The Effect of Photoisomerization on the Antioxidant Properties of Sinapic Acid and Methyl Sinapate in Different Solvents: A DFT/TD-DFT Study
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
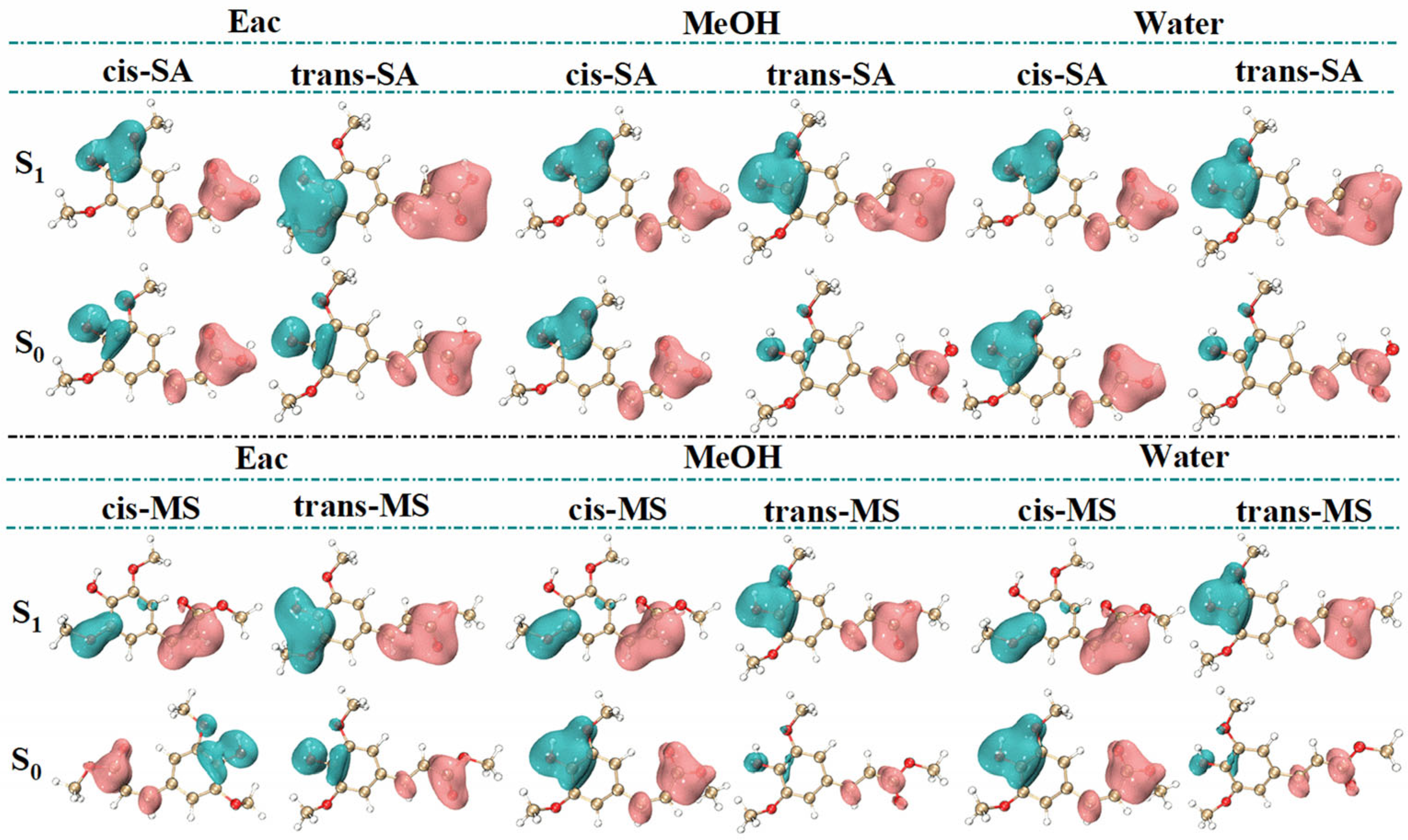
3.1. Spectral Properties and Frontier Molecular Orbitals (FMOs)
3.2. Photoisomerization Process of Two Compounds
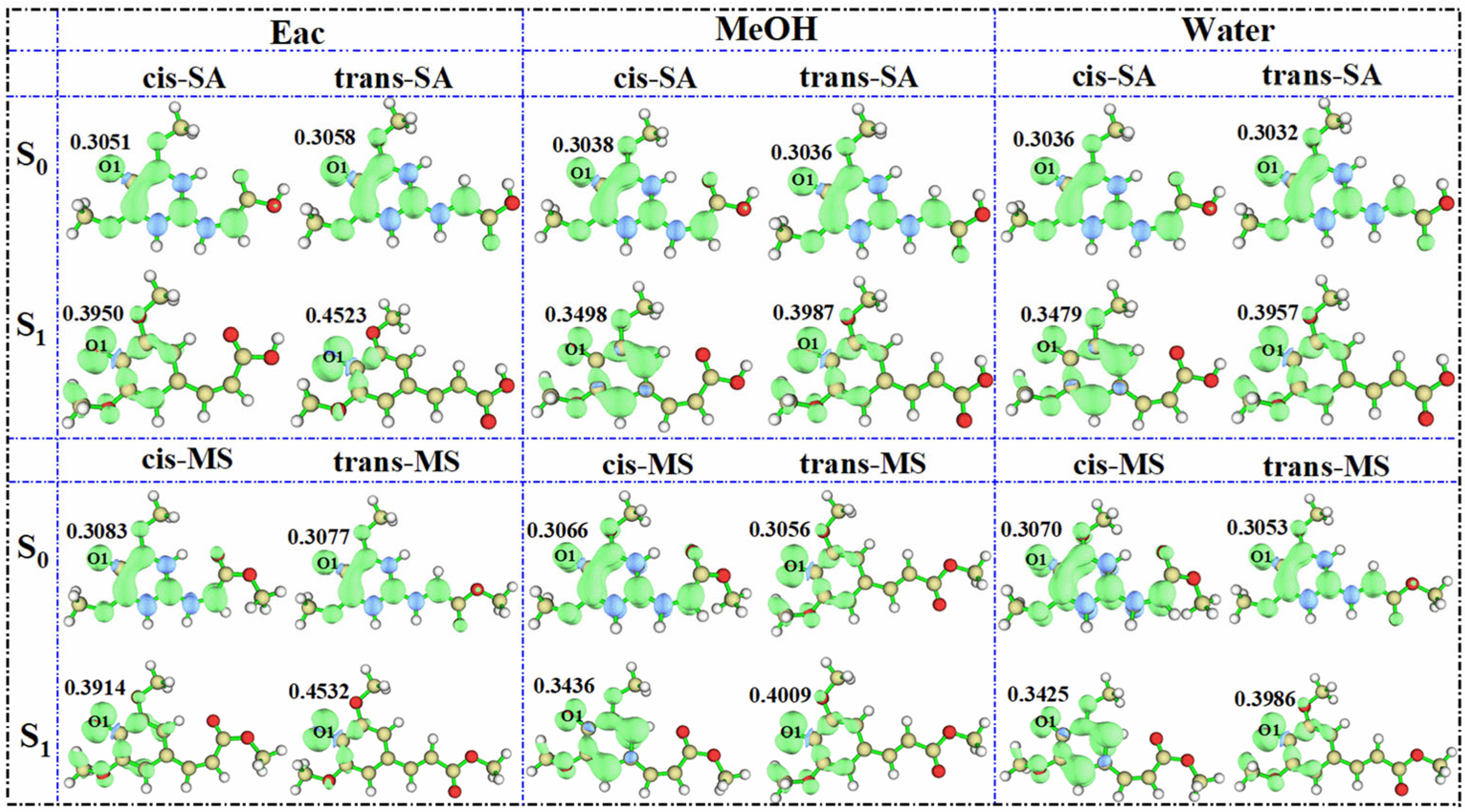
3.3. Active Site
3.4. Global Descriptive Parameters of cis–trans-SA and -MS in Different Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barayeu, U.; Schilling, D.; Eid, M.; da Silva, T.N.X.; Schlicker, L.; Mitreska, N.; Zapp, C.; Gräter, F.; Miller, A.K.; Kappl, R.; et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 2023, 19, 28–37. [Google Scholar] [CrossRef]
- Treml, J.; Smejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Cao, B.F.; Li, K.; Chen, C.G.; Shi, Y. Effects of functional groups on ESIPT and antioxidant activity of apigenin: A non-existent enol* state fluorescein. Spectrochim. Acta Part A 2025, 326, 125287. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, J.; Yang, S.; Han, K. New Insight into the Photoprotection Mechanism of Plant Sunscreens: Adiabatic Relaxation Competing with Nonadiabatic Relaxation in the cis → trans Photoisomerization of Methyl Sinapate. J. Phys. Chem. Lett. 2019, 10, 4197–4202. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Toldo, J.M.; Rocha, W.R.; Barbatti, M. Photoisomerization pathways of trans-resveratrol. Phys. Chem. Chem. Phys. 2024, 26, 24179–24188. [Google Scholar] [CrossRef]
- Welc-Stanowska, R.; Pietras, R.; Mielecki, B.; Sarewicz, M.; Luchowski, R.; Widomska, J.; Grudzinski, W.; Osyczka, A.; Gruszecki, W.I. How Do Xanthophylls Protect Lipid Membranes from Oxidative Damage? J. Phys. Chem. Lett. 2023, 14, 7440–7444. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, Y.; Sun, C.; Huang, Z. Influence of cis–trans isomerization induced by photoexcitation on the antioxidant properties of piceatannol and its derivatives. Chem. Phys. Lett. 2025, 863, 141875. [Google Scholar] [CrossRef]
