Abstract
Male-factor infertility accounts for nearly half of all infertility cases, and mounting evidence points to oxidative stress as a pivotal driver of sperm dysfunction, genetic instability, and epigenetic dysregulation. In particular, the oxidative DNA lesion 8-hydroxy-2′-deoxyguanosine (8-OHdG) has emerged as a central mediator at the interface of DNA damage and epigenetic regulation. We discuss how this lesion can disrupt key epigenetic mechanisms such as DNA methylation, histone modifications, and small non-coding RNAs, thereby influencing fertilization outcomes, embryo development, and offspring health. We propose that the interplay between oxidative DNA damage and epigenetic reprogramming is further exacerbated by aging in both the paternal and maternal germlines, creating a “perfect storm” that increases the risk of heritable (epi)mutations. The consequences of unresolved oxidative lesions can thus persist beyond fertilization, contributing to transgenerational health risks. Finally, we explore the promise and potential pitfalls of antioxidant therapy as a strategy to mitigate sperm oxidative damage. While antioxidant supplementation may hold significant therapeutic value for men with subfertility experiencing elevated oxidative stress, a careful, personalized approach is essential to avoid reductive stress and unintended epigenetic disruptions. Recognizing the dual role of oxidative stress in shaping both the genome and the epigenome underscores the need for integrating redox biology into reproductive medicine, with the aim of improving fertility treatments and safeguarding the health of future generations.
1. Introduction
Global fertility rates are projected to continue declining [1], with male factors recognized as a major contributor, accounting for nearly half of all infertility cases worldwide [2]. The steady decline in key parameters of male reproductive health—including sperm count [3], motility [4], and DNA integrity—raises serious concerns for fertility outcomes and transgenerational well-being [5]. Although numerous factors have been implicated in this decline, the precise molecular mechanisms remain only partially understood [6]. Notably, oxidative stress [7] has emerged as both a driver of sperm dysfunction and a critical determinant of genetic and epigenetic stability [8].
1.1. Redox-Driven Modifications in Spermatozoa
Spermatozoa exhibit a unique susceptibility to oxidative damage because of their structural and functional characteristics. Oxidative stress, defined by the imbalance between reactive oxygen species (ROS) and the body’s antioxidant defenses, generates oxidative DNA lesions such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) [9]. Although more than 20 oxidative DNA base lesions have been identified [10], guanine ring is the most frequently oxidized base due to its low redox potential [11]. Hydroxyl radicals preferentially attack the C8 position of guanine’s imidazole ring, leading to the formation of 8-OHdG. Widely recognized as one of the most prevalent and mutagenic modifications, 8-OHdG has been implicated in de novo mutations [12] originating predominantly from the male germline [13]. Consequently, oxidative stress has been linked to male subfertility and infertility, increased sperm DNA damage, and an elevated miscarriage risk in women [14,15,16,17,18,19].
Beyond its mutagenic capability, 8-OHdG also plays a critical role in epigenetic regulation, illustrating its dual impact on both genomic and epigenomic stability [20]. In the male germline, oxidative stress can disrupt DNA methylation, histone modifications, and the integrity of small non-coding RNAs [21], as well as centromere [22] and telomere [23] integrity, ultimately affecting fertilization, embryo development, and offspring health [24,25]. These disruptions not only threaten genetic integrity but may also propagate epigenetic alterations [26] to subsequent generations.
Although reproductive biologists have devoted considerable effort to identifying genetic and epigenetic biomarkers predictive of male fertility and offspring health, this review focuses on the fundamental molecular processes linking oxidative stress, DNA damage, and epigenetic regulation to reproductive outcomes.
We examine the molecular interplay between oxidative stress, DNA damage, and epigenetic regulation within the context of male fertility. Central to this discussion is the pivotal role of 8-OHdG and its incomplete repair via the base excision repair (BER) pathway in driving genomic and epigenomic instability [27]. By integrating recent advances in redox biology, reproductive epigenetics, and DNA biochemistry, we propose a unifying framework to better understand male reproductive dysfunction and its potential impact on future generations.
1.2. A Roadmap to This Review
This narrative review begins with a concise overview of redox biology and epigenetic mechanisms, highlighting their individual roles in male fertility and early embryonic development (Section 2 and Section 3). It then explores how oxidative DNA lesions and their repair pathways mechanistically alter epigenetic regulation (Section 4). These discussions culminate in the proposal that oxidative stress significantly drives epigenetic changes in spermatozoa (Section 5). Subsequently, the review examines paternal epigenetic influences on embryo development and offspring health (Section 6) and concludes by discussing diagnostic and therapeutic approaches aimed at mitigating oxidative stress-related reproductive risks (Section 7).
2. Oxidative Stress and Male Fertility
An increasingly recognized concept known as ‘redox homeodynamics’ underscores the pivotal role of redox balance in shaping cellular responses to the broader exposome [28]. Oxidative stress emerges when reactive metabolic species (RMS) overwhelm endogenous antioxidant defenses (enzymes and small-molecule micronutrients), leading to cellular dysfunction [29] and molecular damage [30], affecting lipids, proteins, DNA, and RNA, and ultimately, if unchecked, causing cell death [31].
As germ cells mature, their DNA repair capacity declines, worsening further with paternal age [32]. Elevated oxidative stress impairs sperm motility and sperm–oocyte interactions, reducing fertilization rates [33]. Notably, significant sperm DNA damage occurs at oxidative stress levels lower than those required to disrupt fertilization itself [34]. These persistent genetic and epigenetic lesions may compromise offspring fertility and health. Thus, evaluating male reproductive competence should extend beyond fertilization alone, encompassing the potential to support successful pregnancy and healthy progeny.
The causes of oxidative stress in the male reproductive tract, particularly in sperm, are multifactorial. Endogenous contributors include ROS production by seminal leukocytes during immunological responses, sperm mitochondrial dysfunction, and enzymatic activity (e.g., NADPH oxidases, L-amino acid oxidase) [35]. Exogenous influences relate to modern lifestyle factors such as exposure to pollutants, heat, electromagnetic/radiation sources, and habits like smoking and alcohol consumption [36,37,38]. These stressors are further exacerbated by nutrient-deficient diets, a consequence of modern agricultural and food-processing practices [39].
2.1. A Unique Susceptibility
In spermatozoa, ROS have a dual role [40]: low-level production is essential for processes like sperm maturation, capacitation, tyrosine phosphorylation, and cAMP signaling, yet sperm are exceptionally vulnerable to oxidative stress due to their high polyunsaturated fatty acid membrane content (roughly 50% docosahexaenoic acid) [41,42], limited cytoplasmic antioxidant defenses [43], and truncated DNA repair mechanisms [27]. Consequently, these cells depend heavily on antioxidant protection within the seminal plasma and the reproductive tract [44]; any compromise in these defenses can lead to significant oxidative damage [45].
2.2. Far-Reaching Implications
When oxidative DNA lesions persist up to the point of conception and are not efficiently repaired by the oocyte—particularly if the oxidative burden is extensive and/or maternal repair capacity is also diminished—these 8-OHdG lesions may become fixed as de novo mutations/epimutations in the embryo [46]. Paternal age further amplifies this effect, as older fathers exhibit increased levels of oxidative stress and DNA damage, which correlate with elevated risks of neurodevelopmental disorders, psychiatric conditions, and other health vulnerabilities in offspring [47,48]. Recent findings also suggest that specific paternal genomic regions, enriched for genes of interest, are disproportionately susceptible to oxidative insults [49].
Clearly, oxidative damage to sperm extends beyond impairing fertilization, with significant implications for embryonic development and long-term offspring health. In the sections that follow, we will examine how DNA/RNA oxidative lesions disrupt critical epigenetic processes ranging from histone modifications and DNA methylation to the integrity of small non-coding RNAs, and how these disruptions can have profound, enduring consequences for future generations.
3. Epigenetics, Male Fertility, and Embryonic Development
Historically viewed as mere carriers of genetic information, spermatozoa are now recognized as key influencers of offspring health and disease risk through non-genetic mechanisms of inheritance [50,51]. Environmental factors and paternal age, among others, can impart heritable changes on sperm that extend well beyond DNA sequence alterations. Although epigenetic programs influence spermatogenesis and sperm maturation, multiple checkpoints tightly regulate progression from primordial germ cells to mature spermatozoa [52]. Consequently, this review does not focus on testicular epigenetic mechanisms but rather emphasizes post-testicular epigenetic modifications.
These epigenetic changes are mediated by classical processes—most notably histone modifications, DNA methylation, and small non-coding RNAs (sncRNAs)—and understanding how these processes regulate gene expression in ways that can persist through fertilization, escape reprogramming, and ultimately affect developmental and phenotypic outcomes in offspring is a rapidly advancing area of research [53].
