Physicochemical Characterization of Kynurenine Pathway Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. 1H NMR (Nuclear Magnetic Resonance) Spectroscopy Measurements
2.3. pH-Potentiometric Titrations
2.4. UV-pH Titrations
2.5. Statistical Analysis
2.6. Molecular Dynamic and Quantum Chemistry Computations
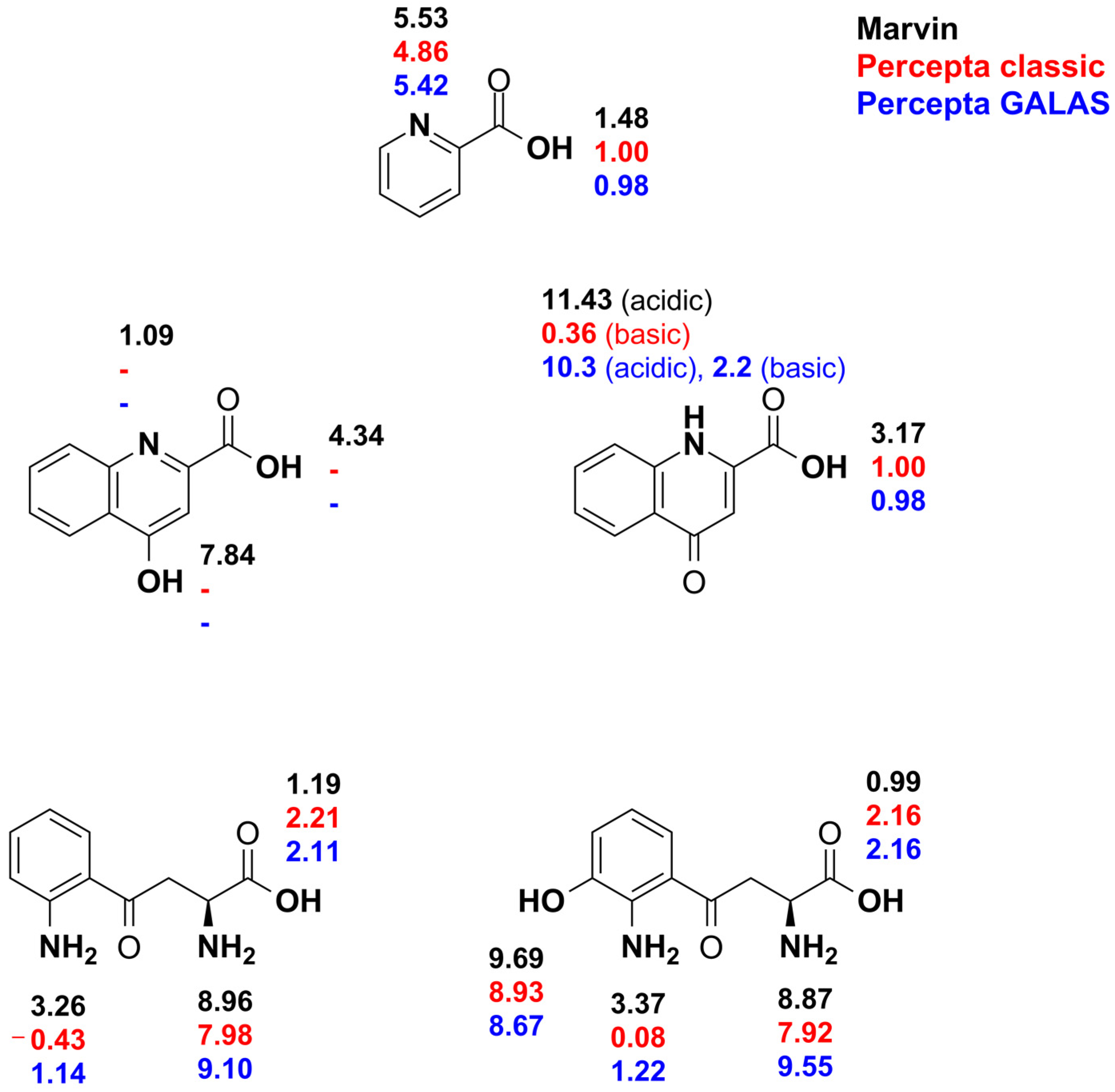
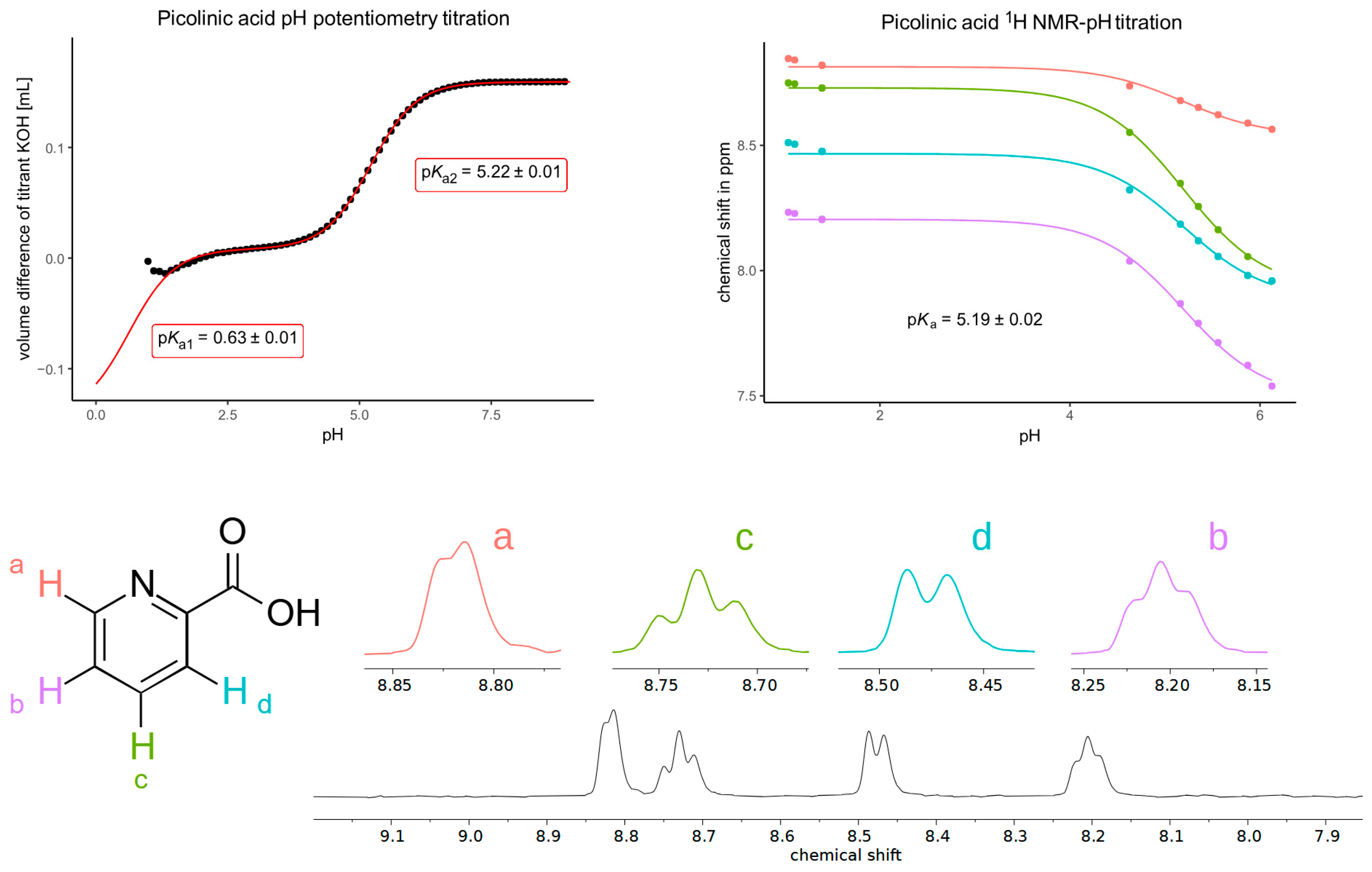
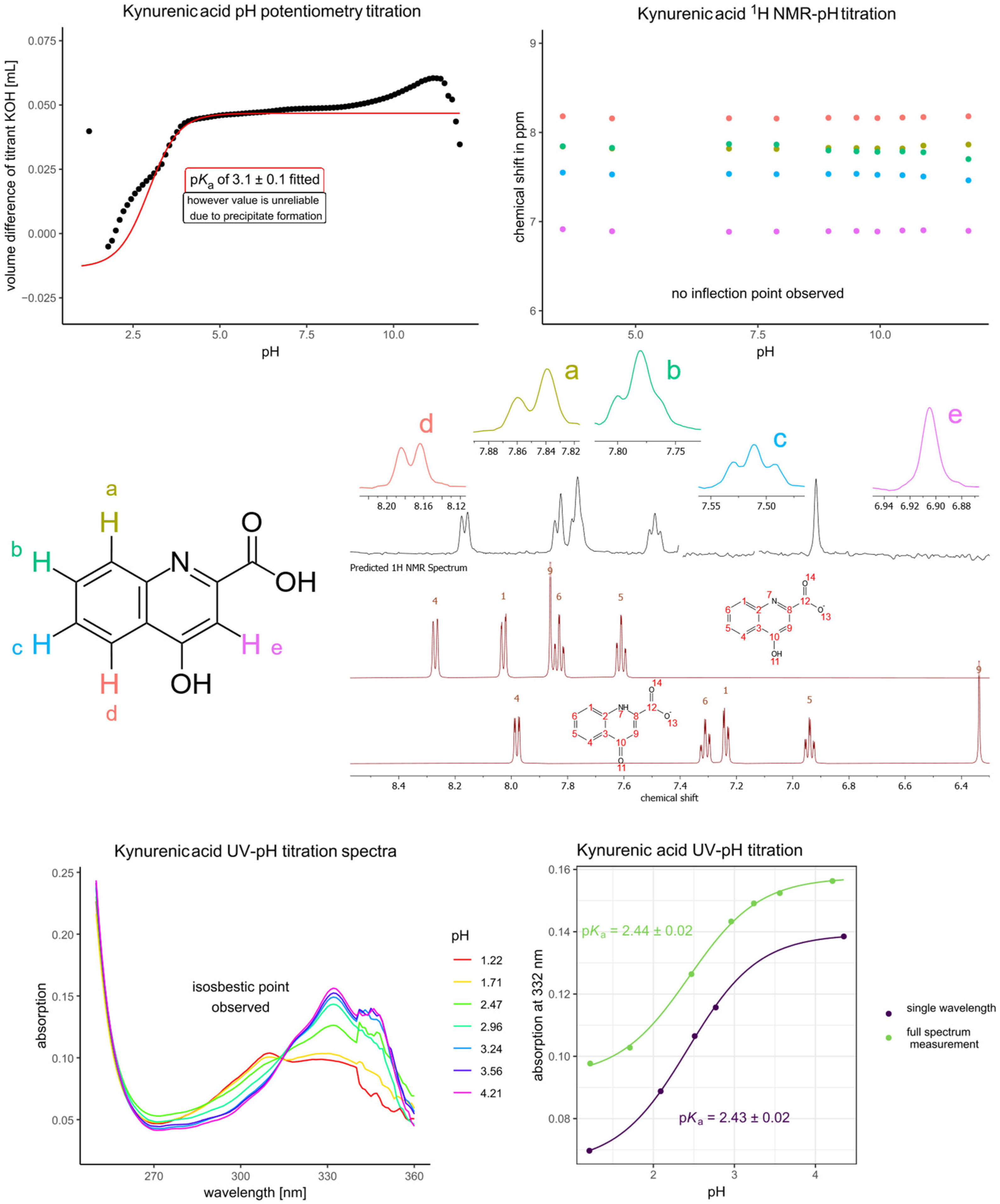
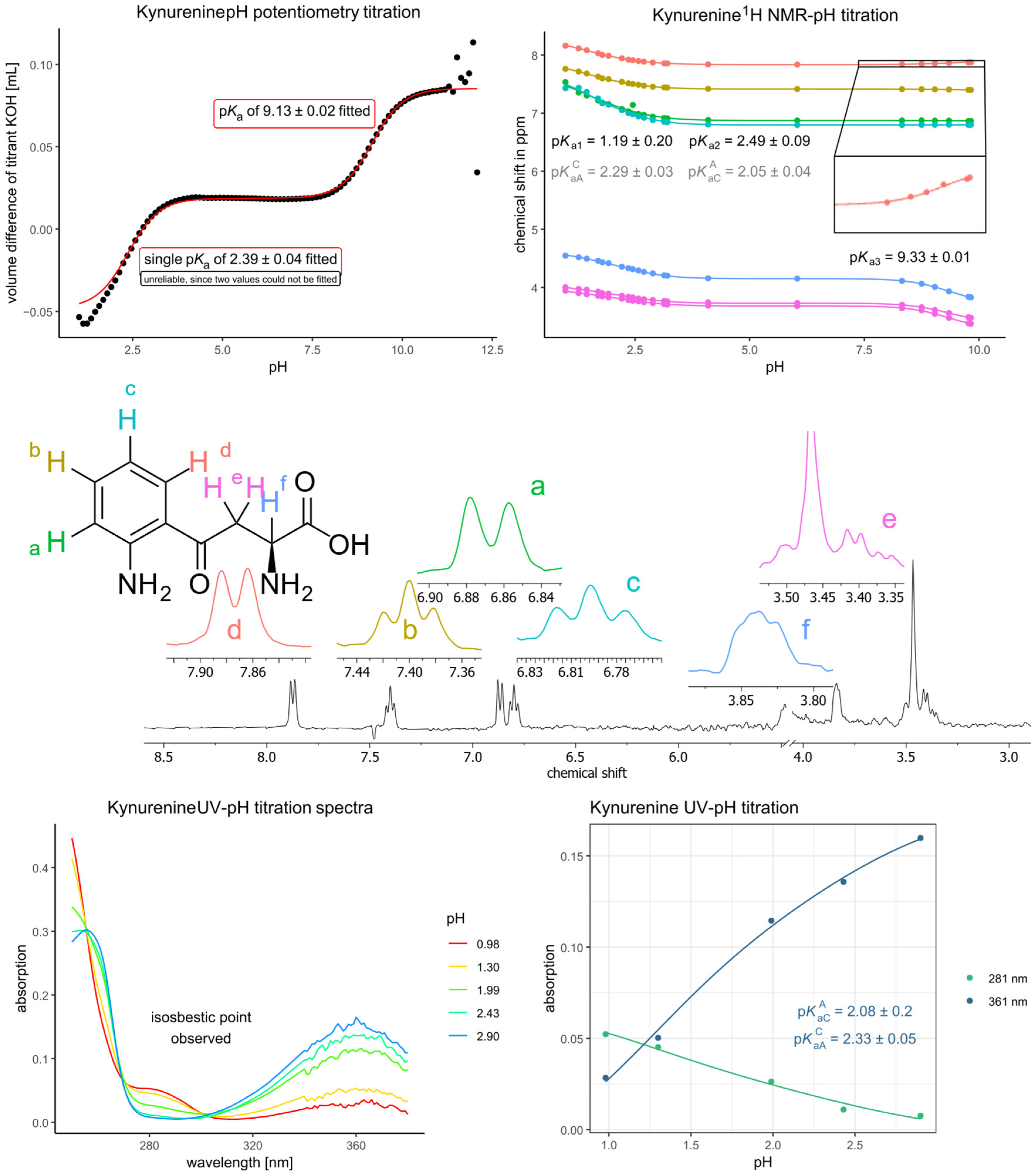
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidelberger, C.; Gullberg, M.E.; Morgan, A.F.; Lepkovsky, S. Tryptophan metabolism. 1. Concerning the mechanism of the mammalian conversion of tryptophan into kynurenine, kynurenic acid, and nicotinic acid. J. Biol. Chem. 1949, 179, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan metabolism and related pathways in psychoneuroimmunology: The impact of nutrition and lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef]
- Thomas, S.R.; Stocker, R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999, 4, 199–220. [Google Scholar] [CrossRef]
- Amaral, M.; Outeiro, T.F.; Scrutton, N.S.; Giorgini, F. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. J. Mol. Med. 2013, 91, 705–713. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine metabolism and Alzheimer’s disease: The potential targets and approaches. Neurochem. Res. 2022, 47, 1459–1476. [Google Scholar] [CrossRef]
- Brown, S.J.; Huang, X.-F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Robotka, H.; Toldi, J.; Vécsei, L. L-kynurenine: Metabolism and mechanism of neuroprotection. Future Neurol. 2008, 3, 169–188. [Google Scholar] [CrossRef]