- Gangadasu, B.; Reddy, M.J.R.; Ravinder, M.; Kumar, S.B.; Raju, B.C.; Kumar, K.P.; Murthy, U.S.N.; Rao, V.J. Synthesis, photochemical E (trans)→Z (cis) isomerization and antimicrobial activity of 2-chloro-5-methylpyridine-3-olefin derivatives. Eur. J. Med. Chem. 2009, 44, 4661–4667. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, Q. Assessment of the antioxidant activities of representative optical and geometric isomers of astaxanthin against singlet oxygen in solution by a spectroscopic approach. Food Chem. 2022, 395, 133584. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Castejón, M.L.; Rubio-Senent, F.; Fernández-Prior, Á.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and structural determination of cis- and trans-p-coumaroyl-secologanoside (comselogoside) from olive oil waste (alperujo). Photoisomerization with ultraviolet irradiation and antioxidant activities. Food Chem. 2024, 432, 137233. [Google Scholar] [CrossRef]
- Joshi, N.K.; Fuyuki, M.; Wada, A. Polarity Controlled Reaction Path and Kinetics of Thermal Cis-to-Trans Isomerization of 4-Aminoazobenzene. J. Phys. Chem. B 2014, 118, 1891–1899. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. 1965, 137, A1697–A1705. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Matsuzawa, N.N.; Ishitani, A.; Dixon, D.A.; Uda, T. Time-dependent density functional theory calculations of photoabsorption spectra in the vacuum ultraviolet region. J. Phys. Chem. A 2001, 105, 4953–4962. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Q.; Lin, W.; Wang, J.; Chen, M.; Sun, M. Surface-enhanced Raman scattering of pyrazine on Au5Al5 bimetallic nanoclusters. RSC Adv. 2017, 7, 12170–12178. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Merchán, M.; Roos, B.O. The electron affinity of NH2: A restricted active-space SCF multi-reference CI study. Chem. Phys. Lett. 1991, 184, 346–352. [Google Scholar] [CrossRef]
- Malmqvist, P.Å.; Roos, B.O.; Schimmelpfennig, B. The restricted active space (RAS) state interaction approach with spin–orbit coupling. Chem. Phys. Lett. 2002, 357, 230–240. [Google Scholar] [CrossRef]
