Antimicrobial Evaluation and Fraction-Based Profiling of Basil Essential Oil Against Vaginal Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Sourcing and Identification
2.2. Essential Oil Extraction
2.3. Phytochemical Analysis
2.3.1. Gas Chromatography–Mass Spectrometry (GC-MS)
2.3.2. Fractionation of BEO by Preparative HPLC
2.4. Antioxidant Activity Assays
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
2.5. Antibacterial Testing
2.5.1. Strains and Culture Conditions
2.5.2. Disk Diffusion Test
2.5.3. Minimum Inhibitory Concentration (MIC)
2.5.4. Relative Microbial Growth Inhibition
2.6. Toxicity Assessment
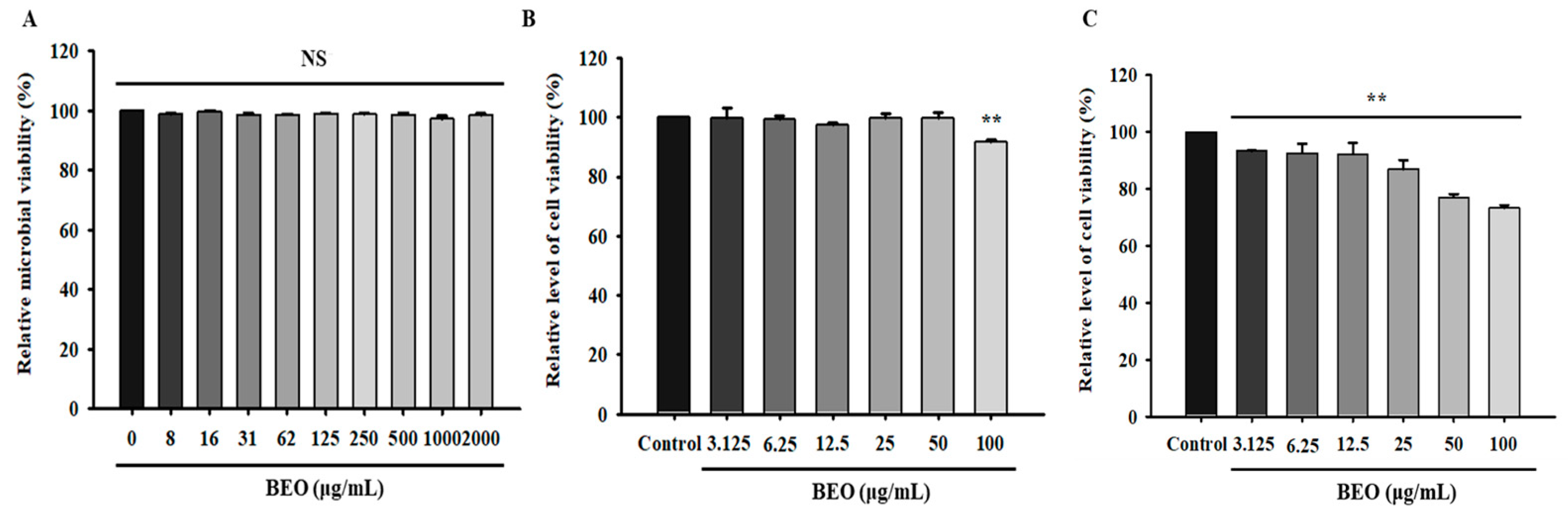
2.6.1. Relative Microbial Viability of Beneficial Bacteria
2.6.2. Cytotoxicity of BEO
2.7. Statistical Analysis
3. Results
3.1. Composition of BEO by GC-MS
3.2. Isolation and Identification of Antimicrobial Compounds
3.3. Antioxidant Activities of BEO
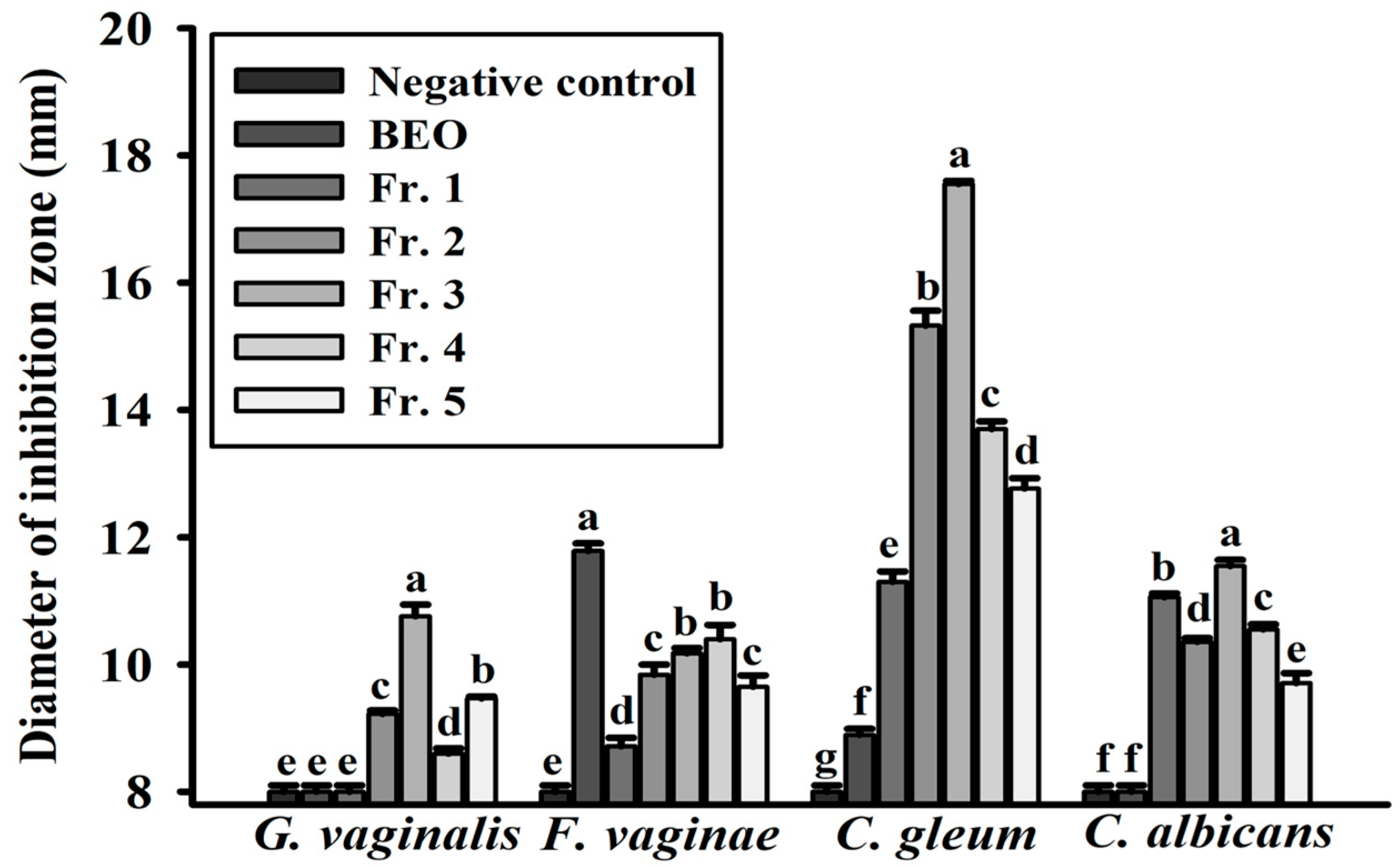
3.4. Disk Diffusion Assays of BEO Against Vaginitis Pathogens
3.5. Evaluation of the MIC Values of BEO
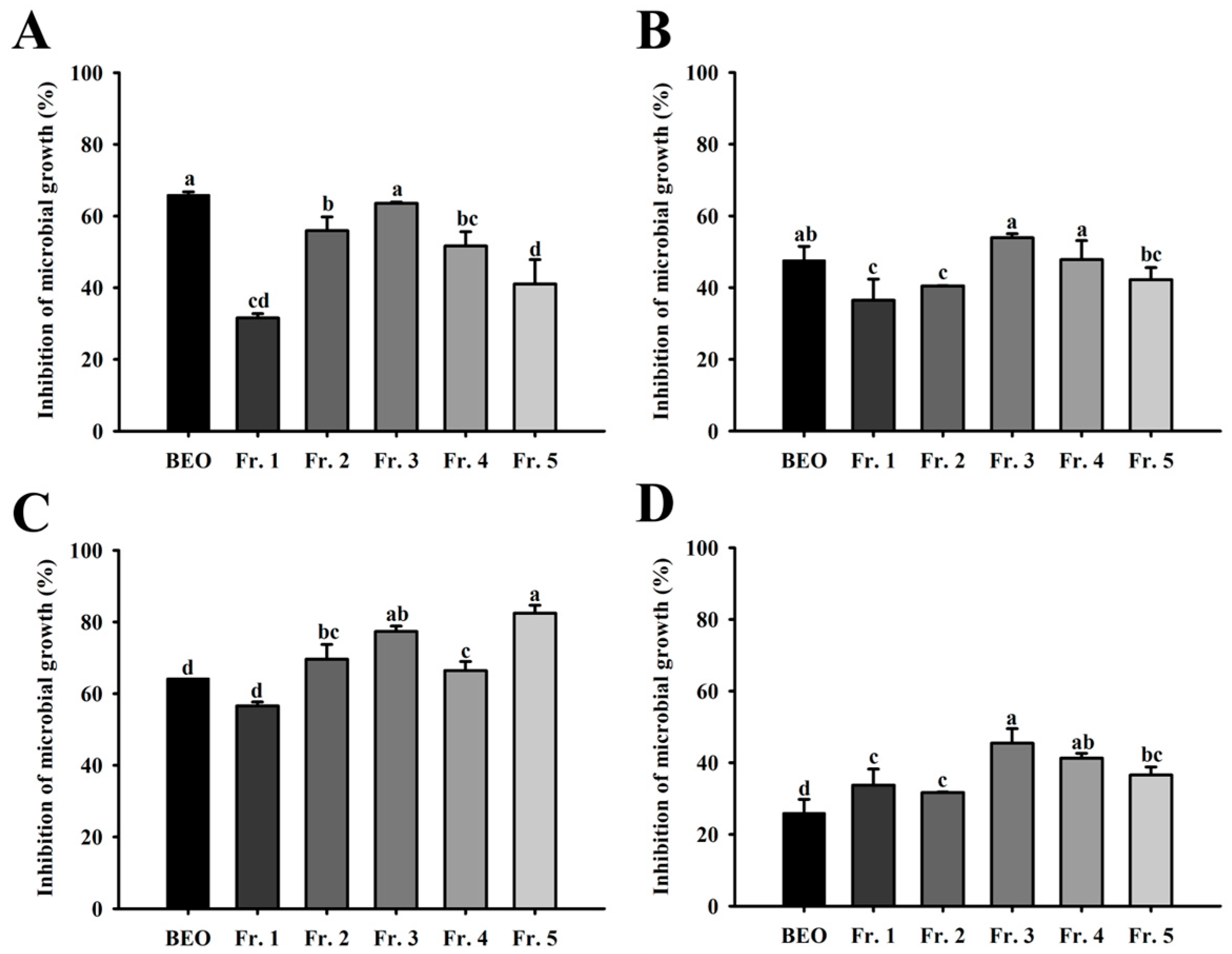
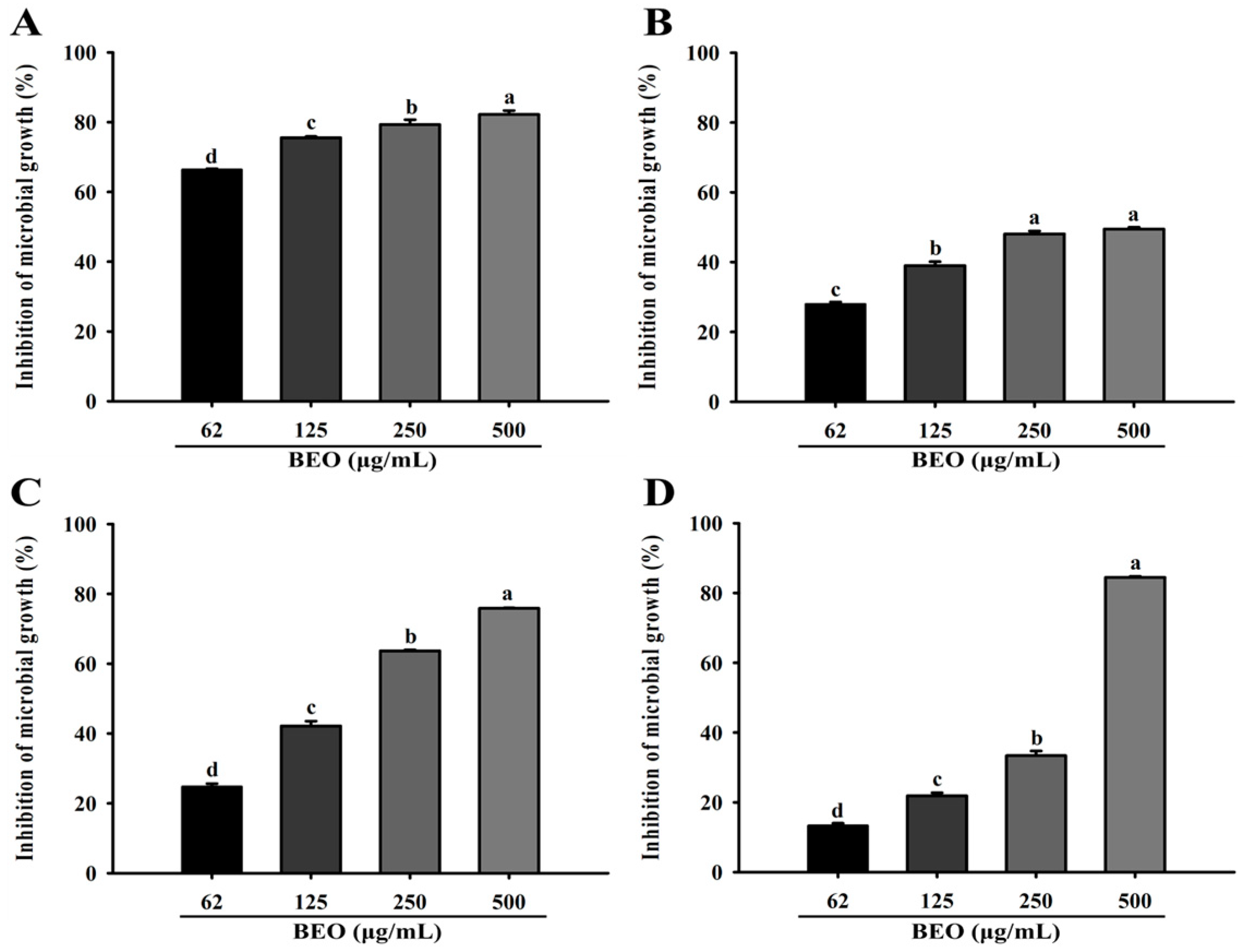
3.6. Relative Microbial Growth Inhibition of Vaginitis Pathogens by BEO
3.7. Safety Assessment of BEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| BEO | Basil essential oil |
| BV | Bacterial vaginosis |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| EC50 | Half-maximal effective concentration |
| FBS | Fetal bovine serum |
| GC-MS | Gas chromatography–mass spectrometry |
| HDF | Human dermal fibroblast |
| NIST | National Institute of Standards and Technology |
