Abstract
Most DNA damage caused by oxidative metabolism consists of single lesions that can accumulate in tissues. This review focuses on two classes of lesions: the two 8-oxopurine (8-oxo-Pu) lesions that are repaired by the base excision repair (BER) enzyme and the four 5′,8-cyclopurine (cPu) lesions that are repaired exclusively by the nucleotide excision repair (NER) enzyme. The aim is to correlate the simultaneous quantification of these two classes of lesions in the context of neurological disorders. The first half is a summary of reactive oxygen species (ROS) with particular attention to the pathways of hydroxyl radical (HO•) formation, followed by a summary of protocols for the quantification of six lesions and the biomimetic chemistry of the HO• radical with double-stranded oligonucleotides (ds-ODN) and calf thymus DNA (ct-DNA). The second half addresses two neurodegenerative diseases: xeroderma pigmentosum (XP) and Cockayne syndrome (CS). The quantitative data on the six lesions obtained from genomic and/or mitochondrial DNA extracts across several XP and CS cell lines are discussed. Oxidative stress contributes to oxidative DNA damage by resulting in the accumulation of cPu and 8-oxo-Pu in DNA. The formation of cPu is the postulated culprit inducing neurological symptoms associated with XP and CS.
1. Introduction
DNA is vulnerable to oxidative insults originating either through the metabolism of oxygen or through environmental sources (e.g., ionizing radiation, UV light, and chemical mutagens), causing DNA strand breaks and chemical modifications of nucleobases [1,2]. Most DNA damage induced through oxidative metabolism consists of single lesions, although multiple lesions, such as tandem or clustered lesions and DNA/DNA or DNA/protein crosslinking, are also reported. Thousands of these lesions accumulate within the mammalian cell genome each day [3]. The damage is particularly deleterious because they cause heritable mutations to daughter cells. Enzymatic systems are known to remove the majority of DNA lesions and maintain the integrity of the genome [3,4]. However, enzymatic deficiencies can give rise to the accumulation of damage to cellular components that are linked to specific pathologies, including neurodegeneration [5]. For a comprehensive overview of oxidatively induced DNA damage, including quantification methods (in vitro and in vivo), repair mechanisms, and biological consequences, please refer to the two-volume set of books recently published [6].
In this review, two groups of purine DNA damage by reactive oxygen species (ROS) are compared, the 8-oxopurine (8-oxo-Pu) and 5′,8-cyclopurine (cPu) lesions, which are repaired by the base excision repair (BER) and nucleotide excision repair (NER) enzymes, respectively. cPu, as transcriptional blocking lesions, have been identified as molecular defects in neurodegenerative processes [7]. The first part of the review highlights the endogenous formation of HO• radicals within the ROS system, the mechanistic aspects for forming these lesions, and their interconnection in biomimetic models. In the second part, emphasis is given to the experimental protocols utilized for quantification of these purine lesions, the role of BER and NER enzymes in repairing these lesions, and the comparison of the literature data of these lesions in some biological models of neurodegenerative disorders.
Molecular oxygen (O2) is not just a benign participant in cellular metabolism and function; it also has a dark side when ROS production becomes potentially harmful. Hypoxia, a condition of reduced oxygen supply, has been observed during the aging process and the onset of neurodegeneration. Hypoxia is associated with the pathogenesis of numerous neurological disorders, including Alzheimer’s, Parkinson’s, and other age-related neurodegenerative diseases. The ROS involvement and the mechanisms by which this occurs are not well understood [8,9]. This review also examines the role of O2 in biomimetic and biological models, highlighting the need for further research in this area.
2. Reactive Oxygen Species (ROS) and Pathways of Hydroxyl Radical Formation
ROS are produced during physiological intracellular metabolism, and their roles in biology are related to redox signaling [10,11,12,13,14]. Various cellular compartments produce ROS, including mitochondria, peroxisomes, cytosol, and the endoplasmic reticulum [15]. ROS encompass various radicals such as superoxide radical anion (O2•−), nitric oxide (NO•), nitrogen dioxide radical (NO2•), hydroxyl radical (HO•), and carbonate radical anion (CO3•−), which operate as one-electron oxidants, as well as small molecules, including hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and hypohalous acids (HOCl or HOBr), which function as two-electron oxidants [16,17,18,19]. At least 90 different genes encode cellular enzymes to handle ROS efficiently. When the ROS system malfunctions, it can contribute to disease. A recent review outlines how ROS may often act as second messengers within a tightly controlled system, rather than solely being toxic precursors of cellular dysfunction, death, and destruction [15].
The event of oxidative stress (OS) is connected with the balance between oxidants and antioxidants; oxidative eustress and oxidative distress are defined under physiological and supra-physiological deviations, respectively [20,21,22,23]. Particularly in oxidative distress, the ROS excess damages various cell components such as proteins, lipids, and DNA, which are implicated in cancer, cardiovascular disease, neurodegenerative disorders, and aging [24]. For example, ROS-induced lipid peroxidation provokes apoptosis, autophagy, ferroptosis [25], two-electron oxidation of methionine moieties in proteins [26], and ~70,000 DNA lesions (chemical modification of DNA) per cell per day in humans [27].
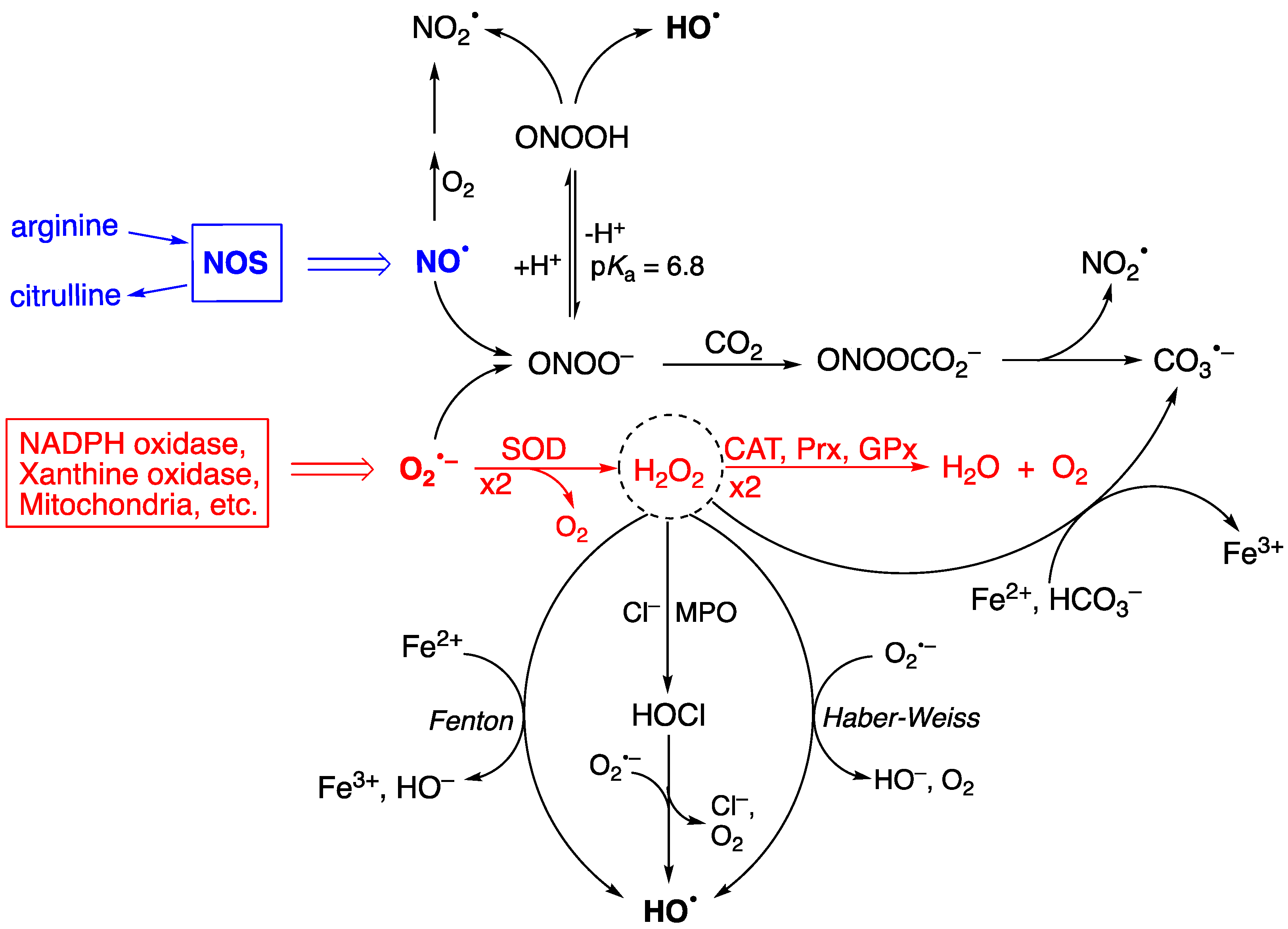
Figure 1 shows the two progenitors of the ROS network [28], which are O2•− and NO•, and how they are chemically connected. Their concentrations are ~0.1 nM O2•− and ~10 nM NO• under physiological conditions but can increase up to 100-fold during inflammatory response, thus affecting severely the integrity of biomolecular heritage. The superoxide dismutase (SOD) enzymes participate in controlling the level of O2•−, causing its dismutation to H2O2 and O2, in order to maintain the steady-state concentration at picomolar levels [29,30]. H2O2 is the most abundant ROS in eukaryotes; the intracellular physiological concentration of H2O2 under oxidative eustress is in the range of 1–10 nM, whereas under oxidative distress, it is larger than 100 nM [31,32]. Catalase (CAT), glutathione peroxidase (GPx), and peroxiredoxin (Prx) participate in the detoxification of H2O2 by transforming it into water and oxygen, depending upon the site of generation and the specific enzymatic equipment (see Figure 1—red color) [33]. H2O2 serves a fundamental regulatory function in metabolism, beyond its role as a damage signal [34,35]. At any given moment, the local concentration of H2O2 in a specific compartment reflects the balance of production and removal dynamics [34].
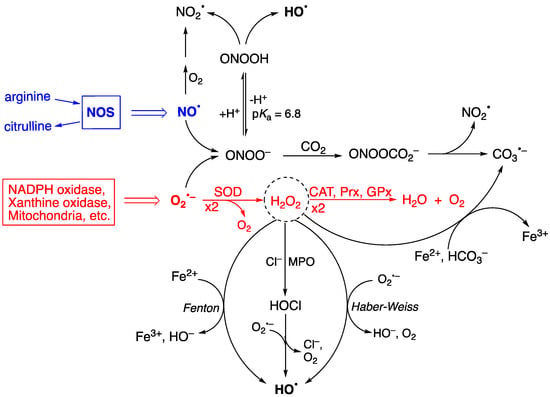
Figure 1.
A summary of chemical transformation of the reactive oxygen species (ROS) network. O2•− is ubiquitous and continuously formed during normal cellular metabolism by a variety of systems, and NO• is produced by a variety of nitric-oxide synthases (NOS). The superoxide dismutase (SOD) enzymes participate in controlling the production of O2•−, and the catalase (CAT), glutathione peroxidase (GPx), and peroxiredoxin (Prx) enzymes in their turn are involved in the detoxification of H2O2 (see reactions in red color). H2O2 is at the crossroads of several pathways for the formation of HO•, the main ones being the Fenton and Haber–Weiss reactions, as well as HOCl formation, mediated by myeloperoxidase (MPO), followed by one-electron oxidation. The protonated form of ONOO− (pKa 6.8) spontaneously decomposes, generating HO•, whereas the ONOO− acts as a nucleophile with CO2 affording an adduct that instantaneously decomposes to NO2• and CO3•−. H2O2 is also a precursor of CO3•− through the reaction with HCO3− in the presence of reduced iron (Fe2+). Adapted from Ref. [28].
Figure 1 also shows the importance of H2O2 in generating radicals. Myeloperoxidase (MPO) uses H2O2 and anions like Cl− or Br− to generate hypochlorous acid (HOCl) or hypobromous acid (HOBr), respectively. The half-life of HOCl in cells is a few seconds, and its biological properties are different from H2O2 [11]. Several pathways are involved in the production of HO•, the main ones being the Fenton reaction (Fe2+ and H2O2), the Haber–Weiss reaction (O2•− and H2O2), and the reduction of hypohalous acids (HOCl or HOBr) by O2•− [28,36,37]. Other biologically critical free radicals can be produced due to ONOO− formation from the diffusion-controlled reaction of O2•− and NO• radicals generated during normal cellular metabolism [38,39,40,41,42]. Spontaneous decomposition of the protonated form (ONOOH, pKa of 6.8) produces HO• and NO2• with a rate constant of 4.5 s−1 at 37 °C [38,41,43,44]. The HO• radical is the most reactive of the common ROS and has close to diffusion-limit rates with numerous biomolecules (e.g., with glutathione, k = 2.3 × 1010 M−1 s−1) [45]. Its diffusion distance is around 1−5 nm, i.e., less than the width of a 100 kDa protein [11]. It must be considered that the HO• radical can be formed from more stable ROS molecules, like H2O2, HOCl, and ONOO−, having a larger diffusion distance. Therefore, the damaging potential of HO• can be expanded by the long-distance reactivity of its progenitors, becoming more than previously thought.
The ubiquity of CO2 and its hydrated form bicarbonate (HCO3−) in biological systems is well documented, e.g., 25 mM HCO3− is in equilibrium with 1.3 mM CO2 at pH 7.4. The reaction of ONOO− with CO2 affords the adduct ONOOCO2− with a relatively high rate constant (k = 5.8 × 104 M−1s−1), followed by a rapid decomposition (<100 ns) to NO2• and CO3•− [38,43,44]. The reduction potential of CO3•−/CO32− is 1.59 V, which makes CO3•− a milder single-electron oxidant. It is also reported that the Fenton reaction, in the presence of HCO3−, also produces CO3•− as the predominant species rather than the HO• radical [46,47,48]. Given the uncertain nature of the cellular labile iron pool [49], the contribution of Fenton chemistry to ROS generation is likely to be context-dependent [11].
A complex and well-developed antioxidant system protects cells from oxidative damage. An antioxidant is defined as “any substance that delays, prevents or removes oxidative damage to a target molecule” [23,36]. The antioxidant network fighting against the damaging potential of high levels of oxidants consists of two classes: (i) antioxidant enzymes and (ii) low-molecular-mass antioxidants [20,22,23].