- Fdez. Galván, I.; Vacher, M.; Alavi, A.; Angeli, C.; Aquilante, F.; Autschbach, J.; Bao, J.J.; Bokarev, S.I.; Bogdanov, N.A.; Carlson, R.K.; et al. OpenMolcas: From Source Code to Insight. J. Chem. Theory Comput. 2019, 15, 5925–5964. [Google Scholar]
- Li Manni, G.; Fdez. Galván, I.; Alavi, A.; Aleotti, F.; Aquilante, F.; Autschbach, J.; Avagliano, D.; Baiardi, A.; Bao, J.J.; Battaglia, S.; et al. The OpenMolcas Web: A Community-Driven Approach to Advancing Computational Chemistry. J. Chem. Theory Comput. 2023, 19, 6933–6991. [Google Scholar] [CrossRef]
- Roos, B.O.; Taylor, P.R.; Sigbahn, P.E.M. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 1980, 48, 157–173. [Google Scholar] [CrossRef]
- Bearpark, M.J.; Ogliaro, F.; Vreven, T.; Boggio-Pasqua, M.; Frisch, M.J.; Larkin, S.M.; Morrison, M.; Robb, M.A. CASSCF calculations for photoinduced processes in large molecules: Choosing when to use the RASSCF, ONIOM and MMVB approximations. J. Photochem. Photobiol. A 2007, 190, 207–227. [Google Scholar] [CrossRef]
- Tomasello, G.; Garavelli, M.; Orlandi, G. Tracking the stilbene photoisomerization in the S1 state using RASSCF. Phys. Chem. Chem. Phys. 2013, 15, 19763–19773. [Google Scholar] [CrossRef]
- Boggio-Pasqua, M.; Robb, M.A.; Bearpark, M.J. Photostability via a sloped conical intersection: A CASSCF and RASSCF study of pyracylene. J. Phys. Chem. A 2005, 109, 8849–8856. [Google Scholar] [CrossRef]
- Krausbeck, F.; Mendive-Tapia, D.; Thom, A.J.W.; Bearpark, M.J. Choosing RASSCF orbital active spaces for multiple electronic states. Comput. Theor. Chem. 2014, 1040–1041, 14–19. [Google Scholar] [CrossRef]
- Roos, B.O.; Andersson, K. Multiconfigurational perturbation theory with level shift—the Cr2 potential revisited. Chem. Phys. Lett. 1995, 245, 215–223. [Google Scholar] [CrossRef]
- Roos, B.O.; Andersson, K.; Fülscher, M.P.; Serrano-Andrés, L.; Pierloot, K.; Merchán, M.; Molina, V. Applications of level shift corrected perturbation theory in electronic spectroscopy. J. Mol. Struct. THEOCHEM 1996, 388, 257–276. [Google Scholar] [CrossRef]