| MIC | Minimum inhibitory concentration |
| PBS | Phosphate-buffered saline |
| PDA | Photodiode-array detector |
| SD | Standard deviation |
| TSB | Tryptic soy broth |
| VVC | Vulvovaginal candidiasis |
References
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.A.; Lupattelli, A.; Koren, G.; Nordeng, H. Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement. Altern. Med. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum linn.). Asian Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kabir, M.J.; Salehuddin, S.M.; Rahman, S.M.; Das, A.K.; Singha, S.K.; Alam, A.; Rahman, A. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharm. Biol. 2010, 48, 504–511. [Google Scholar] [CrossRef]
- Akoto, C.O.; Acheampong, A.; Boakye, Y.D.; Naazo, A.A.; Adomah, D.H. Anti-Inflammatory, Antioxidant, and Anthelmintic Activities of Ocimum basilicum (Sweet Basil) Fruits. J. Chem. 2020, 2020, 2153534. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Teofilović, B.; Grujić-Letić, N.; Gligorić, E.; Rašković, A.; Igić, R.; Vastag, G.; Gadžurić, S. Experimental and computational evaluation of extraction procedure and scavenging capacity of sweet basil extracts (Ocimum basilicum L.). Plant Foods Hum. Nutr. 2021, 76, 240–247. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp.(basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs. Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Zhakipbekov, K.; Turgumbayeva, A.; Akhelova, S.; Bekmuratova, K.; Blinova, O.; Utegenova, G.; Shertaeva, K.; Sadykov, N.; Tastambek, K.; Saginbazarova, A.; et al. Antimicrobial and other pharmacological properties of Ocimum basilicum, Lamiaceae. Molecules 2024, 29, 388. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef]