- Mackay, G.; Forrest, C.; Stoy, N.; Christofides, J.; Egerton, M.; Stone, T.; Darlington, L. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur. J. Neurol. 2006, 13, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.J.; Guillemin, G.J.; Teipel, S.J.; Buerger, K.; Hampel, H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 345–352. [Google Scholar] [CrossRef]
- Sheipouri, D.; Braidy, N.; Guillemin, G.J. Kynurenine pathway in skin cells: Implications for UV-induced skin damage. Int. J. Tryptophan Res. 2012, 5, IJTR-S9835. [Google Scholar] [CrossRef]
- Sherin, P.; Tsentalovich, Y.P.; Snytnikova, O.; Sagdeev, R. Photoactivity of kynurenine-derived UV filters. J. Photochem. Photobiol. B Biol. 2008, 93, 127–132. [Google Scholar] [CrossRef]
- Zarnowski, T.; Rejdak, R.; Zagorski, Z.; Juenemann, A.G.; Zrenner, E.; Kocki, T.; Urbanska, E.M.; Turski, W.A. Content of kynurenic acid and activity of kynurenine aminotransferases in mammalian eyes. Ophthalmic Res. 2004, 36, 124–128. [Google Scholar] [CrossRef]
- Jabs, D.A.; Van Natta, M.L.; Trang, G.; Jones, N.G.; Milush, J.M.; Cheu, R.; Klatt, N.R.; Danis, R.P.; Hunt, P.W. Association of age-related macular degeneration with mortality in patients with acquired immunodeficiency syndrome; role of systemic inflammation. Am. J. Ophthalmol. 2019, 199, 230–237. [Google Scholar] [CrossRef]
- Hu, L.-J.; Li, X.-F.; Hu, J.-Q.; Ni, X.-J.; Lu, H.-Y.; Wang, J.-J.; Huang, X.-N.; Lin, C.-X.; Shang, D.-W.; Wen, Y.-G. A simple HPLC–MS/MS method for determination of tryptophan, kynurenine and kynurenic acid in human serum and its potential for monitoring antidepressant therapy. J. Anal. Toxicol. 2017, 41, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mrštná, K.; Krčmová, L.K.; Švec, F. Advances in kynurenine analysis. Clin. Chim. Acta 2023, 547, 117441. [Google Scholar] [CrossRef]
