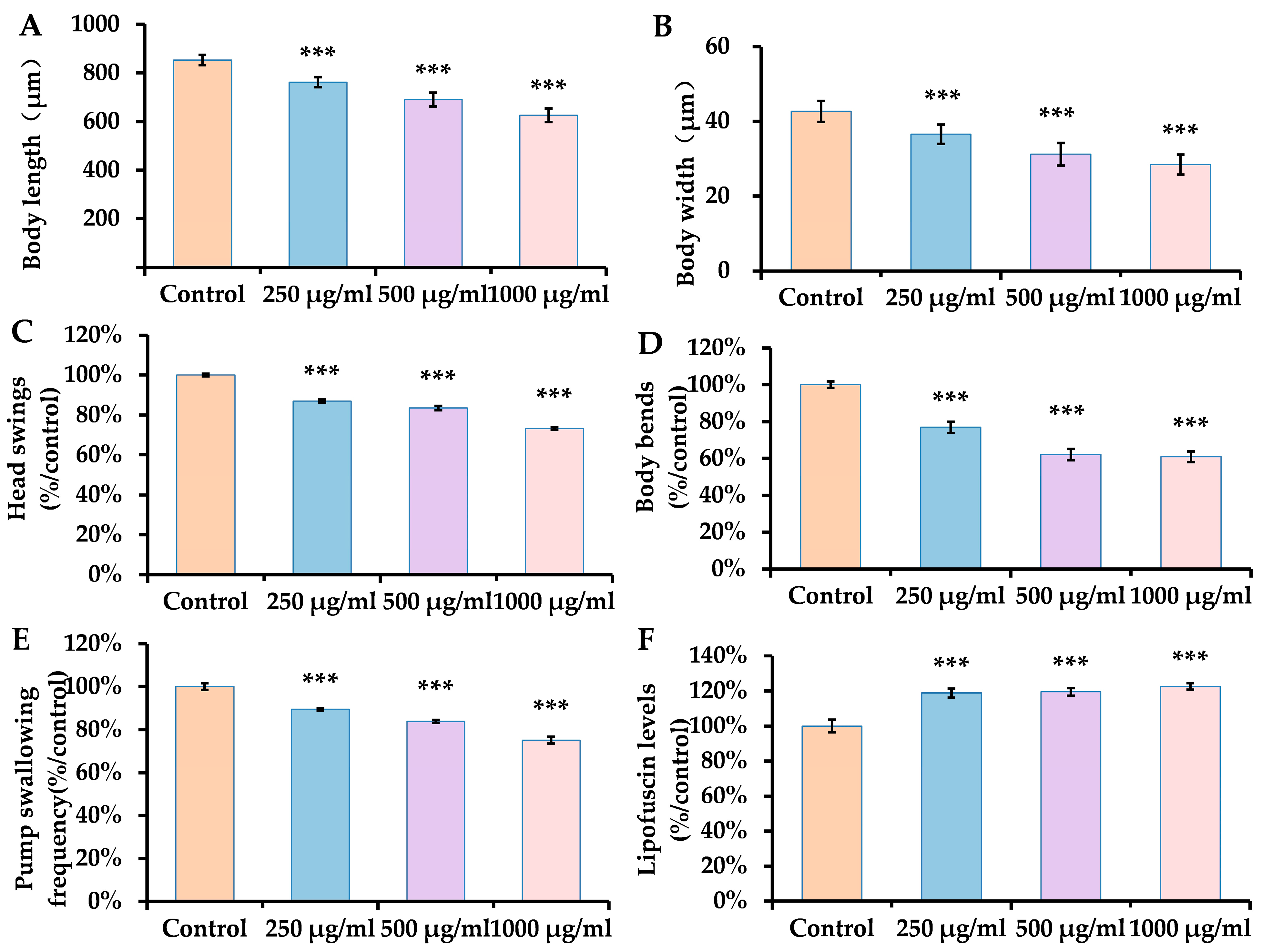
3.1. Dose-Dependent Impairment of Fitness by ACR in C. elegans
3.2. Dose-Dependent Impairment of Oxidative by ACR in C. elegans
3.3. Dose-Dependent Impairment of Sensory Behavior by ACR in C. elegans
3.4. Dose-Dependent Impairment of Neural by ACR in C. elegans
3.5. ACR Changes the Expression of Genes Related to Neurotransmitters and Antioxidant Function in C. elegans
3.6. ACR Exposure Increases DAF-16 and the Expression of SOD-3 and GST-4
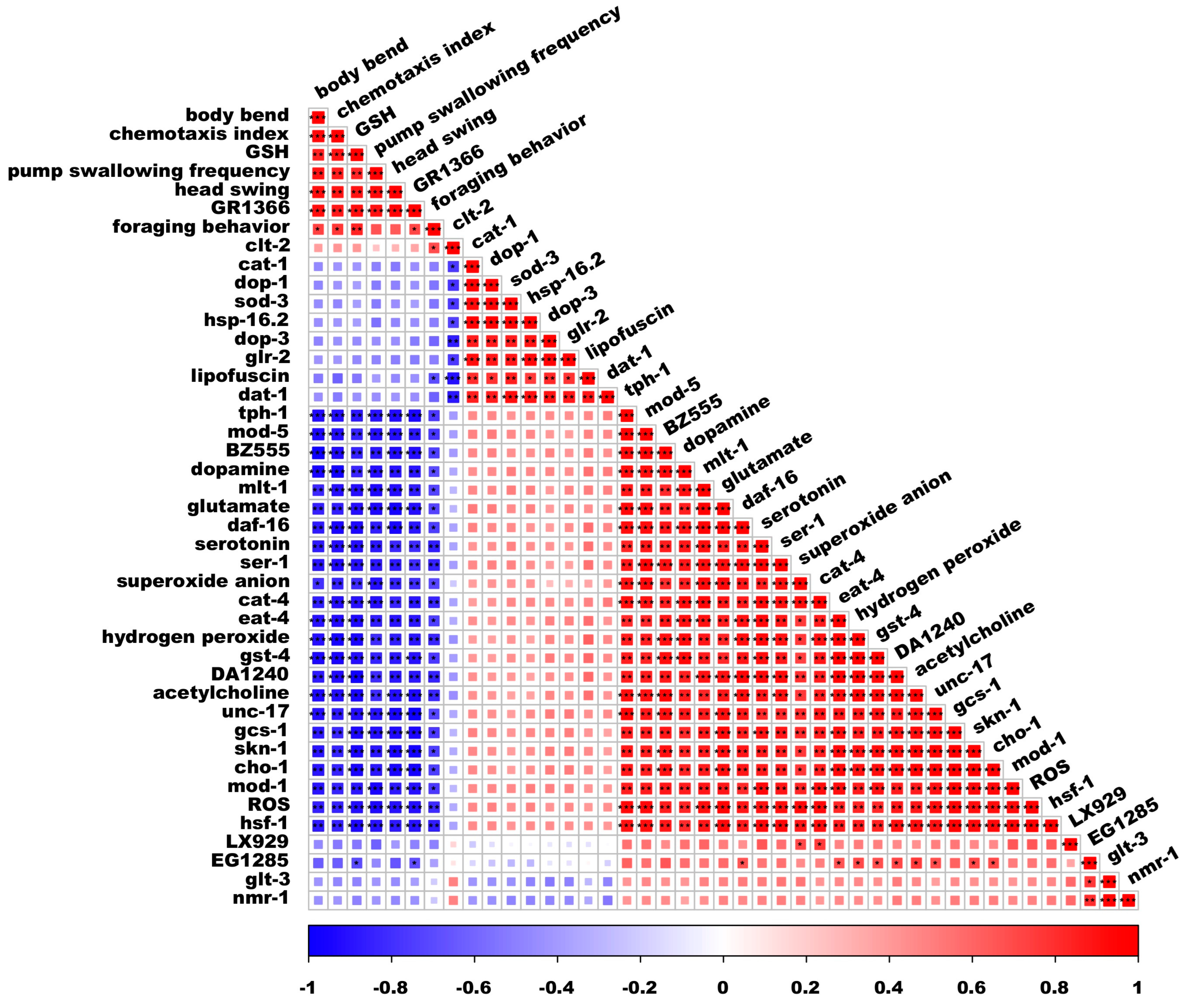
3.7. Spearman’s Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, X.; Jiang, H.; Hu, C.; Xu, J.; Sun, C.; Wei, W.; Han, T.; Jiang, W. The Association Between Exposure to Acrylamide and Mortalities of Cardiovascular Disease and All-Cause Among People with Hyperglycemia. Front. Cardiovasc. Med. 2022, 9, 930135. [Google Scholar] [CrossRef]
- Hashem, M.M.; Abo-El-Sooud, K.; Abd El-Hakim, Y.M.; Badr, Y.A.; El-Metwally, A.E.; Bahy-El-Dien, A. The impact of long-term oral exposure to low doses of acrylamide on the hematological indicators, immune functions, and splenic tissue architecture in rats. Int. Immunopharmacol. 2022, 105, 108568. [Google Scholar] [CrossRef]
