Corynoxeine Supplementation Ameliorates Colistin-Induced Kidney Oxidative Stress and Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
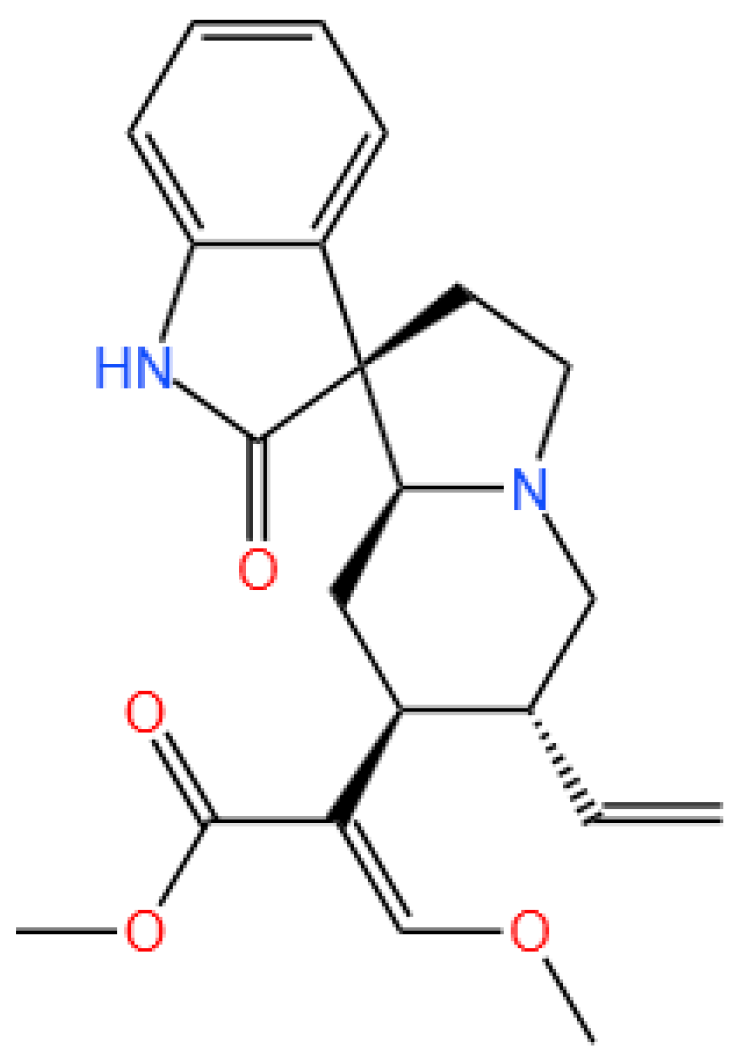
2.1. Chemicals and Reagents
2.2. Animals and Experiment Designs
2.3. Serum Isolation and Biochemical Analysis
2.4. Oxidative Stress Biomarker Measurement
2.5. Measurement of the Activities of Caspases-9 and -3
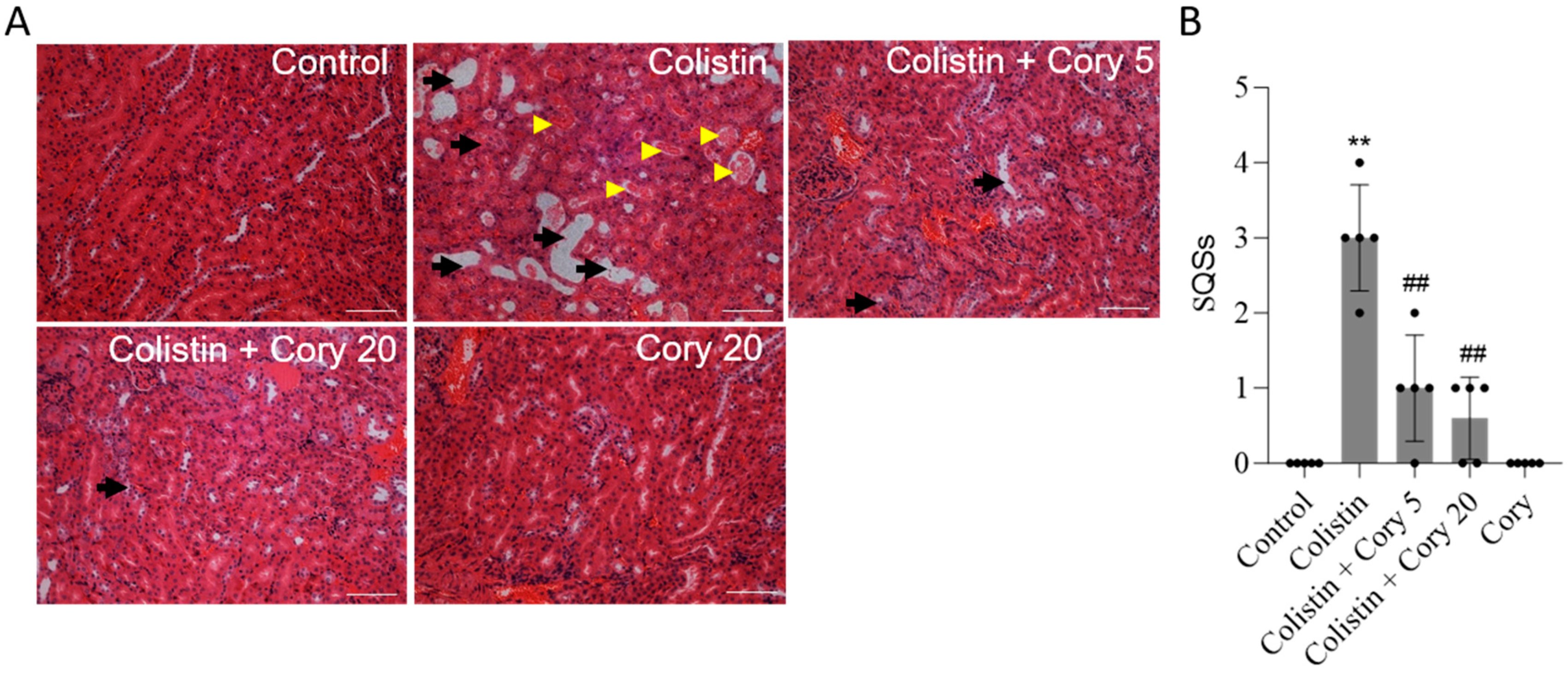
2.6. Histopathological Examination
2.7. Immunohistochemical Examination
2.8. Quantitative Real-Time PCR Analysis
2.9. Western Blot Analysis of Kidney Tissue Proteins
2.10. Statistical Analysis
3. Results
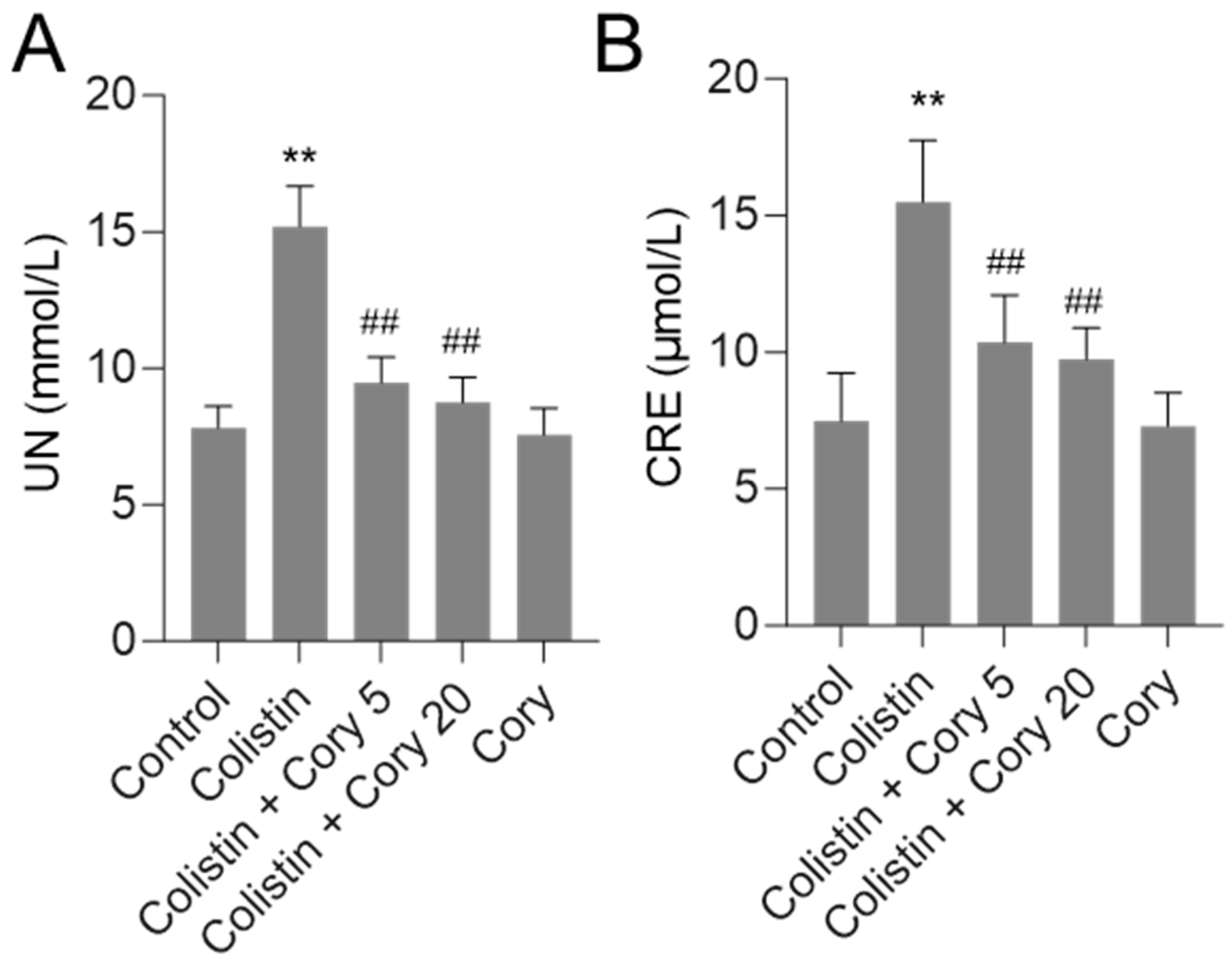
3.1. Cory Supplementation Ameliorates Colistin-Induced Nephrotoxicity in Mice
3.2. Cory Supplementation Ameliorates Colistin Treatment-Caused Oxidative Stress Damage in the Kidneys of Mice
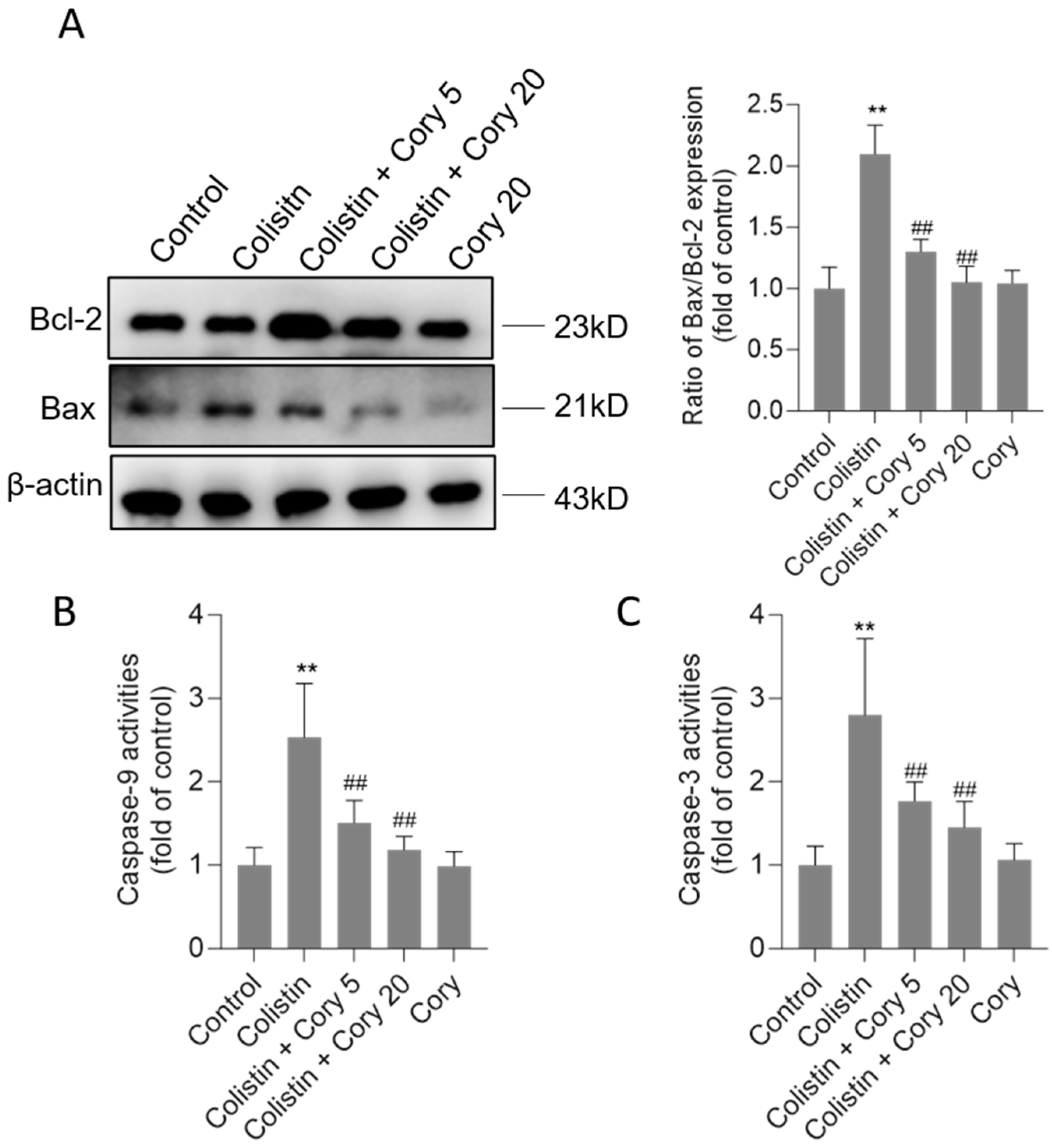
3.3. Cory Supplementation Attenuates Colistin Treatment-Caused Activation of the Mitochondrial Apoptotic Pathway
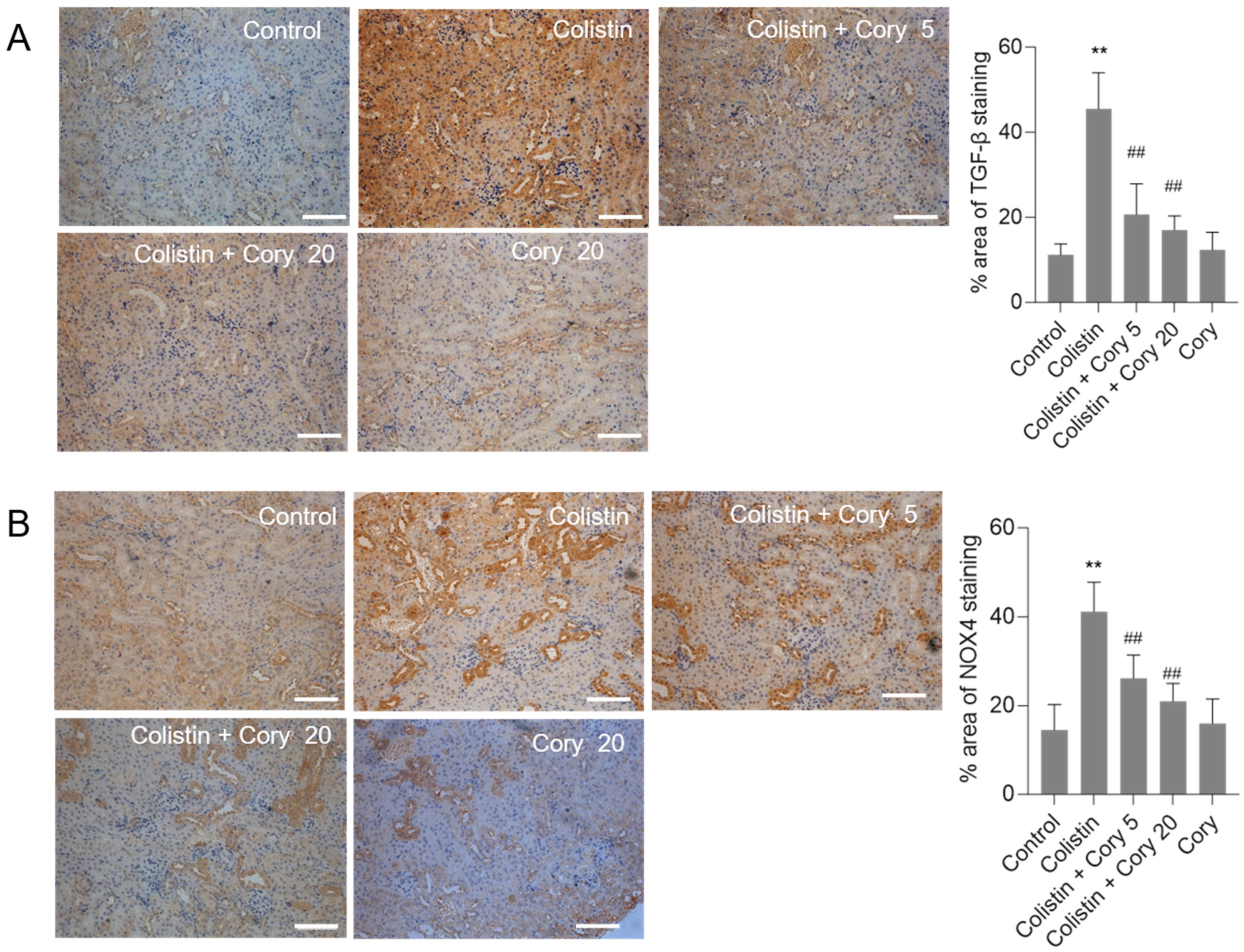
3.4. Cory Supplementation Downregulated the Expression of TGF-β and NOX4 Proteins in the Kidneys
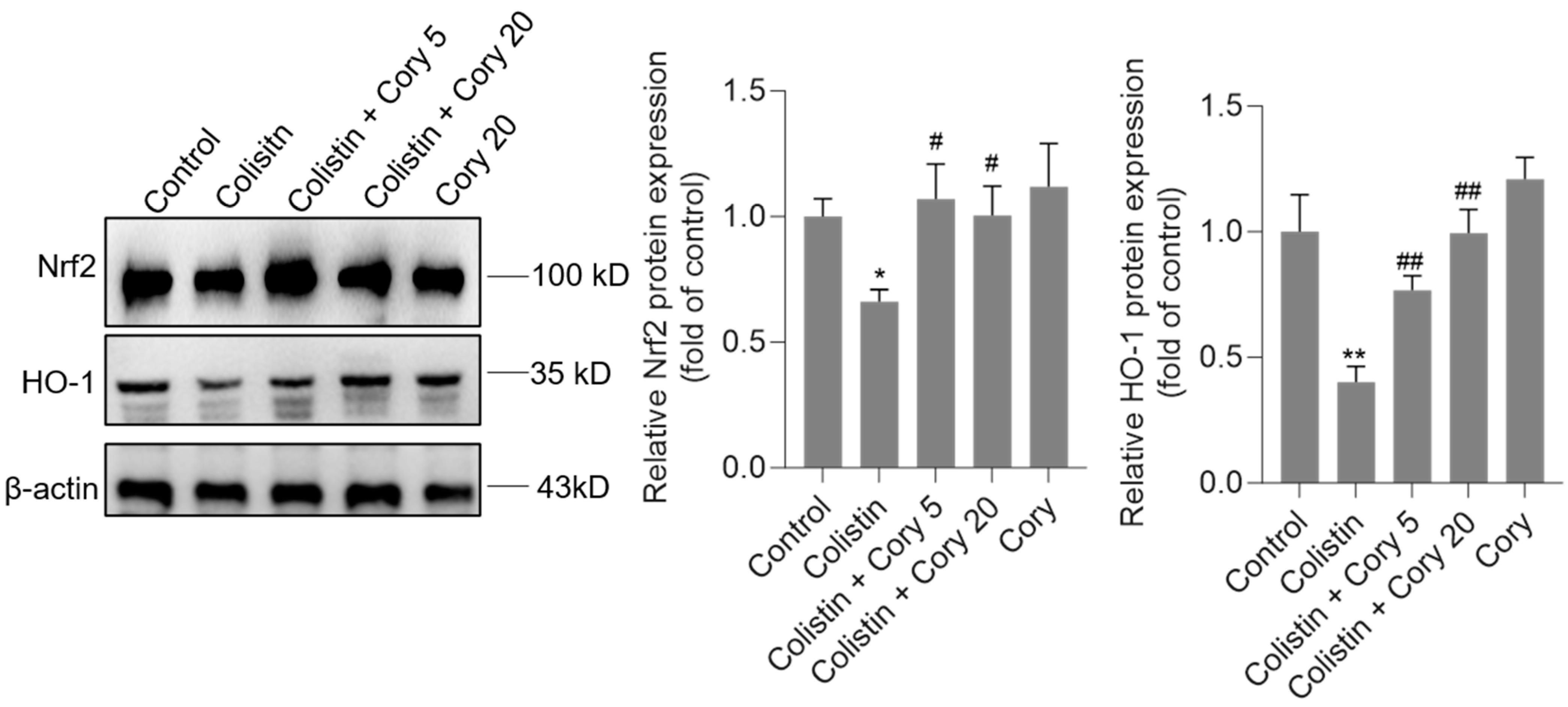
3.5. Cory Supplementation Upregulated the Expression of Nrf2 and HO-1 Proteins in the Kidneys
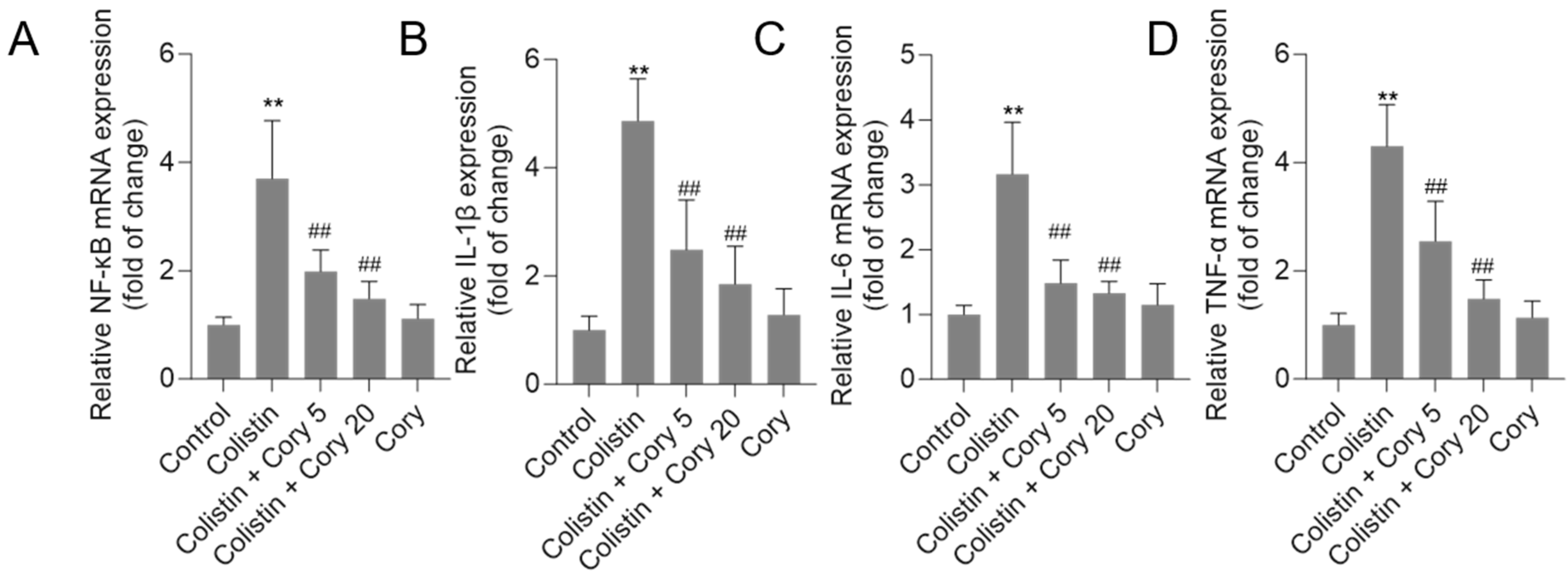
3.6. Cory Treatment Attenuates the Expression of NF-κB, IL-1β, IL-6, and TNF-α mRNAs in the Kidney Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.T.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef]
- Omrani, A.S.; Alfahad, W.A.; Shoukri, M.M.; Baadani, A.M.; Aldalbahi, S.; Almitwazi, A.A.; Albarrak, A.M. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef]
- Ballı, F.N.; Ekinci, P.B.; Kurtaran, M.; Kara, E.; Dizman, G.T.; Sönmezer, M.; Hayran, M.; Demirkan, K.; Metan, G. Battle of polymyxin induced nephrotoxicity: Polymyxin B versus colistin. Int. J. Antimicrob. Agents 2024, 63, 107035. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Roberts, K.D.; Yu, H.H.; Liu, B.; Schofield, A.V.; James, S.A.; Howard, D.L.; Nation, R.L.; Rogers, K.; de Jonge, M.D.; et al. Significant accumulation of polymyxin in single renal tubular cells: A medicinal chemistry and triple correlative microscopy approach. Anal. Chem. 2015, 87, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Azad, M.A.; Nowell, C.J.; Nation, R.L.; Thompson, P.E.; Roberts, K.D.; Velkov, T.; Li, J. Cellular Uptake and Localization of Polymyxins in Renal Tubular Cells Using Rationally Designed Fluorescent Probes. Antimicrob. Agents Chemother. 