Preventive Effect of Probiotic Leuconostoc mesenteroides H40 Against Cognitive Disorder by Anti-Inflammatory, Synaptic Plasticity Regulation, and Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
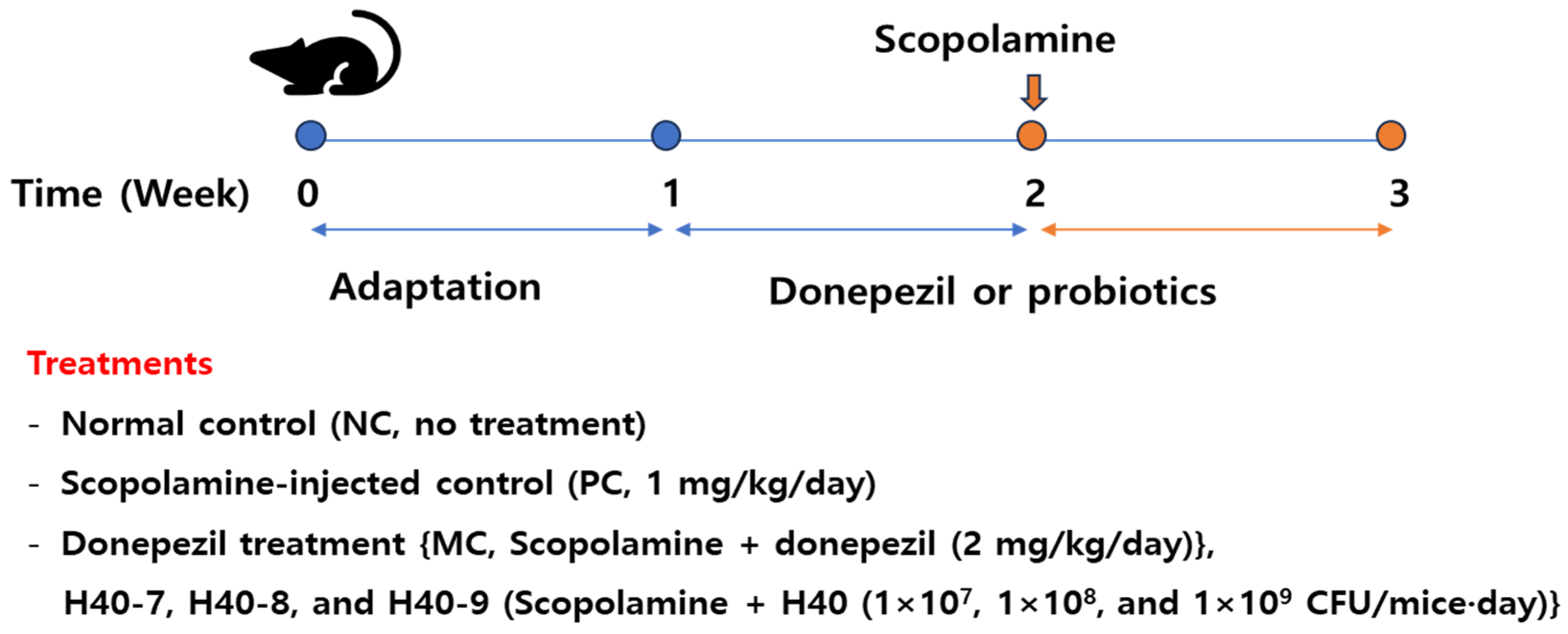
2.2. Experimental Design for Examining the Cognitive Abilities
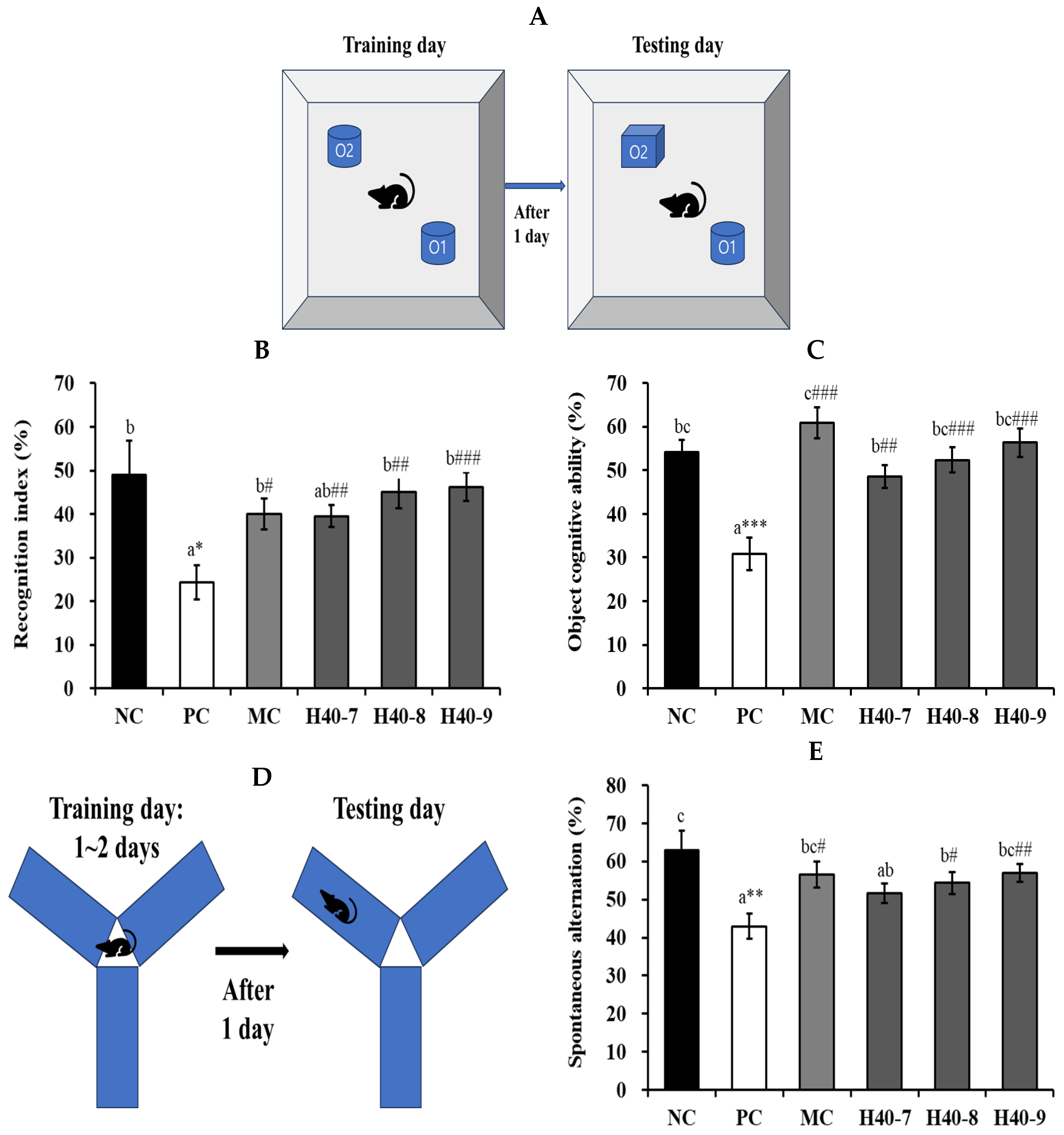