- Park, J.W. Single-State Single-Reference and Multistate Multireference Zeroth-Order Hamiltonians in MS-CASPT2 and Conical Intersections. J. Chem. Theory Comput. 2019, 15, 3960–3973. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.-W.; Wang, X.-T.-H.; Xia, S.-H. Photocyclization and Photoisomerization Mechanisms of an Indolylfulgide Derivative in Acetonitrile Solution by Using the QM (MS-CASPT2)/MM Method. J. Phys. Chem. A 2024, 128, 8190–8197. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Roohi, H.; Mohtamedifar, N.; Hejazi, F. Intramolecular photoinduced proton transfer in 2-(2′-hydroxyphenyl)benzazole family: A TD-DFT quantum chemical study. Chem. Phys. 2014, 444, 66–76. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef]
- Wang, L.L.; Yang, F.J.; Zhao, X.H.; Li, Y.Z. Effects of nitro- and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha, D.; Sadasivam, K.; Praveena, R.; Jayaprakasam, R. DFT study of glycosyl group reactivity in quercetin derivatives. J. Mol. Struct. 2016, 1120, 15–24. [Google Scholar] [CrossRef]
- Sadasivam, K.; Kumaresan, R. Antioxidant behavior of mearnsetin and myricetin flavonoid compounds—A DFT study. Spectrochim. Acta Part A 2011, 79, 282–293. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. The dual descriptor potential. J. Math. Chem. 2024, 62, 1094–1112. [Google Scholar] [CrossRef]
- Halliday, G.M.; Byrne, S.N.; Damian, D.L. Ultraviolet A Radiation: Its Role in Immunosuppression and Carcinogenesis. Semin. Cutaneous Med. Surg. 2011, 30, 214–221. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision B.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radical Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef]
- Liu, X.D.; Feng, J.K.; Ren, A.M.; Yang, L.; Yang, B.; Ma, Y.G. Theoretical studies of electronic structures, absorption and emission spectra in cyclometalated phenylpyridine Ir(III) complex and its derivatives using density functional theory. Opt. Mater. 2006, 29, 231–238. [Google Scholar] [CrossRef]
- Chen, C.; Wang, K.Y.; Jiang, P.; Song, G.L.; Zhu, H.J. Synthesis, crystal structures and photophysical properties of novel copper(I) complexes with 4-diphenylphosphino-1,5-naphthyridine ligands. Inorg. Chem. Commun. 2012, 17, 116–119. [Google Scholar] [CrossRef]