2015, 59, 7489–7496. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Park, S.R.; Cho, S.; Yu, S.L.; Lee, H.Y.; Park, C.G.; Kang, J.; Jung, D.Y.; Park, M.H.; Hwang, W.M.; et al. TGF-β-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018, 73, 962–972. [Google Scholar] [CrossRef]
- Dai, C.; Li, M.; Sun, T.; Zhang, Y.; Wang, Y.; Shen, Z.; Velkov, T.; Tang, S.; Shen, J. Colistin-induced pulmonary toxicity involves the activation of NOX4/TGF-β/mtROS pathway and the inhibition of Akt/mTOR pathway. Food Chem. Toxicol. 2022, 163, 112966. [Google Scholar] [CrossRef]
- Worakajit, N.; Thipboonchoo, N.; Chaturongakul, S.; Jutabha, P.; Soontornniyomkij, V.; Tuchinda, P.; Soodvilai, S. Nephroprotective potential of Panduratin A against colistin-induced renal injury via attenuating mitochondrial dysfunction and cell apoptosis. Biomed. Pharmacother. 2022, 148, 112732. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X. Rapamycin Confers Neuroprotection against Colistin-Induced Oxidative Stress, Mitochondria Dysfunction, and Apoptosis through the Activation of Autophagy and mTOR/Akt/CREB Signaling Pathways. ACS Chem. Neurosci. 2018, 9, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Miao, Y.; Muhammad, I.; Tian, E.; Hu, W.; Wang, J.; Wang, B.; Li, R.; Li, J. Colistin-induced autophagy and apoptosis involves the JNK-Bcl2-Bax signaling pathway and JNK-p53-ROS positive feedback loop in PC-12 cells. Chem. Biol. Interact. 2017, 277, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liu, Y.; Chen, C.; Velkov, T.; Tang, S.; Shen, J.; Dai, C. Colistin Induces Oxidative Stress and Apoptotic Cell Death through the Activation of the AhR/CYP1A1 Pathway in PC12 Cells. Antioxidants 2024, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, M.Z.; Neamtalllah, T.; Alshibani, M.; Bagalagel, A.A.; Noor, A.O.; Bakhsh, H.T.; Abdel-Naim, A.B. Caffeic acid phenethyl ester ameliorates colistin-induced nephrotoxicity in rats via modulation of FOXO1/Nrf2/Sirt1 axis. Clin. Exp. Pharmacol. Physiol. 2024, 51, e70000. [Google Scholar] [CrossRef]