2.3. Novel Object Recognition and Y-Maze Test
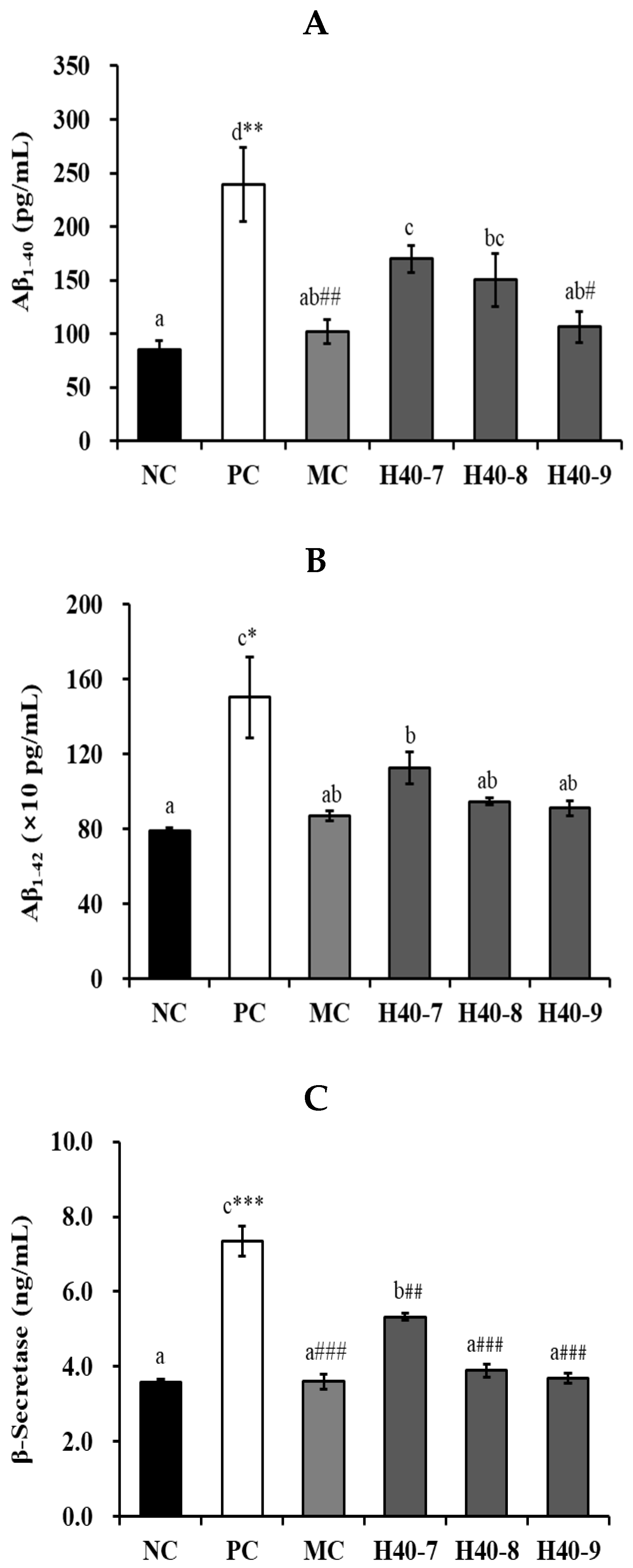
2.4. β-Secretase, Amyloid β, Acetylcholine, Acetylcholinesterase, and Choline Acetyltransferase
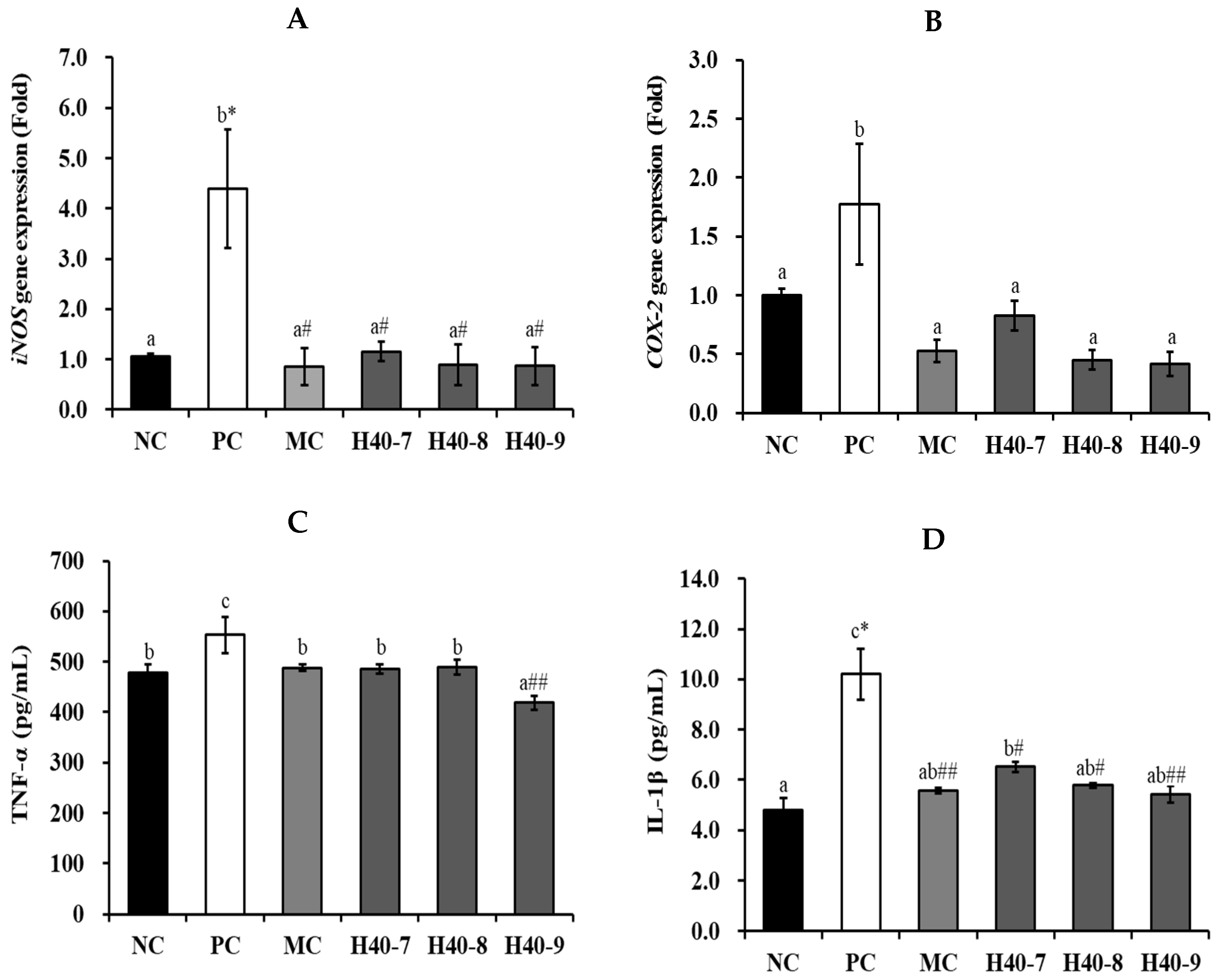
2.5. Cytokines and Factors Related to Neuroinflammatory Effect
2.6. BDNF and CREB Production in Brain Tissue
2.7. Antioxidant Activity
2.8. Statistical Evaluation