3. Biomimetic Chemistry of Oxidative DNA Damage: cPu vs. 8-oxo-Pu
DNA is vulnerable to the attack of HO• radicals, causing chemical modifications of nucleobases or nucleotides, abasic sites, and DNA strand breaks, which have been identified in living organisms [6]. These modifications are similar to those observed in vitro. Biomimetic experiments can be instrumental in understanding such processes’ dynamics and competitiveness issues [6]. The rate constant for the reactions of HO• radicals with DNA has been reported to be ca. 2.5 × 108 M−1 s−1 [50]. Recently, a rate constant of 1.4 × 1010 M−1 s−1 was determined for the reaction of HO• with the double-stranded oligonucleotide (ds-ODN) containing GC pairs, i.e., the palindromic 5′-d(GCGCGC)-3′), and a value of 2.3 × 109 M−1 s−1 can be estimated for the reaction of HO• with the G:C pair, which is ~5-fold smaller than the sum of the two single nucleoside rate constants participating in the pair [51]. The site of attack is both the H-atom abstraction from the 2′-deoxyribose units and the addition to the base moieties, the latter accounting for ~85% of attacks in naked DNA [52]. The order of reactivity of HO• radicals towards the various H-atoms of the 2′-deoxyribose moiety in DNA is widely accepted to be H5′ > H4′ > H3′ ≈ H2′ ≈ H1′, which goes along with the exposure of H-atoms to the solvent [53,54]. It is estimated that ca. 7% of HO• radicals abstract the H5′ in naked DNA [55]. The fate of the resulting C5′ radical depends on its surrounding environment. Several studies have focused on the selective generation of C5′ radicals, yielding valuable quantitative data [56].
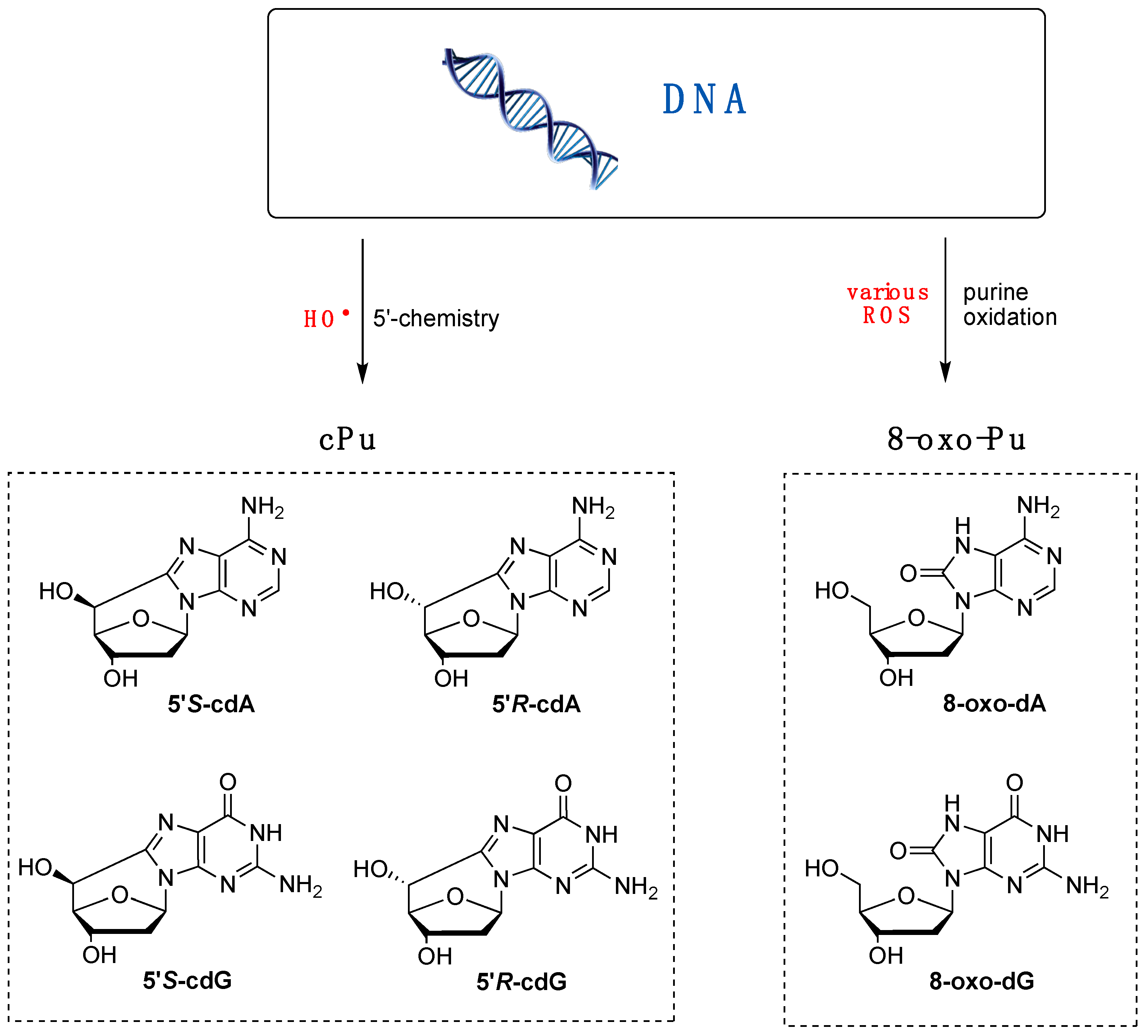
Various purine (Pu) lesions were observed in vitro due to oxidatively generated DNA damage [1,2]. Among these are the two 8-oxo-Pu lesions (8-oxo-dA and 8-oxo-dG) deriving from the oxidation of the base and the four cPu lesions (cdA and cdG in their 5′R and 5′S diastereoisomeric forms) originating from the C5′ radical chemistry of 2′-deoxyribose unit (Figure 2) [57,58]. The relative yields of these lesions depend on the reaction context, as evidenced by several comparative studies with various oxidants. For example, in isolated or commercial DNA, the 8-oxo-Pu and cPu lesions are detected in biomimetic experiments by specific generation of HO• radicals, whereas 8-oxo-Pu is the product of other non-radical ROS.
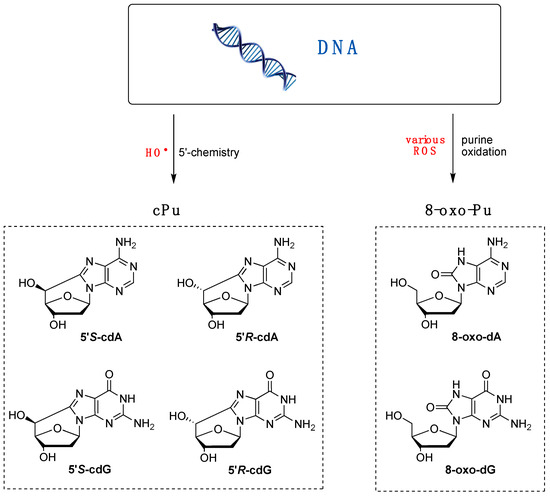
Figure 2.
Purine lesions are formed after oxidatively induced DNA damage in vivo and in vitro; (left part) structures of 5′,8-cyclo-2′-deoxyguanosine (cdG) and 5′,8-cyclo-2′-deoxyadenosine (cdA) in their 5′S and 5′R diastereomeric forms generated by H5′ abstraction from HO• radical; (right part) structures of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) and 8-oxo-7,8-dihydro-2′-deoxyadenosine (8-oxo-dA) generated by ROS-mediated oxidation, also including the HO• radical.
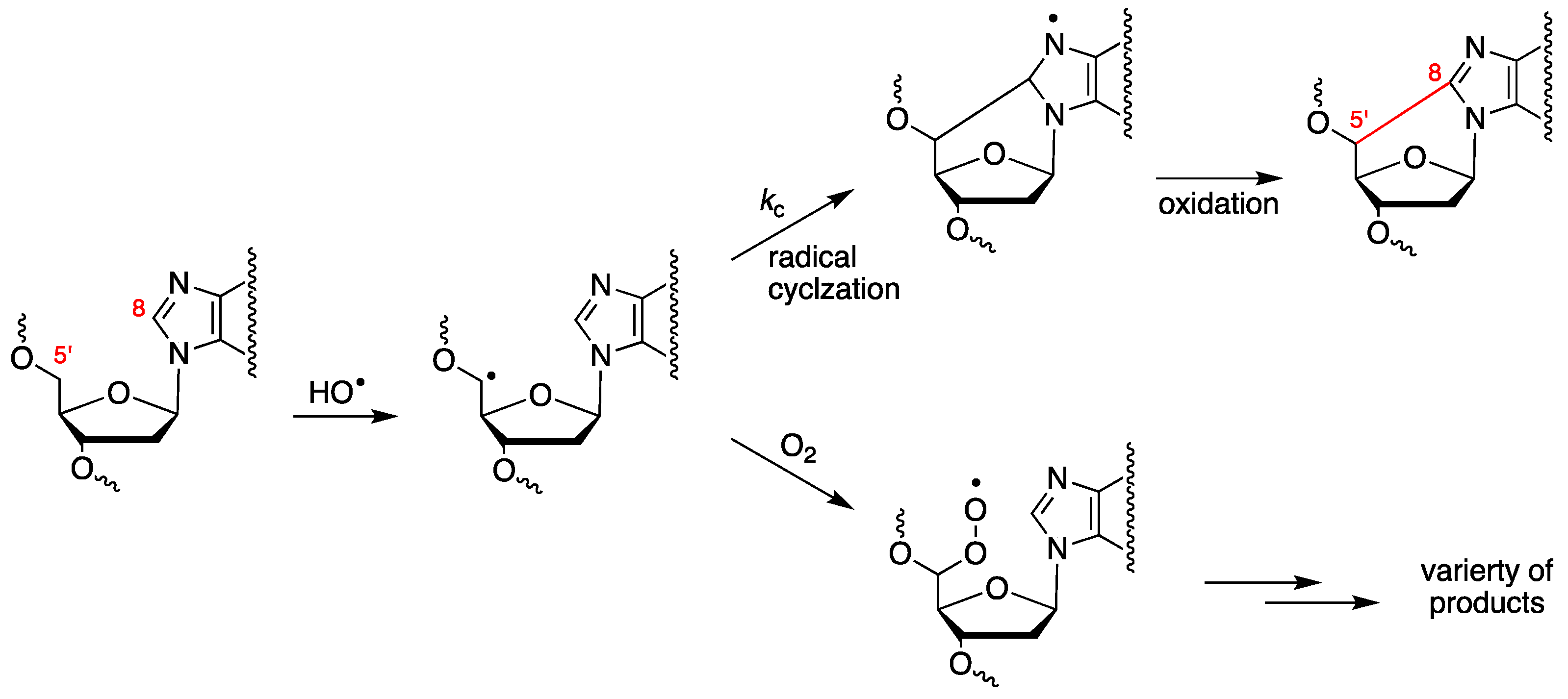
The abstraction of H5′ of purine moieties of DNA by HO• radicals is estimated to be ca. 3% of all possible DNA attack sites [52]. The fate of the resulting C5′ radical is oxygen-dependent (see Figure 3). Under anaerobic conditions, the C5′ radical attacks the adenine or guanine moiety at the C8 position, forming a new covalent bond C5′–C8, followed by oxidation that leads to 5′,8-cyclopurines (cPu) [28,58]. By increasing the oxygen concentration, the cPu lesions decrease and the major pathway for DNA damage results in direct strand scission via the corresponding peroxyl radical [59,60,61].
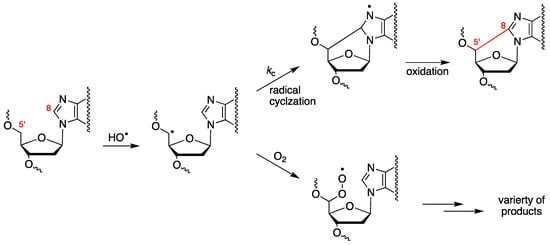
Figure 3.
Purine 2′-deoxynucleotide moiety reacts with the HO• radical, yielding the C5′ radical, whose fate is oxygen-dependent. Cyclization of the C5′ radical followed by oxidation leads to cPu lesions, whereas the reaction of the C5′ radical with oxygen provides the peroxyl radical that undergoes further transformations, including strand scission.
Are ROS other than the HO• radical able to generate C5′ radicals in DNA? O2•− is unreactive in H-atom abstraction, the bond dissociation enthalpy (BDE) of C5′–H being 91.3 kcal/mol and that of −O2–H being ca. 60 kcal/mol. Its conjugate acid, HOO• (pKa ~4.8), has a H-atom-abstracting ability comparable to that of the alkylperoxyl radicals (ROO•), i.e., 88–89 kcal/mol; however, it has been demonstrated that ROO• cannot induce DNA strand scission [62,63]. Furthermore, HOO• is present at only ca. 0.2% of the O2•− concentration at pH 7.4.
HO• radicals react with guanine and adenine moieties, leading to a variety of products that include the 8-oxo-Pu family (Figure 2) [28]. Other ROS transients, such as H2O2, ONOO−, singlet oxygen, or ROO• radicals, can generate 8-oxo-Pu lesions [64,65]. The 8-oxo-dG is a major DNA lesion caused by oxidative stress, and it is further oxidized to other deleterious secondary DNA lesions [28]. 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) and 8-hydroxy-2′-deoxyguanosine (8-HO-dG) are the same molecule in two different tautomeric forms. Both acronyms, 8-oxo-dG and 8-HO-dG, are used in the literature, although NMR studies on a 12-mer ds-ODN indicated that 8-oxo-dG is the exclusive species with respect to its tautomer [66]. In this review, the acronyms 8-oxo-dG and 8-oxo-dA are used.