3.1. Chromatin Modifications
During spermatogenesis, sperm DNA undergoes extensive reorganization, replacing most histones with protamines to compact the genome, protect it from oxidative insult, and maintain transcriptional silence [54,55,56]. A small proportion of histones remains, typically at genes essential for early embryonic development. These retained histones bear post-translational modifications, such as H3K4 methylation (active) and H3K27 methylation (repressive), that shape paternal epigenetic inheritance [57,58,59]. Protamines also undergo modifications (e.g., acetylation, phosphorylation) which influence sperm functionality and chromatin remodeling after fertilization [60]. Faulty protamine incorporation or abnormal P1/P2 ratios may impair pregnancy rates [61,62]; however, it remains unclear whether environmental influences on the paternal germline can alter protamine modifications sufficiently to impact embryonic development.
3.2. DNA Methylation
In sperm, DNA methylation involves the addition of methyl groups to cytosine bases, primarily at CpG dinucleotides, a process orchestrated by DNA methyltransferases (DNMTs) during spermatogenesis [63]. Ten-eleven translocation (TET) enzymes then refine these patterns by removing or modifying methylation marks [64]. Perturbations in sperm DNA methylation have been linked to infertility and reduced reproductive potential, underscoring the sensitivity of this epigenetic mechanism to environmental and physiological stressors [65,66,67,68]. Both histone/protamine modifications and DNA methylation patterns undergo two major waves of reprogramming, one during gametogenesis and another shortly after fertilization, to help safeguard the embryo from parental epigenetic disturbances [69,70]. Certain imprinted genomic regions, however, are exempt from these reprogramming events, limiting but not eliminating their transgenerational influence [71].
3.3. Small Non-Coding RNAs
Small non-coding RNAs, including microRNAs, piRNAs, and tRNA-derived fragments, represent another crucial layer of epigenetic regulation in sperm [72]. As regulators of chromatin architecture and gene expression, sncRNAs support fertilization, guide embryonic development, and establish broader developmental trajectories [73]. Unlike histone modifications and DNA methylation, sncRNAs are not extensively reprogrammed during gametogenesis or early embryogenesis, allowing them to directly convey environmental cues from the father to the embryo [74].
Growing evidence points to sncRNAs as potent mediators of transgenerational inheritance, linking paternal exposures, ranging from stress and diet to toxicants, with offspring phenotypes [75,76,77,78]. Through these molecular messengers, paternal experiences can influence the zygote’s genetic regulatory landscape and ultimately shape the health and development of subsequent generations.
The mechanisms underlying epigenetic alterations remain a critical area of investigation. Many factors implicated in epigenetic modifications—such as aging, environmental pollutants, and lifestyle choices—also drive oxidative stress, hinting at a shared redox-based mechanism. Over the past decade, this interplay between oxidative DNA damage, the cellular response, and epigenetic regulation has attracted significant attention. In the following section, we explore these specific processes, shedding light on the central role of redox stress in epigenetic regulation.
4. Crosstalk Between Oxidative Damage, Response, and Epigenetic Alterations
Beyond reproductive biology, there is broad scientific consensus that oxidative DNA lesions, particularly 8-OHdG, do more than simply mark DNA damage; they actively shape epigenetic regulation. This occurs via the direct actions of 8-OHdG, its processing by DNA repair enzymes, and their overlapping interactions with established epigenetic marks and processes [79].
4.1. 8-OHdG and Structural DNA Biochemistry
The BER pathway is critical for repairing oxidative DNA lesions. In somatic cells, 8-oxoguanine DNA glycosylase 1 (OGG1) initiates this pathway by excising 8-OHdG and leaving behind apurinic (AP) sites, which are then further processed by apurinic/apyrimidinic endonuclease 1 (APE1) and X-ray repair cross-complementing protein 1 (XRCC1) [80]. In addition to initiating repair, OGG1 and related BER enzymes help recruit transcriptional machinery, thereby influencing gene regulation [81,82].
When 8-OHdG in guanine-rich promoter regions is excised by OGG1, the resulting abasic site destabilizes the duplex, allowing the PQS to fold into a G-quadruplex (G4) and act as an epigenetic signal that activates transcription. Furthermore, when OGG1 engages 8-OHdG, it inserts catalytic residues, flips the lesion and its opposing cytosine out of the helix, and bends the DNA by ~70°. This structural remodeling creates a recognition platform that promotes the assembly of transcription-initiation complexes, thereby linking DNA repair to gene activation [82,83,84]. Genome-wide analyses also show that AP sites, OGG1, and APE1 are enriched within G4 sequences. Depleting APE1 abolishes cellular G4 formation, indicating that APE1 binding, and its acetylation-dependent residence time, promotes and stabilizes G4 folding. The resulting APE1–G4 complex then facilitates transcription-factor loading at promoters, revealing that oxidized-base repair not only safeguards genome integrity but also shapes higher-order DNA structures to modulate gene expression [85,86].
Beyond transcription, persistent 8-OHdG lesions and AP sites also signal broader epigenetic changes [87]. OGG1 and APE1 collaborate with chromatin-remodeling complexes during repair [88,89,90,91], modifying genomic accessibility and facilitating other epigenetic processes, including methylation and demethylation. See Figure 1A,B for a summary of these processes. Differences in how the BER pathway functions in male versus female germ lines—as well as early embryos— reveal that while oxidative stress-driven epigenetic changes in sperm do not generally impair sperm functionality, they can dramatically alter gene expression patterns in the developing embryo and potentially compromise fertilization outcomes.
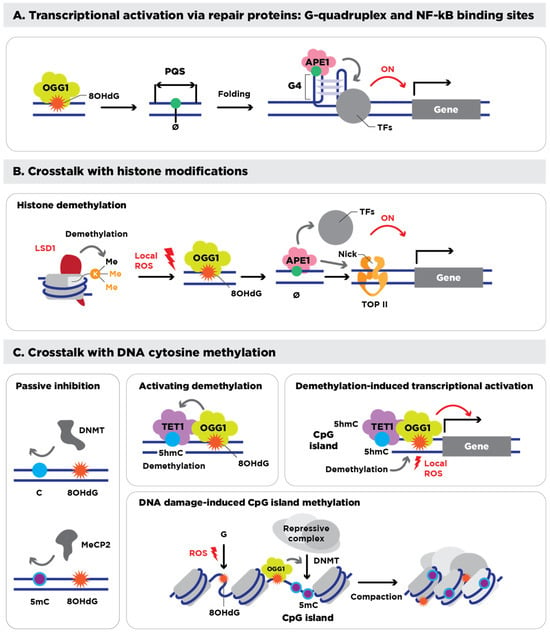
Figure 1.
Epigenetic roles of 8-OHdG and its processing. (A) Transcriptional regulation mediated by 8-OHdG, its repair intermediate, AP site (Ø), and its repair proteins. 8-OHdG, bound by OGG1, and its intermediate AP site induce folding of quadruplex-forming sequences (PQSs) into a G4, which recruits various transcription factors (TFs) to transcriptionally activate downstream genes. (B) Interplay of 8-OHdG with epigenetic histone modifications. During the histone demethylation reaction, Lysine-specific demethylase 1 (LSD1) generates local ROS (H2O2) that lead to the formation of 8-OHdG and AP sites in the promoter, which are occupied by OGG1 and APE1. Then, APE1 recruits other TFs, and its nick formation associates with topoisomerase II (TOP II), eventually activating the transcription of downstream genes. (C) Interplay of 8-OHdG with DNA cytosine methylation (5mC). 8-OHdG near CpG islands inhibits the binding of DNMTs and methyl-CpG-binding protein 2 (MeCP2), thus passively interfering with 5mC (left panel). OGG1, which is associated with 8-OHdG, interacts with TET1, which oxidizes adjacent 5mC to 5-hydroxymethylcytosine (5hmC) for DNA demethylation (upper middle panel). During the DNA demethylation process of CpG islands, TET1 generates local ROS, which induce 8-OHdG associated with OGG1, thus activating the transcription of downstream genes (upper right panel). Oxidative DNA damage triggers the formation of the 8-OHdG and OGG1 complex, which recruits repressive complexes, including DNMT, and induces methylation of CpG islands, finally resulting in chromatin condensation and silencing of damaged DNA regions. Figure and legend adapted and reproduced from Hahm et al. [79].
4.2. Redox-Mediated Methylation Processes
Oxidative stress also interferes with DNA methylation, a principal epigenetic mechanism [92]. DNMTs mediate the addition of methyl groups to cytosines in CpG islands, either preserving patterns during replication (Dnmt1) or establishing new marks (Dnmt3a and Dnmtb) [93]. Crosstalk between oxidative DNA damage and DNA methylation reveals a complex and multifaceted regulatory relationship involving 8-OHdG [94]. The presence of 8-OHdG can decrease DNMT binding affinity, indirectly inhibiting methylation, and in already-methylated CpG regions, can disrupt the binding of methyl-CpG binding proteins (MBPs) [95,96]. When 8-OHdG is converted to an AP site within potential G4 sequences, these structural changes can further modify local methylation dynamics [97].