3. Results
3.1. Effect of Probiotic H40 on Recognition Index, Object Cognitive Ability, and Y-Maze Tests
3.2. Effect of Probiotic H40 on Amyloid b40, Amyloid b42, and b-Secretase in Hippocampal Tissue
3.3. Effect of Probiotic H40 on Anti-Neuroinflammatory Effect in Hippocampal Tissue
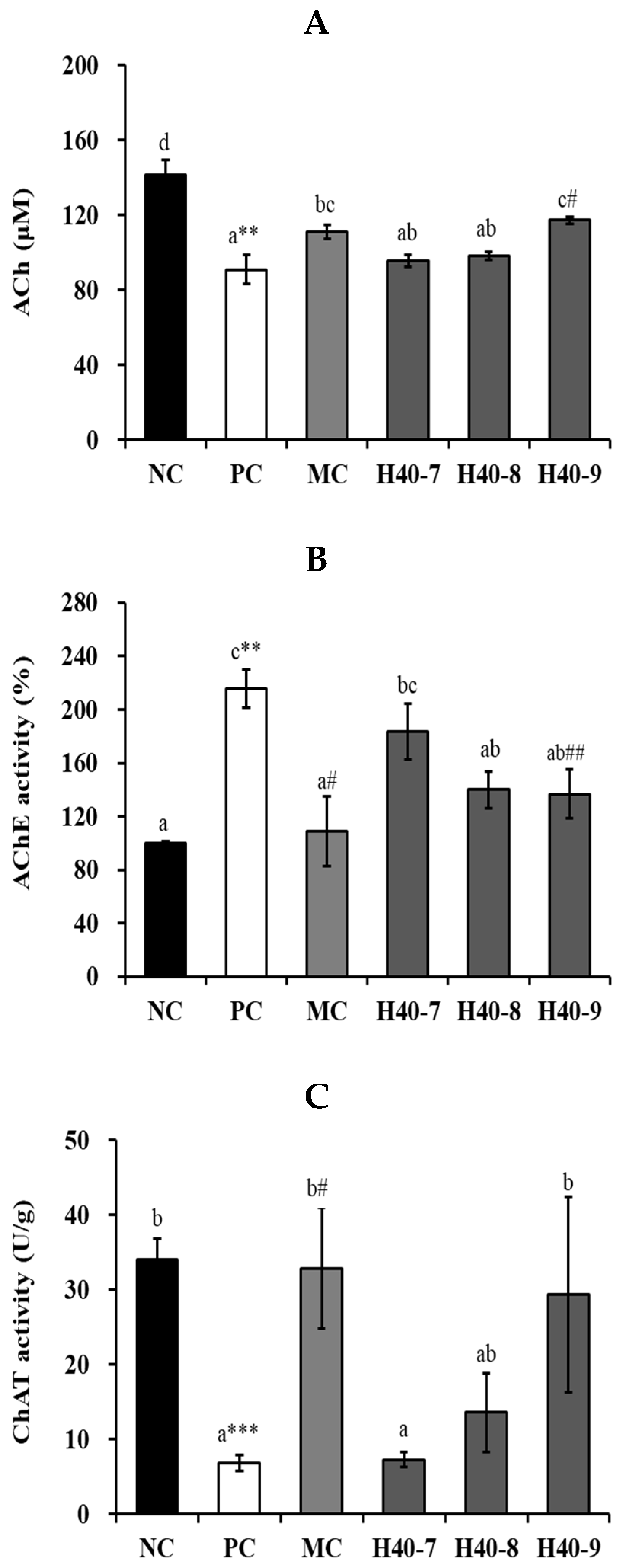