- Narsaria, A.K.; Poater, J.; Guerra, C.F.; Ehlers, A.W.; Hamlin, T.A.; Lammertsma, K.; Bickelhaupt, F.M. Distortion-Controlled Redshift of Organic Dye Molecules. Chem.-Eur. J. 2020, 26, 2080–2093. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Li, C.; Liu, Y.; Jiang, K. Asymmetric substitution changes the UV-induced nonradiative decay pathway and the spectra behaviors of β-diketones. Spectrochim. Acta Part A 2019, 207, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wang, L.; Guo, X.G.; Zhang, J.L. A CASSCF/CASPT2 Insight into Excited-State Intramolecular Proton Transfer of Four Imidazole Derivatives. J. Comput. Chem. 2015, 36, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Zhao, X.Y.; Wang, C.; Wang, Y.; Zhang, S.; Li, P.; Feng, X.; Jin, B.; Yuan, M.H.; Cui, S.; et al. Ultrafast Nonadiabatic Photoisomerization Dynamics Mechanism for the UV Photoprotection of Stilbenoids in Grape Skin. Chem.-Asian J. 2020, 15, 1478–1483. [Google Scholar] [CrossRef]
- Zhou, P.W.; Liu, J.Y.; Han, K.L.; He, G.Z. The Photoisomerization of 11-cis-Retinal Protonated Schiff Base in Gas Phase: Insight from Spin-Flip Density Functional Theory. J. Comput. Chem. 2014, 35, 109–120. [Google Scholar] [CrossRef]
- Galego, J.; Garcia-Vidal, F.J.; Feist, J. Suppressing photochemical reactions with quantized light fields. Nat. Commun. 2016, 7, 13841. [Google Scholar] [CrossRef]
- Polli, D.; Altoè, P.; Weingart, O.; Spillane, K.M.; Manzoni, C.; Brida, D.; Tomasello, G.; Orlandi, G.; Kukura, P.; Mathies, R.A.; et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 2010, 467, 440–443. [Google Scholar] [CrossRef]
- von Conta, A.; Tehlar, A.; Schletter, A.; Arasaki, Y.; Takatsuka, K.; Wörner, H.J. Conical-intersection dynamics and ground-state chemistry probed by extreme-ultraviolet time-resolved photoelectron spectroscopy. Nat. Commun. 2018, 9, 3162. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.A.A.; Fleming, G.R. Following Coupled Electronic-Nuclear Motion through Conical Intersections in the Ultrafast Relaxation of β-Apo-8′-carotenal. J. Phys. Chem. B 2015, 119, 11428–11441. [Google Scholar] [CrossRef]