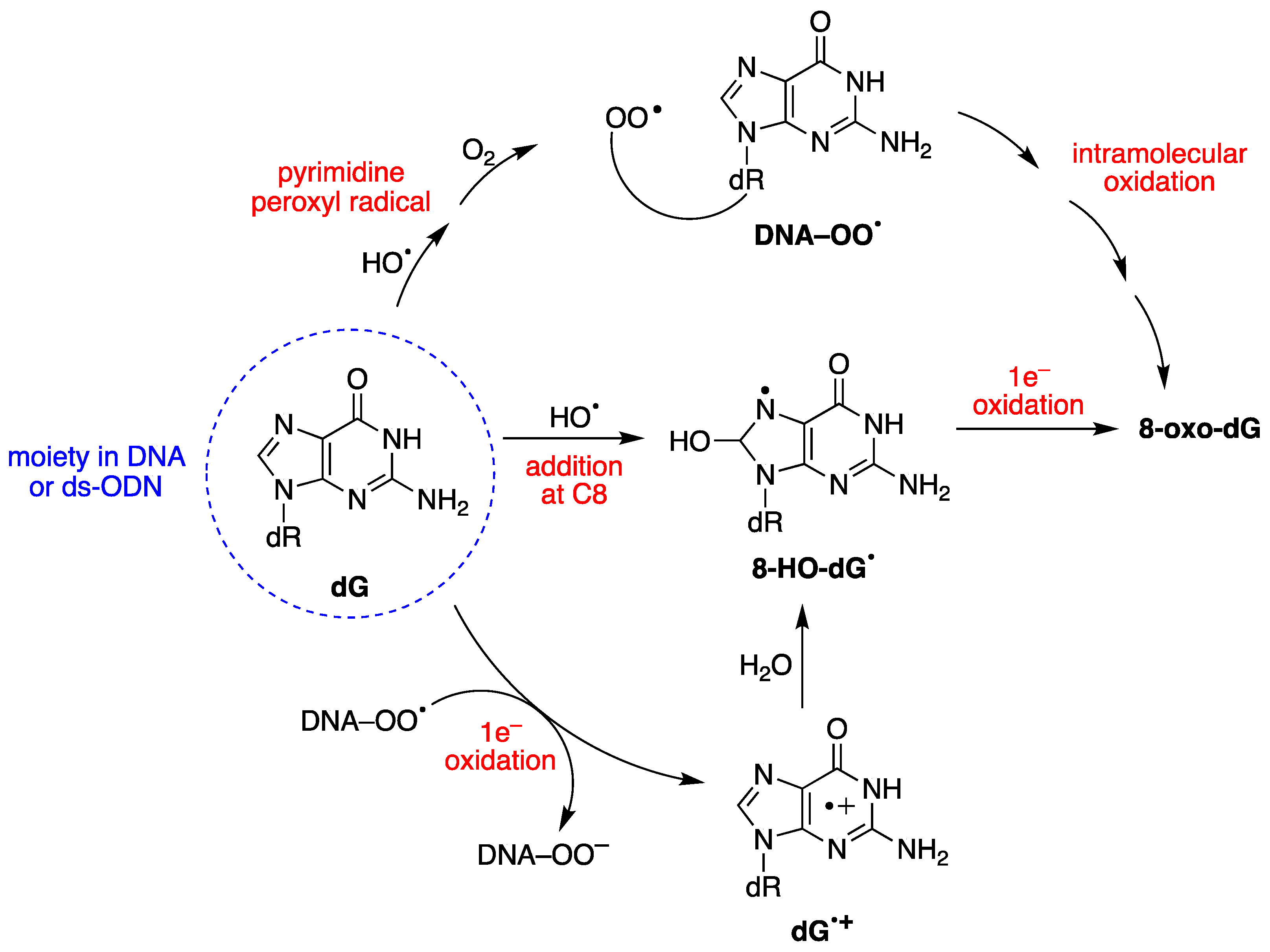
8-oxo-dG formation results from the initial attack by HO• radicals or through a one-electron oxidation step [28]. The mechanism of 8-oxo-dG formation by the reaction of HO• with ct-DNA [67,68], as well as with 21-mer ds-ODN [69,70], has been investigated in detail, in the absence or presence of different oxygen concentrations. It was demonstrated that three pathways contribute to forming 8-oxo-dG moiety, the two major ones being oxygen-dependent and a minor one being oxygen-independent, as shown in Figure 4 [67,68,70]. The minor path (5–10%) is the direct addition of HO• to the C8 position of the guanine moiety to form 8-HO-dG•, followed by one-electron oxidation. The HO• attacks are mostly non-selective (90–95%), generating diverse carbon-centered radicals that, in the presence of O2, are converted into corresponding peroxyl radicals, DNA–OO• [52]. DNA–OO• radicals produce two types of reactions in approximately equal amounts: (i) an intramolecular oxidation through addition of the peroxyl radical onto the C8 of a vicinal guanine base (see Figure 4) [67,68]; (ii) an intramolecular electron transfer leading to DNA•+, followed by hole transfer to [G:C]•+ pair, hydrolysis to give the 8-HO-dG•, and further oxidization to 8-oxo-dG [71]. Model studies on ODNs have reported the transient generation of pyrimidine peroxyl radicals, which add to the C8 position of a vicinal guanine base [72,73]. In these experiments, the formation of 8-oxo-dA was several times lower than that of 8-oxo-dG [67,69]. This lower yield of 8-oxo-dA formation was attributed to the absence of a one-electron oxidation pathway, and to a lesser extent, to the involvement of an intramolecular DNA–OO• radical on the adenine base.
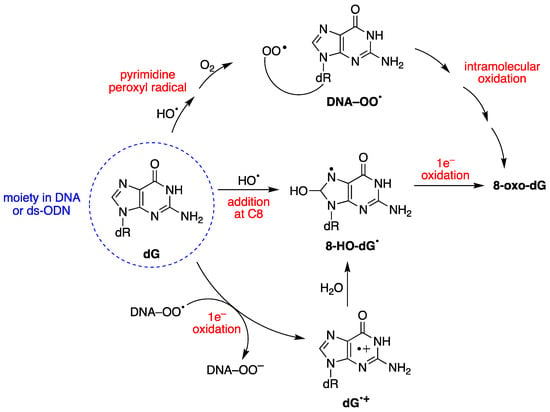
Figure 4.
Mechanism for the oxidatively generated 8-oxo-dG in DNA or ds-ODNs; three pathways contribute to forming 8-oxo-dG, the two major ones being oxygen-dependent.
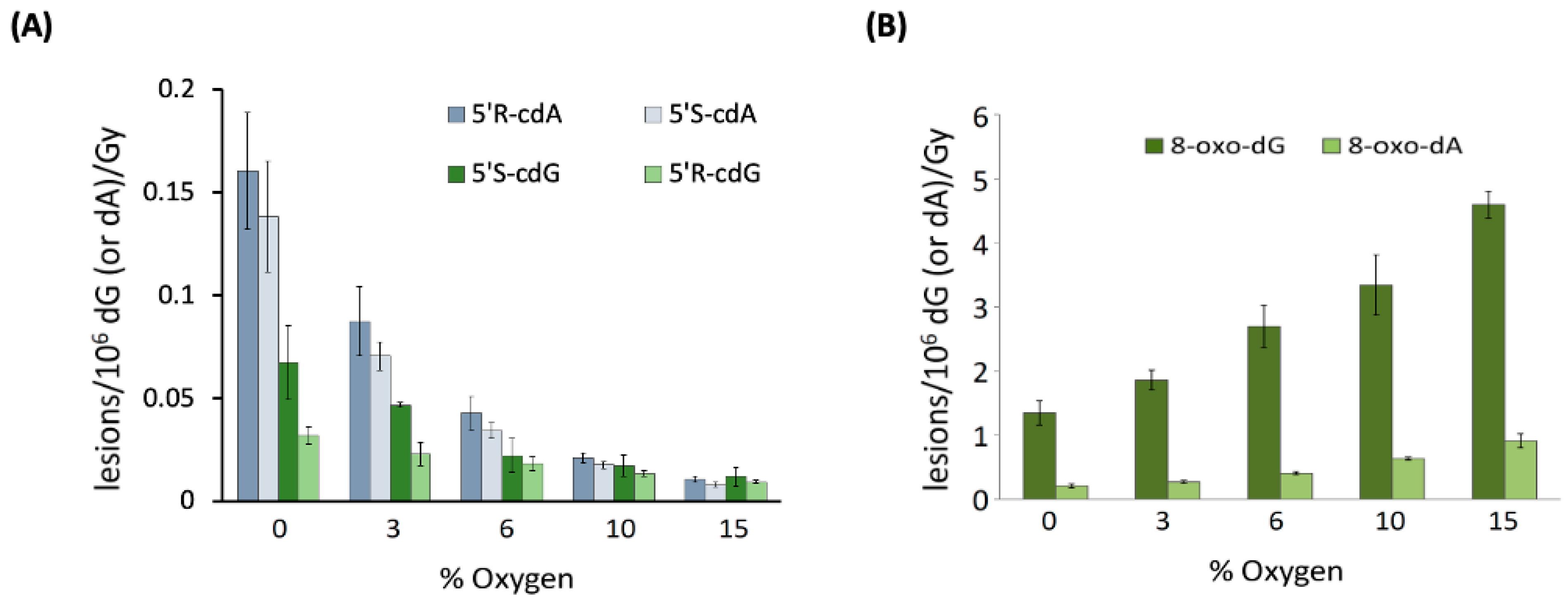
When examining 21-mer ds-ODNs [70], it was observed that increasing oxygen concentrations significantly reduced the levels of cdA and cdG. Figure 5A illustrates the variations in the levels of each of the four distinct cPu lesions per 106 nucleosides (either dA or dG) per Gy of irradiation as oxygen concentration increases. This finding supports the notion that the C5′ radical has competing pathways—either cyclization or addition to molecular oxygen (see Figure 3). Rate constants for the cyclization steps were found to be in the range of 0.8–4.9 × 104 s−1 through competition between the C5′ radical cyclization and the oxygen trapping. Moreover, it is noteworthy that the oxygen concentration did not influence the 5′R/5′S ratio for both cdG and cdA. As illustrated in Figure 5B, the reaction of HO• radicals with 21-mer ds-ODNs results in an increased production of both 8-oxo-dG and 8-oxo-dA as the concentration of O2 rises. The levels of 8-oxo-dG were found to be six times higher than those of 8-oxo-dA. It is worth highlighting that increasing O2 concentrations led to reduced amounts of the four cPu lesions (Figure 5A) and excess amounts of the two 8-oxo-Pu lesions (Figure 5B). The 8-oxo-Pu lesions were only four times higher than the cPu ones in the absence of O2 but became 130 times more than cPu lesions at 15% O2, which corresponds to an O2 concentration of 2 × 10−4 M [70].
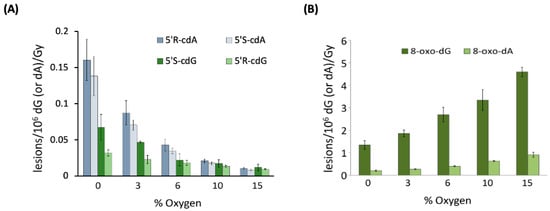
Figure 5.
Oxygen-dependent radiation induced formation of purine lesions in ds-(ODN1/ODN2), where ODN1: 5′-GGG TTA GGG TTA GGG TTA GGG-3′ and ODN2: 5′-CCC TAA CCC TAA CCC TAA CCC-3′; cPu (A) and 8-oxo-Pu (B) lesions per 106 dG (or 106 dA) per Gy of γ-irradiation (in the presence of N2O) in the range of 0 to 60 Gy. Adapted from ref. [70] with permission of the American Chemical Society.
Comparison of the HO• radical reactivity towards mtDNA and tDNA in an “isolated” context was addressed by the simultaneous measurement of 8-oxo-Pu and cPu lesions. Total (t) DNA is equal to nuclear (n) DNA plus mitochondrial (mt) DNA. The amount of 8–oxo–Pu and cPu lesions for the model reactivity of HO• radical towards mtDNA and tDNA, isolated from wild-type CS1AN–wtCSB cells, under deoxygenated conditions was found to be (units of lesions/107 nucleosides/Gy) 40.3 for 8–oxo–Pu and 19.0 for cPu in tDNA, and 4.6 for 8–oxo–Pu and 2.5 for cPu in mtDNA [74]. The 8–oxo–Pu was nearly twice the cPu values in both genomic materials, whereas 8–oxo–Pu and cPu were 8.8 and 7.6 times higher in tDNA than in mtDNA. These results under biomimetic conditions contribute to a better understanding of HO• radical reactivity in distinct genetic pools, such as mtDNA and nDNA, highlighting the effects of different helical topology. In this respect, purine oxidation by the HO• radical is highly dependent on tertiary ds-DNA helical forms having a supercoiled, open circular, and linear conformation, and that greater damage occurs towards the extended B-DNA topology for both 8-oxo-Pu and cPu [75].
4. The Protocol for the Simultaneous Quantification of Six Purine Lesions in DNA
Experimental protocols quantifying DNA lesions are numerous, the mass-spectrometry-based platforms being the most representative [65,76,77]. The use of genetic material, going down to the single nucleoside level by hydrolysis, and liquid chromatography–tandem mass spectrometry (LC–MS/MS), using isotopically labeled reference compounds [78,79], confer the advantage of specificity and repeatability, providing lower limits of detection (LOD) and quantification (LOQ) as well as precise measurement of DNA lesions [80]. Major efforts were devoted to the simultaneous quantification of purine lesions (four cPu and two 8-oxo-Pu) in cellular DNA, with the choice of the most appropriate assays, including suitable conditions of DNA extraction, hydrolysis, and base quantification [81,82,83]. Initially, we developed a protocol for the four cPu lesions [81,82] and later included the two 8-oxo-Pu lesions [83].