- Antonio, M.A.; Hawes, S.E.; Hillier, S.L. The identification of vaginal lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 1999, 180, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Boskey, R.; Telsch, K.M.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 1999, 67, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, P.; Alleyn, H.N.; Raphael, E.; Krupp, K.; Ravi, K.; Nebhrajani, R.; Anjali, A.R.; Reingold, L.W.; Klausner, J.D. Identification of culturable vaginal lactobacillus species among reproductive age women in Mysore, India. J. Med. Microbiol. 2015, 64, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.T.; Lee, I.A.; Hyun, Y.J.; Kim, D.H. Artemisia princeps pamp. essential oil and its constituents eucalyptol and α-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-κB activation. Planta Medica 2011, 77, 1996–2002. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial vaginosis: What do we currently know? Front. Cell. Infect. Microbiol. 2022, 11, 1393. [Google Scholar] [CrossRef]
- Jang, J.; Kang, D.H.; Yoon, J.; Kim, H.S. Separation and purification of antimicrobial substance from Syzygium aromaticum Merrill et Perry for treatment of microbial vaginosis. KSBB J. 2018, 33, 285–292. [Google Scholar] [CrossRef]
- Pendharkar, S.; Brandsborg, E.; Hammarström, L.; Marcotte, H.; Larsson, P.G. Vaginal colonization by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 2015, 15, 255. [Google Scholar] [CrossRef]