- Andersson, K.; Malmqvist, P.A.; Roos, B.O.; Sadlej, A.J.; Wolinski, K. Second-order perturbation theory with a CASSCF reference function. J. Phys. Chem. 1990, 94, 5483–5488. [Google Scholar] [CrossRef]
- Olsen, J.; Roos, B.O.; Jo/rgensen, P.; Jensen, H.J.r.A. Determinant based configuration interaction algorithms for complete and restricted configuration interaction spaces. J. Chem. Phys. 1988, 89, 2185–2192. [Google Scholar] [CrossRef]
- Medimagh, M.; Ben Mleh, C.; Issaoui, N.; Kazachenko, A.S.; Roisnel, T.; Al-Dossary, O.M.; Marouani, H.; Bousiakoug, L.G. DFT and molecular docking study of the effect of a green solvent (water and DMSO) on the structure, MEP, and FMOs of the 1-ethylpiperazine-1,4-diium bis(hydrogenoxalate) compound. J. Mol. Liq. 2023, 369, 120851. [Google Scholar] [CrossRef]
- Uludag, N.; Serdaroglu, G.; Sugumar, P.; Rajkumar, P.; Colak, N.; Ercag, E. Synthesis of thiophene derivatives: Substituent effect, antioxidant activity, cyclic voltammetry, molecular docking, DFT, and TD-DFT calculations. J. Mol. Struct. 2022, 1257, 132607. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shang, C.J.; Sun, C.F.; Wang, L.L. Simultaneously regulating absorption capacities and antioxidant activities of four stilbene derivatives utilizing substitution effect: A theoretical and experimental study against UVB radiation. Spectrochim. Acta Part A 2024, 304, 123325. [Google Scholar] [CrossRef] [PubMed]
- Madala, N.E.; Kabanda, M.M. LC-MS based validation and DFT investigation on the antioxidant properties of clovamide: •OH and •OOH scavenging and Cu(II) chelation mechanisms. J. Mol. Struct. 2021, 1236, 130349. [Google Scholar] [CrossRef]
- Cao, B.; Li, Y.; Zhou, Q.; Li, B.; Su, X.; Yin, H.; Shi, Y. Synergistically improving myricetin ESIPT and antioxidant activity via dexterously trimming atomic electronegativity. J. Mol. Liq. 2021, 325, 115272. [Google Scholar] [CrossRef]
- Hu, G.; Guo, M.; Li, Q.; Zhao, J.; Zhang, L.; Yang, J.; Yin, H.; Han, J.; Shi, Y. The substituent effect on the ESIPT and antioxidant activity of dual proton-transfer-site salicylaldehyde azine derivatives. Chem. Phys. Lett. 2025, 865, 141933. [Google Scholar] [CrossRef]

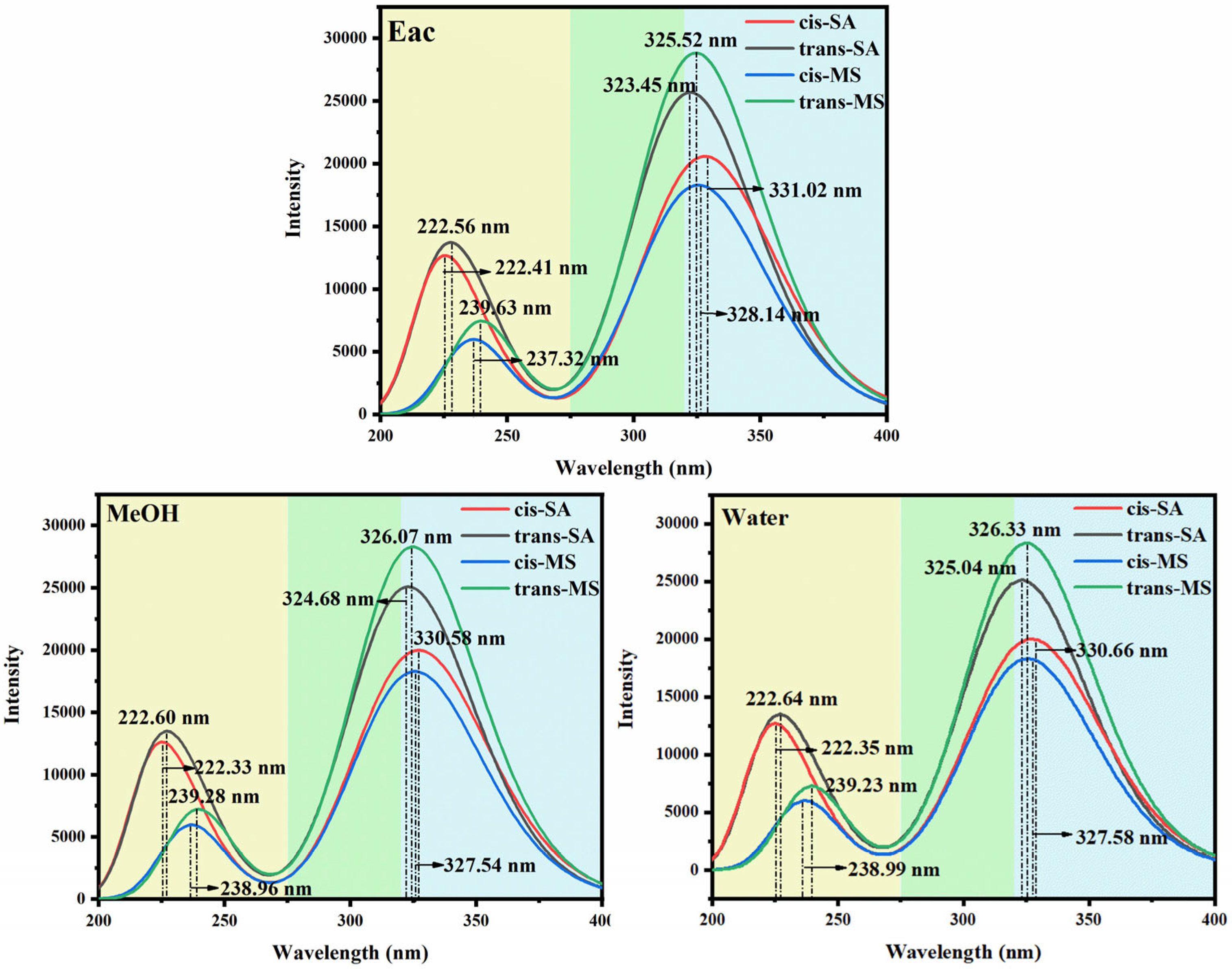