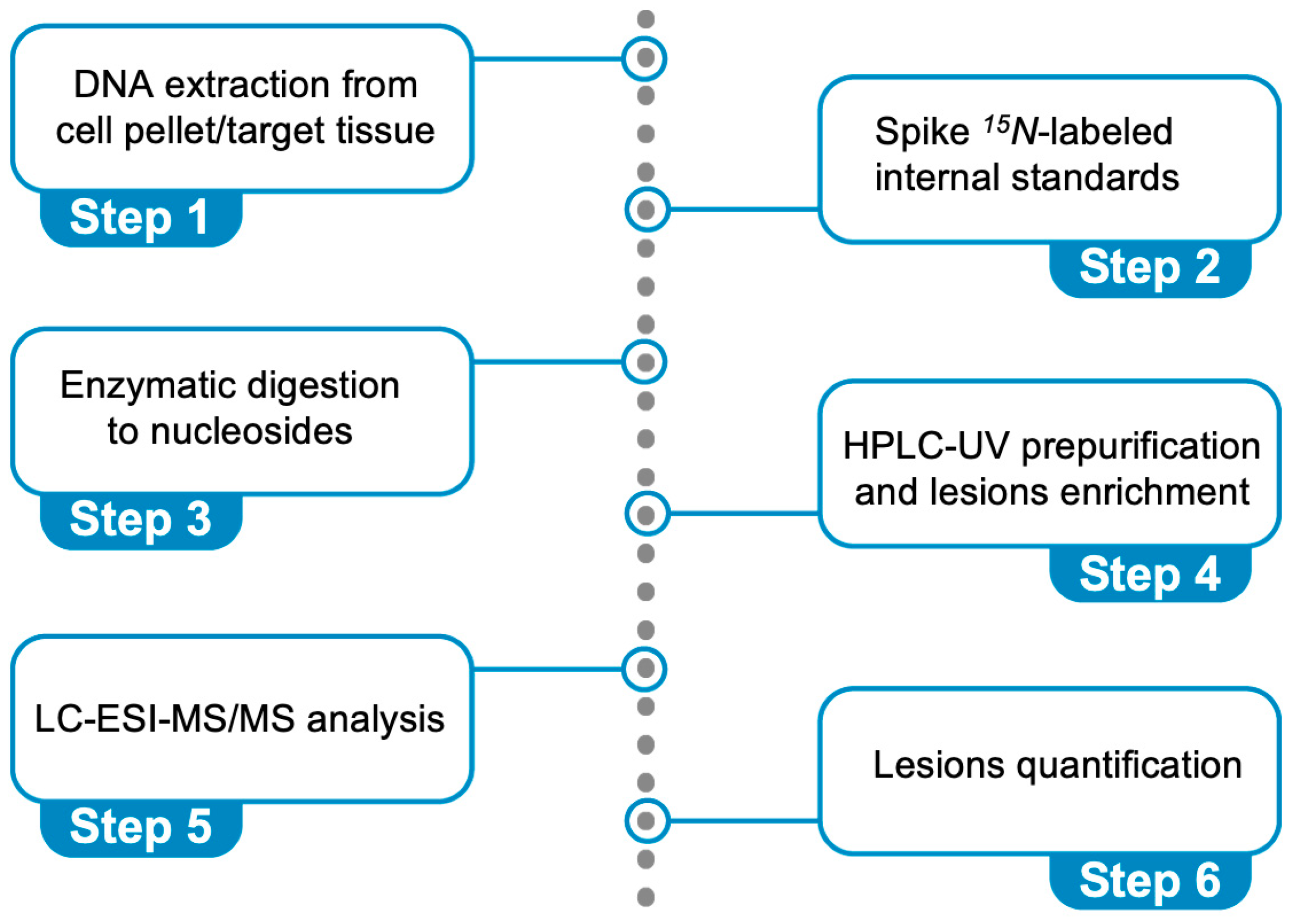
Figure 6 describes the steps: (i) DNA extraction from a cell pellet or a target tissue (addition of chelating agents and antioxidants), (ii) spiking 15N-labeled internal standards (addition of deaminase inhibitors and antioxidants), (iii) enzymatic digestion of DNA to nucleosides, (iv) HPLC-UV pre-purification and lesion enrichment (fraction collection), (v) quantification through liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis, and (vi) lesion quantification. It is worth underlining that the cPu lesions are stable compared with other oxidatively generated DNA adducts and cannot be artificially produced during DNA isolation. We optimized this protocol using model experiments of HO• radical reactions with ds-ODNs or commercially available ct-DNA [69,70]. The data reported in Figure 5A,B refer to cPu and 8-oxo-Pu, respectively, for the reaction of HO• radicals (generated by γ-radiolysis) with the 21-mer ds-(ODN1/ODN2), using our analytical protocol [70]. The reaction of HO• radicals (generated by γ-radiolysis) with ct-DNA in aqueous solutions in the absence of O2 shows the level of total 8-oxo-Pu (8-oxo-dG/8-oxo-dA = 7.7) to be 40-fold in excess of total cPu lesions (5′R-cdG/5′S-cdG = 4.5 and 5′R-cdA/5′S-cdA = 1.2) [69]. In a recent article [83], we described the protocol in detail, including limitations and troubleshooting.
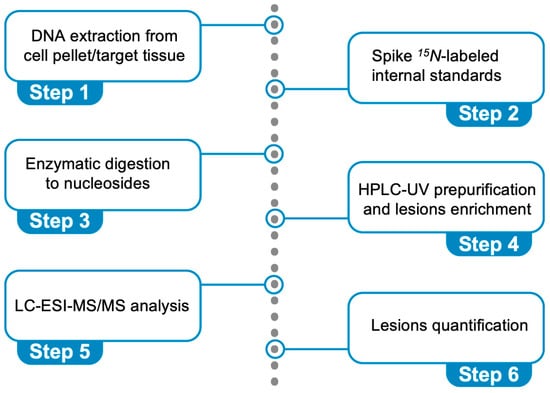
Figure 6.
Protocol for the simultaneous quantification of the six oxidative purine lesions (see Figure 2) in DNA using LC-MS/MS analysis.
To determine the levels of six specific DNA lesions, we used this protocol on genomic and/or mitochondrial DNA extracted from cell cultures of various neurodegenerative disorders (further discussed in Section 6). Additionally, we applied it to DNA from estrogen-receptor-alpha-positive (ER-α) MCF-7 and triple-negative MDA-MB-231 breast cancer cell lines [84]. We also compared lesion levels in tissues (liver, kidney, and brain) of immunodeficient (SCID) xenografted mice with and without tumor implantation at different ages (4 and 17 weeks) [85]. Furthermore, we analyzed genetic material from tissue biopsies of patients with inflammatory bowel diseases (IBDs) and severely obese individuals in comparison to control samples [86].
5. Repair and Mutagenicity Studies: The Role of Nucleotide Excision Repair (NER)
Cells have developed a complex DNA repair network to manage oxidative insults and restore genome integrity [3,4,5]. DNA repair defects are linked with a wide spectrum of diseases, including cancer, premature aging, and neurological disorders [87]. BER and NER are cellular pathways for recognizing and repairing oxidatively induced DNA lesions [3,87]. BER is the primary system that processes base damage by creating an abasic site, e.g., the 8-oxo-dG lesion [88,89]. NER machinery is a widespread mechanism for repairing various helix-distorting DNA lesions, such as bulky DNA adducts caused by ultraviolet radiation and intra-strand crosslinks [90,91,92]. Evidence has also been provided on removing some oxidation-induced purine DNA lesions involving the overlap between these pathways [93]. NER is split into two sub-pathways: transcription-coupled (TC-NER), which repairs transcriptionally active domains, and global genome (GG-NER), which removes lesions from the whole genome [94,95]. Recent research using patient cells with specific NER gene defects and newly created knockout models has helped define the roles of GG-NER and TC-NER in repairing synthetic cdA and cdG lesions [96]. Defects in these sub-pathways may lead to clinical conditions such as xeroderma pigmentosum (XP) and Cockayne syndrome (CS). The NER pathway comprises many proteins (including the XP proteins (A through G) and the CSB proteins).
Biochemical studies demonstrated that the cPu lesions are not BER substrates but fairly good substrates of NER [97,98,99,100,101]. The cPu lesions cause structural deformation [102], and their transcription-blocking potential towards human RNA polymerase II have been reported [103]. The relative NER incision efficiencies of the four cPu lesions were measured in a 147-mer ds-ODN under the same sequence context in human HeLa cell extracts for a direct comparison [104]. The cdA and cdG lesions were excised with similar efficiencies by NER, the 5′R diastereomeric lesions being about twice as efficient as the 5′S diastereomeric lesions. Molecular modeling and dynamics simulations show that the cPu lesions cause more significant local distortion of the DNA backbone and a greater disruption of local van der Waals stacking interactions in 5′R than in 5′S diastereomers. The locally impaired stacking interaction energies correlate with relative NER incision efficiencies. Overall, NER is 2–4 times less effective at repairing cPu lesions than other bulky DNA adducts [104]. The relative NER efficiencies towards the cPu at the level of nucleosomes have also been addressed; the complete NER resistance of the cPu lesions in human HeLa cell extracts is attributed to the size of the cPu, which is similar to natural nucleotides [105]. Such a resistance means an accumulation of these lesions in the genome, which leads to the inhibition of DNA and RNA polymerase activities, DNA binding of transcription factors, replication fork stalling, and transcription termination [97,106,107]. Cells have evolved mechanisms to bypass these lesions through DNA repair, translesion DNA polymerases, and RNA polymerase II to address the challenges posed by cPu lesions. The DNA repair polymerases (pol) that can bypass cdA lesions include pol β, pol η, pol ι, and pol ζ [108,109,110,111,112,113]. Nonetheless, bypassing cPu lesions by DNA and RNA polymerases can also incorporate wrong nucleotides or result in the stalling of DNA and RNA synthesis [108,110,111,113,114,115]. For example, pol β can get stuck after inserting a dT opposite cdA, generating single-strand breaks [110]. On the other hand, RNA polymerase II and pol β can also cause multi-nucleotide deletion and repeat deletion to bypass cdA lesions during transcription and BER [109,111]. Recent findings show that human repair DNA polymerases pol β and pol η can incorporate the 5′R-cdA and 5′S-cdA lesions and create a cdA:dC mismatch [116]. Furthermore, the incorporation of these two lesions can be fully extended by pol β and ligated by LIG I, suggesting that cPu lesions can be embedded in the human genome from the damaged nucleotide pools [116].
In summary, the accumulation of cPu lesions in the human genome can lead to mutations and genome instability, which are linked to various diseases and conditions, including aging, inflammation, cancer development, and neurodegenerative disorders [57,117,118,119].
6. Oxidative Stress Causes Purine DNA Damage in Biological Models of Neurodegenerative Disorders
6.1. Xeroderma Pigmentosum (XP)
Xeroderma pigmentosum (XP-complementation group A–G) is an autosomal recessive genetic disorder associated with NER deficiency. As indicated in the previous section, NER is fundamental in repairing various helix-distorting or bulky DNA damage [91,92]. For example, the sunlight-induced photoproducts remain unrepaired, increasing the incidence of skin cancers [120,121,122]. The most severe cases of XP neurological disease are seen in XP-A patients, where defects emerge between the ages of 2 and 8. These cases present microcephaly, mild cognitive loss, followed by cerebellar variations and, ultimately, neuropathy [123]. XP-A is one of the 30 proteins involved in GG-NER and TC-NER; XP-A plays a central role in both sub-pathways, which cause different disease phenotypes [7,124,125]. However, many XP patients also display early-onset neurodegeneration, which leads to premature death. The mechanism of neurodegeneration is unknown.
Since exogenous ultraviolet radiation cannot reach the human brain, endogenous DNA lesions like the cPu, which are repaired exclusively by NER, have been assumed to elicit XP-related neurodegeneration [97,98,126]. cPu lesions either completely block RNAPII, causing transcription inactivation and neuronal death, or partially lead to mutant RNA transcripts and a deficient translation process [117,127]. Regarding the 8-oxo-Pu, in particular 8-oxo-dG lesions, earlier studies have shown that XPA-silenced cells, compared to the wild type (wt), are characterized by enhanced steady-state ROS levels and accumulation of 8-oxo-dG in their genome [128,129].
The enzyme-linked immunosorbent assay (ELISA) has been extensively employed for different biological applications, including identifying DNA adducts. However, the ELISA approaches regarding 8-oxo-dG overestimate the levels and therefore are inappropriate for an accurate quantification [130]. Using an XP group-A gene-knockout (Xpa−/−) mouse model, an ELISA test for the quantification of cdA (5′R-cdA and 5′S-cdA together) in DNA was generated by a monoclonal antibody (CdA-1) [131,132]. Initially, the detection level of the ELISA was as low as 10 lesions/106 Nu in a 0.5 μg DNA sample, which was inadequate to evaluate different levels of cdA in tissues between Xpa−/− and control wt mice [131]. Further optimization increased the ELISA sensitivity to a detection limit of 1 cdA lesion/106 Nu [132]. Indeed, the improved ELISA showed that cdA lesions accumulate with age in the liver, kidney, and brain tissues of Xpa−/− and of wt mice, but there were significantly more cdA lesions in Xpa−/− mice than in wt mice at 6, 24, and 29 months of age [132]. These findings suggest that the brain’s age-dependent accumulation of endogenous cPu lesions may be critical for XP neurological abnormalities. However, a significant disadvantage of ELISA is the absence of structural information and potential lesion overestimation compared with mass spectrometry approaches.
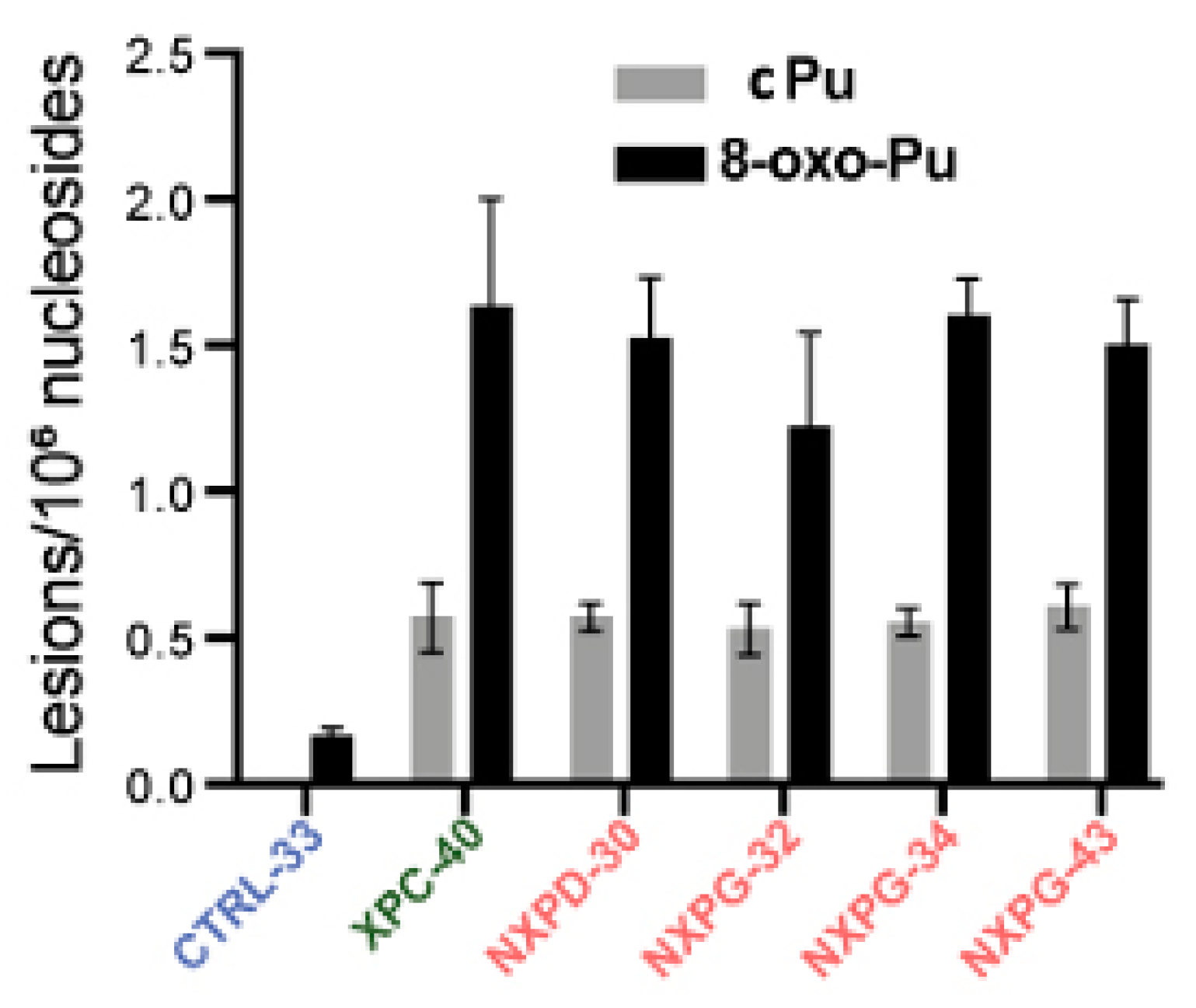
A recent investigation reported on XP neurodegeneration using human-induced pluripotent stem cells (hiPSC) derived from multiple XP complementation patients and healthy relatives, performing functional multi-omics on samples during neuronal differentiation [133]. Evidence is given of endoplasmic reticulum stress and marked oxidative DNA damage in XP neurons. The hiPSCs were obtained from one healthy control and four XP patients (groups D and G) with different cerebral disabilities but no signs of accelerated aging. After differentiation into neurons in triplicate, DNA was isolated and analyzed for the cPu and 8-oxo-Pu lesions, using the protocol mentioned in Section 4, on neurons from XP patients with or without neurodegeneration and healthy controls [133]. Figure 7 illustrates the substantial accumulation of oxidized nucleosides cPu and 8-oxo-Pu on several XP cell lines (XPC-40, NXPD-30, NXPG-32, NXPG-43, and NXPD-93). Compared with controls (CTRL-33), substantially elevated cPu lesions were observed in XP neurons with (NXPD or NXPG) or without (XPC-40) neurodegeneration. Increased 8-oxo-Pu in XP neurons was also found, the ratio 8-oxo-dG/8-oxo-dA being ~5. However, the fold increase was more significant for cPu than 8-oxo-Pu, resulting in an 8-oxo-Pu/cPu ratio of 2–3. For comparison, in the biomimetic chemistry of HO• radicals with ct-DNA, the level of 8-oxo-Pu was found to be ~40-fold in excess of cPu in the absence of O2 [69], and in ds-ODN, the 8-oxo-Pu was 130 times more than cPu at 15% O2 concentration (see Figure 5) [70]. The 8-oxo-Pu lesions observed in controls agree with prior reports that hiPSCs are exposed to oxidative damage [134,135].