- Hurley, R.; de Louvois, J. Candida vaginitis. Postgrad. Med. J. 1979, 55, 645. [Google Scholar] [CrossRef]
- Fachriyah, E.; Wibawa, P.J.; Awaliyah, A. Antibacterial activity of basil oil (Ocimum basilicum L.) and basil oil nanoemulsion. J. Phys. Conf. Ser. 2020, 1524, 12–60. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- De Beer, E.J.; Sherwood, M.B. The paper-disc agar-plate method for the assay of antibiotic substances. J. Bacteriol. 1945, 50, 459–467. [Google Scholar] [CrossRef]
- Liu, M.; Seidel, V.; Katerere, D.R.; Gray, A.I. Colorimetric broth microdilution method for the antifungal screening of plant extracts against yeasts. Methods 2007, 42, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.; Marei, G.I.; Rabea, E.I.; Taktak, N.E. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Neelam, K.A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, Z.; Liang, Y. Comparative analysis of plant essential oils by GC-MS coupled with integrated chemometric resolution methods. Anal. Methods 2010, 2, 359–367. [Google Scholar] [CrossRef]
- Gawron, G.; Krzyczkowski, W.; Lemke, K.; Ołdak, A.; Kadziński, L.; Banecki, B. Nigella sativa seed extract applicability in preparations against methicillin-resistant Staphylococcus aureus and effects on human dermal fibroblasts viability. J. Ethnopharmacol. 2019, 244, 112–135. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Özcan, M.M. Comparative essential oil composition of flowers, leaves and stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008, 110, 501–503. [Google Scholar] [CrossRef]