- Kabel, A.M.; Salama, S.A. Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 1767–1780. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the Nrf2/HO-1 pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef]
- Edrees, N.E.; Galal, A.A.A.; Abdel Monaem, A.R.; Beheiry, R.R.; Metwally, M.M.M. Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018, 294, 56–64. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, H.; Liu, X.; Wei, D.; Ren, C.; Cui, L.; Li, Y.; Wang, L.; Wang, J.; Zhao, Z.; et al. Elucidating the anti-hypertensive mechanisms of Uncaria rhynchophylla-Alisma plantago-aquatica L: An integrated network pharmacology, cluster analysis, and molecular docking approach. Front. Chem. 2024, 12, 1356458. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Xie, J. The beneficial pharmacological effects of Uncaria rhynchophylla in neurodegenerative diseases: Focus on alkaloids. Front. Pharmacol. 2024, 15, 1436481. [Google Scholar] [CrossRef]
- Xie, L.; Wang, T.; Lin, S.; Lu, Z.; Wang, Y.; Shen, Z.; Cheng, Y.; Shen, A.; Peng, J.; Chu, J. Uncaria Rhynchophylla attenuates angiotensin II-induced myocardial fibrosis via suppression of the RhoA/ROCK1 pathway. Biomed. Pharmacother. 2022, 146, 112607. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhu, M.; Gu, H.; Tao, Y.; Huang, H.; Chen, L. Alkaloids in Uncaria rhynchophylla improves AD pathology by restraining CD4(+) T cell-mediated neuroinflammation via inhibition of glycolysis in APP/PS1 mice. J. Ethnopharmacol. 2024, 331, 118273. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R.; Kim, M.J.; Lee, J.A.; Roh, S.S. Effect of Uncaria rhynchophylla against Thioacetamide-Induced Acute Liver Injury in Rat. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5581816. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, C.H.; Park, S.Y.; Lee, S.J.; Kim, Y. Uncaria rhynchophylla inhibits the production of nitric oxide and interleukin-1β through blocking nuclear factor κB, Akt, and mitogen-activated protein kinase activation in macrophages. J. Med. Food 2010, 13, 1133–1140. [Google Scholar] [CrossRef]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Hu, Z.; Zhao, M.; Che, C.T.; Ip, S.P. Bioassay-Guided Isolation of Neuroprotective Compounds from Uncaria rhynchophylla against Beta-Amyloid-Induced Neurotoxicity. Evid. Based Complement. Altern. Med. 2012, 2012, 802625. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Yu, X.; Lu, J.; Jia, W.; Song, J.; Liu, L.; Wang, Y.; Huang, Y.; Xie, J.; et al. Corynoxine Protects Dopaminergic Neurons Through Inducing Autophagy and Diminishing Neuroinflammation in Rotenone-Induced Animal Models of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 642900. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Lee, J.H.; Lee, J.J.; Yu, J.Y.; Hwang, B.Y.; Ye, S.K.; Shujuan, L.; Gao, L.; Pyo, M.Y.; Yun, Y.P. Corynoxeine isolated from the hook of Uncaria rhynchophylla inhibits rat aortic vascular smooth muscle cell proliferation through the blocking of extracellular signal regulated kinase 1/2 phosphorylation. Biol. Pharm. Bull. 2008, 31, 2073–2078. [Google Scholar] [CrossRef]
- Sugiura, Y.; Hiramatsu, K.; Hamauzu, R.; Motoki, T.; Miyazaki, M.; Uto, H.; Tsubouchi, H.; Tanaka, S.; Gohda, E. Mitogen-activated protein kinases-dependent induction of hepatocyte growth factor production in human dermal fibroblasts by the antibiotic polymyxin B. Cytokine 2012, 60, 205–211. [Google Scholar] [CrossRef]
- Kagi, T.; Inoue, A.; Noguchi, T.; Suzuki, W.; Takano, S.; Otani, K.; Naganuma, R.; Sekiguchi, Y.; Hirata, Y.; Shindo, S.; et al. The NLRP3 Inflammasome Is a Major Cause of Acute Renal Failure Induced by Polypeptide Antibiotics. J. Immunol. 2024, 212, 1807–1818. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, L.; Xu, J.; Liu, W.; Feng, F.; Qu, W. Neuroprotective Effects of Rhynchophylline Against Aβ(1-42)-Induced Oxidative Stress, Neurodegeneration, and Memory Impairment Via Nrf2-ARE Activation. Neurochem. Res. 2021, 46, 2439–2450. [Google Scholar] [CrossRef]
- Long, H.; Ruan, J.; Zhang, M.; Wang, C.; Huang, Y. Rhynchophylline Attenuates Tourette Syndrome via BDNF/NF-κB Pathway In Vivo and In Vitro. Neurotox. Res. 2019, 36, 756–763. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Matsumoto, K.; Tabata, K.; Takayama, H.; Kitajima, M.; Watanabe, H. Effects of Uncaria tomentosa total alkaloid and its components on experimental amnesia in mice: Elucidation using the passive avoidance test. J. Pharm. Pharmacol. 