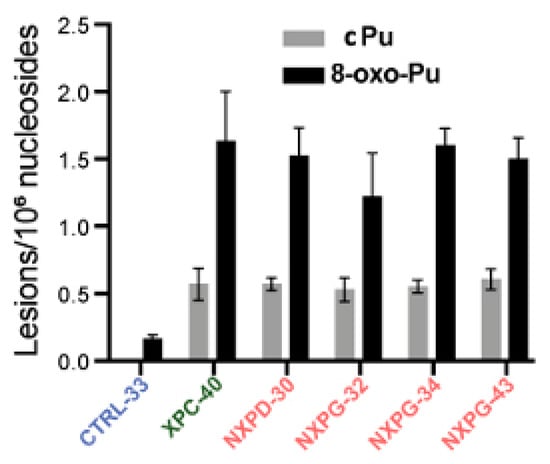
Figure 7.
Comparison between cPu and 8-oxo-Pu levels (lesions/106 nucleosides) in XP samples with neurodegeneration (red; NXP with XPD or XPG mutant), XP without neurodegeneration (green; XPC-40) and healthy controls (blue; CTRL-33). Adapted from ref. [133] with permission from Elsevier.
The cPu and 8-oxo-Pu were simultaneously quantified in wt (EUE-pBD650) human embryonic epithelial cell lines and the same line, where the XPA gene was silenced by 80% (EUE-siXPA), under different oxygen tensions [136]. Table 1 reports the mean values (lesions/106 Nu) of the six purine lesions under aerobic (21% of O2) and hypoxic (1% of O2) conditions. Figure 8A compares the sum of four cPu and the sum of two 8-oxo-Pu lesions. The 8-oxo-Pu levels were only ~1.7-fold higher than the cPu in both cell types, and both wt and silenced cell lines accumulated high levels of lesions under hypoxic conditions. Interestingly, the iron (Fe) levels were significantly higher in XPA-defective compared to wild-type cells (see Section 1 for the role of Fe2+ in the chemistry of ROS).
The results presented above on XP indicate that cPu lesions contribute to DNA damage in NER-deficient cells. This unrepaired oxidative DNA damage accumulates over time in terminally differentiated post-mitotic cells such as neurons and has deleterious effects on transcription and apoptosis regulation, resulting in neurodegeneration [137].
6.2. Cockayne Syndrome (CS)
Cockayne syndrome (CS) is a multi-system disorder associated in many patients with defects in NER [94]. CS provokes early aging and gradual neurological dysfunctions. Over 90% of CS cases are due to mutations in the CSA or CSB genes. A key aspect of CS cells is the defect in the TC-NER sub-pathway, which is responsible for removing lesions in the transcribed strand of actively transcribed genes [94]. Even though CSA and CSB patients present similar clinical characteristics, the proteins involved have distinct activities and functions in the TC-NER process. A key insight into the severe neurological abnormalities associated with CS was the discovery that the defect in TC-NER affects lesions repaired by NER and extends to oxidative DNA lesions, which are typically repaired by BER [95].
The sensitivity of CS cells to oxidatively generated DNA damage underscores the crucial role of CSA and CSB proteins in removing these lesions. Research has shown that the CSA protein in untreated human fibroblasts regulates the steady state level of 8-oxo-dG [138] and that keratinocytes from CSA patients accumulated 5′S-cdA under standard atmospheric oxygen tension [139]. Accumulation of 5′S-cdA in organs of CSB knockout mice has also been reported [140]. Furthermore, the CSB protein has been shown to enhance APE1 activity and to protect against agents that induce BER intermediates [141,142]. These findings highlight the importance of CS proteins in the recognition, signaling, and processing of single-strand breaks (SSBs) and double-strand breaks (DSBs), which are relevant lesions in neurodegenerative disorders [143].
Cells from CS patients, with mutations in CSA or CSB genes, present elevated levels of ROS and are defective in the repair by NER of various helix-distorting DNA lesions. The cPu and the 8-oxo-Pu lesions were ascertained in both wild-type (CS3BE–wtCSA) and (CS1AN–wtCSB) cell lines, and their defective counterparts CS3BE and CS1AN [144]. Like in XPA cells described above, the role of oxygen concentration in DNA damage was also reported for CSB and CSA cells. Table 1 reports the mean values (lesions/106 Nu) of the six purine lesions under aerobic (21% of O2) and hypoxic (1% of O2) conditions. Figure 8B,C illustrate the sum of four cPu and two 8-oxo-Pu lesions for CSB and CSA, respectively. cPu levels were comparable to 8-oxo-Pu levels in all cases, i.e., within the range of 2–6 lesions/106 Nu, and there was a substantial increase from aerobic to hypoxia. Comparing the accumulations, it can be seen that (i) the 8-oxo-Pu level is 1.3–1.9-fold higher than cPu for CS1AN-wtCSB and CS1AN cells (Figure 8B), and (ii) the 8-oxo-Pu level is 1.3–1.7-fold higher than cPu for CS3BE–wtCSA and CS3BE cells (Figure 8C). In hypoxic conditions, CSB- and CSA-defective cells showed higher levels of the four cPu lesions, compared to regular counterparts, and a significant increase in 8-oxo-Pu [144].

Table 1.
The levels (lesions/106 nucleosides) of four cPu and two 8-oxo-Pu lesions in DNA isolated from EUE-pBD650 (wt) and EUE-siXPA (XPA gene silenced by 80%), CS1AN-wtCSB and CS1AN (deficient), and CS3BE-wtCSA and CS3BE (deficient) cells in aerobic (21% O2) and hypoxic (1% O2) conditions *.
Table 1.
The levels (lesions/106 nucleosides) of four cPu and two 8-oxo-Pu lesions in DNA isolated from EUE-pBD650 (wt) and EUE-siXPA (XPA gene silenced by 80%), CS1AN-wtCSB and CS1AN (deficient), and CS3BE-wtCSA and CS3BE (deficient) cells in aerobic (21% O2) and hypoxic (1% O2) conditions *.
| Cell Type | O2 | 5′R-cdG/5′S-cdG | 5′R-cdA/5′S-cdA | 8-oxo-dG/8-oxo-dA | |
|---|---|---|---|---|---|
| XPA | EUE-pBD650 | 21% | 0.3/0.9 | 0.5/0.2 | 2.6/0.5 |
| 1% | 0.3/1.0 | 0.6/0.2 | 3.2/0.7 | ||
| EUE-siXPA | 21% | 0.3/0.9 | 0.5/0.2 | 3.0/0.5 | |
| 1% | 0.4/1.2 | 0.7/0.2 | 3.1/0.6 | ||
| CSB | CS1AN-wtCSB | 21% | 0.5/0.9 | 0.6/0.2 | 2.7/0.6 |
| 1% | 0.7/1.3 | 0.8/0.3 | 3.3/0.7 | ||
| CS1AN | 21% | 0.5/0.9 | 0.7/0.2 | 3.8/0.6 | |
| 1% | 0.9/1.5 | 1.0/0.4 | 4.8/1.1 | ||
| CSA | CS3BE–wtCSA | 21% | 0.4/0.9 | 0.6/0.2 | 2.0/0.6 |
| 1% | 0.4/0.9 | 0.6/0.3 | 2.7/0.7 | ||
| CS3BE | 21% | 0.4/0.9 | 0.7/0.2 | 2.7/0.8 | |
| 1% | 0.8/1.2 | 1.3/0.4 | 4.4/1.4 | ||
* Mean values of three sample measurements; for the standard deviation, see the original articles [136,144].
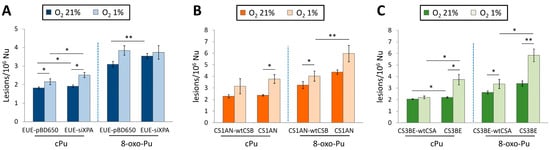
Figure 8.
Comparison between cPu and 8-oxo-Pu levels (lesions/106 nucleosides) in DNA extracted from various cells in aerobic (21% O2) and hypoxic (1% O2) conditions; (A) EUE-pBD650 (wt) and EUEsiXPA (XPA gene silenced by 80%) cells; (B) CS1AN-wtCSB and CS1AN (deficient) cells; (C) CS3BE-wtCSA and CS3BE (deficient) cells. * Denotes a statistically significant difference (p < 0.05) between the groups, ** denotes a statistically significant difference (p < 0.01) between the groups. Data from references [136,144].
Figure 8.
Comparison between cPu and 8-oxo-Pu levels (lesions/106 nucleosides) in DNA extracted from various cells in aerobic (21% O2) and hypoxic (1% O2) conditions; (A) EUE-pBD650 (wt) and EUEsiXPA (XPA gene silenced by 80%) cells; (B) CS1AN-wtCSB and CS1AN (deficient) cells; (C) CS3BE-wtCSA and CS3BE (deficient) cells. * Denotes a statistically significant difference (p < 0.05) between the groups, ** denotes a statistically significant difference (p < 0.01) between the groups. Data from references [136,144].
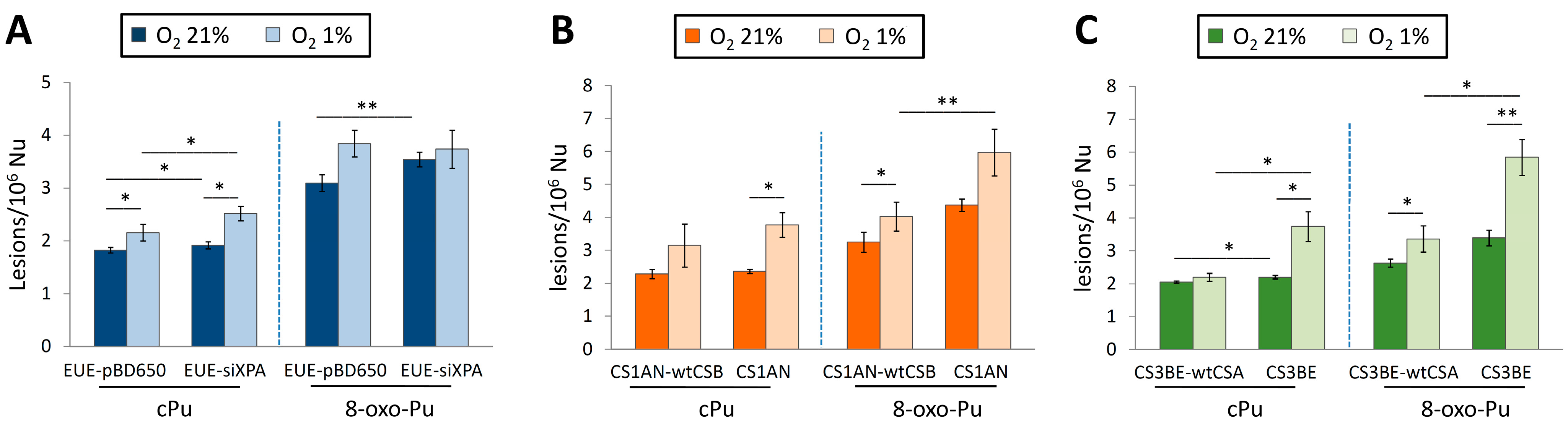
A similar trend of increased levels of cPu and 8-oxo-Pu lesions was observed in defective CSB (CS1AN) and CSA (CS3BE) compared to normal fibroblasts CS1AN-wtCSB and CS3BE-wtCSA (Figure 8B,C), as well as EUE-siXPA (XPA gene was silenced by 80%) compared to wt human embryonic epithelial cell lines EUE-pBD650 (Figure 8A) under aerobic conditions (O2 21%). It is noticeable that the relatively small differences in the levels of cPu vs. 8-oxo-Pu and the increased levels of cPu and 8-oxo-Pu lesions go from aerobic (21% O2) and hypoxic (1% O2) conditions (Figure 8). The same trends are observed for the six individual lesions (Table 1). Several studies support the role of oxidative DNA damage in neurodegenerative diseases and aging processes, and the aim was to elucidate the role of oxygen incubation conditions when cellular repair capacity is affected. It is worth mentioning that there is a link between hypoxia and neurodegeneration, but the mechanisms by which this occurs are not yet completely understood [9,145]. Hypoxia has been reported to increase ROS generation and induce neuroinflammation [145,146]. Moreover, hypoxia (1% O2) is known to cause a rapid increase in the expression of several genes and, subsequently, induce increased levels of intracellular ROS and DNA damage accumulation. The accumulation of cPu lesions observed under hypoxic conditions indicates that XP and CS proteins are involved in cPu repair through the NER pathway. cPu lesions are transcription-blocking, and the build-up of this type of oxidative DNA damage in actively transcribed genes has been linked with neuronal death [147]. It is well established that the neurological defects observed in CS patients differ significantly from those in XPA patients, characterized by extensive neurodegeneration [148]. Thus, the accumulation of cPu lesions plays a crucial role in the neurological symptoms of these syndromes. Moreover, the abundance of cPu lesions in CSB and CSA cells, like in XPA, endorses their role as candidate markers for pathologies with NER defects.