- Kavoosi, G.; Amirghofran, Z. Chemical composition, radical scavenging and antioxidant capacity of Ocimum basilicum essential oil. J. Essent. Oil Res. 2017, 29, 189–199. [Google Scholar] [CrossRef]
- Krokidi, K.M.; Turner, M.A.; Pearcy, P.A.; Stavros, V.G. A systematic approach to methyl cinnamate photodynamics. Mol. Phys. 2021, 119, e1811910. [Google Scholar] [CrossRef]
- Rosas, J.F.; Zoghbi, M.D.G.B.; Andrade, E.H.A.; van den Berg, M.E. Chemical composition of a methyl-(E)-cinnamate Ocimum micranthum Willd. from the Amazon. Flavour Fragr. J. 2005, 20, 161–163. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lee, M.H.; Hsu, C.C.; Wei, C.L.; Tsai, Y.C. Methyl cinnamate inhibits adipocyte differentiation via activation of the CaMKK2–AMPK pathway in 3T3-L1 preadipocytes. J. Agric. Food Chem. 2012, 60, 955–963. [Google Scholar] [CrossRef]
- Huang, Q.S.; Zhu, Y.J.; Li, H.L.; Zhuang, J.X.; Zhang, C.L.; Zhou, J.J.; Li, W.G.; Chen, Q.X. Inhibitory effects of methyl trans-cinnamate on mushroom tyrosinase and its antimicrobial activities. J. Agric. Food Chem. 2009, 57, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Lee, H.; Cho, M.; Yun, H.M. A phytochemical constituent, (E)-methyl-cinnamate isolated from Alpinia katsumadai Hayata suppresses cell survival, migration, and differentiation in pre-osteoblasts. Int. J. Mol. Sci. 2020, 21, 3700. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Jang, J.; Kang, D.H.; Yoon, J.; Kim, H.S. Isolation and characterization of an antibacterial substance from Rheum palmatum for treatment of bacterial vaginosis. KSBB J. 2017, 32, 133–139. [Google Scholar] [CrossRef]