2000, 52, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Zhu, S.; Xu, T.; Lu, J.; Han, H.; Zhou, C.; Yan, J. The role of rhynchophylline in alleviating early brain injury following subarachnoid hemorrhage in rats. Brain Res. 2016, 1631, 92–100. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Tang, S.; Li, J.; Xiao, X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 2014, 58, 4075–4085. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, S.; Velkov, T.; Shen, J.; Dai, C. Copper exposure induces mitochondrial dysfunction and hepatotoxicity via the induction of oxidative stress and PERK/ATF4 -mediated endoplasmic reticulum stress. Environ. Pollut. 2024, 352, 124145. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liang, X.; Zhao, X.; Shi, Y.; Zhuo, F.; Tong, X.; Yang, X.; Zhai, Q.; Wang, J.; Guo, Q.; et al. Uncaria rhynchophylla alkaloid extract exerts neuroprotective activity against Parkinson’s disease via activating mitophagy with the involvement of UCHL1. J. Ethnopharmacol. 2025, 338, 119009. [Google Scholar] [CrossRef]
- Alford, A.S.; Moreno, H.L.; Benjamin, M.M.; Dickinson, C.F.; Hamann, M.T. Exploring the Therapeutic Potential of Mitragynine and Corynoxeine: Kratom-Derived Indole and Oxindole Alkaloids for Pain Management. Pharmaceuticals 2025, 18, 222. [Google Scholar] [CrossRef]
- Lei, S.; Lu, J.; Cheng, A.; Hussain, Z.; Tidgewell, K.; Zhu, J.; Ma, X. Identification of PXR Activators from Uncaria Rhynchophylla (Gou Teng) and Uncaria Tomentosa (Cat’s Claw). Drug Metab. Dispos. Biol. Fate Chem. 2023, 51, 629–636. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Shin, S.J.; Prajapati, R.; Shin, S.M.; Kim, M.J.; Soo Kim, H.; Leem, S.H.; Kim, T.J.; Park, Y.H.; et al. Dual modulators of aggregation and dissociation of amyloid beta and tau: In vitro, in vivo, and in silico studies of Uncaria rhynchophylla and its bioactive components. Biomed. Pharmacother. 2022, 156, 113865. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, J.; Zhuo, L.; Zhang, W.; Lv, L.; Zhu, L.; Zhang, J.; Feng, F.; Liu, W.; Han, L.; et al. The TLR4/NF-κB/NLRP3 and Nrf2/HO-1 pathways mediate the neuroprotective effects of alkaloids extracted from Uncaria rhynchophylla in Parkinson’s disease. J. Ethnopharmacol. 2024, 333, 118391. [Google Scholar] [CrossRef]