6.3. Mitochondria DNA (mtDNA) in Cockayne Syndrome (CS)
mtDNA is a circular molecule of approximately 16.5 kb in length. It exists in a compacted DNA–protein complex known as the mitochondrial nucleoid, which may provide some protection against ROS produced by the mitochondrial respiratory chain [149,150]. Similar to nuclear DNA (nDNA), mtDNA is highly susceptible to ROS, leading to accumulation of DNA modifications [151,152,153]. Increased oxidative damage in mtDNA has been linked to neurological degeneration, inflammasomes, tumor development, and cancer progression [154,155]. Among the various mtDNA repair pathways, the BER pathway has been well studied and shown to effectively remove certain types of oxidative DNA damage in the mitochondria, comparable to its efficiency in the nuclei [152]. Implications of other repair pathways remain unclear, although it is well established that NER does not occur in mitochondria [147]. Despite the constant exposure to ROS and the less protective pathways available in mitochondria, it remains unclear how the integrity of genetic information in this compartment is preserved [151,152,153]. However, mitochondria have evolved a unique mechanism which maintains mtDNA integrity through degradation of excessively damaged genomes followed by replication of intact/repaired mtDNA. This mechanism is not present in the nucleus and is enabled by multiple copies of mtDNA present in mitochondria. In mitochondria, a unique pathway occurs that is enabled by high redundancy of the mitochondrial DNA and allows for the disposal of damaged DNA molecules operating in this organelle [156].
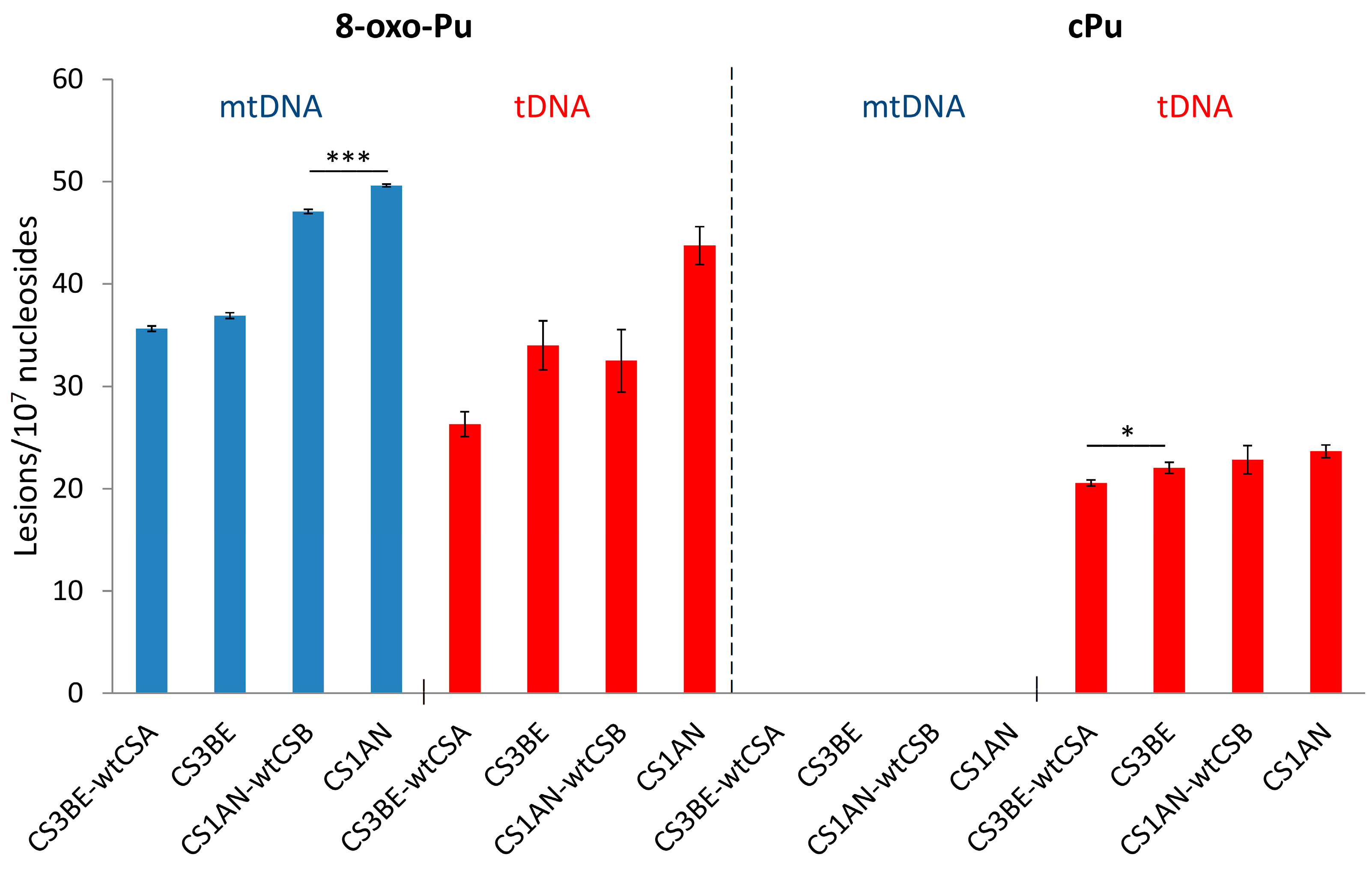
The cPu and 8–oxo–Pu lesions were identified in the mtDNA of wtCSA and wtCSB cells, along with their defective counterparts CS3BE and CS1AN, in comparison to the corresponding total (t) DNA (t = n + mt). Figure 9 (left side) shows that the 8–oxo–Pu levels are comparable in both mtDNA and tDNA in defective cells and the wt counterparts of CSA and CSB cells. Figure 9 (right side) shows the presence of cPu lesions in the tDNA and their absence in mtDNA (at least a 100 times lower level than in tDNA), indicating an absence of HO• radical reactivity within mtDNA [74]. The nonexistence of cPu lesions in the mtDNA of CS cells may involve specific mechanisms devoted to maintaining the mitochondrial genome integrity in view of the absence of NER in the mitochondria [155]. In the biomimetic model examining HO• radicals with purified tDNA and mtDNA, it was found that the levels of all six purine lesions in tDNA were approximately eight times higher than those in mtDNA. This disparity suggests that HO• radicals have different accessibility to mtDNA compared to nDNA, which can be attributed to variations in their helical topologies (cf. Section 3) [75]. In this respect, purine tDNA lesion levels in defective CSA cells overexpressing Parkin, a mechanism devoted to the removal of dysfunctional mitochondria [157,158], have an effect on 8-oxo-Pu rather than cPu [143], indicating that mitochondria do not play a role in the accumulation of cPu lesions.
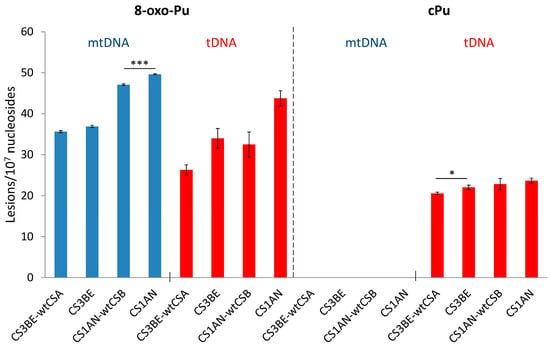
Figure 9.
Comparison between 8-oxo-Pu and cPu levels (lesions/107 Nu) in mtDNA (blue) and tDNA (red) samples extracted from CS1AN-wtCSB, CS1AN, CS3BE-wtCSA, and CS3BE cells. * Denotes a statistically significant difference (p < 0.05) between the groups, *** denotes a statistically significant difference (p < 0.001) between the groups. Taken from ref. [74].
6.4. 5′R and 5′S Diastereomers of cPu Lesions and the 8-oxo-dG/8-oxo-dA Ratio
The 8-oxo-dG and 8-oxo-dA measured across biological models of XP and CS cell lines indicate a higher accumulation of such lesions, going from aerobic to hypoxic conditions, with 8-oxo-dA increasing a little more than 8-oxo-dG (see Table 1). In all described cell lines, the 8-oxo-dG/8-oxo-dA ratio varies from 3.4 to 6.3, the majority being close to 5, which indicate a substantial amount of 8-oxo-dA. Although the 8-oxo-G is the most abundant and comprehensively studied DNA lesion resulting from oxidative damage, the analogous information of 8-oxo-dA on the cellular level is much less available. The accumulation of 8-oxo-dG, along with 8-oxo-dA, represents a significant threat to genomic stability [159].
The diastereomeric ratio (5′R/5′S) of cdG and cdA is a fascinating subject of mechanistic investigation on the occurrence of this free radical damage. The information reported in Table 2 for XPA, CSB, and CSA cells can be obtained from Table 1. In all cases, independently of the cell line type (wt or defective) and oxygen concentration, in cdG the 5′S configuration is always more abundant than the 5′R one, whereas in cdA, the 5′R is always more abundant than 5′S. For comparison, in the case of in vivo damage to the brain of tumor-bearing SCID mice (17 weeks) and colon tissue from inflammatory bowel diseases (IBD) patients, determined by the same analytical performance and uncertainty of the measurements, levels of cPu and 8-oxo-Pu similar to the in vitro models were reported, but with the diastereoisomeric ratios equal or in favor of 5′S (Table 2). At least two factors play a role in determining the diastereomeric ratios of cdG and cdA: (a) the local conformations at the reactive sites prior to C5′ radical cyclization, which increases the prevalence of one type of diastereoisomer [70], and (b) the NER efficiency for the repair of the diastereoisomers [104].

Table 2.
Diastereomers ratios of cPu lesions.
The cdA and cdG lesions are removed with comparable efficiency by NER; however the formation of the 5′R-diastereoisomers of cdA and cdG in the DNA backbone causes more significant distortion, becoming better substrates of NER than the corresponding S ones [104]. The measured values of the 5′R/5′S ratio for cPu lesions in cells (XPA, CSB, CSA, and XP neurons) compared with tissues reflect connections and distinct pathways between neurodegenerative diseases and pathologies with deficiencies in the NER process.
7. Conclusions
Most DNA damage in induced by oxidative metabolism, emphasizing the role of nucleic acid oxidation in the etiology and treatment of various diseases and aging processes. The accumulation of cPu as specific markers of HO• radical damage and comparison with 8-oxo-Pu arising by various ROS are discussed with the results from several neurological cell types. Considering that cPu and 8-oxoPu DNA lesions do not suffer from stability issues and artifacts of other oxidatively generated DNA lesions, this review describes how the simultaneous detection of cPu and 8-oxoPu can be determinant to depicting the scenario of DNA exposure, highlighting the conditions where nucleotide excision repair (NER) is defective. A significant connection has been described between defective enzymatic repair mechanisms for oxidative DNA damage and oxygen concentration in various XP- and CS-deficient cell lines conditions. These results support the hypothesis that defective repair of oxidative DNA damage, particularly cPu, is involved in the clinical outcomes of neurodegenerative disorders.
Funding
This research received no external funding.