- Marcas, J.; Romero, L.; Tipiani, O.; Loyola, S.; Tamariz, J. In vitro antimicrobial activity of Bixa orellana L. leaves extract against anaerobic bacteria associated to bacterial vaginosis and Lactobacillus spp. Rev. Peru Med. Exp. Salud Pública 2023, 39, 408–414. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Aliahmadi, A.; Toliat, T.; Dalimi, A.; Momeni, Z.; Rahimi, R. Evaluation of antimicrobial activity of Trachyspermum ammi (L.) sprague essential oil and its active constituent, thymol, against vaginal pathogens. Trad. Integr. Med. 2020, 5, 49–58. [Google Scholar] [CrossRef]
- Murina, F.; Vicariotto, F.; Di Francesco, S. Thymol, eugenol and lactobacilli in a medical device for the treatment of bacterial vaginosis and vulvovaginal candidiasis. New Microbiol. 2018, 41, 220–224. [Google Scholar] [PubMed]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal activity of natural compounds vs. Candida spp.: A mixture of cinnamaldehyde and eugenol shows promising in vitro results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Shariati, A. Eugenol: A novel therapeutic agent for the inhibition of Candida species infection. Front. Pharmacol. 2022, 13, 872127. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the antioxidant, cytoprotective and antityrosinase effects of Schisandra chinensis Extracts and their applicability in skin care product. Molecules 2022, 27, 8877. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T. Saponins as natural raw materials for increasing the safety of bodywash cosmetic use. JSD 2018, 21, 67–776. [Google Scholar] [CrossRef]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385. [Google Scholar] [CrossRef]