Additionally, oxidative stress generates 8-OHdG lesions that recruit OGG1; OGG1, in turn, binds TET1 and delivers it to the damaged site, triggering local DNA demethylation. Cells lacking OGG1 resist this oxidative demethylation, whereas OGG1 over-expression enhances it, showing that BER machinery not only repairs 8-oxoG but also channels the demethylation signal to TET enzymes [98]. TET1 also initiates iterative oxidation reactions that demethylate 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) and subsequent oxidation products [99,100]. This process affects local DNA methylation patterns and modulates epigenetic reprogramming [101]. See Figure 1C for a summary of these processes. Altogether, these interconnected events showcase the duality of 8-OHdG as both a lesion in the genome and epigenetic modulator of methylation, blurring the lines between its genetic and epigenetic influences on embryo development and offspring health [102,103].
4.3. Implications for RNA Integrity
Because RNA is single-stranded and often lacks the protective proteins or robust repair mechanisms of DNA, it is inherently more vulnerable to oxidative damage. Guanine oxidation in RNA can affect both coding and non-coding transcripts and may be accompanied by strand breaks or crosslink formation—especially in small regulatory RNAs [104]. Site-specific oxidation of miRNAs or other sncRNAs can disrupt their structural integrity or binding specificities, ultimately dysregulating gene expression pathways linked to disease [105,106,107]. APE1—which is central to the BER pathway—also processes damaged RNA, including oxidized or apurinic forms, and can regulate oncogenic miRNAs [108]. Such oxidative modifications to RNA stem from a host of factors—exercise, drug abuse, toxins, inflammation, poor nutrition, and obesity—that are known to reshape the sncRNA landscape in sperm. These changes correlate with diverse offspring phenotypes, supporting a multi-layered network by which oxidative stress can propagate epigenetic changes across generations [109].
Taken together, these observations emphasize the pivotal role of 8-OHdG and DNA damage repair pathways in linking oxidative stress to epigenetic regulation. Through their influence on histone modifications, DNA methylation, and RNA integrity, oxidative lesions and their repair responses orchestrate dynamic changes that shape both immediate fertility outcomes and long-term heritable effects.
5. Oxidative Stress as the Key Driver of Epigenetic Change in Sperm
Accumulating evidence points to oxidative stress as a central player in genetic damage and altered epigenetic profiles in spermatozoa. Here, we argue that the distinct structural and functional characteristics of spermatozoa position oxidative stress as the primary mechanism shaping male subfertility and transgenerational health outcomes. This proposal rests on two foundational principles:
- Inherent Susceptibility to Oxidative Damage: Spermatozoa exhibit an elevated vulnerability to oxidative insult due to their high polyunsaturated fatty acid content and limited antioxidant defenses. These features render their membranes, intracellular components (DNA, RNA, proteins), and epigenetic factors such as methylation and sncRNA signatures, exceptionally prone to oxidative damage or alteration [110]. In most cells, oxidative damage becomes problematic only when stress levels overwhelm robust repair pathways; spermatozoa, however, lack this, providing a fresh twist on the classic “two-hit” hypothesis.
- Truncated BER Pathway: Unlike somatic cells, sperm rely on a partial BER process to address oxidative DNA lesions. Although they retain OGG1 for excising 8-OHdG, the lack of critical downstream enzymes such as APE1 and XRCC1 leads to the accumulation of apurinic sites and incomplete repair in the paternal genome [27]. Interestingly, these lesions may not prevent fertilization, yet they can still impact embryonic genetic and epigenetic programming, as their repair relies on the oocyte’s BER machinery.
The simultaneous presence of high oxidative damage and incomplete repair interrupts two key redox-mediated mechanisms observed in somatic cells: the direct effects of 8-OHdG and its downstream processing. As a result, oxidative stress can trigger the following epigenetic disruptions in sperm:
- Impact of Increased Oxidative Damage:
- Chromatin Architecture: Altered histone-to-protamine ratios can increase the susceptibility of interlinker DNA regions to oxidative assault [111], and escalating the consequence of these epigenetic modifications to further localized DNA oxidation, increased DNA fragmentation, lower fertilization rates, and poorer embryo quality [112,113].
- G-Quadruplex Structures: The disruption of potential G4-forming sequences affects chromatin topology and the binding of epigenetic regulators [114,115]. Recent evidence indicates that G-quadruplex DNA adds another cis-regulatory layer to transcriptional control during embryonic development [116].
- Methylation Dynamics: Perturbations in DNA methylation patterns and the balance between 5mC and 5hmC [117,118]. During early embryo development, the paternal genome undergoes rapid active DNA demethylation driven by TET enzymes, 5mC to 5hmC and then to 5-formylcytosine (5fC)/5-carboxylcytosine (5caC); Thymine DNA Glycosylase (TDG) excises these oxidized bases, and BER restores unmodified cytosine. Because this TET-TDG-BER machinery also repairs oxidative lesions, an excess of paternally inherited 5hmC or 8-OHdG could divert or saturate the pathway, reshaping the normal methylation-reprogramming wave and, in turn, altering embryonic gene regulation [119].
- RNA Integrity: Up to 18,000 mRNAs and sncRNAs delivered by sperm may be compromised, influencing embryonic gene expression [120,121]. Recent studies confirm that specific sncRNA profiles correlate strongly with sperm quality and could serve as biomarkers to improve IVF success rates [122], alter the transcriptomic profiles of early embryos [123], and even predict contributions to regulation of gene expression in offspring development [124].
- Consequences of APE1 Deficiency:
- Chromatin Remodeling: Impaired recruitment of chromatin-modifying complexes hinders the proper establishment of histone marks and DNA methylation status [125,126,127,128]. APE1 spatiotemporal activity impacts both.
- G4 Sequence Resolution: Prevalent oxidized bases, together with APE1, actively shape higher-order DNA structures that modulate transcription—an influence that extends beyond APE1′s traditional role in genome maintenance. Insufficient APE1 activity prevents the formation or resolution of G4 structures, further altering epigenetic regulation [23,85].
- RNA Damage Processing: Emerging evidence shows that APE1, beyond its DNA repair duties, also participates in RNA metabolism: during genotoxic stress, APE1 associates with the DROSHA complex to influence miRNA processing and stability [129]. Deficient capacity to process oxidatively damaged RNA potentially alters transgenerational inheritance [130,131].
When these oxidative and epigenetic disruptions converge—particularly under conditions of advanced maternal age, where oocyte repair capacity is often diminished—the potential harm to embryonic development and offspring health is magnified. Collectively, the multifaceted role of 8-OHdG in both genetic and epigenetic processes underscore its significance as a key driver of male fertility issues and transgenerational health risks [132].
6. The “Perfect Storm” of Aging: Oxidative Damage, Impaired Repair, and Resulting (Epi)mutations
Spermatozoa, once they have matured, lose most of their capacity for DNA repair and possess minimal antioxidant defenses. As a result, genomic damage from a combination of endogenous, environmental, and lifestyle factors can accumulate over time with no inherent mechanism to rectify these lesions [27,133,134]. Consequently, the oocyte’s active DNA repair programs become essential for safeguarding the embryo from paternal mutagenesis [135,136]. The repair of the paternal genome requires the re-establishment of the nucleosomal chromatin architecture—replacing protamines with transition proteins and eventually histones [137]—thereby granting repair factors access to damaged DNA [138].
6.1. Oxidative Damage and Repair Post-Fertilization
For oxidative DNA lesions, repair in the zygote is initiated by 8-OHdG excision by OGG1 [139]. However, oocytes typically express OGG1 at relatively low levels, which limits this crucial initial step [139]. Intriguingly, fertilizing sperm deliver a significant complement of OGG1 to the zygote, thereby boosting its total repair capacity [27,138]. The subsequent steps—processing of apurinic sites—depend on APE1 and XRCC1, both abundant in oocytes but absent in sperm. Although this gametic collaboration theoretically curbs the risk of inheriting oxidative lesions, it also introduces a critical window where the same BER enzymes influence epigenetic processes. Paternal and maternal oxidative stress can thus have a profound impact on early embryonic development [140,141].
Nevertheless, an oocyte’s capacity to repair DNA diminishes with advanced maternal age [142,143], largely due to declining levels of key repair enzymes, including OGG1 [144]. The convergence of paternal and maternal aging thus creates a “perfect storm”—amplifying oxidative DNA damage in sperm while limiting the oocyte’s ability to repair it. This leads to higher risks of genomic instability in the embryo, as apurinic sites and 8-OHdG lesions may remain unresolved following fertilization [145,146,147]. Although the “post-meiotic collusion hypothesis” provides a compelling framework, direct experimental evidence detailing how oxidative stress in sperm affects DNA repair in the oocyte and early embryo remains limited, and the precise role of the oocyte’s DNA damage response fidelity has yet to be fully elucidated.