- Virág, D.; Király, M.; Drahos, L.; Édes, A.E.; Gecse, K.; Bagdy, G.; Juhász, G.; Antal, I.; Klebovich, I.; Kiss, B.D. Development, validation and application of LC–MS/MS method for quantification of amino acids, kynurenine and serotonin in human plasma. J. Pharm. Biomed. Anal. 2020, 180, 113018. [Google Scholar] [CrossRef] [PubMed]
- Hornok, V.; Amin, K.W.K.; Kovács, A.N.; Juhász, Á.; Katona, G.; Balogh, G.T.; Csapó, E. Increased blood-brain barrier permeability of neuroprotective drug by colloidal serum albumin carriers. Colloids Surf. B Biointerfaces 2022, 220, 112935. [Google Scholar] [CrossRef]
- Juhász, Á.; Ungor, D.; Varga, N.; Katona, G.; Balogh, G.T.; Csapó, E. Lipid-Based nanocarriers for delivery of neuroprotective kynurenic acid: Preparation, characterization, and BBB transport. Int. J. Mol. Sci. 2023, 24, 14251. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Snytnikova, O.A.; Forbes, M.D.; Chernyak, E.I.; Morozov, S.V. Photochemical and thermal reactivity of kynurenine. Exp. Eye Res. 2006, 83, 1439–1445. [Google Scholar] [CrossRef]
- Pileni, M.; Giraud, M.; Santus, R. Kynurenic acid—I. spectroscopic properties. Photochem. Photobiol. 1979, 30, 251–256. [Google Scholar] [CrossRef]
- Zhuravleva, Y.S.; Tsentalovich, Y.P. Acid-alkaline properties of triplet state and radical of kynurenic acid. J. Photochem. Photobiol. A Chem. 2018, 365, 7–12. [Google Scholar] [CrossRef]
- Zhuravleva, Y.S.; Sherin, P.S. Influence of pH on radical reactions between kynurenic acid and amino acids tryptophan and tyrosine. Part II. Amino acids within the protein globule of lysozyme. Free Radic. Biol. Med. 2021, 174, 211–224. [Google Scholar] [CrossRef]