3.4. Effect of Probiotic H40 on Acetylcholine, Acetylcholine Esterase, and Choline Acetyltransferase in Hippocampal Tissue
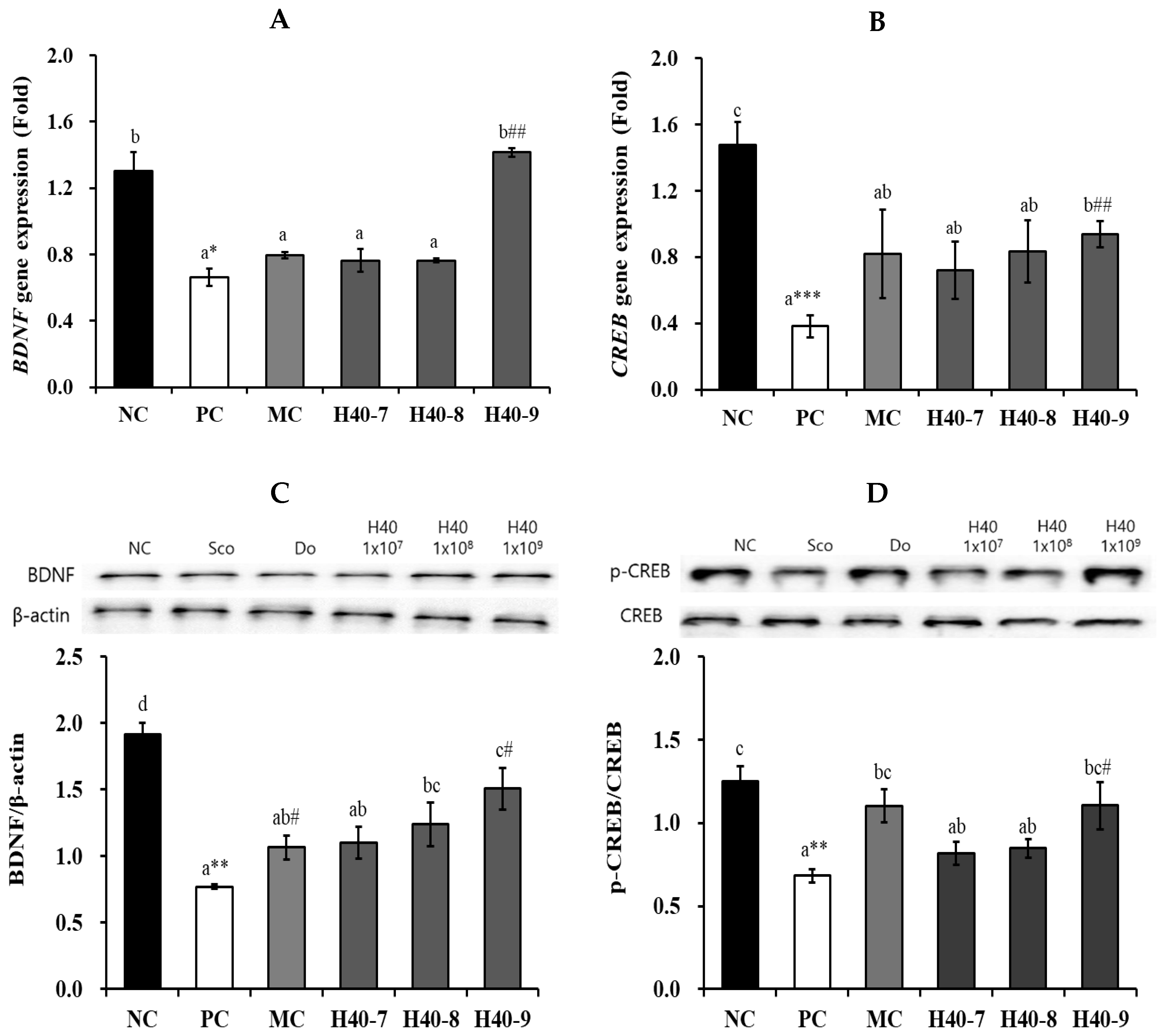
3.5. Effect of Probiotic H40 on BDNF and CREB Expression in Hippocampal Tissue
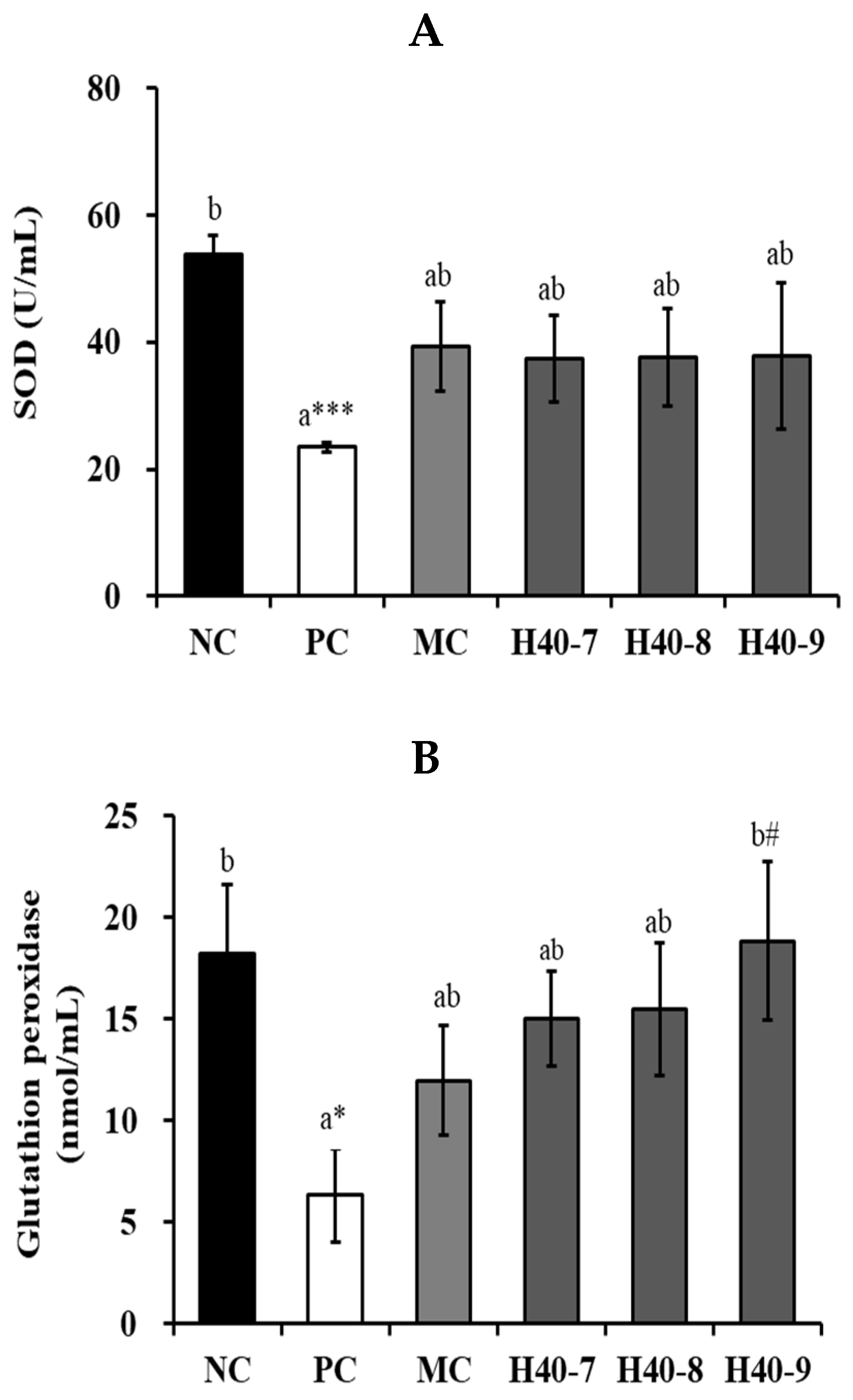
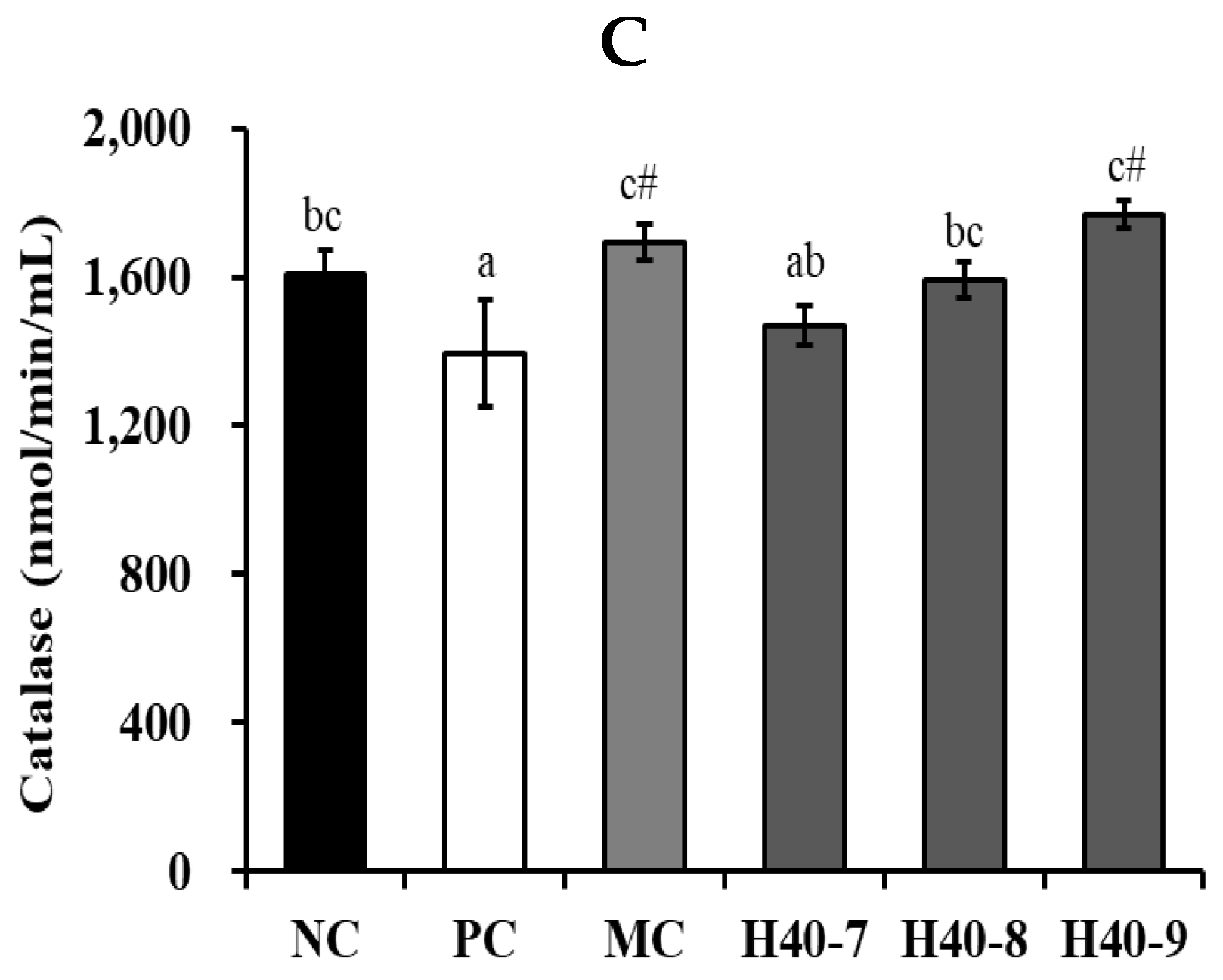
3.6. Antioxidant Effect of Probiotic H40 in Hippocampal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP response element-binding protein |
| AD | Alzheimer’s disease |
| mAChRs | Muscarinic acetylcholine receptors |
| LPS | Lipopolysaccharide |
| PBS | Phosphate buffered saline |
| ELISA | Enzyme-linked immunosorbent assay |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ChAT | Choline acetyltransferase |
| SOD | Superoxide dismutase |
| ANOVA | One-way analysis of variance |
| GSH-Px | Glutathione peroxidase |
References
- WHO (World Health Organization). Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; WHO: Geneva, Switzerland, 2020; pp. 1–96. Available online: https://www.who.int/publications/i/item/9789241550543 (accessed on 23 November 2023).