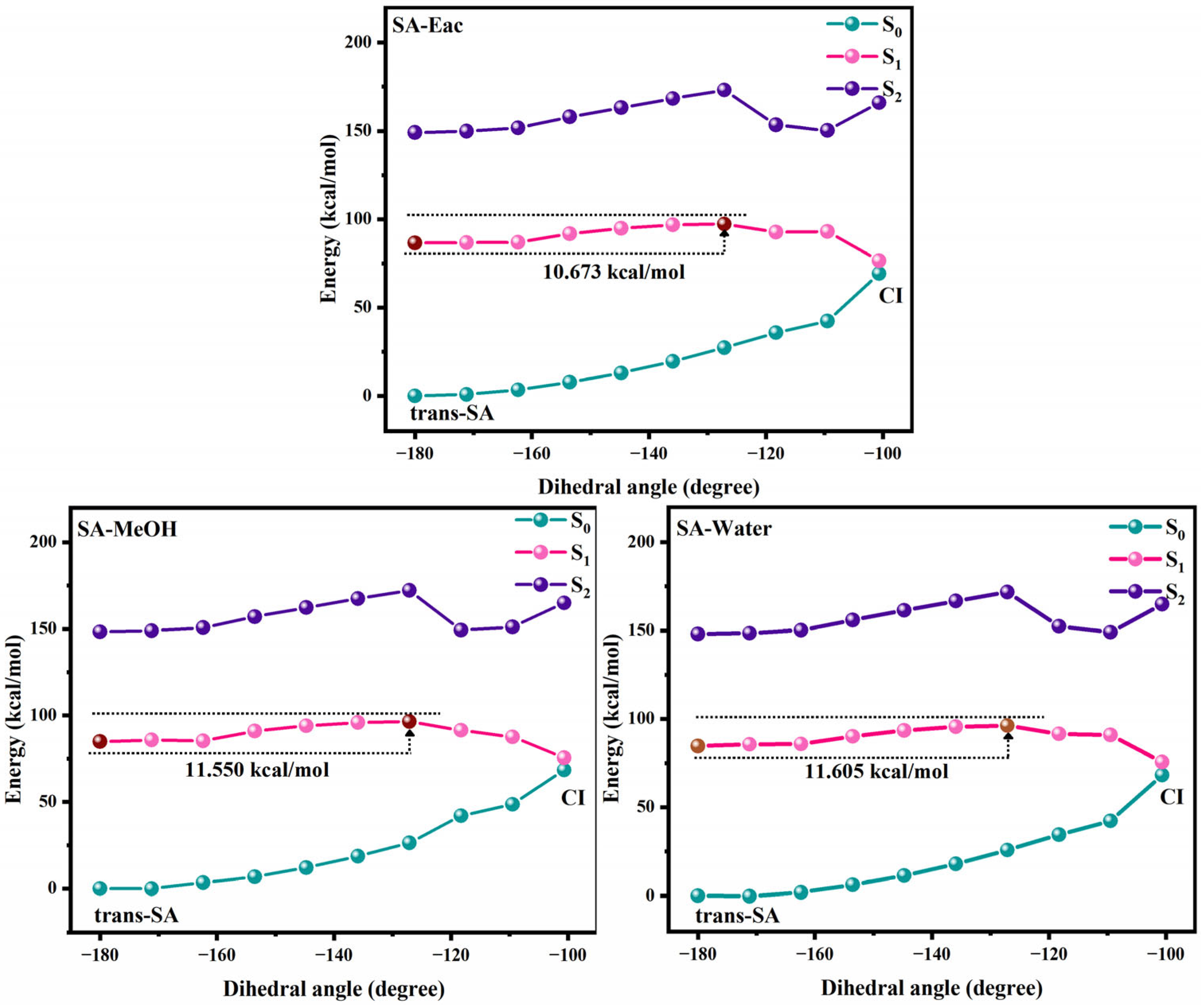
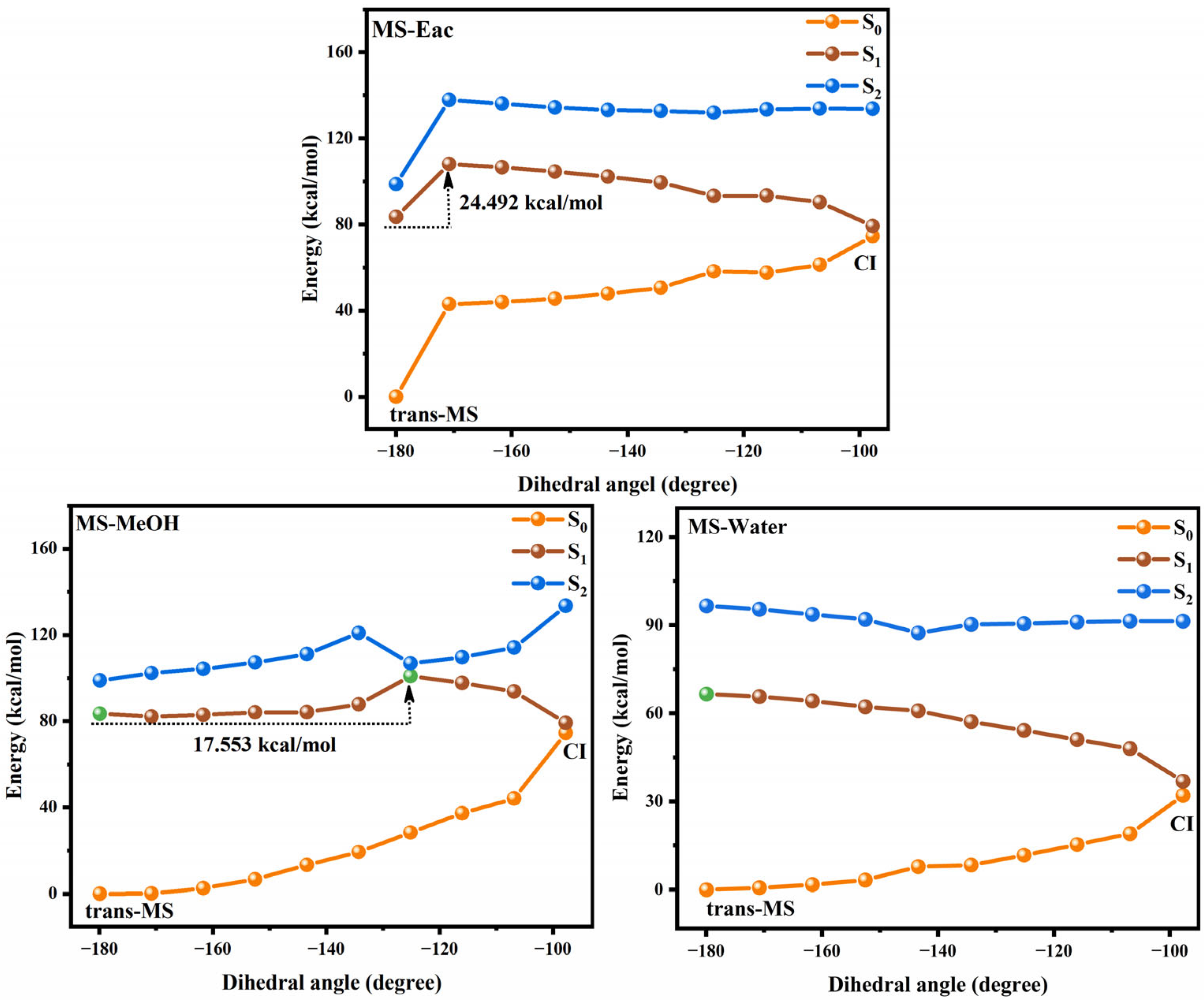
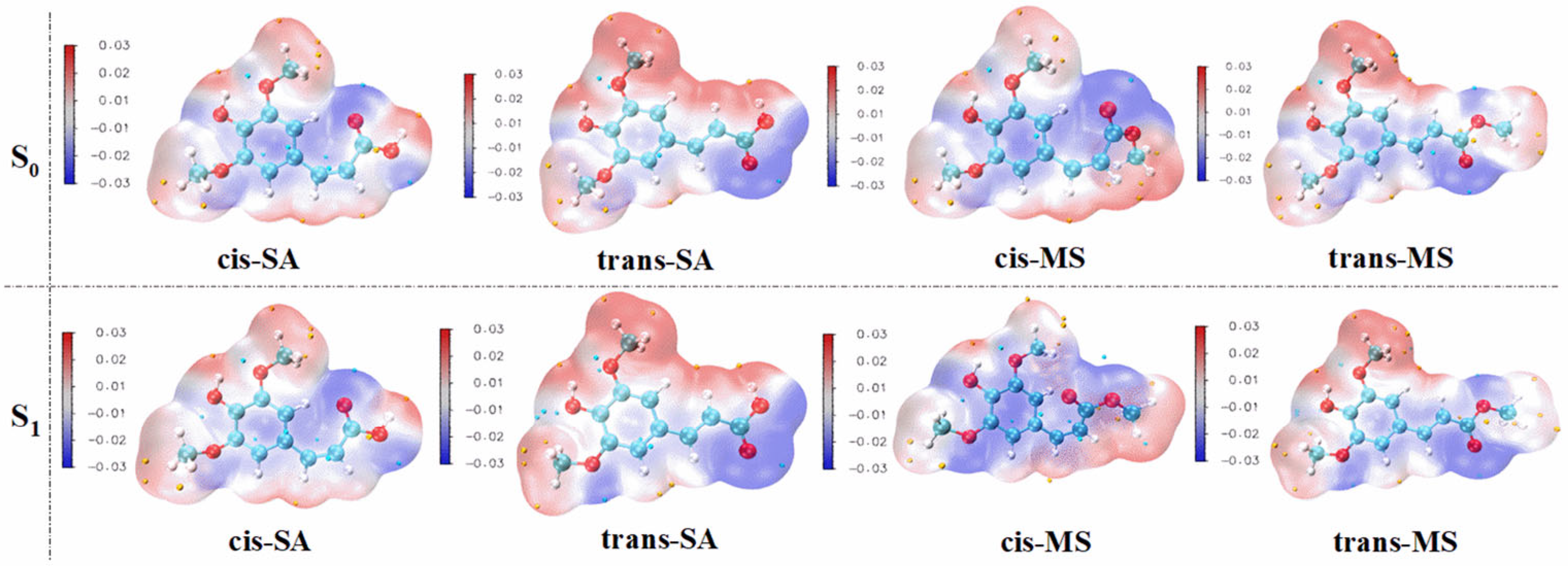
| cis-SA | trans-SA | cis-MS | trans-MS | |||
|---|---|---|---|---|---|---|
| S0 | Eac | D3-1-2-4 | 0.120 | −179.944 | 1.053 | −179.923 |
| D1-2-4-o | −0.195 | −0.006 | 5.506 | −0.022 | ||
| MeOH | D3-1-2-4 | 0.142 | −179.990 | 2.851 | −179.934 | |
| D1-2-4-o | −0.241 | 0.291 | 18.798 | 0.060 | ||
| Water | D3-1-2-4 | 0.145 | 179.993 | 2.938 | −179.947 | |
| D1-2-4-o | −0.253 | 0.350 | 18.855 | 0.085 | ||
| S1 | Eac | D3-1-2-4 | −0.013 | −179.990 | 11.79 | 179.991 |
| D1-2-4-o | −0.047 | 0.019 | 2.291 | −0.010 | ||
| MeOH | D3-1-2-4 | 0.525 | −179.706 | 13.199 | −179.693 | |
| D1-2-4-o | 0.163 | −0.254 | 2.551 | −0.096 | ||
| Water | D3-1-2-4 | 0.638 | −179.689 | 13.445 | −179.714 | |
| D1-2-4-o | 0.181 | −0.101 | 2.603 | −0.118 |
| cis-SA | trans-SA | cis-MS | trans-MS | |||
|---|---|---|---|---|---|---|
| Eac | S0 | H1 | 0.49140 | 0.49161 | 0.49071 | 0.49104 |
| S1 | 0.49708 | 0.50481 | 0.49373 | 0.50512 | ||
| S0 | H2 | 0.48647 | 0.47932 | |||
| S1 | 0.47101 | 0.46309 | ||||
| MeOH | S0 | H1 | 0.49371 | 0.49405 | 0.49261 | 0.49354 |
| S1 | 0.50263 | 0.50556 | 0.49666 | 0.50543 | ||
| S0 | H2 | 0.48980 | 0.48564 | |||
| S1 | 0.47428 | 0.47325 | ||||
| Water | S0 | H1 | 0.49405 | 0.49440 | 0.49283 | 0.49391 |
| S1 | 0.50607 | 0.50608 | 0.49713 | 0.50597 | ||
| S0 | H2 | 0.49028 | 0.48658 | |||
| S1 | 0.47759 | 0.47417 |
| IP (eV) | EA (eV) | η (eV) | ω (eV) | S (eV−1) | μ (eV) | |||
|---|---|---|---|---|---|---|---|---|
| cis-SA | Eac | S0 | 5.9671 | 1.5191 | 4.4480 | 1.5750 | 0.2248 | −3.7431 |
| S1 | 5.8363 | 1.8024 | 4.0338 | 1.8081 | 0.2479 | −3.8193 | ||
| MeOH | S0 | 5.7512 | 1.8169 | 3.9343 | 1.8197 | 0.2542 | −3.7840 | |
| S1 | 5.6010 | 2.1023 | 3.4987 | 2.1201 | 0.2858 | −3.8517 | ||
| Water | S0 | 5.7225 | 1.8578 | 3.8646 | 1.8586 | 0.2588 | −3.7902 | |
| S1 | 5.5516 | 2.0998 | 3.4518 | 2.1201 | 0.2897 | −3.8257 | ||
| trans-SA | Eac | S0 | 6.0332 | 1.5276 | 4.5056 | 1.5859 | 0.2219 | −3.7804 |
| S1 | 5.7569 | 1.7732 | 3.9837 | 1.7792 | 0.2510 | −3.7651 | ||