- Zapała, L.; Kalembkiewicz, J.; Sitarz-Palczak, E. Studies on equilibrium of anthranilic acid in aqueous solutions and in two-phase systems: Aromatic solvent–water. Biophys. Chem. 2009, 140, 91–98. [Google Scholar] [CrossRef]
- Christopherson, M.J.; Yoder, K.J.; Hill, J.T. Hydrophilic interaction liquid chromatographic (HILIC)/ion exchange separation of picolinic and nicotinic acids. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2545–2558. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, J.; Rubab, M.; Feng, Y.-l.; Poon, R. An analytical method for the measurement of acid metabolites of tryptophan-NAD pathway and related acids in urine. J. Chromatogr. B 2010, 878, 1003–1006. [Google Scholar] [CrossRef]
- Orgován, G.; Noszál, B. Electrodeless, accurate pH determination in highly basic media using a new set of 1H NMR pH indicators. J. Pharm. Biomed. Anal. 2011, 54, 958–964. [Google Scholar] [CrossRef]
- Szakács, Z.; Hägele, G.; Tyka, R. 1H/31P NMR pH indicator series to eliminate the glass electrode in NMR spectroscopic pKa determinations. Anal. Chim. Acta 2004, 522, 247–258. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pinheiro, J.; Bates, D. Mixed-effects models in S and S-PLUS. Int. J. Soc. Res. Methodol. 2000, 17, 197–214. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D. OPLS4: Improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Balduf, T.; Beachy, M.D.; Bennett, M.C.; Bochevarov, A.D.; Chien, A.; Dub, P.A.; Dyall, K.G.; Furness, J.W.; Halls, M.D. Quantum chemical package Jaguar: A survey of recent developments and unique features. J. Chem. Phys. 2024, 161, 052502. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Cao, Y.; Beachy, M.D.; Braden, D.A.; Morrill, L.; Ringnalda, M.N.; Friesner, R.A. Nuclear-magnetic-resonance shielding constants calculated by pseudospectral methods. J. Chem. Phys. 2005, 122, 224116. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Orgován, G.; Hosztafi, S.; Noszál, B. The complete microspeciation of ovothiol A, the smallest octafarious antioxidant biomolecule. Anal. Bioanal. Chem. 2014, 406, 2377–2387. [Google Scholar] [CrossRef]
- Szakacs, Z.; Kraszni, M.; Noszal, B. Determination of microscopic acid-base parameters from NMR-pH titrations. Anal. Bioanal. Chem. 2004, 378, 1428–1448. [Google Scholar] [CrossRef]
- Szakacs, Z.; Noszal, B. Protonation microequilibrium treatment of polybasic compounds with any possible symmetry. J. Math. Chem. 1999, 26, 139–155. [Google Scholar] [CrossRef]
- Borkovec, M.; Koper, G.J. A cluster expansion method for the complete resolution of microscopic ionization equilibria from NMR titrations. Anal. Chem. 2000, 72, 3272–3279. [Google Scholar] [CrossRef]
- Borkovec, M.; Koper, G.J.; Spiess, B. The intrinsic view of ionization equilibria of polyprotic molecules. New J. Chem. 2014, 38, 5679–5685. [Google Scholar] [CrossRef]
- Kalydi, E.; Malanga, M.; Ujj, D.; Benkovics, G.; Szakács, Z.; Béni, S. Fully symmetric cyclodextrin polycarboxylates: How to determine reliable protonation constants from NMR titration data. Int. J. Mol. Sci. 2022, 23, 14448. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Orgovan, G.; Toth, G.; Hosztafi, S.; Noszal, B. The complete microspeciation of ovothiol A disulfide: A hexabasic symmetric biomolecule. J. Pharm. Biomed. Anal. 2015, 107, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Noszal, B.; Sandor, P. Rota-Microspeciation of Aspartic-Acid and Asparagine. Anal. Chem. 1989, 61, 2631–2637. [Google Scholar] [CrossRef]