Acknowledgments
I am especially grateful for the privilege of collaboration and friendship to the colleagues who have worked with me over the years on this subject and published papers together, as reported in the references.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BER | Base excision repair |
| CAT | Catalase |
| CS | Cockayne syndrome |
| cdA | 5′,8-cyclo-2′-deoxyadenosine |
| cdG | 5′,8-cyclo-2′-deoxyguanosine |
| cPu | 5′,8-cyclopurine |
| ct-DNA | Calf thymus DNA |
| ds-ODN | Double-stranded oligonucleotide |
| ELISA | Enzyme-linked immunosorbent assay |
| GG-NER | Global genome nucleotide excision repair |
| GPx | Glutathione peroxidase |
| hiPSC | Human-induced pluripotent stem cells |
| MPO | Myeloperoxidase |
| mtDNA | Mitochondrial DNA |
| NER | Nucleotide excision repair |
| NOS | Nitric-oxide synthases |
| OS | Oxidative stress |
| 8-oxo-Pu | 8-oxopurine |
| Prx | Peroxiredoxin |
| Pu | Purine |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TC-NER | Transcription-coupled nucleotide excision repair |
| wt | Wild type |
| XP | Xeroderma pigmentosum |
References
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.G.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Iyama, T.; Wilson, D.M., III. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Lloyd, R.S. (Eds.) DNA Damage, DNA Repair and Disease; Royal Society of Chemistry: Croydon, UK, 2021. [Google Scholar]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef]
- Peers, C.; Dallas, M.L.; Boycott, H.E.; Scragg, J.L.; Pearson, H.A.; Boyle, J.P. Hypoxia and neurodegeneration. Ann. N. Y. Acad. Sci. 2009, 1177, 169–177. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- D’Autreáux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C.C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.T.; Rhodes, C.J. Reactive Oxygen Species (ROS) in Metabolic Disease—Don’t Shoot the Metabolic Messenger. Int. J. Mol. Sci. 2025, 26, 2622. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological chemistry of reactive oxygen species. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 3, pp. 1260–1281. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Geacintov, N.E.; Shafirovich, V. Reactions of small reactive species with DNA. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 3, pp. 1284–1317. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. (Ed.) Oxidative Stress: Eustress and Distress; Academic Press: London, UK, 2020; pp. 1–844. [Google Scholar]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Azzi, A. Oxidative stress: What is it? can it be measured? where is it located? can it be good or bad? can it be prevented? can it be cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Long. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Barnes, D.E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Vašková, J.; Kocan, L.; Vaško, L.; Perjési, P. Glutathione-related enzymes and proteins: A review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Sies, H. Dynamics of intracellular and intercellular redox communication. Free Radic. Biol. Med. 2024, 225, 933–939. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Haliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Zhao, Z. Hydroxyl radical generation from the physiologically relevant Fenton-like reactions. Free Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Bounds, P.L.; Koppenol, W.H. Peroxynitrite: A tale of two radicals. Redox Biochem. Chem. 2024, 10, 100038. [Google Scholar] [CrossRef]
- Möller, M.N.; Denicola, A. Diffusion of peroxynitrite, its precursors, and derived reactive species, and the effect of cell membranes. Redox Biochem. Chem. 2024, 9, 100033. [Google Scholar] [CrossRef]
- Mezyk, S.P. Rate constant determination for the reaction of hydroxyl and glutathione thiyl radicals with glutathione in aqueous solution. J. Phys. Chem. 1996, 100, 8861–8866. [Google Scholar] [CrossRef]
- Illes, E.; Patra, S.G.; Marks, V.; Mizrahi, A.; Meyerstein, D. The FeII(citrate) Fenton reaction under physiological conditions. J. Inorg. Biochem. 2020, 206, 111018. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.G.; Mizrahi, A.; Meyerstein, D. The role of carbonate in catalytic oxidations. Acc. Chem. Res. 2020, 53, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Dingman, J.C.; Burrows, C.J. CO2 protects cells from iron-Fenton oxidative DNA damage in Escherichia coli and humans. Proc. Natl. Acad. Sci. USA 2024, 121, e2419175121. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Masi, A.; Capobianco, A.; Bobrowski, K.; Peluso, A.; Chatgilialoglu, C. Hydroxyl radical vs. one-electron oxidation reactivities in an alternating GC double-stranded oligonucleotide: A new type electron hole stabilization. Biomolecules 2023, 13, 1493. [Google Scholar] [CrossRef]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- Chan, W.; Chen, B.; Wang, L.; Taghizadeh, K.; Demott, M.S.; Dedon, P.C. Quantification of the 2-deoxyribonolactone and nucleoside 5′-aldehyde products of 2-deoxyribose oxidation in DNA and cells by isotope-dilution gas chromatography mass spectrometry: Differential effects of γ-radiation and Fe2+–EDTA. J. Am. Chem. Soc. 2010, 132, 6145–6153. [Google Scholar] [CrossRef]
- Aydogan, B.; Marshall, D.T.; Swarts, S.G.; Turner, J.E.; Boone, A.J.; Richards, N.G.; Bolch, W.E. Site-specific OH attack to the sugar moiety of DNA: A comparison of experimental data and computational simulation. Radiat. Res. 2002, 157, 38–44. [Google Scholar] [CrossRef]
- Chatgilialoglu, C. Reactivity of nucleic acid sugar radicals. In Radical and Radical Ion Reactivity in Nucleic Acid Chemistry; Greenberg, M.M., Ed.; Wiley: Hoboken, NJ, USA, 2009; Chapter 4; pp. 99–133. [Google Scholar]
- Jaruga, P.; Dizdaroglu, M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: Mechanisms of formation, measurement, repair and biological effects. DNA Repair 2008, 7, 1413–1425. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Geacintov, N.E.; Krokidis, M.G.; Liu, Y.; Masi, A.; Shafirovich, V.; Terzidis, M.A.; Tsegay, P.S. 5′,8-Cyclopurine lesions in DNA damage: Chemical, analytical, biological and diagnostic significance. Cells 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem. Res. Toxicol. 2008, 21, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Pitié, M.; Pratviel, G. Activation of DNA carbon−hydrogen bonds by metal complexes. Chem. Rev. 2010, 110, 1018–1059. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.M. Reactivity of nucleic acid radicals. Adv. Phys. Org. Chem. 2016, 50, 119–202. [Google Scholar]
- Paul, T.; Hill, M.J.; Young, I.E.; Ingold, K.U. Strand cleavage of supercoiled DNA by water-soluble peroxyl radicals. The overlooked importance of peroxyl radical charge. Biochemistry 2000, 39, 4129–4135. [Google Scholar] [CrossRef]
- Ingold, K.U. Reactions of water-soluble alkylperoxyl radicals and superoxide with DNA, lipoproteins and phospholipid vesicles: The role played by electrostatic forces. Curr. Med. Chem. 2003, 10, 2631–2642. [Google Scholar] [CrossRef]
- Cui, L.; Ye, W.; Prestwich, E.G.; Wishnok, J.S.; Taghizadeh, K.; Dedon, P.C.; Tannenbaum, S.R. Comparative analysis of four oxidized guanine lesions from reactions of DNA with peroxynitrite, single oxygen, and –radiation. Chem. Res. Toxicol. 2013, 26, 195–202. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.; Cui, Y.; Wang, Y. Chemical analysis of DNA damage. Anal. Chem. 2018, 90, 556–576. [Google Scholar] [CrossRef]
- Oda, Y.; Uesugi, S.; Ikehara, M.; Nishimura, S.; Kawase, Y.; Ishikawa, H.; Inoue, H.; Ohtsuka, E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991, 19, 1407–1412. [Google Scholar] [CrossRef]
- Bergeron, F.; Auvré, F.; Radicella, J.P.; Ravanat, J.-L. HO• radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc. Natl. Acad. Sci. USA 2010, 107, 5528–5533. [Google Scholar] [CrossRef]
- Ravanat, J.-L. Endogenous natural and radiation-induced DNA lesions: Differences and similarities and possible implications for human health and radiological protection. Radioprotection 2018, 53, 241–248. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Terzidis, M.A.; Szreder, T.; Bobrowski, K. New insights into the reaction paths of hydroxyl radicals with purine moieties in DNA and double-stranded oligodeoxynucleotides. Molecules 2019, 24, 3860. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Eriksson, L.A.; Krokidis, M.G.; Masi, A.; Wang, S.-D.; Zhang, R. Oxygen dependent purine lesions in double-stranded oligodeoxynucleotides: Kinetic and computational studies highlight the mechanism for 5′,8-cyplopurine formation. J. Am. Chem. Soc. 2020, 142, 5825–5833. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Peluso, A. Hole transfer and the resulting DNA damage. Biomolecules 2025, 15, 29. [Google Scholar] [CrossRef]
- Robert, G.; Wagner, J.R. Tandem lesions arising from 5-(uracilyl)methyl peroxyl radical addition to guanine: Product analysis and mechanistic studies. Chem. Res. Toxicol. 2020, 33, 565–575. [Google Scholar] [CrossRef]
- Robert, G.; Wagner, J.R.; Cadet, J. Oxidatively generated tandem DNA modifications by pyrimidinyl and 2-deoxyribosyl peroxyl radicals. Free Radic. Biol. Med. 2023, 196, 22–36. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Pascucci, B.; D’Errico, M. Assessing the Formation of Purine Lesions in Mitochondrial DNA of Cockayne Syndrome Cells. Biomolecules 2022, 12, 1630. [Google Scholar] [CrossRef]
- Terzidis, M.A.; Prisecaru, A.; Molphy, Z.; Barron, N.; Randazzo, A.; Dumont, E.; Krokidis, M.G.; Kellett, A.; Chatgilialoglu, C. Radical-induced purine lesion formation is dependent on DNA helical topology. Free Radic. Res. 2016, 50, S91–S101. [Google Scholar] [CrossRef]
- Gonzalez-Hunt, C.P.; Wadhwa, M.; Sanders, L.H. DNA damage by oxidative stress: Measurement strategies for two genomes. Curr. Opin. Toxicol. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Coskun, E.; Jaruga, P. Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free Radic. Res. 2015, 49, 525–548. [Google Scholar] [CrossRef]
- Morton, T.H. Isotopic labelling in mass spectrometry. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D., Eds.; Elsevier: Oxford, UK, 2017; pp. 501–511. [Google Scholar]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, biological consequences, and human health relevance of oxidative stress-induced DNA damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y. Mass spectrometry for the assessment of the occurrence and biological consequences of DNA adducts. Chem. Soc. Rev. 2015, 44, 7829–7854. [Google Scholar] [CrossRef] [PubMed]
- Terzidis, M.A.; Chatgilialoglu, C. An ameliorative protocol for the quantification of purine 5′,8-cyclo-2′-deoxynucleosides in oxidized DNA. Front. Chem. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C. Cyclopurine (cPu) lesions: What, how and why? Free Radic. Res. 2019, 53, 941–943. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Krokidis, M.G.; Terzidis, M.A. Protocol for the simultaneous quantification of oxidative purine lesions in DNA using LC-MS/MS analysis. STAR Protoc. 2024, 5, 103191. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Terzidis, M.A.; Efthimiadou, E.; Zervou, S.; Kordas, G.; Papadopoulos, K.; Hiskia, A.; Kletsas, D.; Chatgilialoglu, C. Purine 5′,8-cyclo-2′-deoxynucleoside lesions: Formation by radical stress and repair in human breast epithelial cancer cells. Free Radic. Res. 2017, 51, 470–482. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Prasinou, P.; Efthimiadou, E.K.; Boari, A.; Ferreri, C.; Chatgilialoglu, C. Effects of aging and disease conditions in brain of tumor-bearing mice: Evaluation of purine DNA damages and fatty acid pool changes. Biomolecules 2022, 12, 1075. [Google Scholar] [CrossRef]
- Masi, A.; Fortini, P.; Krokidis, M.G.; Romeo, E.F.; Bascietto, C.; De Angelis, P.; Guglielmi, V.; Chatgilialoglu, C. Increased levels of 5′,8-cyclopurine DNA lesions in inflammatory bowel diseases. Redox Biol. 2020, 34, 101562. [Google Scholar] [CrossRef]
- D’Errico, M.; Pascucci, B.; Parlanti, E.; Simonelly, V.; Dogliotti, E. DNA repair in the development of human diseases and therapy. In DNA Damage, DNA Repair and Disease; Dizdaroglu, M., Lloyd, R.S., Eds.; Royal Society of Chemistry: Croydon, UK, 2021; Chapter 15; pp. 348–378. [Google Scholar]
- Whitaker, A.M.; Schaich, M.A.; Smith, M.; Flynn, T.; Freudenthal, B.D. Base excision repair of oxidative DNA damage: From mechanism to disease. Front. Biosci. 2017, 22, 1493–1522. [Google Scholar]
- Kumar, N.; Raja, S.; Van Houten, B. The involvement of nucleotide excision repair proteins in the removal of oxidative DNA damage. Nucleic Acids Res. 2020, 48, 11227–11243. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Gaul, L.; Svejstrup, J.Q. Transcription-coupled repair and the transcriptional response to UV-Irradiation. DNA Repair 2021, 107, 103208. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Murphy, D.L.; Sweasy, J.B. Base excision repair and cancer. Cancer Lett. 2012, 327, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Shafirovich, V.; Geacintov, N.E. Removal of oxidatively generated DNA damage by overlapping repair pathways. Free Radic. Biol. Med. 2017, 107, 53–61. [Google Scholar] [CrossRef]
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell. Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef]
- Guo, J.; Hanawalt, P.C.; Spivak, G. Comet-FISH with strand-specific probes reveals transcription-coupled repair of 8-oxoGuanine in human cells. Nucleic Acids Res. 2013, 41, 7700–7712. [Google Scholar] [CrossRef]
- Sarmini, L.; Meabed, M.; Emmanouil, E.; Atsaves, G.; Robeska, E.; Karwowski, B.T.; Campalans, A.; Gimisis, T.; Khobta, A. Requirement of transcription-coupled nucleotide excision repair for the removal of a specific type of oxidatively induced DNA damage. Nucleic Acids Res. 2023, 51, 4982–4994. [Google Scholar] [CrossRef]
- Brooks, P.J.; Wise, D.S.; Berry, D.A.; Kosmoski, J.V.; Smerdon, M.J.; Somers, R.L.; Mackie, H.; Spoonde, A.Y.; Ackerman, E.J.; Coleman, K.; et al. The oxidative DNA lesion 8,5′-(S)-cyclo-2′-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J. Biol. Chem. 2000, 275, 22355–22362. [Google Scholar] [CrossRef]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 50,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef]