| MeOH | S0 | 5.7841 | 1.8112 | 3.9729 | 1.8150 | 0.25107 | −3.7976 | |
| S1 | 5.6093 | 2.0858 | 3.5235 | 2.1007 | 0.2838 | −3.8475 | ||
| Water | S0 | 5.7504 | 1.8498 | 3.9007 | 1.8511 | 0.2564 | −3.8001 | |
| S1 | 5.5752 | 2.1263 | 3.4489 | 2.1498 | 0.2900 | −3.8508 | ||
| cis-MS | Eac | S0 | 5.9706 | 1.5788 | 4.3918 | 1.6222 | 0.2277 | −3.7747 |
| S1 | 5.1760 | 1.9203 | 3.2557 | 1.9334 | 0.3072 | −3.5481 | ||
| MeOH | S0 | 5.7510 | 1.8325 | 3.9185 | 1.8346 | 0.2552 | −3.7917 | |
| S1 | 5.0366 | 2.2297 | 2.8069 | 2.3513 | 0.3563 | −3.6331 | ||
| Water | S0 | 5.7225 | 1.8734 | 3.8491 | 1.8737 | 0.2598 | −3.7980 | |
| S1 | 5.0200 | 2.2730 | 2.7470 | 2.4203 | 0.3640 | −3.6465 | ||
| trans-MS | Eac | S0 | 5.9500 | 1.4242 | 4.5258 | 1.5019 | 0.2210 | −3.6871 |
| S1 | 5.6675 | 1.6847 | 3.9828 | 1.6965 | 0.2511 | −3.6761 | ||
| MeOH | S0 | 5.7271 | 1.7231 | 4.0039 | 1.7328 | 0.2498 | −3.7251 | |
| S1 | 5.5433 | 2.0227 | 3.5206 | 2.0325 | 0.2840 | −3.7830 | ||
| Water | S0 | 5.6977 | 1.7643 | 3.9333 | 1.7695 | 0.2542 | −3.7310 | |
| S1 | 5.5127 | 2.0656 | 3.4471 | 2.0825 | 0.2901 | −3.7891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Sun, C.; Wang, L. The Effect of Photoisomerization on the Antioxidant Properties of Sinapic Acid and Methyl Sinapate in Different Solvents: A DFT/TD-DFT Study. Antioxidants 2025, 14, 633. https://doi.org/10.3390/antiox14060633
Wang L, Sun C, Wang L. The Effect of Photoisomerization on the Antioxidant Properties of Sinapic Acid and Methyl Sinapate in Different Solvents: A DFT/TD-DFT Study. Antioxidants. 2025; 14(6):633. https://doi.org/10.3390/antiox14060633
Chicago/Turabian StyleWang, Lei, Chaofan Sun, and Lingling Wang. 2025. "The Effect of Photoisomerization on the Antioxidant Properties of Sinapic Acid and Methyl Sinapate in Different Solvents: A DFT/TD-DFT Study" Antioxidants 14, no. 6: 633. https://doi.org/10.3390/antiox14060633
APA StyleWang, L., Sun, C., & Wang, L. (2025). The Effect of Photoisomerization on the Antioxidant Properties of Sinapic Acid and Methyl Sinapate in Different Solvents: A DFT/TD-DFT Study. Antioxidants, 14(6), 633. https://doi.org/10.3390/antiox14060633