Although few clinical or in vivo studies have examined how advancing paternal and maternal age jointly influence heritable (epi)mutations, pregnancy data show a clear additive age effect: live-birth rates decline most when both partners are older [148]. Meta-analyses further indicate that advanced paternal age worsens ART outcomes when autologous oocytes are used, but not with donor oocytes—implicating co-existing maternal age as the main driver of these deleterious results [149].
6.2. The Paternal Origins of Health and Disease
Unresolved paternal genetic damage not only compromises embryonic developmental stability [150,151] but also has far-reaching implications for offspring health [152,153]. De novo mutations in paternal chromosome 15, a region notably susceptible to oxidative damage [49], are linked to numerous conditions, including developmental delays, autism spectrum disorders, epilepsy, schizophrenia, bipolar disorder, and multiple congenital anomalies [154,155,156,157,158,159,160,161,162,163,164,165]. The whole-genome sequencing of parent-offspring trios further reveals that children of older fathers exhibit higher frequencies of C>T transitions at CpG sites than those of younger fathers [166], reinforcing oxidative stress as a key driver of adverse transgenerational inheritance. Because the male germ line has a lower baseline mutation rate than somatic tissues, yielding only about two de novo mutations per year of paternal age, epigenetic disturbances largely explain the broader spectrum of outcomes triggered by oxidative damage in spermatozoa [167].
Beyond direct genomic instability, the interplay between advancing parental age, unrepaired oxidative damage, and decreased repair capacity disrupts the extensive epigenetic reprogramming critical for early embryogenesis [168]. During this stage, DNA methylation and histone modifications are restructured to establish a pluripotent state. The tight interconnection among oxidative stress, BER, and methylation/demethylation has been recognized as central to germline reprogramming events [169]. Specifically, the paternal genome becomes a primary target for TET enzymes—which facilitate active DNA demethylation via 5mC oxidation to 5hmC—but can be hindered by unresolved 8-OHdG lesions [170]. Similarly, methyltransferase machinery (e.g., DNMT1/2), dependent on chromodomain helicase DNA-binding protein 4 (CHD4) and OGG1 co-localization [98,171], may be diverted by ongoing oxidative repair processes. Recent experiments in bovine models also revealed that oxidative DNA lesions in the paternal genome impair active zygotic DNA demethylation through the preferential recruitment of XRCC1. This finding indicates that oxidative DNA damage repair is prioritized, potentially at the expense of concurrent epigenetic reprogramming processes [172]. Clearly, oxidative damage and its processing may contribute to aberrant epigenetic landscapes in the developing embryo [173].
A wide range of environmental and lifestyle factors known to induce oxidative stress—such as heat [78], poor diet [174], alcohol use [175], pollutants, endocrine-disrupting chemicals (e.g., phthalates) [176], and smoking [177]—are also implicated in altering sperm small non-coding RNA profiles. These changes have been linked to disruptions in embryonic development and DNA methylation as well as a host of offspring disorders, including metabolic, neurological, and behavioral abnormalities [178,179,180,181].
Taken together, these genomic and epigenetic instabilities highlight the profound influence of preconception oxidative stress on offspring health (Figure 2), highlighting the urgent need for preventative and therapeutic strategies [182]. Addressing oxidative stress in both parents before conception may prove instrumental for improving reproductive outcomes and promoting long-term transgenerational well-being.
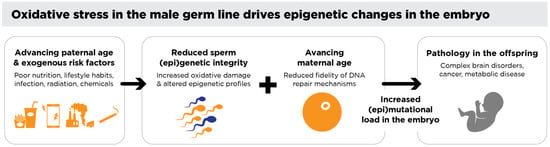
Figure 2.
Oxidative stress in the male germ line drives epigenetic changes in the embryo. Environmental, lifestyle, and medical factors contribute to oxidative stress in the male germ line, leading to DNA damage and altered epigenetic profiles in spermatozoa. When a spermatozoon with significant oxidative DNA damage fertilizes an oocyte, the oocyte attempts to repair this damage during the critical period between fertilization and the initiation of the S-phase of the first mitotic division. Errors in this repair process can fix paternal oxidative DNA damage as mutations in the embryo and/or disrupt normal epigenetic reprogramming. Such damage may affect genomic regions, such as chromosome 15, associated with offspring pathologies including brain disorders, imprinting errors, and cancers—all of which are known to have a strong paternal component. Additionally, altered sperm-borne small non-coding RNAs (sncRNAs) have been shown to influence offspring health, further underscoring the transgenerational impact of oxidative stress in the male germ line.
8. Conclusions
Oxidative stress represents a critical yet underappreciated threat to male reproductive health, exerting profound effects on both the genome and epigenome. At the heart of this process is 8-OHdG, which functions both as a marker of oxidative damage and an active epigenetic regulator. This dual capacity underscores its importance in shaping heritable traits, as 8-OHdG-driven alterations can propagate through multiple generations.
The heightened vulnerability of spermatozoa to oxidative stress, combined with its truncated base excision repair pathway, magnifies the impact of 8-OHdG-induced damage. Disruptions to DNA methylation, post-translational modifications of histones and protamines, and the integrity of small non-coding RNAs all converge to undermine classical epigenetic regulation and reprogramming, ultimately affecting embryonic development and long-term offspring health.
Clinically, these insights make a compelling case for embracing redox biology within reproductive medicine. By systematically identifying and mitigating oxidative stress, via precision-based antioxidant therapy and other targeted interventions, healthcare professionals can substantially improve fertility outcomes and safeguard the well-being of future generations. This holistic integration of oxidative stress management into standard clinical practice stands to reshape the landscape of fertility treatments and holds transformative potential for advancing transgenerational health.
Author Contributions
Conceptualization, Visualization, Writing—original draft preparation, Writing—review and editing, A.M.; Writing—review and editing, F.S.; Writing—review and editing, J.R.D.; Conceptualization, Supervision, Writing—review and editing, R.J.A.; Conceptualization, Supervision, Writing—review and editing, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to apologize to those investigators whose pertinent work was not cited in the interest of providing a concise perspective on the most recent overlapping advancements in the fields of oxidative stress, epigenetics, and male infertility. The authors would like to thank Laura Moazamian for creating the drawings used in Figure 1 and Figure 2.