- Wiels, W.A.; Wittens, M.M.J.; Zeeuws, D.; Baeken, C.; Engelborghs, S. Neuropsychiatric symptoms in mild cognitive impairment and dementia due to AD: Relation with disease stage and cognitive deficits. Front. Psychiatry 2021, 12, 707580. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Liu, Y.; Zhang, X.; Wang, J.; Zhao, J.; He, J.; Zhou, H.; Mei, L.; Liang, X. Discovery of new muscarinic acetylcholine receptor antagonists from Scopolia tangutica. Sci. Rep. 2017, 7, 46067. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The impact of microbiota on the gut-brain axis: Examining the complex interplay and implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, Y.; Park, J.Y.; Park, E.; Paik, H.D. Heat-killed Lactococcus lactis KC24 ameliorates scopolamine-induced memory impairment in ICR mice. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Matin, S.; Dadkhah, M. BDNF/CREB signaling pathway contribution in depression pathogenesis: A survey on the non-pharmacological therapeutic opportunities for gut microbiota dysbiosis. Brain Res. Bull. 2024, 207, 110882. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Gu, M.; Nguyen, H.T.; Cho, J.H.; Suh, J.W.; Cheng, J. Characterization of Leuconostoc mesenteroides MJM60376 as an oral probiotic and its antibiofilm activity. Mol. Oral Microbiol. 2023, 38, 145–157. [Google Scholar] [CrossRef]
- Park, J.; Heo, S.; Lee, G.; Hong, S.W.; Jeong, D.W. Bacterial diversity of baechu-kimchi with seafood based on culture-independent investigation. Food Sci. Biotechnol. 2024, 33, 1661–1670. [Google Scholar] [CrossRef]
- Schifano, E.; Tomassini, A.; Preziosi, A.; Montes, J.; Aureli, W.; Mancini, P.; Miccheli, A.; Uccelletti, D. Leuconostoc mesenteroides strains isolated from carrots show probiotic features. Microorganisms 2021, 9, 2290. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Kim, E.H.; Reaney, M.J.T.; Shim, Y.Y.; Chung, M.J. Immunomodulatory activity of extracellular vesicles of kimchi-derived lactic acid bacteria (Leuconostoc mesenteroides, Latilactobacillus curvatus, and Lactiplantibacillus plantarum). Foods 2022, 11, 313. [Google Scholar] [CrossRef]
- Lee, N.K.; Lim, S.M.; Cheon, M.J.; Paik, H.D. Physicochemical analysis of yogurt produced by Leuconostoc mesenteroides H40 and its effects on oxidative stress in neuronal cells. Food Sci. Anim. Resour. 2021, 41, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Lee, Y.; Hwang, J.; Park, E.; Paik, H.D. Heat-killed Leuconostoc mesenteroides H40 alleviates cognitive impairment by anti-inflammation and antioxidant effects in a scopolamine-induced mouse model. J. Microbiol. Biotechnol. 2025, 35, e2411013. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Park, Y.S.; Kang, D.K.; Paik, H.D. Paraprobiotics: Definition, manufacturing methods, and functionality. Food Sci. Biotechnol. 2023, 32, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, N.; Aghamohammad, S.; Gholizadeh Khiavi, E.H.A.; Pourshafie, M.R.; Talebi, M.; Rohani, M. Comparison of novel native probiotics and paraprobiotics in modulating oxidative stress and inflammation in DSS-induced colitis: Implications for enhanced therapeutic strategies in high fat diet. BMC Immunol. 2024, 25, 85. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Koo, B.N.; Kim, S.Y.; Kam, E.H.; Nam, J.; Kim, E.J. Scopolamine promotes neuroinflammation and delirium-like neuropsychiatric disorder in mice. Sci. Rep. 2021, 11, 8376. [Google Scholar] [CrossRef]
- DArgenio, V.; Sarnataro, D. Probiotics, prebiotics and their role in Alzheimer’s disease. Neural. Regen. Res. 2021, 16, 1768–1769. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. β-amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef]
- Zuliani, G.; Trentini, A.; Brombo, G.; Rosta, V.; Guasti, P.; Romagnoli, T.; Polastri, M.; Marabini, L.; Pedrini, D.; Pistolesi, C.; et al. Serum beta-secretase 1 (BACE1) activity increases in patients with mild cognitive impairment. J. Neurochem. 2021, 159, 629–637. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.G.; Michikawa, M. Probiotic Bifidobacterium breve prevents memory impairment through the reduction of both amyloid-β production and microglia activation in app knock-in mouse. J. Alzheimer’s Dis. 2022, 85, 1555–1571. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, T.; Fu, J.; Fu, S.; Hu, C.; Sun, B.; Fan, X.; Zhu, J. Lactobacillus acidophilus exerts neuroprotective effects in mice with traumatic brain injury. J. Nutr. 2019, 149, 1543–1552. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflammation 2014, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor alpha signaling during peripheral organ inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.; Rai-Bhogal, R.; Crawford, D.A. Abnormal microglial density and morphology in the brain of cyclooxygenase 2 knockin mice. Neuroscience 2023, 534, 66–81. [Google Scholar] [CrossRef]