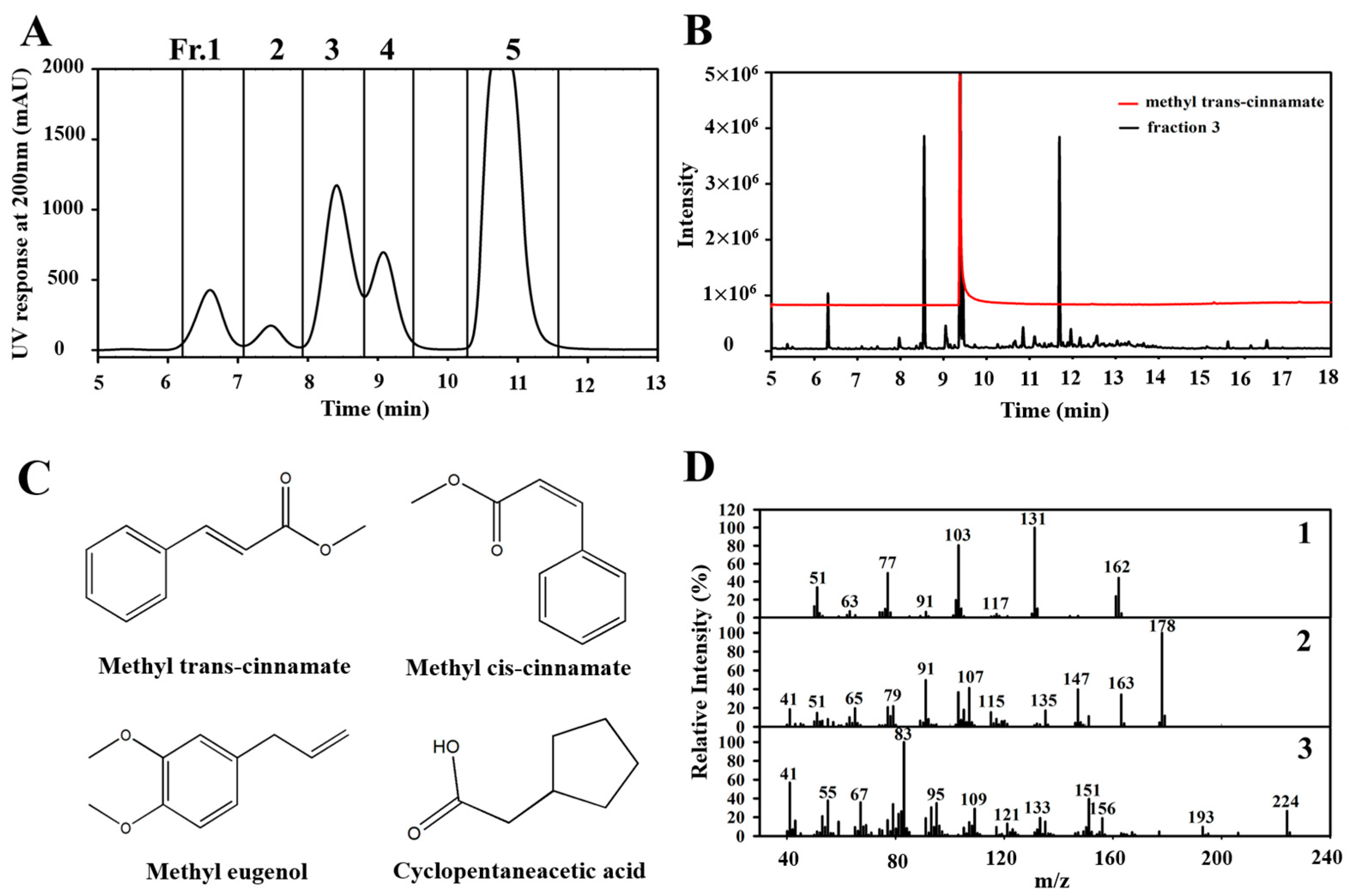
| No. | Compound | Formula | Rt (min) | Area (%) |
|---|---|---|---|---|
| 1 | Bicyclo[3.1.1]heptane | C7H12 | 5.080 | 0.67 |
| 2 | 2-Isopropyltoluene | C10H14 | 5.586 | 0.48 |
| 3 | l-Limonene | C10H16 | 5.647 | 0.45 |
| 4 | Eucalyptol | C10H18O | 5.714 | 6.33 |
| 5 | Linalool | C10H18O | 6.440 | 19.60 |
| 6 | Camphor | C10H16O | 7.059 | 0.37 |
| 7 | Estragole | C10H12O | 7.593 | 29.48 |
| 8 | (-)-Carvone | C10H14O | 8.057 | 0.38 |
| 9 | Bornyl acetate | C12H20O2 | 8.479 | 1.21 |
| 10 | Methyl cinnamate | C10H10O2 | 8.642 | 2.37 |
| 11 | Eugenol | C10H12O2 | 9.141 | 0.44 |
| 12 | Methyl cinnamate | C10H10O2 | 9.519 | 16.72 |
| 13 | Bicyclo[5.2.0]nonane | C15H24 | 9.549 | 1.72 |
| 14 | Caryophyllene | C15H24 | 9.933 | 1.07 |
| 15 | trans-alpha-Bergamotene | C15H24 | 9.973 | 7.67 |
| 16 | alpha-Humulene | C15H24 | 10.269 | 0.53 |
| 17 | n.i | - | 10.470 | 0.48 |
| 18 | Germacrene-D | C15H24 | 10.493 | 1.88 |
| 19 | n.i | - | 10.627 | 0.42 |
| 20 | n.i | - | 10.650 | 0.46 |
| 21 | gamma-Amorphene | C15H24 | 10.759 | 2.59 |
| 22 | (E)-alpha-Bisabolene | C15H24 | 10.904 | 0.80 |
| 23 | (-)-Caryophyllene oxide | C15H24O | 11.436 | 0.30 |
| 24 | n.i | - | 11.673 | 0.64 |
| 25 | Epi-cadinol | C15H26O | 11.883 | 2.94 |
| 100.00 |
| MIC | BEO | Fr. 1 | Fr. 2 | Fr. 3 | Fr. 4 | Fr. 5 | |
|---|---|---|---|---|---|---|---|
| G. vaginalis | (μg/mL) | 125 | 125 | 62 | 31 | 62 | 125 |
| F. vaginae | (μg/mL) | 31 | 250 | 250 | 31 | 125 | 125 |
| C. gleum | (μg/mL) | 31 | 125 | 62 | 16 | 62 | 16 |
| C. albicans | (μg/mL) | 500 | 250 | 250 | 62 | 125 | 125 |
| DPPH | ABTS |