- Ji, K.; Kang, S.; Lee, G.; Lee, S.; Jo, A.; Kwak, K.; Kim, D.; Kho, D.; Lee, S.; Kim, S. Urinary levels of N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA), an acrylamide metabolite, in Korean children and their association with food consumption. Sci. Total Environ. 2013, 456, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, A.; Alhasani, R.H.A.; Biswas, L.; Reilly, J.; Shu, X. Protective effect of carnosic acid against acrylamide-induced toxicity in RPE cells. Food Chem. Toxicol. 2017, 108, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Cheng, C.; Yang, Y.; Chang, Y.; Li, C.; Wu, S.; Lin, C.; Wu, H.; Suen, J. Acrylamide, an air pollutant, enhances allergen-induced eosinophilic lung inflammation via group 2 innate lymphoid cells. Mucosal Immunol. 2024, 17, 13–24. [Google Scholar] [CrossRef]
- Mueller, N.P.F.; Carloni, P.; Alfonso-Prieto, M. Molecular determinants of acrylamide neurotoxicity through covalent docking. Front. Pharmacol. 2023, 14, 1125871. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Luo, Y.; Dong, L.; Chen, F. Acrylamide-induced hepatotoxicity through oxidative stress: Mechanisms and interventions. Antioxid. Redox Signal. 2023, 38, 1122–1137. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, J.; Ren, C.; Gao, Y.; Zhang, T.; Long, Y.; Wei, W.; Hou, S.; Sun, C.; Wang, C. The association between biomarkers of acrylamide and cancer mortality in US adult population: Evidence from NHANES 2003–2014. Front. Oncol. 2022, 12, 970021. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, P.; Jiao, J.; Wang, J.; Zhang, Y. Association of acrylamide hemoglobin biomarkers with obesity, abdominal obesity and overweight in general US population: NHANES 2003–2006. Sci. Total Environ. 2018, 631, 589–596. [Google Scholar] [CrossRef]
- Chen, J.; Yang, C.; Wang, Y.; Lee, J.; Cheng, C.; Chou, C. Acrylamide-induced mitochondria collapse and apoptosis in human astrocytoma cells. Food Chem. Toxicol. 2013, 51, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Xu, C.; Holck, A.L.; Liu, R. Acrylamide inhibits autophagy, induces apoptosis and alters cellular metabolic profiles. Ecotoxicol. Environ. Saf. 2021, 208, 111543. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Ye, J.; Liu, W.; Zhao, B.; Shi, X.; Zhang, C.; Liu, Z.; Liu, X. Acrylamide aggravates cognitive deficits at night period via the gut–brain axis by reprogramming the brain circadian clock. Arch. Toxicol. 2019, 93, 467–486. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, P.; Zhu, Y.; Liu, X.; Hu, X.; Chen, F. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 2015, 6, 3006–3012. [Google Scholar] [CrossRef]
- World Health Organization. Chemical Fact Sheets: Acrylamide. March 2022. Available online: https://www.who.int/publications/m/item/chemical-fact-sheets--acrylamide (accessed on 4 March 2022).