- Ferreira de Santana, J.; Mirzahosseini, A.; Mándity, B.; Bogdán, D.; Mándity, I.; Noszál, B. Close correlation between thiolate basicity and certain NMR parameters in cysteine and cystine microspecies. PLoS ONE 2022, 17, e0264866. [Google Scholar] [CrossRef]
- Pálla, T.; Fogarasi, E.; Noszál, B.; Tóth, G. Characterization of the Site-Specific Acid-Base Equilibria of 3-Nitrotyrosine. Chem. Biodivers. 2019, 16, e1900358. [Google Scholar] [CrossRef] [PubMed]
- Mazák, K.; Dóczy, V.; Kökösi, J.; Noszál, B. Proton speciation and microspeciation of serotonin and 5-hydroxytryptophan. Chem. Biodivers. 2009, 6, 578–590. [Google Scholar] [CrossRef]
- Szakács, Z.; Hägele, G. Accurate determination of low pK values by 1H NMR titration. Talanta 2004, 62, 819–825. [Google Scholar] [CrossRef]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood–brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with Neuroactive and Redox Properties: Relevance to Aging and Brain Diseases. Oxidative Med. Cell Longev. 2014, 2014, 646909. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverrí, J.; Sánchez Chapul, L. On the antioxidant properties of L-kynurenine: An efficient ROS scavenger and enhancer of rat brain antioxidant defense. Antioxidants 2021, 11, 31. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining roles: A paradigm shift in tryptophan–kynurenine metabolism for innovative clinical applications. Int. J. Mol. Sci. 2024, 25, 12767. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.; Torres, I.; Pinzón, E.; Ortiz-Islas, E. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.J.; Fenwick, P.K.; Kirk, H. An in vitro study of the effect of picolinic acid on metal translocation across lipid bilayers. J. Nutr. 1989, 119, 1432–1437. [Google Scholar] [CrossRef]
- Han, Q.; Li, J.; Li, J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur. J. Biochem. 2004, 271, 4804–4814. [Google Scholar] [CrossRef]