- Lim, H.B.; Lee, H.R. Safety and biological activity evaluation of Uncaria rhynchophylla ethanolic extract. Drug Chem. Toxicol. 2022, 45, 907–918. [Google Scholar] [CrossRef]
- Nasrullah, M.Z.; Eljaaly, K.; Neamatallah, T.; Fahmy, U.A.; Alamoudi, A.J.; Bakhsh, H.T.; Abdel-Naim, A.B. Omeprazole Prevents Colistin-Induced Nephrotoxicity in Rats: Emphasis on Oxidative Stress, Inflammation, Apoptosis and Colistin Accumulation in Kidneys. Pharmaceuticals 2022, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Aslan, T.; Guler, E.M.; Cakir, A.; Dundar, T.; Gulgec, A.S.; Huseyinbas, O.; Celikten, M.; Coban, G.; Hakyemez, I.N.; Kocyigit, A.; et al. Dexpanthenol and ascorbic acid ameliorate colistin-induced nephrotoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Behnamrad, S.; Khodami, Z.; Ommati, M.M.; Azarpira, N.; Vazin, A. The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomed. Pharmacother. 2019, 109, 103–111. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Ding, W.; Jiang, G.; Lu, Z.; Li, L.; Wang, J.; Li, J.; Li, J. Autophagy regulates colistin-induced apoptosis in PC-12 cells. Antimicrob. Agents Chemother. 2015, 59, 2189–2197. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Zhou, T.; Wang, C.; Zhang, H.; Wang, H. Colistin-induced apoptosis in PC12 cells: Involvement of the mitochondrial apoptotic and death receptor pathways. Int. J. Mol. Med. 2014, 33, 1298–1304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, C.; Tang, S.; Velkov, T.; Xiao, X. Colistin-Induced Apoptosis of Neuroblastoma-2a Cells Involves the Generation of Reactive Oxygen Species, Mitochondrial Dysfunction, and Autophagy. Mol. Neurobiol. 2016, 53, 4685–4700. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Xiao, X.; Velkov, T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017, 72, 1635–1645. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Küçükler, S. The effects of hesperidin on colistin-induced reproductive damage, autophagy, and apoptosis by reducing oxidative stress. Andrologia 2021, 53, e13900. [Google Scholar] [CrossRef]
- Azad, M.A.; Akter, J.; Rogers, K.L.; Nation, R.L.; Velkov, T.; Li, J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob. Agents Chemother. 2015, 59, 2136–2143. [Google Scholar] [CrossRef]