- Wu, C.W.; Wang, Y.; Choe, K.P. F-Box Protein XREP-4 Is a New Regulator of the Oxidative Stress Response in Caenorhabditis elegans. Genetics 2017, 206, 859–871. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Yang, Y.; Xu, T.; Li, P.; He, D. Acrylamide induces locomotor defects and degeneration of dopamine neurons in Caenorhabditis elegans. J. Appl. Toxicol. 2016, 36, 60–67. [Google Scholar] [CrossRef]
- Murray, S.M.; Waddell, B.M.; Wu, C. Neuron-specific toxicity of chronic acrylamide exposure in C. elegans. Neurotoxicol. Teratol. 2020, 77, 106848. [Google Scholar] [CrossRef]
- Chen, H.; Hua, X.; Yang, Y.; Wang, C.; Jin, L.; Dong, C.; Chang, Z.; Ding, P.; Xiang, M.; Li, H. Chronic exposure to UV-aged microplastics induces neurotoxicity by affecting dopamine, glutamate, and serotonin neurotransmission in Caenorhabditis elegans. J. Hazard. Mater. 2021, 419, 126482. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Zhang, J.; Wang, L.; Zhao, X.; Luo, Y. Xanthotoxin induced photoactivated toxicity, oxidative stress and cellular apoptosis in Caenorhabditis elegans under ultraviolet A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 251, 109217. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D. Polyethylene nanoparticles at environmentally relevant concentrations enhances neurotoxicity and accumulation of 6-PPD quinone in Caenorhabditis elegans. Sci. Total Environ. 2024, 918, 170760. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Zeng, K.; Wang, Q. Mercuric sulfide nanoparticles suppress the neurobehavioral functions of Caenorhabditis elegans through a Skp1-dependent mechanism. Food Chem. Toxicol. 2024, 186, 114576. [Google Scholar] [CrossRef]
- Wang, B.; Yin, Z.; Liu, J.; Tang, C.; Zhang, Y.; Wang, L.; Li, H.; Luo, Y. Diquat Induces Cell Death and dopamine Neuron Loss via Reactive Oxygen Species Generation in Caenorhabditis elegans. Environ. Sci. Technol. 2025, 59, 152–162. [Google Scholar] [CrossRef]
- Teng, J.; Yu, T.; Yan, F. GABA attenuates neurotoxicity of zinc oxide nanoparticles due to oxidative stress via DAF-16/FoxO and SKN-1/Nrf2 pathways. Sci. Total Environ. 2024, 934, 173214. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, J.; Han, R.; Ma, Q.; Wu, Z.; Fu, P.; Ma, A.; Feng, N. Melatonin derivative 6a protects Caenorhabditis elegans from formaldehyde neurotoxicity via ADH5. Free. Radic. Biol. Med. 2024, 223, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Piggott, B.J.; Liu, J.; Feng, Z.; Wescott, S.A.; Xu, X.S. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 2011, 147, 922–933. [Google Scholar] [CrossRef]
- Li, W.; Kang, L.; Piggott, B.J.; Feng, Z.; Xu, X.S. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat. Commun. 2011, 2, 315. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Dong, C.; Chen, X.; Gu, Y.; Jiang, Y.; Cui, J.; Chen, H. Behavioral and Molecular Neurotoxicity of Thermally Degraded Polystyrene in Caenorhabditis elegans. J. Hazard. Mater. 2025, 487, 137212. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Zhang, J.; Wang, R.; Yin, D. Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicol. Environ. Saf. 2013, 88, 178–184. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, M.; Hu, J.; Li, Z.; Wu, T.; Bao, J.; Wu, S.; Lei, L.; He, D. Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere 2017, 181, 55–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Rui, Q.; Wang, D. Toxicity induction of nanopolystyrene under microgravity stress condition in Caenorhabditis elegans. Sci. Total Environ. 2020, 703, 135623. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Y.; Sun, J.; Wang, J.; Tang, L.; Xu, Y.; Ji, J.; Sun, X. Abnormal neurotransmission of GABA and serotonin in Caenorhabditis elegans induced by Fumonisin B1. Environ. Pollut. 2022, 304, 119141. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Chen, H.; Li, H.; Tariq, M.; Wang, C.; Zhou, Y.; Liu, Y. Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ. Pollut. 2019, 244, 947–957. [Google Scholar] [CrossRef]
- Saeki, S.; Yamamoto, M.; Iino, Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 2001, 204, 1757–1764. [Google Scholar] [CrossRef]