- Zhuravlev, A.V.; Zakharov, G.A.; Shchegolev, B.F.; Savvateeva-Popova, E.V. Antioxidant properties of kynurenines: Density functional theory calculations. PLoS Comput. Biol. 2016, 12, e1005213. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.V.; Thong, N.M.; Le Huyen, T.; Nam, P.C.; Tam, N.M.; Hoa, N.T.; Mechler, A. A thermodynamic and kinetic study of the antioxidant activity of natural hydroanthraquinones. RSC Adv. 2020, 10, 20089–20097. [Google Scholar] [CrossRef]
- Van Bay, M.; Hoa, N.T.; Mechler, A.; Vo, Q.V. The radical scavenging activity of glycozolidol in physiological environments: A quantum chemical study. RSC Adv. 2022, 12, 32693–32699. [Google Scholar]
| Kynurenine | Histidine a | Asparagine b | ||||||
|---|---|---|---|---|---|---|---|---|
| αCH | βCH2,a | βCH2,b | αCH | βCH2,a | βCH2,b | αCH | βCH2,a | βCH2,b |
| 0.427 | 0.333 | 0.405 | 0.480 | - | - | 0.459 | 0.302 | 0.418 |
| 0.471 | 0.313 | 0.290 | 0.407 | - | - | 0.387 | 0.154 | 0.162 |
| Cysteine c | 3-nitrotyrosine d | Serotonin e | ||||||
| αCH | βCH2,a | βCH2,b | αCH | βCH2,a | βCH2,b | αCH | βCH2,a | βCH2,b |
| 0.532 | 0.247 | 0.286 | 0.474 | - | - | 0.471 | 0.318 | 0.376 |
| 0.380 | 0.090 | 0.107 | 0.376 | - | - | 0.342 | 0.062 | 0.178 |
| Picolinic Acid | Kynurenic Acid | ||
| pKa1 (by extrapolation of potentiometry) | 0.63 | pKa1 (UV) | 2.43 |
| pKa2 (mean of potentiometry and NMR) | 5.21 | ||
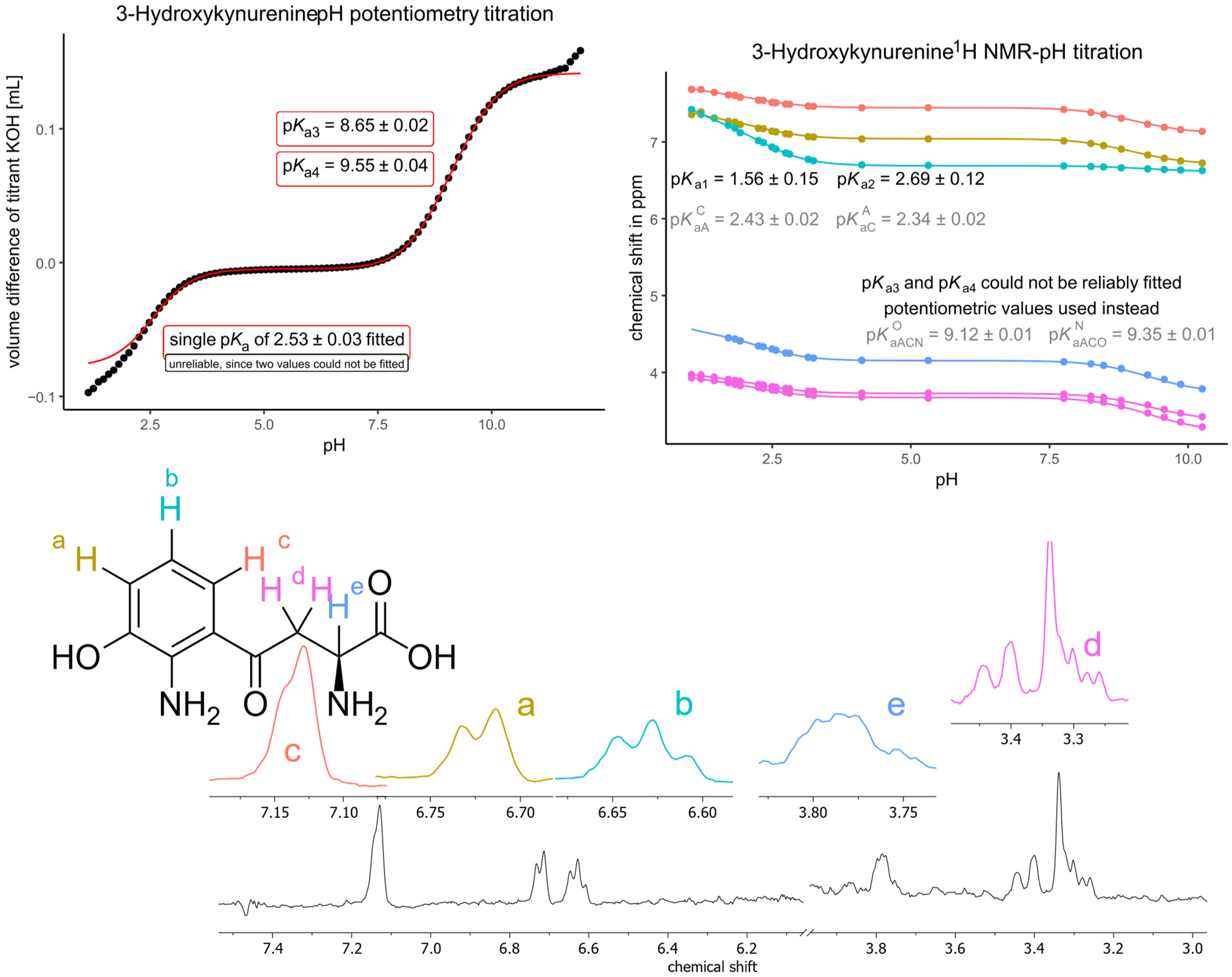
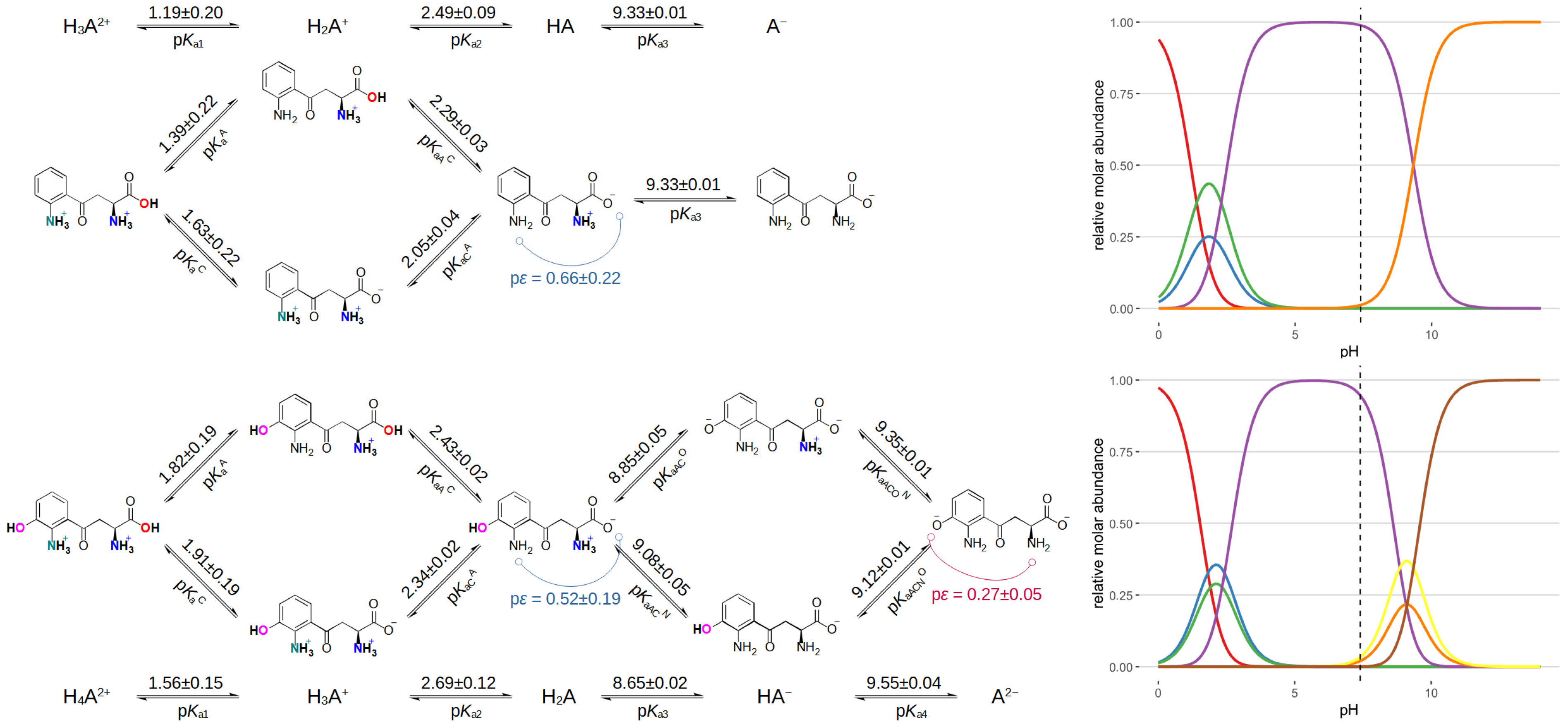
| Kynurenine (NMR) | 3-Hydroxykynurenine (NMR, unless stated otherwise) | ||
| pKa1 | 1.19 | pKa1 | 1.56 |
| pKa2 | 2.49 | pKa2 | 2.69 |
| pKa3 | 9.33 | pKa3 (potentiometry) | 8.65 |
| pKaA | 1.39 | pKa4 (potentiometry) | 9.55 |