|---|---|
| EC50 (μg/mL) | |
| 115.36 ± 2.19 | 54.77 ± 0.29 |
| Diameter of Inhibition Zone (mm) | MIC | |||||
|---|---|---|---|---|---|---|
| Test Strains | mg/disk | μg/mL | ||||
| 0 | 0.5 | 1 | 2 | 5 | ||
| G. vaginalis | 8.02 ± 0.01 a | 8.03 ± 0.02 c | 9.91 ± 0.35 c | 11.79 ± 0.20 b | 13.75 ± 0.39 d | 125 |
| F. vaginae | 8.03 ± 0.01 a | 12.97 ± 0.11 a | 19.34 ± 0.46 a | 22.71 ± 0.33 a | 25.88 ± 0.52 a | 31 |
| C. gleum | 8.03 ± 0.01 a | 8.09 ± 0.01 b | 11.48 ± 0.13 b | 13.06 ± 0.02 b | 17.74 ± 0.25 b | 31 |
| C. albicans | 8.02 ± 0.01 a | 8.02 ± 0.02 c | 9.03 ± 0.10 d | 11.07 ± 0.30 d | 13.95 ± 0.11 c | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Park, J.; Hwang, D.Y.; Park, S.; Lee, H. Antimicrobial Evaluation and Fraction-Based Profiling of Basil Essential Oil Against Vaginal Pathogens. Antioxidants 2025, 14, 628. https://doi.org/10.3390/antiox14060628
Park M, Park J, Hwang DY, Park S, Lee H. Antimicrobial Evaluation and Fraction-Based Profiling of Basil Essential Oil Against Vaginal Pathogens. Antioxidants. 2025; 14(6):628. https://doi.org/10.3390/antiox14060628
Chicago/Turabian StylePark, Minkyoung, Jumin Park, Dae Youn Hwang, Sohae Park, and Heeseob Lee. 2025. "Antimicrobial Evaluation and Fraction-Based Profiling of Basil Essential Oil Against Vaginal Pathogens" Antioxidants 14, no. 6: 628. https://doi.org/10.3390/antiox14060628
APA StylePark, M., Park, J., Hwang, D. Y., Park, S., & Lee, H. (2025). Antimicrobial Evaluation and Fraction-Based Profiling of Basil Essential Oil Against Vaginal Pathogens. Antioxidants, 14(6), 628. https://doi.org/10.3390/antiox14060628