- Wong, C.T.; Ussyshkin, N.; Ahmad, E.; Rai-Bhogal, R.; Li, H.; Crawford, D.A. Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression. J. Neurosci. Res. 2016, 94, 759–775. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Camuso, S.; La Rosa, P.; Fiorenza, M.T.; Canterini, S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 2022, 163, 105606. [Google Scholar] [CrossRef]
- Kalivarathan, J.; Kalaivanan, K.; Chandrasekara, S.P.; Nanda, D.; Ramachandran, V.; Venkatraman, A.C. Apigenin modulates hippocampal CREB-BDNF signaling in high fat, high fructose diet-fed rats. J. Funct. Foods 2020, 68, 103898. [Google Scholar] [CrossRef]
- Ortega-Martínez, S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB family transcription factors are major mediators of BDNF transcriptional autoregulation in cortical neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar] [CrossRef]
- Ranuh, R.; Athiyyah, A.F.; Darma, A.; Risky, V.P.; Riawan, W.; Surono, I.S.; Sudarmo, S.M. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iran J. Microbiol. 2019, 11, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Webberley, T.S.; Masetti, G.; Bevan, R.J.; Kerry-Smith, J.; Jack, A.A.; Michael, D.R.; Thomas, S.; Glymenaki, M.; Li, J.; McDonald, J.A.K.; et al. The impact of probiotic supplementation on cognitive, pathological and metabolic markers in a transgenic mouse model of Alzheimer’s disease. Front. Neurosci. 2022, 16, 843105. [Google Scholar] [CrossRef] [PubMed]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New insights into oxidative stress and inflammatory response in neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Simeonova, R.; Atanasova, M.; Stavrakov, G.; Philipova, I.; Doytchinova, I. Ex vivo antioxidant and cholinesterase inhibiting effects of a novel galantamine-curcumin hybrid on scopolamine-induced neurotoxicity in mice. Int. J. Mol. Sci. 2022, 23, 14843. [Google Scholar] [CrossRef]
- Cullen, N.C.; Leuzy, A.; Palmqvist, S.; Janelidze, S.; Stomrud, E.; Pesini, P.; Sarasa, L.; Allué, J.A.; Proctor, N.K.; Zetterberg, H.; et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat. Aging 2021, 1, 114–123. [Google Scholar] [CrossRef]
- Arévalo-Caro, C.; López, D.; Sánchez Milán, J.A.; Lorca, C.; Mulet, M.; Arboleda, H.; Losada Amaya, S.; Serra, A.; Gallart-Palau, X. Periodontal indices as predictors of cognitive decline: Insights from the PerioMind Colombia cohort. Biomedicines 2025, 13, 205. [Google Scholar] [CrossRef]
- Shen, X.N.; Lu, X.; Tan, C.T.Y.; Liu, L.Y.; Yu, J.T.; Feng, L.; Larbi, A. Identification of inflammatory and vascular markers associated with mild cognitive impairment. Aging 2019, 11, 2403–2419. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.-K.; Lee, Y.; Won, M.; Kim, N.; Park, E.; Paik, H.-D. Preventive Effect of Probiotic Leuconostoc mesenteroides H40 Against Cognitive Disorder by Anti-Inflammatory, Synaptic Plasticity Regulation, and Antioxidant Effects. Antioxidants 2025, 14, 565. https://doi.org/10.3390/antiox14050565
Lee N-K, Lee Y, Won M, Kim N, Park E, Paik H-D. Preventive Effect of Probiotic Leuconostoc mesenteroides H40 Against Cognitive Disorder by Anti-Inflammatory, Synaptic Plasticity Regulation, and Antioxidant Effects. Antioxidants. 2025; 14(5):565. https://doi.org/10.3390/antiox14050565
Chicago/Turabian StyleLee, Na-Kyoung, Yunjung Lee, Minhye Won, Nayeong Kim, Eunju Park, and Hyun-Dong Paik. 2025. "Preventive Effect of Probiotic Leuconostoc mesenteroides H40 Against Cognitive Disorder by Anti-Inflammatory, Synaptic Plasticity Regulation, and Antioxidant Effects" Antioxidants 14, no. 5: 565. https://doi.org/10.3390/antiox14050565
APA StyleLee, N.-K., Lee, Y., Won, M., Kim, N., Park, E., & Paik, H.-D. (2025). Preventive Effect of Probiotic Leuconostoc mesenteroides H40 Against Cognitive Disorder by Anti-Inflammatory, Synaptic Plasticity Regulation, and Antioxidant Effects. Antioxidants, 14(5), 565. https://doi.org/10.3390/antiox14050565