Conflicts of Interest
Authors A.M. and P.G. are employed by the company CellOxess Biotechnology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| RMS | Reactive metabolic species |
| sncRNAs | Small non-coding RNAs |
| DNMTs | DNA methyltransferases |
| TET | Ten-eleven translocation |
| OGG1 | 8-oxoguanine DNA glycosylase 1 |
| APE1 | Apurinic/apyrimidinic endonuclease 1 |
| XRCC1 | X-ray repair cross-complementing protein 1 |
| MBPs | Methyl-CpG binding proteins |
| 5mC | 5-methylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| 5fC | 5-formylcytosine |
| 5caC | 5-carboxylcytosine |
| TDG | Thymine DNA Glycosylase |
| CHD4 | Chromodomain helicase DNA-binding protein 4 |
References
- GBD 2021 Fertility and Forecasting Collaborators. Global fertility in 204 countries and territories, 1950–2021, with forecasts to 2100: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2057–2099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lassen, E.; Pacey, A.; Skytte, A.-B.; Montgomerie, R. Recent decline in sperm motility among donor candidates at a sperm bank in Denmark. Hum. Reprod. 2024, 39, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Jonge, C.J.; Barratt, C.L.R.; Aitken, R.J.; Anderson, R.A.; Baker, P.; Chan, D.Y.L.; Connolly, M.P.; Eisenberg, M.L.; Garrido, N.; Jørgensen, N.; et al. Current global status of male reproductive health. Hum. Reprod. Open 2024, 2024, hoae017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J. What is driving the global decline of human fertility? Need for a multidisciplinary approach to the underlying mechanisms. Front. Reprod. Health 2024, 6, 1364352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Baker, M.A. The Role of Genetics and Oxidative Stress in the Etiology of Male Infertility—A Unifying Hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Hernández, C.E.; Close, D.M.; Gorb, L.; Leszczynski, J. Determination of redox potentials for the Watson—Crick base pairs, DNA nucleosides, and relevant nucleoside analogues. J. Phys. Chem. B 2007, 111, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Sakumi, K.; Fukumura, R.; Furuichi, M.; Iwasaki, Y.; Hokama, M.; Ikemura, T.; Tsuzuki, T.; Gondo, Y.; Nakabeppu, Y. 8-oxoguanine causes spontaneous de novo germline mutations in mice. Sci. Rep. 2014, 4, 4689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vorilhon, S.; Brugnon, F.; Kocer, A.; Dollet, S.; Bourgne, C.; Berger, M.; Janny, L.; Pereira, B.; Aitken, R.J.; Moazamian, A.; et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 2018, 33, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Lewis, S.E.M. DNA damage in testicular germ cells and spermatozoa. When and how is it induced? How should we measure it? What does it mean? Andrology 2023, 11, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- du Fossé, N.A.; van der Hoorn, M.-L.P.; van Lith, J.M.M.; le Cessie, S.; Lashley, E.E.L.O. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 650–669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behdarvandian, P.; Nasr-Esfahani, A.; Tavalaee, M.; Pashaei, K.; Naderi, N.; Darmishonnejad, Z.; Hallak, J.; Aitken, R.J.; Gharagozloo, P.; Drevet, J.R.; et al. Sperm chromatin structure assay (SCSA®) and flow cytometry-assisted TUNEL assay provide a concordant assessment of sperm DNA fragmentation as a function of age in a large cohort of approximately 10,000 patients. Basic Clin. Androl. 2023, 33, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inversetti, A.; Bossi, A.; Cristodoro, M.; Larcher, A.; Busnelli, A.; Grande, G.; Salonia, A.; Di Simone, N. Recurrent pregnancy loss: A male crucial factor—A systematic review and meta-analysis. Andrology 2023, 13, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, L.; Cheng, Y.; Xiong, Y.; Qi, D.; Li, B.; Zhang, X.; Zheng, F. Identification and validation of oxidative stress-related diagnostic markers for recurrent pregnancy loss: Insights from machine learning and molecular analysis. Mol. Divers. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Scala, G.; Cooke, M.S.; Majello, B.; Amente, S. Towards a comprehensive view of 8-oxo-7,8-dihydro-2′-deoxyguanosine: Highlighting the intertwined roles of DNA damage and epigenetics in genomic instability. DNA Repair 2021, 97, 103027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, S.; Sengupta, P.; Mottola, F.; Das, S.; Hussain, A.; Ashour, A.; Rocco, L.; Govindasamy, K.; Rosas, I.M.; Roychoudhury, S. Crosstalk Between Oxidative Stress and Epigenetics: Unveiling New Biomarkers in Human Infertility. Cells 2024, 13, 1846. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, M.; Aitken, R.J. Exposure of spermatozoa to dibutyl phthalate induces abnormal embryonic development in a marine invertebrate Galeolaria caespitosa (Polychaeta: Serpulidae). Aquat. Toxicol. 2017, 191, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: Sperm telomeres, oxidative stress, and infertility. Reproduction 2022, 164, F125–F133. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, J.; Savani, S.; Krempley, B.D.; Nguyen, M.; Kitlinska, J.B. Influence of paternal preconception exposures on their offspring: Through epigenetics to phenotype. Am. J. Stem Cells 2016, 5, 11–18. [Google Scholar] [PubMed] [PubMed Central]
- Fleming, A.M.; Burrows, C.J. Chemistry of ROS-mediated oxidation to the guanine base in DNA and its biological consequences. Int. J. Radiat. Biol. 2022, 98, 452–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126 Pt 6, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, J.; Baumgartner, A.; Schmid, T.; Brinkworth, M. Responses to genotoxicity in mouse testicular germ cells and epididymal spermatozoa are affected by increased age. Toxicol. Lett. 2019, 310, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa1. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Aurich, C. “Fine feathers make fine birds”—The mammalian sperm plasma membrane lipid composition and effects on assisted reproduction. Anim. Reprod. Sci. 2021, 246, 106884. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [PubMed]
- O’flaherty, C.; Scarlata, E. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The protection of mammalian spermatozoa against oxidative stress. Reproduction 2022, 164, F67–F78. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.R.; Hallak, J.; Nasr-Esfahani, M.-H.; Aitken, R.J. Reactive Oxygen Species and Their Consequences on the Structure and Function of Mammalian Spermatozoa. Antioxid. Redox Signal. 2022, 37, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Role of sperm DNA damage in creating de-novo mutations in human offspring: The ‘post-meiotic oocyte collusion’ hypothesis. Reprod. Biomed. Online 2022, 45, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Male reproductive ageing: A radical road to ruin. Hum. Reprod. 2023, 38, 1861–1871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; De Iuliis, G.N.; Nixon, B. The Sins of Our Forefathers: Paternal Impacts on De Novo Mutation Rate and Development. Annu. Rev. Genet. 2020, 54, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Scott, R.J.; Drevet, J.R.; Aitken, R.J. Paternal impacts on development: Identification of genomic regions vulnerable to oxidative DNA damage in human spermatozoa. Hum. Reprod. 2019, 34, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: Implications for offspring health and development. Hum. Reprod. Update 2023, 29, 24–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lismer, A.; Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development. Nat. Commun. 2023, 14, 2142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, W.W.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Balder, P.; Jones, C.; Coward, K.; Yeste, M. Sperm chromatin: Evaluation, epigenetic signatures and relevance for embryo development and assisted reproductive technology outcomes. Eur. J. Cell Biol. 2024, 103, 151429. [Google Scholar] [CrossRef] [PubMed]
- Gatewood, J.M.; Cook, G.R.; Balhorn, R.; Bradbury, E.M.; Schmid, C.W. Sequence-specific packaging of DNA in human sperm chromatin. Science 1987, 236, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Moritz, L.; Hammoud, S.S. The Art of Packaging the Sperm Genome: Molecular and Structural Basis of the Histone-To-Protamine Exchange. Front. Endocrinol. 2022, 13, 895502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wykes, S.M.; Krawetz, S.A. The Structural Organization of Sperm Chromatin. J. Biol. Chem. 2003, 278, 29471–29477. [Google Scholar] [CrossRef] [PubMed]
- Luense, L.J.; Wang, X.; Schon, S.B.; Weller, A.H.; Shiao, E.L.; Bryant, J.M.; Bartolomei, M.S.; Coutifaris, C.; Garcia, B.A.; Berger, S.L. Comprehensive analysis of histone post-translational modifications in mouse and human male germ cells. Epigenetics Chromatin 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siklenka, K.; Erkek, S.; Godmann, M.; Lambrot, R.; McGraw, S.; Lafleur, C.; Cohen, T.; Xia, J.; Suderman, M.; Hallett, M.; et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 2015, 350, aab2006. [Google Scholar] [CrossRef] [PubMed]
- Lismer, A.; Dumeaux, V.; Lafleur, C.; Lambrot, R.; Brind’amour, J.; Lorincz, M.C.; Kimmins, S. Histone H3 lysine 4 trimethylation in sperm is transmitted to the embryo and associated with diet-induced phenotypes in the offspring. Dev. Cell 2021, 56, 671–686.e6. [Google Scholar] [CrossRef] [PubMed]