- Chu, Q.; Chen, W.; Jia, R.; Ye, X.; Li, Y.; Liu, Y.; Jiang, Y.; Zheng, X. Tetrastigma hemsleyanum leaves extract against acrylamide-induced toxicity in HepG2 cells and Caenorhabditis elegans. J. Hazard. Mater. 2020, 393, 122364. [Google Scholar] [CrossRef]
- Sun, M.; Chen, X.; Cao, J.; Cui, X.; Wang, H. Polygonum multiflorum Thunb extract extended the lifespan and healthspan of Caenorhabditis elegans via DAF-16/SIR-2.1/SKN-1. Food Funct. 2021, 12, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, S.; Zu, S.; Michiyo, S.; Yuichi, I.; Toshio, T. Forward and backward locomotion patterns in C. elegans generated by a connectome-based model simulation. Sci. Rep. UK 2021, 11, 13737. [Google Scholar] [CrossRef]
- Kaplan, H.S.; Zimmer, M. Sensorimotor Integration for Decision Making: How the Worm Steers. Neuron 2018, 97, 258–260. [Google Scholar] [CrossRef]
- Brenner, I.R.; Raizen, D.M.; Fang-Yen, C. Pharyngeal timing and particle transport defects in Caenorhabditis elegans feeding mutants. J. Neurophysiol. 2022, 128, 302–309. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, X.; Zheng, X.; Yan, F. FoxO signaling pathway stimulation by Bacillus smithii XY1 contributes to alleviating copper-induced neurotoxicity. J. Hazard. Mater. 2024, 465, 133345. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, S.; Chen, M.; Han, W.; Jia, R.; Chen, W.; Zheng, X. Cherry anthocyanins regulate NAFLD by promoting autophagy pathway. Oxid. Med. Cell Longev. 2019, 2019, 4825949. [Google Scholar] [CrossRef]
- Azari, A.; Shokrzadeh, M.; Zamani, E.; Amani, N.; Shaki, F. Cerium oxide nanoparticles protects against acrylamide induced toxicity in HepG2 cells through modulation of oxidative stress. Drug Chem. Toxicol. 2019, 42, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, M.P.; Salfelder, F.; Sanders, T.; Umuerri, O.; Cohen, N.; Jansen, G. Plasticity in gustatory and nociceptive neurons controls decision making in C. Elegans salt navigation. Commun. Biol. 2021, 4, 1053. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M. TRPM2 cation channels, oxidative stress and neurological diseases: Where are we now? Neurochem. Res. 2011, 36, 355–366. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C. Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L. The mechanism of acrylamide-induced neurotoxicity: Current status and future perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef]
- Kniazeva, M.; Zhu, H.; Sewell, A.K.; Han, M. A lipid-TORC1 pathway promotes neuronal development and foraging behavior under both fed and fasted conditions in C. elegans. Dev. Cell 2015, 33, 260–271. [Google Scholar] [CrossRef]
- Wu, T.; He, K.; Zhan, Q.; Ang, S.; Ying, J.; Zhang, S.; Zhang, T.; Xue, Y.; Tang, M. MPA-capped CdTe quantum dots exposure causes neurotoxic effects in nematode Caenorhabditis elegans by affecting the transporters and receptors of glutamate, serotonin and dopamine at the genetic level, or by increasing ROS, or both. Nanoscale 2015, 7, 20460–20473. [Google Scholar] [CrossRef]
- Wang, D.; Xing, X. Pre-treatment with mild metal exposure suppresses the neurotoxicity on locomotion behavior induced by the subsequent severe metal exposure in Caenorhabditis elegans. Environ. Toxicol. Phar 2009, 28, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Merino Serrais, P.; Plaza Alonso, S.; Hellal, F.; Valero Freitag, S.; Kastanauskaite, A.; Plesnila, N.; DeFelipe, J. Structural changes of CA1 pyramidal neurons after stroke in the contralesional hippocampus. Brain Pathol. 2024, 34, e13222. [Google Scholar] [CrossRef]
- Jorge-Oliva, M.; van Weering, J.R.; Scheper, W. Structurally and Morphologically Distinct Pathological Tau Assemblies Differentially Affect GVB Accumulation. Int. J. Mol. Sci. 2023, 24, 10865. [Google Scholar] [CrossRef]
- Tiantian, X.; Ping, L.; Siyu, W.; Lili, L.; Defu, H. Tris(2-chloroethyl) phosphate (TCEP) and tris(2-chloropropyl) phosphate (TCPP) induce locomotor deficits and dopaminergic degeneration in Caenorhabditis elegans. Toxicol. Res. UK 2017, 6, 63–72. [Google Scholar] [CrossRef]
- Itzhak, M.; Sarah, S.; Monica, D. Caenorhabditis elegans glutamate transporters influence synaptic function and behavior at sites distant from the synapse. J. Biol. Chem. 2007, 282, 34412–34419. [Google Scholar] [CrossRef]