- Das, R.S.; Samaraweera, M.; Morton, M.; Gascon, J.A.; Basu, A.K. Stability of N-glycosidic bond of (5′S)-8,5′-cyclo-2′-deoxyguanosine. Chem. Res. Toxicol. 2012, 25, 2451–2461. [Google Scholar] [CrossRef]
- Pande, P.; Das, R.S.; Shepard, C.; Kow, Y.W.; Basu, A.K. Repair efficiency of (5′S)-8,5-cyclo-2′-deoxyguanosine and (5′S)-8,5′-cyclo-2′-deoxyadenosine depends on the complementary base. DNA Repair 2012, 11, 926–931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basu, A.K.; Colis, L.C.; Bacurio, J.H.T. Mutagenesis and Repair of γ-Radiation- and Radical-Induced Tandem DNA Lesions. DNA 2024, 4, 154–169. [Google Scholar] [CrossRef]
- Huang, H.; Das, R.S.; Basu, A.K.; Stone, M.P. Structure of (5′S)-8,5′-cyclo-2′-deoxyguanosine in DNA. J. Am. Chem. Soc. 2011, 133, 20357–20368. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Dai, X.; Yuan, B.; Wang, J.; Wang, J.; Brooks, P.J.; Niedernhofer, L.J.; Wang, Y. A quantitative assay for assessing the effects of DNA lesions on transcription. Nat. Chem. Biol. 2012, 8, 817–822. [Google Scholar] [CrossRef]
- Kropachev, K.; Ding, S.; Terzidis, M.A.; Masi, A.; Liu, Z.; Cai, Y.; Kolbanovskiy, M.; Chatgilialoglu, C.; Broyde, S.; Geacintov, N.E.; et al. Structural basis for the recognition of diastereomeric 5′,8-cyclo-2′-deoxypurine lesions by the human nucleotide excision repair system. Nucleic Acids Res. 2014, 42, 5020–5032. [Google Scholar] [CrossRef]
- Shafirovich, V.; Kolbanovskiy, M.; Kropachev, K.; Liu, Z.; Cai, Y.; Terzidis, M.A.; Masi, A.; Chatgilialoglu, C.; Amin, S.; Dadali, A.; et al. Nucleotide Excision Repair and Impact of Site-Specific 5′,8-Cyclopurine and Bulky DNA Lesions on the Physical Properties of Nucleosomes. Biochemistry 2019, 58, 561–574. [Google Scholar] [CrossRef]
- Marietta, C.; Gulam, H.; Brooks, P.J. A single 8,5′-cyclo-2′-deoxyadenosine lesion in a TATA box prevents binding of the TATA binding protein and strongly reduces transcription in vivo. DNA Repair 2002, 1, 967–975. [Google Scholar] [CrossRef]
- Abraham, J.; Brooks, P.J. Divergent effects of oxidatively induced modification to the C8 of 2′-deoxyadenosine on transcription factor binding: 8,5′(S)-cyclo-2′- deoxyadenosine inhibits the binding of multiple sequence specific transcription factors, while 8-oxo-2′-deoxyadenosine increases binding of CREB and NF-kappa B to DNA. Environ. Mol. Mutagen. 2011, 52, 287–295. [Google Scholar]
- You, C.; Swanson, A.L.; Dai, X.; Yuan, B.; Wang, J.; Wang, Y. Translesion synthesis of 8,5′-cyclopurine-2′-deoxynucleosides by DNA polymerases η, ι, and ζ. J. Biol. Chem. 2013, 288, 28548–28556. [Google Scholar] [CrossRef]
- Xu, M.; Lai, Y.; Jiang, Z.; Terzidis, M.A.; Masi, A.; Chatgilialoglu, C.; Liu, Y. A 5′, 8-cyclo-2′-deoxypurine lesion induces trinucleotide repeat deletion via a unique lesion bypass by DNA polymerase β. Nucleic Acids Res. 2014, 42, 13749–13763. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, M.; Lai, Y.; Laverde, E.E.; Terzidis, M.A.; Masi, A.; Chatgilialoglu, C.; Liu, Y. Bypass of a 5′,8-cyclopurine-2′-deoxynucleoside by DNA polymerase b during DNA replication and base excision repair leads to nucleotide misinsertions and DNA strand breaks. DNA Repair 2015, 33, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Walmacq, C.; Wang, L.; Chong, J.; Scibelli, K.; Lubkowska, L.; Gnatt, A.; Brooks, P.J.; Wang, D.; Kashlev, M. Mechanism of RNA polymerase II bypass of oxidative cyclopurine DNA lesions. Proc. Natl. Acad. Sci. USA 2015, 112, E410–E419. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.J.; Gao, Y.; Gregory, M.T.; Wang, P.; Wang, Y.; Yang, W. Bypassing a 8,5′-cyclo-2′-deoxyadenosine lesion by human DNA polymerase η at atomic resolution. Proc. Natl. Acad. Sci. USA 2018, 115, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Robins, P.; Masutani, C.; Hanaoka, F.; Gasparutto, D.; Cadet, J.; Wood, R.D.; Lindahl, T. Oxygen free radical damage to DNA. Translesion synthesis by human DNA polymerase. J. Biol. Chem. 2001, 276, 49283–49288. [Google Scholar] [CrossRef]
- Swanson, A.L.; Wang, J.; Wang, Y. Accurate and efficient bypass of 8,5′-cyclopurine-2′-deoxynucleosides by human. Chem. Res. Toxicol. 2012, 25, 1682–1691. [Google Scholar] [CrossRef][Green Version]
- Pednekar, V.; Weerasooriya, S.; Jasti, V.P.; Basu, A.K. Mutagenicity and genotoxicity of (5′S)-8,5′-cyclo-2′-deoxyadenosine. Chem. Res. Toxicol. 2014, 27, 200–210. [Google Scholar] [CrossRef]
- Tsegay, P.S.; Hernandez, D.; Brache, C.; Chatgilialoglu, C.; Krokidis, M.G.; Yang, W.; Chapagain, P.; Liu, Y. Incorporation of 5′,8-cyclo-2′-deoxyadenosines by DNA repair polymerases via base excision repair. DNA Repair 2022, 109, 103258. [Google Scholar] [CrossRef]
- Brooks, P.J. The 8,5′-cyclopurine-2′-deoxynucleosides: Candidate neurodegenerative DNA lesions in xeroderma pigmentosum, and unique probes of transcription and nucleotide excision repair. DNA Repair 2008, 7, 1168–1179. [Google Scholar] [CrossRef]
- Wang, J.; Clauson, C.L.; Robbins, P.D.; Niedernhofer, L.J.; Wang, Y. The oxidative DNA lesions 8,5′-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 2012, 11, 714–716. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, B.; Guerrero, C.; Bahde, R.; Gupta, S.; Wang, Y. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. Anal. Chem. 2011, 83, 2201–2209. [Google Scholar] [CrossRef]
- Friedberg, E.C. How nucleotide excision repair protects against cancer. Nat. Rev. Cancer 2001, 1, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.H.; Patronas, N.J.; Schiffmann, R.; Brooks, B.P.; Tamura, D.; Di Giovanna, J.J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience 2007, 145, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Rapin, I.; Lindenbaum, Y.; Dickson, D.W.; Kraemer, K.H.; Robbins, J.H. Cockayne syndrome and xeroderma pigmentosum. Neurology 2000, 55, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Anttinen, A.; Koulu, L.; Nikoskelainen, E.; Portin, R.; Kurki, T.; Erkinjuntti, M.; Jaspers, N.G.; Raams, A.; Green, M.H.; Lehmann, A.R.; et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain 2008, 131, 1979–1989. [Google Scholar] [CrossRef]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Spivak, G. Nucleotide excision repair in humans. DNA Repair 2015, 36, 13–18. [Google Scholar] [CrossRef]
- Reardon, J.T.; Bessho, T.; Kung, H.C.; Bolton, P.H.; Sancar, A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: Possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. USA 1997, 94, 9463–9468. [Google Scholar] [CrossRef]
- Marietta, C.; Brooks, P.J. Transcriptional bypass of bulky DNA lesions causes new mutant RNA transcripts in human cells. EMBO Rep. 2007, 8, 388–393. [Google Scholar] [CrossRef]
- Brooks, P.J. The case for 8,5′-cyclopurine-2′-deoxynucleosides as endogenous DNA lesions that cause neurodegeneration in xeroderma pigmentosum. Neuroscience 2007, 145, 1407–1417. [Google Scholar] [CrossRef]
- D’Errico, M.; Parlanti, E.; Teson, M.; Bernardes de Jesus, B.M.; Degan, P.; Calcagnile, A.; Jaruga, P.; Bjørås, M.; Crescenzi, M.; Pedrini, A.M.; et al. New functions of XPC in the protection of human skin cells from oxidative damage. Embo J. 2006, 25, 4305–4315. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Long. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Brooks, P.J.; Nishiwaki, T.; Nishimura, K.; Kobayashi, N.; Sugiura, S.; Mori, T. Quantitative and in situ detection of oxidatively generated DNA damage 8,5′-cyclo-2′-deoxyadenosine using an immunoassay with a novel monoclonal antibody. Photochem. Photobiol. 2014, 90, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Nakane, H.; Iwamoto, T.; Krokidis, M.G.; Chatgilialoglu, C.; Tanaka, K.; Kaidoh, T.; Hasegawa, M.; Sugiura, S. High levels of oxidatively generated DNA damage 8,5′-cyclo-2′-deoxyadenosine accumulate in the brain tissues of xeroderma pigmentosum group A gene-knockout mice. DNA Repair 2019, 80, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Badja, C.; Momen, S.; Koh, G.C.C.; Boushaki, S.; Roumeliotis, T.I.; Kozik, Z.; Jones, I.; Bousgouni, V.; Dias, J.M.L.; Krokidis, M.G.; et al. Insights from multi-omic modeling of neurodegeneration in xeroderma pigmentosum using an induced pluripotent stem cell system. Cell Rep. 2024, 43, 114243. [Google Scholar] [CrossRef]
- Rouhani, F.J.; Zou, X.; Danecek, P.; Badja, C.; Amarante, T.D.; Koh, G.; Wu, Q.; Memari, Y.; Durbin, R.; Martincorena, I.; et al. Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat. Genet. 2022, 54, 1406–1416. [Google Scholar] [CrossRef]
- Kuijk, E.; Jager, M.; van der Roest, B.; Locati, M.D.; Van Hoeck, A.; Korzelius, J.; Janssen, R.; Besselink, N.; Boymans, S.; van Boxtel, R.; et al. The mutational impact of culturing human pluripotent and adult stem cells. Nat. Commun. 2020, 11, 2493. [Google Scholar] [CrossRef]
- Krokidis, M.G.; Parlanti, E.; D’Errico, M.; Pascucci, B.; Pino, A.; Alimonti, A.; Pietraforte, D.; Masi, A.; Ferreri, C.; Chatgilialoglu, C. Purine DNA Lesions at Different Oxygen Concentration in DNA Repair-Impaired Human Cells (EUE-siXPA). Cells 2019, 8, 1377. [Google Scholar] [CrossRef]
- Abeti, R.; Zeitlberger, A.; Peelo, C.; Fassihi, H.; Sarkany, R.P.E.; Lehmann, A.R.; Giunti, P. Xeroderma pigmentosum: Overview of pharmacology and novel therapeutic strategies for neurological symptoms. Br. J. Pharmacol. 2019, 176, 4293–4301. [Google Scholar] [CrossRef]
- Tuo, J.; Müftüoglu, M.; Chen, C.; Jaruga, P.; Selzer, R.R.; Brosh, R.M., Jr.; Rodriguez, H.; Dizdaroglu, M.; Bohr, V.A. The Cockayne Syndrome group B gene product is involved in general genome base excision repair of 8-hydroxyguanine in DNA. J. Biol. Chem. 2001, 276, 45772–45779. [Google Scholar] [CrossRef]
- D’Errico, M.; Parlanti, E.; Teson, M.; Degan, P.; Lemma, T.; Calcagnile, A.; Iavarone, I.; Jaruga, P.; Ropolo, M.; Pedrini, A.M.; et al. The role of CSA in the response to oxidative DNA damage in human cells. Oncogene 2007, 26, 4336–4343. [Google Scholar] [CrossRef]
- Kirkali, G.; de Souza-Pinto, N.C.; Jaruga, P.; Bohr, V.A.; Dizdaroglu, M. Accumulation of (5′S)-8,5′-cyclo-2′-deoxyadenosine in organs of Cockayne syndrome complementation group B gene knockout mice. DNA Repair 2009, 8, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Muftuoglu, M.; Beck, G.; Imam, S.Z.; Bohr, V.A.; Wilson, D.M., 3rd. Cockayne syndrome B protein stimulates apurinic endonuclease 1 activity and protects against agents that introduce base excision repair intermediates. Nucleic Acids Res. 2007, 35, 4103–4113. [Google Scholar] [CrossRef] [PubMed]
- Khobta, A.; Kitsera, N.; Speckmann, B.; Epe, B. 8-oxoguanine DNA glycosylase (Ogg1) causes a transcriptional inactivation of damaged DNA in the absence of functional Cockayne syndrome B (Csb) protein. DNA Repair 2009, 8, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, B.; Fragale, A.; Marabitti, V.; Leuzzi, G.; Calcagnile, A.S.; Parlanti, E.; Franchitto, A.; Dogliotti, E.; D’Errico, M. CSA and CSB play a role in the response to DNA breaks. Oncotarget 2018, 9, 11581–11591. [Google Scholar] [CrossRef]
- Krokidis, M.G.; D’Errico, M.; Pascucci, B.; Parlanti, E.; Masi, A.; Ferreri, C.; Chatgilialoglu, C. Oxygen-Dependent Accumulation of Purine DNA Lesions in Cockayne Syndrome Cells. Cells 2020, 9, 1671. [Google Scholar] [CrossRef]
- Dakal, T.C.; Choudhary, K.; Tiwari, I.; Yadav, V.; Maurya, P.K.; Sharma, N.K. Unraveling the triad: Hypoxia, oxidative stress and inflammation in neurodegenerative disorders. Neuroscience 2024, 552, 126–141. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Brooks, P.J. The cyclopurine deoxynucleosides: DNA repair, biological effects, mechanistic insights, and unanswered questions. Free Radic. Biol. Med. 2017, 107, 90–100. [Google Scholar] [CrossRef]
- Brooks, P.J. Blinded by the UV light: How the focus on transcription-coupled NER has distracted from understanding the mechanisms of Cockayne syndrome neurologic disease. DNA Repair 2013, 12, 656–671. [Google Scholar] [CrossRef]
- Farge, G.; Falkenberg, M. Organization of DNA in mammalian mitochondria. Int. J. Mol. Sci. 2019, 20, 2770. [Google Scholar] [CrossRef]
- Mandavilli, B.S.; Santos, J.H.; Van Houten, B. Mitochondrial DNA repair and ageing. Mutat. Res. 2002, 509, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, Y.; Zhang, Y. Mitochondrial reactive oxygen species cause major oxidative mitochondrial DNA damages and repair pathways. J. Biosci. 2020, 45, 84. [Google Scholar] [CrossRef]
- Muftuoglu, M.; Mori, M.P.; de Souza–Pinto, N.C. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion 2014, 17, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, J.H.K.; Stewart, J.B. Mitochondrial DNA: Radically free of free–radical driven mutations. Biochim. Biophys. Acta 2015, 1847, 1354–1361. [Google Scholar] [CrossRef]
- Kopinski, P.K.; Singh, L.N.; Zhang, S.; Lott, M.T.; Wallace, D.C. Mitochondrial DNA variation and cancer. Nat. Rev. 2021, 21, 431–445. [Google Scholar] [CrossRef]
- D’Errico, M.; Parlanti, E.; Pascucci, B.; Filomeni, G.; Mastroberardino, P.G.; Dogliotti, E. The interplay between mitochondrial functionality and genome integrity in the prevention of human neurologic diseases. Arch. Biochem. Biophys. 2021, 710, 108977. [Google Scholar] [CrossRef]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The maintenance of mitochondrial DNA integrity—Critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef]
- Pascucci, B.; D’Errico, M.; Romagnoli, A.; De Nuccio, C.; Savino, M.; Pietraforte, D.; Lanzafame, M.; Calcagnile, A.S.; Fortini, P.; Baccarini, S.; et al. Overexpression of parkin rescues the defective mitochondrial phenotype and the increased apoptosis of Cockayne syndrome A cells. Oncotarget 2016, 8, 102852–102867. [Google Scholar] [CrossRef][Green Version]
- Peeva, V.; Blei, D.; Trombly, G.; Corsi, S.; Szukszto, M.J.; Rebelo-Guiomar, P.; Gammage, P.A.; Kudin, A.P.; Becker, C.; Altmüller, J.; et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018, 9, 1727. [Google Scholar] [CrossRef]
- Kruchinin, A.A.; Kamzeeva, P.N.; Zharkov, D.O.; Aralov, A.V.; Makarova, A.V. 8-oxoadenine: A «New» Player of the Oxidative Stress in Mammals? Int. J. Mol. Sci. 2024, 25, 1342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).