- Torres-Flores, U.; Hernández-Hernández, A. The Interplay Between Replacement and Retention of Histones in the Sperm Genome. Front. Genet. 2020, 11, 780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Mateo, S.; Gázquez, C.; Guimerà, M.; Balasch, J.; Meistrich, M.L.; Ballescà, J.L.; Oliva, R. Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil. Steril. 2009, 91, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Pandya, R.K.; Jijo, A.; Cheredath, A.; Uppangala, S.; Salian, S.R.; Lakshmi, V.R.; Kumar, P.; Kalthur, G.; Gupta, S.; Adiga, S.K. Differential sperm histone retention in normozoospermic ejaculates of infertile men negatively affects sperm functional competence and embryo quality. Andrology 2024, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Oakes, C.C.; La Salle, S.; Smiraglia, D.J.; Robaire, B.; Trasler, J.M. A unique configuration of genome-wide DNA methylation patterns in the testis. Proc. Natl. Acad. Sci. USA 2007, 104, 228–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kohli, R.M.; Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aston, K.I.; Uren, P.J.; Jenkins, T.G.; Horsager, A.; Cairns, B.R.; Smith, A.D.; Carrell, D.T. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 2015, 104, 1388–1397.e1-5. [Google Scholar] [CrossRef] [PubMed]
- Greeson, K.W.; Crow, K.M.S.; Edenfield, R.C.; Easley, C.A. Inheritance of paternal lifestyles and exposures through sperm DNA methylation. Nat. Rev. Urol. 2023, 20, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Pollard, C.A.; Brogaard, K.R.; Olson, A.C.; Barney, R.C.; Lipshultz, L.I.; Johnstone, E.B.; Ibrahim, Y.O.; Hotaling, J.M.; Schisterman, E.F.; et al. Tissue-specific DNA methylation variability and its potential clinical value. Front. Genet. 2023, 14, 1125967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, R.H.; DeVilbiss, E.A.; Brogaard, K.R.; Norton, C.R.; Pollard, C.A.; Emery, B.R.; Aston, K.I.; Hotaling, J.M.; Jenkins, T.G. Epigenetic determinants of reproductive potential augment the predictive ability of the semen analysis. Fertil. Steril. Sci. 2023, 4, 279–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burton, A.; Torres-Padilla, M.-E. Epigenetic reprogramming and development: A unique heterochromatin organization in the preimplantation mouse embryo. Brief. Funct. Genom. 2010, 9, 444–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Zhang, J.; Duan, J.; Gao, X.; Zhu, W.; Lu, X.; Yang, L.; Zhang, J.; Li, G.; Ci, W.; et al. Programming and inheritance of parental DNA methylomes in mammals. Cell 2014, 157, 979–991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barlow, D.P.; Bartolomei, M.S. Genomic Imprinting in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, U. Paternal Contributions to Offspring Health: Role of Sperm Small RNAs in Intergenerational Transmission of Epigenetic Information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kretschmer, M.; Gapp, K. Deciphering the RNA universe in sperm in its role as a vertical information carrier. Environ. Epigenetics 2022, 8, dvac011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trigg, N.A.; Conine, C.C. Epididymal acquired sperm microRNAs modify post-fertilization embryonic gene expression. Cell Rep. 2024, 43, 114698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sharma, U. Sperm RNA Payload: Implications for Intergenerational Epigenetic Inheritance. Int. J. Mol. Sci. 2023, 24, 5889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomar, A.; Gomez-Velazquez, M.; Gerlini, R.; Comas-Armangué, G.; Makharadze, L.; Kolbe, T.; Boersma, A.; Dahlhoff, M.; Burgstaller, J.P.; Lassi, M.; et al. Epigenetic inheritance of diet-induced and sperm-borne mitochondrial RNAs. Nature 2024, 630, 720–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trigg, N.; Schjenken, J.E.; Martin, J.H.; Skerrett-Byrne, D.A.; Smyth, S.P.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Simpson, E.N.A.; Tomar, A.; et al. Subchronic elevation in ambient temperature drives alterations to the sperm epigenome and accelerates early embryonic development in mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2409790121. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.Y.; Park, J.; Jang, E.-S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krokan, H.E.; Bjørås, M. Base Excision Repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, A.M.; Ding, Y.; Burrows, C.J. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. USA 2017, 114, 2604–2609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, A.M.; Burrows, C.J. 8-Oxo-7,8-dihydroguanine, friend and foe: Epigenetic-like regulator versus initiator of mutagenesis. DNA Repair 2017, 56, 75–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, A.M.; Zhu, J.; Ding, Y.; Burrows, C.J. 8-Oxo-7,8-dihydroguanine in the Context of a Gene Promoter G-Quadruplex Is an On–Off Switch for Transcription. ACS Chem. Biol. 2017, 12, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roychoudhury, S.; Pramanik, S.; Harris, H.L.; Tarpley, M.; Sarkar, A.; Spagnol, G.; Sorgen, P.L.; Chowdhury, D.; Band, V.; Klinkebiel, D.; et al. Endogenous oxidized DNA bases and APE1 regulate the formation of G-quadruplex structures in the genome. Proc. Natl. Acad. Sci. USA 2020, 117, 11409–11420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, A.M.; Burrows, C.J. Oxidative stress-mediated epigenetic regulation by G-quadruplexes. NAR Cancer 2021, 3, zcab038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giorgio, M.; Dellino, G.I.; Gambino, V.; Roda, N.; Pelicci, P.G. On the epigenetic role of guanosine oxidation. Redox Biol. 2020, 29, 101398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perillo, B.; Ombra, M.N.; Bertoni, A.; Cuozzo, C.; Sacchetti, S.; Sasso, A.; Chiariotti, L.; Malorni, A.; Abbondanza, C.; Avvedimento, E.V. DNA Oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 2008, 319, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Amente, S.; Bertoni, A.; Morano, A.; Lania, L.; Avvedimento, E.V.; Majello, B. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene 2010, 29, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Zhang, Y.; Wan, X.; Zhang, C.; Huang, X.; Huang, W.; Pu, H.; Pei, C.; Wu, H.; et al. KDM1A triggers androgen-induced miRNA transcription via H3K4me2 demethylation and DNA oxidation. Prostate 2015, 75, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Pezone, A.; Taddei, M.L.; Tramontano, A.; Dolcini, J.; Boffo, F.L.; De Rosa, M.; Parri, M.; Stinziani, S.; Comito, G.; Porcellini, A.; et al. Targeted DNA oxidation by LSD1–SMAD2/3 primes TGF-β1/ EMT genes for activation or repression. Nucleic Acids Res. 2020, 48, 8943–8958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donkena, K.V.; Young, C.Y.F.; Tindall, D.J. Oxidative Stress and DNA Methylation in Prostate Cancer. Obstet. Gynecol. Int. 2010, 2010, 302051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.L.; Fernandez, J.; Koerperich, Z.; Andersen, M.P.; Kotandeniya, D.; Nguyen, M.E.; Sham, Y.Y.; Tretyakova, N.Y. Maintenance DNA Methyltransferase Activity in the Presence of Oxidized Forms of 5-Methylcytosine: Structural Basis for Ten Eleven Translocation-Mediated DNA Demethylation. Biochemistry 2018, 57, 6061–6069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valinluck, V.; Tsai, H.H.; Rogstad, D.K.; Burdzy, A.; Bird, A.; Sowers, L.C. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 2004, 32, 4100–4108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maltseva, D.V.; Baykov, A.A.; Jeltsch, A.; Gromova, E.S. Impact of 7,8-Dihydro-8-oxoguanine on Methylation of the CpG Site by Dnmt3a. Biochemistry 2009, 48, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, L.; Lei, L.; Fu, C.; Huang, J.; Hu, Y.; Dong, Y.; Chen, J.; Zeng, Q. Crosstalk between G-quadruplex and ROS. Cell Death Dis. 2023, 14, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Zhuang, Z.; Wang, W.; He, L.; Wu, H.; Cao, Y.; Pan, F.; Zhao, J.; Hu, Z.; Sekhar, C.; et al. OGG1 is essential in oxidative stress induced DNA demethylation. Cell. Signal. 2016, 28, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Wang, C.; Wang, X. TET (Ten-eleven translocation) family proteins: Structure, biological functions and applications. Signal Transduct. Target. Ther. 2023, 8, 297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasasya, R.D.; Caldwell, B.A.; Liu, Z.; Wu, S.; Leu, N.A.; Fowler, J.M.; Cincotta, S.A.; Laird, D.J.; Kohli, R.M.; Bartolomei, M.S. Iterative oxidation by TET1 is required for reprogramming of imprinting control regions and patterning of mouse sperm hypomethylated regions. Dev. Cell 2024, 59, 1010–1027.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, P.W.S.; Leitch, H.G.; Requena, C.E.; Sun, Z.; Amouroux, R.; Roman-Trufero, M.; Borkowska, M.; Terragni, J.; Vaisvila, R.; Linnett, S.; et al. Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte. Nature 2018, 555, 392–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hug, E.; Renaud, Y.; Guiton, R.; Ben Sassi, M.; Marcaillou, C.; Moazamian, A.; Gharagozloo, P.; Drevet, J.R.; Saez, F. Exploring the epigenetic landscape of spermatozoa: Impact of oxidative stress and antioxidant supplementation on DNA methylation and hydroxymethylation. Antioxidants 2024, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. DNA modifications walk a fine line between epigenetics and mutagenesis. Nat. Rev. Mol. Cell Biol. 2023, 24, 449–450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent Advances: Molecular Mechanism of RNA Oxidation and Its Role in Various Diseases. Front. Mol. Biosci. 2020, 7, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, Q.; Lin, C.-L.G. Oxidative damage to RNA: Mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 2010, 67, 1817–1829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poulsen, H.E.; Specht, E.; Broedbaek, K.; Henriksen, T.; Ellervik, C.; Mandrup-Poulsen, T.; Tonnesen, M.; Nielsen, P.E.; Andersen, H.U.; Weimann, A. RNA modifications by oxidation: A novel disease mechanism? Free. Radic. Biol. Med. 2012, 52, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Lee, H.; Lee, S.; Ahn, S.H.; Lee, H.-S.; Kim, G.-W.D.; Peak, J.; Park, J.; Cho, Y.K.; Jeong, Y.; et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nature 2020, 584, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Antoniali, G.; Codrich, M.; Tell, G. Coping with RNA damage with a focus on APE1, a BER enzyme at the crossroad between DNA damage repair and RNA processing/decay. DNA Repair 2021, 104, 103133. [Google Scholar] [CrossRef] [PubMed]