- Brownlee, D.J.; Fairweather, I. Exploring the neurotransmitter labyrinth in nematodes. Trends Neurosci. 1999, 22, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yau, M.; Lei, E.N.; Ng, I.H.; Yuen, C.K.; Lam, J.C.; Lam, M.H. Changes in the neurotransmitter profile in the central nervous system of marine medaka (Oryzias melastigma) after exposure to brevetoxin PbTx-1–A multivariate approach to establish exposure biomarkers. Sci. Total Environ. 2019, 673, 327–336. [Google Scholar] [CrossRef]
- Ishita, Y.; Chihara, T.; Okumura, M. Serotonergic modulation of feeding behavior in Caenorhabditis elegans and other related nematodes. Neurosci. Res. 2020, 154, 9–19. [Google Scholar] [CrossRef]
- Sze, J.Y.; Victor, M.; Loer, C.; Shi, Y.; Ruvkun, G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 2000, 403, 560–564. [Google Scholar] [CrossRef]
- Ranganathan, R.; Cannon, S.C.; Horvitz, H.R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 2000, 408, 470–475. [Google Scholar] [CrossRef]
- Ranganathan, R.; Sawin, E.R.; Trent, C.; Horvitz, H.R. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and-independent activities of fluoxetine. J. Neurosci. 2001, 21, 5871–5884. [Google Scholar] [CrossRef] [PubMed]
- Yinxia, L.; Shunhui, Y.; Qiuli, W.; Meng, T.; Dayong, W. Transmissions of serotonin, dopamine, and glutamate are required for the formation of neurotoxicity from Al2O3-NPs in nematode Caenorhabditis elegans. Nanotoxicology 2013, 7, 1004–1013. [Google Scholar] [CrossRef]
- Chou, S.; Chen, Y.; Liao, C.; Pan, C. A role for dopamine in C. elegans avoidance behavior induced by mitochondrial stress. Neurosci. Res. 2022, 178, 87–92. [Google Scholar] [CrossRef]
- Hobert, O. A map of terminal regulators of neuronal identity in Caenorhabditis elegans. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 474–498. [Google Scholar] [CrossRef]
- Okuda, T.; Haga, T.; Kanai, Y.; Endou, H.; Ishihara, T.; Katsura, I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Duerr, J.S.; Varoqui, H.; McManus, J.R.; Rand, J.B.; Erickson, J.D. Analysis of point mutants in the Caenorhabditis elegans vesicular acetylcholine transporter reveals domains involved in substrate translocation. J. Biol. Chem. 2001, 276, 41580–41587. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, Q.; Yang, L.; Zha, J. Comparison of the toxicity effects of tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) with tributyl phosphate (TNBP) reveals the mechanism of the apoptosis pathway in Asian freshwater clams (Corbicula fluminea). Environ. Sci. Technol. 2020, 54, 6850–6858. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Russell, T.M.; Richardson, D.R. The good Samaritan glutathione-S-transferase P1: An evolving relationship in nitric oxide metabolism mediated by the direct interactions between multiple effector molecules. Redox Biol. 2023, 59, 102568. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Chen, X.D.; Gill, T.A.; Nguyen, C.D.; Killiny, N.; Pelz-Stelinski, K.S.; Stelinski, L.L. Insecticide toxicity associated with detoxification enzymes and genes related to transcription of cuticular melanization among color morphs of Asian citrus psyllid. Insect Sci. 2018, 26, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yunli, Z.; Ling, J.; Yuxin, C.; Jing, Y.; Quan, Z.; Huazhang, W. Fine Particulate Matter Leads to Unfolded Protein Response and Shortened Lifespan by Inducing Oxidative Stress in C. elegans. Oxid. Med. Cell Longev. 2019, 2019, 2492368. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Ma, L.; Zhang, Y. Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model. Antioxidants 2025, 14, 641. https://doi.org/10.3390/antiox14060641
Ma Z, Ma L, Zhang Y. Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model. Antioxidants. 2025; 14(6):641. https://doi.org/10.3390/antiox14060641
Chicago/Turabian StyleMa, Zhonglian, Liang Ma, and Yuhao Zhang. 2025. "Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model" Antioxidants 14, no. 6: 641. https://doi.org/10.3390/antiox14060641
APA StyleMa, Z., Ma, L., & Zhang, Y. (2025). Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model. Antioxidants, 14(6), 641. https://doi.org/10.3390/antiox14060641