| pKaC | 1.63 | pKaA | 1.82 |
| pKaCA | 2.05 | pKaC | 1.91 |
| pKaAC | 2.29 | pKaCA | 2.34 |
| pKaAC | 2.43 | ||
| pKaACO | 8.85 | ||
| pKaACN | 9.08 | ||
| pKaACNO | 9.12 | ||
| pKaACON | 9.35 | ||
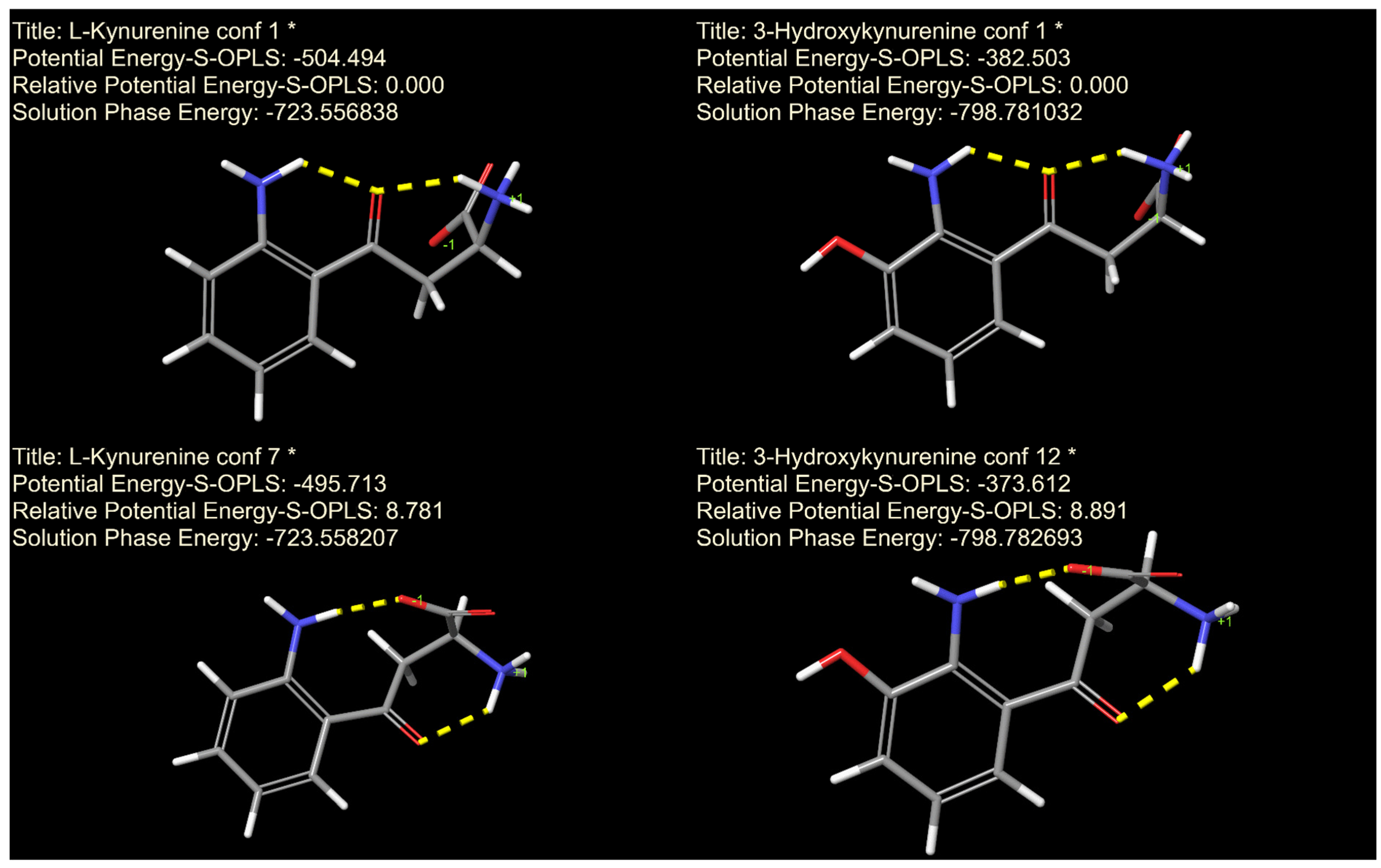
| Chemical Shifts (ppm) | MestReNova | Jaguar | |||
|---|---|---|---|---|---|
| 1H Signal Numbering According to Figure 3 | Experimental | Phenol Tautomer | Keto Tautomer | Phenol Tautomer | Keto Tautomer |
| doublet (4) | 8.18 | 8.27 | 7.98 | 7.92 | 8.13 |
| doublet (1) | 7.86 | 8.03 | 7.24 | 7.93 | 7.44 |
| triplet (6) | 7.70 | 7.83 | 7.31 | 7.79 | 7.63 |
| triplet (4) | 7.46 | 7.61 | 6.94 | 7.69 | 7.34 |
| singlet (9) | 6.90 | 7.86 | 6.34 | 7.70 | 6.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzásy, L.; Mazák, K.; Balogh, B.; Simon, B.; Vincze, A.; Balogh, G.T.; Pálla, T.; Mirzahosseini, A. Physicochemical Characterization of Kynurenine Pathway Metabolites. Antioxidants 2025, 14, 589. https://doi.org/10.3390/antiox14050589
Buzásy L, Mazák K, Balogh B, Simon B, Vincze A, Balogh GT, Pálla T, Mirzahosseini A. Physicochemical Characterization of Kynurenine Pathway Metabolites. Antioxidants. 2025; 14(5):589. https://doi.org/10.3390/antiox14050589
Chicago/Turabian StyleBuzásy, Luca, Károly Mazák, Balázs Balogh, Balázs Simon, Anna Vincze, György Tibor Balogh, Tamás Pálla, and Arash Mirzahosseini. 2025. "Physicochemical Characterization of Kynurenine Pathway Metabolites" Antioxidants 14, no. 5: 589. https://doi.org/10.3390/antiox14050589
APA StyleBuzásy, L., Mazák, K., Balogh, B., Simon, B., Vincze, A., Balogh, G. T., Pálla, T., & Mirzahosseini, A. (2025). Physicochemical Characterization of Kynurenine Pathway Metabolites. Antioxidants, 14(5), 589. https://doi.org/10.3390/antiox14050589