- Conine, C.C.; Rando, O.J. Soma-to-germline RNA communication. Nat. Rev. Genet. 2022, 23, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.; Hamad, M.; Montenarh, M.; Fischer-Hammadeh, C. Protamine contents and P1/P2 ratio in human spermatozoa from smokers and non-smokers. Hum. Reprod. 2010, 25, 2708–2720. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Castillo, J.; Oliva, R.; Lewis, S.E. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod. Biomed. Online 2011, 23, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, A.M.; Burrows, C.J. Interplay of Guanine Oxidation and G-Quadruplex Folding in Gene Promoters. J. Am. Chem. Soc. 2020, 142, 1115–1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorini, F.; Ambrosio, S.; Lania, L.; Majello, B.; Amente, S. The Intertwined Role of 8-oxodG and G4 in Transcription Regulation. Int. J. Mol. Sci. 2023, 24, 2031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, A.P.; Margarit, E.; Domizi, P.; Banchio, C.; Armas, P.; Calcaterra, N.B. G-quadruplexes as novel cis-elements controlling transcription during embryonic development. Nucleic Acids Res. 2016, 44, 4163–4173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Parfenyev, S.E.; Mekina, I.D.; Komarova, E.M.; Mazilina, M.A.; Daev, E.V.; Chiryaeva, O.G.; Galembo, I.A.; et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget 2017, 8, 88294–88307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seddon, A.R.; Liau, Y.; Pace, P.E.; Miller, A.L.; Das, A.B.; Kennedy, M.A.; Hampton, M.B.; Stevens, A.J. Genome-wide impact of hydrogen peroxide on maintenance DNA methylation in replicating cells. Epigenetics Chromatin 2021, 14, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, K.; Lyu, Z.; Chen, J.; Chen, G. 5-Hydroxymethylcytosine: Far Beyond the Intermediate of DNA Demethylation. Int. J. Mol. Sci. 2024, 25, 11780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostermeier, G.C.; Miller, D.; Huntriss, J.D.; Diamond, M.P.; Krawetz, S.A. Delivering spermatozoan RNA to the oocyte. Nature 2004, 429, 154. [Google Scholar] [CrossRef] [PubMed]
- Rassoulzadegan, M.; Grandjean, V.; Gounon, P.; Vincent, S.; Gillot, I.; Cuzin, F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 2006, 441, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Liu, W.; Chen, Y.; Zhang, F.; Xu, B.; Liu, S.; Chen, G.; Shi, H.; Wu, L. Identification of small non-coding RNAs as sperm quality biomarkers for in vitro fertilization. Cell Discov. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trigg, N.A.; Skerrett-Byrne, D.A.; Xavier, M.J.; Zhou, W.; Anderson, A.L.; Stanger, S.J.; Katen, A.L.; De Iuliis, G.N.; Dun, M.D.; Roman, S.D.; et al. Acrylamide modulates the mouse epididymal proteome to drive alterations in the sperm small non-coding RNA profile and dysregulate embryo development. Cell Rep. 2021, 37, 109787. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Curto, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Early embryo development: What Does Daddy Do? Endocrinology 2025, 166, bqaf065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Braganza, A.; Sobol, R.W. Base excision repair facilitates a functional relationship between Guanine oxidation and histone demethylation. Antioxid. Redox Signal. 2013, 18, 2429–2443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, K.; Hada, M.; Fukuda, Y.; Inoue, E.; Makino, Y.; Katou, Y.; Shirahige, K.; Okada, Y. Re-evaluating the Localization of Sperm-Retained Histones Revealed the Modification-Dependent Accumulation in Specific Genome Regions. Cell Rep. 2018, 23, 3920–3932. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.M.; Hoitsma, N.M.; Spencer, J.J.; Gakhar, L.; Schnicker, N.J.; Freudenthal, B.D. Structural basis for APE1 processing DNA damage in the nucleosome. Nat. Commun. 2022, 13, 5390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, M.; Gut, F.; Fan, Y.; Ma, J.; Shan, X.; Yikilmazsoy, A.; Likhodeeva, M.; Hopfner, K.-P.; Zhou, C. Structural basis for human OGG1 processing 8-oxodGuo within nucleosome core particles. Nat. Commun. 2024, 15, 9407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antoniali, G.; Serra, F.; Lirussi, L.; Tanaka, M.; D’Ambrosio, C.; Zhang, S.; Radovic, S.; Dalla, E.; Ciani, Y.; Scaloni, A.; et al. Mammalian APE1 controls miRNA processing and its interactome is linked to cancer RNA metabolism. Nat. Commun. 2017, 8, 797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Powanda, P.; Robaire, B. Oxidative Stress and Reproductive Function in the Aging Male. Biology 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derijck, A.; van der Heijden, G.; Giele, M.; Philippens, M.; de Boer, P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum. Mol. Genet. 2008, 17, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, E.V.; Fesenko, Z.S.; Sopova, J.V.; Leonova, E.I. Features of DNA Repair in the Early Stages of Mammalian Embryonic Development. Genes 2020, 11, 1138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barral, S.; Morozumi, Y.; Tanaka, H.; Montellier, E.; Govin, J.; de Dieuleveult, M.; Charbonnier, G.; Couté, Y.; Puthier, D.; Buchou, T.; et al. Histone Variant H2A.L.2 Guides Transition Protein-Dependent Protamine Assembly in Male Germ Cells. Mol. Cell 2017, 66, 89–101.e8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.; Scherthan, H.; De Rooij, D.G. DNA Double Strand Break Response and Limited Repair Capacity in Mouse Elongated Spermatids. Int. J. Mol. Sci. 2015, 16, 29923–29935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lord, T.; Aitken, R.J. Fertilization stimulates 8-hydroxy-2′-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev. Biol. 2015, 406, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Musson, R.; Gąsior, Ł.; Bisogno, S.; Ptak, G.E. DNA damage in preimplantation embryos and gametes: Specification, clinical relevance and repair strategies. Hum. Reprod. Update 2022, 28, 376–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, W.S.W.; Solomon, B.D.; Bodian, D.L.; Kothiyal, P.; Eley, G.; Huddleston, K.C.; Baker, R.; Thach, D.C.; Iyer, R.K.; Vockley, J.G.; et al. New observations on maternal age effect on germline de novo mutations. Nat. Commun. 2016, 7, 10486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horta, F.; Catt, S.; Ramachandran, P.; Vollenhoven, B.; Temple-Smith, P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum. Reprod. 2020, 35, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Aitken, R.J.; Bromfield, E.G.; Nixon, B. DNA damage and repair in the female germline: Contributions to ART. Hum. Reprod. Update 2019, 25, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.; Catt, S.; Vining, B.; Vollenhoven, B.; Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: A systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Essers, J.; Kanaar, R.; Wyrobek, A.J. Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations. Proc. Natl. Acad. Sci.USA 2007, 104, 17725–17729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Meyer, D.H.; Schumacher, B. Inheritance of paternal DNA damage by histone-mediated repair restriction. Nature 2023, 613, 365–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McPherson, N.O.; Zander-Fox, D.; Vincent, A.D.; Lane, M. Combined advanced parental age has an additive negative effect on live birth rates—data from 4057 first IVF/ICSI cycles. J. Assist. Reprod. Genet. 2018, 35, 279–287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, G.; Mavrelos, D.; Theodorou, E.; Campbell-Forde, M.; Cansfield, D.; Yasmin, E.; Sangster, P.; Saab, W.; Serhal, P.; Seshadri, S. Effect of paternal age on outcomes in assisted reproductive technology cycles: Systematic review and meta-analysis. Fertil. Steril. Rev. 2020, 1, 16–34. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.B.; Liu, L.; Emery, B.; Aston, K.I.; Hotaling, J.; Carrell, D.T. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp, S.; van Tol, H.T.A.; Spierings, D.C.J.; Boymans, S.; Guryev, V.; Roelen, B.A.J.; Lansdorp, P.M.; Cuppen, E.; Kuijk, E.W. Sperm DNA damage causes genomic instability in early embryonic development. Sci. Adv. 2020, 6, eaaz7602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, M.J.; Roman, S.D.; Aitken, R.J.; Nixon, B. Transgenerational inheritance: How impacts to the epigenetic and genetic information of parents affect offspring health. Hum. Reprod. Update 2019, 25, 518–540. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen-Brady, K.; Robison, R.; Cannon, D.; Varvil, T.; Villalobos, M.; Pingree, C.; Leppert, M.F.; Miller, J.; McMahon, W.M.; Coon, H. Genome-wide linkage in Utah autism pedigrees. Mol. Psychiatry 2010, 15, 1006–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappi, C.; Hounie, A.G.; Mariani, D.B.; Diniz, J.B.; Silva, A.R.T.; Reis, V.N.S.; Busso, A.F.; Silva, A.G.; Fidalgo, F.; Rogatto, S.R.; et al. An Inherited Small Microdeletion at 15q13.3 in a Patient with Early- Onset Obsessive-Compulsive Disorder. PLoS ONE 2014, 9, e110198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isles, A.R.; Ingason, A.; Lowther, C.; Walters, J.; Gawlick, M.; Stöber, G.; Rees, E.; Martin, J.; Little, R.B.; Potter, H.; et al. Parental Origin of Interstitial Duplications at 15q11.2-q13.3 in Schizophrenia and Neurodevelopmental Disorders. PLoS Genet. 2016, 12, e1005993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, K.J.; Fanous, A.H.; Chen, J.; Kendler, K.S.; Chen, X. Variants in the 15q25 gene cluster are associated with risk for schizophrenia and bipolar disorder. Psychiatr. Genet. 2013, 23, 20–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jian, X.; Chen, J.; Li, Z.; Fahira, A.; Shao, W.; Zhou, J.; Wang, K.; Wen, Y.; Zhang, J.; Yang, Q.; et al. Common variants in FAN1, located in 15q13.3, confer risk for schizophrenia and bipolar disorder in Han Chinese. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109973. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Ruggieri, M.; Marino, S.D.; Corsello, G.; Pappalardo, X.; Polizzi, A.; Parano, E.; Romano, C.; Praticò, A.D.; Falsaperla, R. Chromosome 15q BP3 to BP5 deletion is a likely locus for speech delay and language impairment: Report on a four-member family and an unrelated boy. Mol. Genet. Genom. Med. 2020, 8, e1109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soemedi, R.; Wilson, I.J.; Bentham, J.; Darlay, R.; Töpf, A.; Zelenika, D.; Cosgrove, C.; Setchfield, K.; Thornborough, C.; Granados-Riveron, J.; et al. Contribution of global rare copy-number variants to the risk of sporadic congenital heart disease. Am. J. Hum. Genet. 2012, 91, 489–501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vazza, G.; Bertolin, C.; Scudellaro, E.; Vettori, A.; Boaretto, F.; Rampinelli, S.; De Sanctis, G.; Perini, G.; Peruzzi, P.; Mostacciuolo, M.L. Genome-wide scan supports the existence of a susceptibility locus for schizophrenia and bipolar disorder on chromosome 15q26. Mol. Psychiatry 2007, 12, 87–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitney, R.; Nair, A.; McCready, E.; Keller, A.E.; Adil, I.S.; Aziz, A.S.; Borys, O.; Siu, K.; Shah, C.; Meaney, B.F.; et al. The spectrum of epilepsy in children with 15q13.3 microdeletion syndrome. Seizure 2021, 92, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Purmann, C.; Ma, S.; Shrestha, A.; Davis, K.N.; Ho, M.; Huang, Y.; Pattni, R.; Wong, W.H.; et al. Network Effects of the 15q13.3 Microdeletion on the Transcriptome and Epigenome in Human-Induced Neurons. Biol. Psychiatry 2021, 89, 497–509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziats, M.N.; Goin-Kochel, R.P.; Berry, L.N.; Ali, M.; Ge, J.; Guffey, D.; Rosenfeld, J.A.; Bader, P.; Gambello, M.J.; Wolf, V.; et al. The complex behavioral phenotype of 15q13.3 microdeletion syndrome. Genet. Med. 2016, 18, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Shojaeisaadi, H.; Schoenrock, A.; Meier, M.J.; Williams, A.; Norris, J.M.; Palmer, N.D.; Yauk, C.L.; Marchetti, F. Mutational signature analyses in multi-child families reveal sources of age-related increases in human germline mutations. Commun. Biol. 2024, 7, 1451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J. Paternal age, de novo mutations, and offspring health? New directions for an ageing problem. Hum. Reprod. 2024, 39, 2645–2654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klutstein, M.; Gonen, N. Epigenetic aging of mammalian gametes. Mol. Reprod. Dev. 2023, 90, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Hajkova, P.; Jeffries, S.J.; Lee, C.; Miller, N.; Jackson, S.P.; Surani, M.A. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science 2010, 329, 78–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montgomery, T.; Uh, K.; Lee, K. TET enzyme driven epigenetic reprogramming in early embryos and its implication on long-term health. Front. Cell Dev. Biol. 2024, 12, 1358649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell. Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative stress in sperm affects the epigenetic reprogramming in early embryonic development. Epigenetics Chromatin 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Q.; Stöger, R.; Alberio, R. A Lexicon of DNA Modifications: Their Roles in Embryo Development and the Germline. Front. Cell Dev. Biol. 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klastrup, L.K.; Bak, S.T.; Nielsen, A.L. The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Mol. Genet. Genom. 2019, 294, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roach, A.N.; Bhadsavle, S.S.; Higgins, S.L.; Derrico, D.D.; Basel, A.; Thomas, K.N.; Golding, M.C. Alterations in sperm RNAs persist after alcohol cessation and correlate with epididymal mitochondrial dysfunction. Andrology 2024, 12, 1012–1023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Shi, J.; Hernandez, R.; Li, X.; Konchadi, P.; Miyake, Y.; Chen, Q.; Zhou, T.; Zhou, C. Paternal phthalate exposure-elicited offspring metabolic disorders are associated with altered sperm small RNAs in mice. Environ. Int. 2023, 172, 107769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marczylo, E.L.; Amoako, A.A.; Konje, J.C.; Gant, T.W.; Marczylo, T.H. Smoking induces differential miRNA expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenetics 2012, 7, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.; Ben Maamar, M.; Skinner, M.K. Integration of sperm ncRNA-directed DNA methylation and DNA methylation-directed histone retention in epigenetic transgenerational inheritance. Epigenetics Chromatin 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Chen, Z.-P.; Hu, H.; Lei, J.; Zhou, Z.; Yao, B.; Chen, L.; Liang, G.; Zhan, S.; Zhu, X.; et al. Sperm microRNAs confer depression susceptibility to offspring. Sci. Adv. 2021, 7, eabd7605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, X.; Anwar, A.; Wang, Y.; Hu, H.; Liang, G.; Zhang, C. Paternal environmental exposure-induced spermatozoal small noncoding RNA alteration meditates the intergenerational epigenetic inheritance of multiple diseases. Front. Med. 2022, 16, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, Q. Father’s diet influences son’s metabolic health through sperm RNA. Nature 2024, 630, 571–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.J.M. Male infertility and antioxidants: One small step for man, no giant leap for andrology? Reprod. Biomed. Online 2019, 39, 879–883. [Google Scholar] [CrossRef] [PubMed]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 5, CD007411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J. Antioxidant trials—The need to test for stress. Hum. Reprod. Open 2021, 3, hoab007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turgut, G.; Abban, G.; Turgut, S.; Take, G. Effect of Overdose Zinc on Mouse Testis and Its Relation with Sperm Count and Motility. Biol. Trace Elem. Res. 2003, 96, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y., Jr.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clément, P.; Benkhalifa, M. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Shirahata, T.; Yusa, J.; Sakurai, Y.; Ito, H.; Oshio, S. Long-term dietary intake of excessive vitamin A impairs spermatogenesis in mice. J. Toxicol. Sci. 2019, 44, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-J.; Liu, M.; Niu, Q.-J.; Huang, Y.-X.; Zhao, L.; Lei, X.G.; Sun, L.-H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free. Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Li, X.; Zhou, G.; Sang, Y.; Zhang, M.; Gao, L.; Xue, J.; Zhao, M.; Yu, H.; et al. Dietary selenium excess affected spermatogenesis via DNA damage and telomere-related cell senescence and apoptosis in mice. Food Chem. Toxicol. 2022, 171, 113556. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Zhao, R.; Hu, G.; Dai, G.; Yao, Q.; Chen, C.; Liu, X.; Xue, B. Chronic oral administration of L-carnitine induces testicular injury: In vivo evidence. Int. Urol. Nephrol. 2025, 57, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, A.; Hug, E.; Villeneuve, P.; Bravard, S.; Geurtsen, R.; Saez, F.; Aitken, R.J.; Gharagozloo, P.; Drevet, J.R. The dual nature of micronutrients on fertility: Too much of a good thing? Fertil. Steril. Sci. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Wilkins, A.; Harrison, N.; Kobarfard, K.; Lambourne, S. Towards the Development of Novel, Point-of-Care Assays for Monitoring Different Forms of Antioxidant Activity: The RoXstaTM System. Antioxidants 2024, 13, 1379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gharagozloo, P.; Gutiérrez-Adán, A.; Champroux, A.; Noblanc, A.; Kocer, A.; Calle, A.; Pérez-Cerezales, S.; Pericuesta, E.; Polhemus, A.; Moazamian, A.; et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: Promising preclinical evidence from animal models. Hum. Reprod. 2016, 31, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Hug, E.; Villeneuve, P.; Bravard, S.; Chorfa, A.; Damon-Soubeyrand, C.; Somkuti, S.G.; Moazamian, A.; Aitken, R.J.; Gharagozloo, P.; Drevet, J.R.; et al. Loss of Nuclear/DNA Integrity in Mouse Epididymal Spermatozoa after Short-Term Exposure to Low Doses of Dibutyl Phthalate or Bisphenol AF and Its Mitigation by Oral Antioxidant Supplementation. Antioxidants 2023, 12, 1046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).