- Azad, M.A.; Finnin, B.A.; Poudyal, A.; Davis, K.; Li, J.; Hill, P.A.; Nation, R.L.; Velkov, T.; Li, J. Polymyxin B Induces Apoptosis in Kidney Proximal Tubular Cells. Antimicrob. Agents Chemother. 2013, 57, 4329–4335. [Google Scholar] [CrossRef] [PubMed]
- Shafik, M.S.; Bishr, A.; El-Tanbouly, D.M.; Attia, A.S. Modulation of miR-205/EGLN2 by rosuvastatin mitigates colistin-induced nephrotoxicity in rats: Involvement of ATF4/CHOP and Nrf2 pathways. Biomed. Pharmacother. 2023, 157, 114042. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Ulusoy, S.; Orem, A.; Alkanat, M.; Mungan, S.; Yulug, E.; Yucesan, F.B. How does colistin-induced nephropathy develop and can it be treated? Antimicrob. Agents Chemother. 2013, 57, 3463–3469. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin Attenuates Colistin-Induced Neurotoxicity in N2a Cells via Anti-inflammatory Activity, Suppression of Oxidative Stress, and Apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Oktan, M.A.; Heybeli, C.; Ural, C.; Kocak, A.; Bilici, G.; Cavdar, Z.; Ozbal, S.; Arslan, S.; Yilmaz, O.; Cavdar, C. Alpha-lipoic acid alleviates colistin nephrotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 761–771. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, G.S.; Dusting, G.J.; Chan, E.C. NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol. 2014, 2, 267–272. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Li, M.Z.; Zhao, Y.; Dai, X.Y.; Talukder, M.; Li, J.L. Lycopene ameliorates DEHP exposure-induced renal pyroptosis through the Nrf2/Keap-1/NLRP3/Caspase-1 axis. J. Nutr. Biochem. 2023, 113, 109266. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Zhang, T.; Li, X.; Wu, C.; Zhao, Z.; Tang, J.; Tan, X.; Hu, Q.; Liao, W. Astragaloside IV attenuates cadmium induced nephrotoxicity in rats by activating Nrf2. Sci. Rep. 2025, 15, 2028. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, J.; Cao, Z.; Ji, Q.; Han, Y.; Song, M.; Shao, B.; Li, Y. Lycopene attenuates AFB(1)-induced renal injury with the activation of the Nrf2 antioxidant signaling pathway in mice. Food Funct. 2018, 9, 6427–6434. [Google Scholar] [CrossRef]
- Wang, J.; Ishfaq, M.; Fan, Q.; Chen, C.; Li, J. 7-Hydroxycoumarin Attenuates Colistin-Induced Kidney Injury in Mice Through the Decreased Level of Histone Deacetylase 1 and the Activation of Nrf2 Signaling Pathway. Front. Pharmacol. 2020, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kagi, T.; Naganuma, R.; Inoue, A.; Noguchi, T.; Hamano, S.; Sekiguchi, Y.; Hwang, G.W.; Hirata, Y.; Matsuzawa, A. The polypeptide antibiotic polymyxin B acts as a pro-inflammatory irritant by preferentially targeting macrophages. J. Antibiot. 2022, 75, 29–39. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Suk, K.; Kim, S.Y.; Leem, K.; Kim, Y.O.; Park, S.Y.; Hur, J.; Baek, J.; Lee, K.J.; Zheng, H.Z.; Kim, H. Neuroprotection by methanol extract of Uncaria rhynchophylla against global cerebral ischemia in rats. Life Sci. 2002, 70, 2467–2480. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Ho, T.T.; Ding, X.; Mo, Y.Y. ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogenesis 2016, 5, e279. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Chatterjee, B.; Sengupta, P.; Tekade, R.K. Chapter 8—Pharmacokinetic Characterization of Drugs and New Product Development. In Biopharmaceutics and Pharmacokinetics Considerations; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 195–277. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, R.; Velkov, T.; Shen, J.; Tang, S.; Dai, C. Corynoxeine Supplementation Ameliorates Colistin-Induced Kidney Oxidative Stress and Inflammation in Mice. Antioxidants 2025, 14, 593. https://doi.org/10.3390/antiox14050593
Liu Y, Zhang R, Velkov T, Shen J, Tang S, Dai C. Corynoxeine Supplementation Ameliorates Colistin-Induced Kidney Oxidative Stress and Inflammation in Mice. Antioxidants. 2025; 14(5):593. https://doi.org/10.3390/antiox14050593
Chicago/Turabian StyleLiu, Yue, Ruichen Zhang, Tony Velkov, Jianzhong Shen, Shusheng Tang, and Chongshan Dai. 2025. "Corynoxeine Supplementation Ameliorates Colistin-Induced Kidney Oxidative Stress and Inflammation in Mice" Antioxidants 14, no. 5: 593. https://doi.org/10.3390/antiox14050593
APA StyleLiu, Y., Zhang, R., Velkov, T., Shen, J., Tang, S., & Dai, C. (2025). Corynoxeine Supplementation Ameliorates Colistin-Induced Kidney Oxidative Stress and Inflammation in Mice. Antioxidants, 14(5), 593. https://doi.org/10.3390/antiox14050593






