Dietary 5-Aminolevulinic Acid Alleviates Heat Stress-Induced Renal Injury in Laying Hens by Improving Mitochondrial Quality and Enhancing Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diet, and Experimental Design
2.3. Blood Collection and Analysis
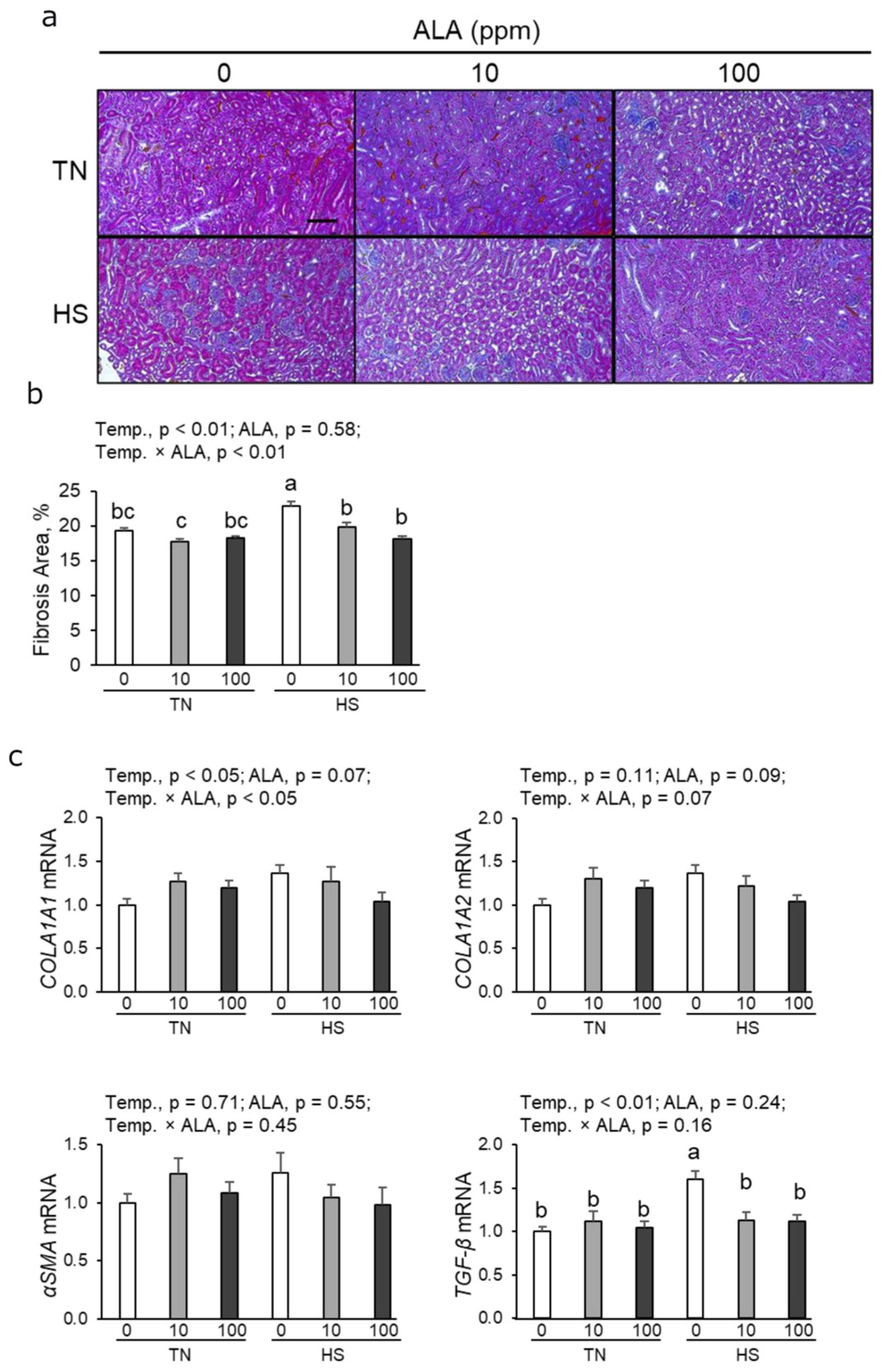
2.4. Histological Analysis of the Fibrotic Area
2.5. Total RNA Isolation, cDNA Synthesis, and Real-Time PCR
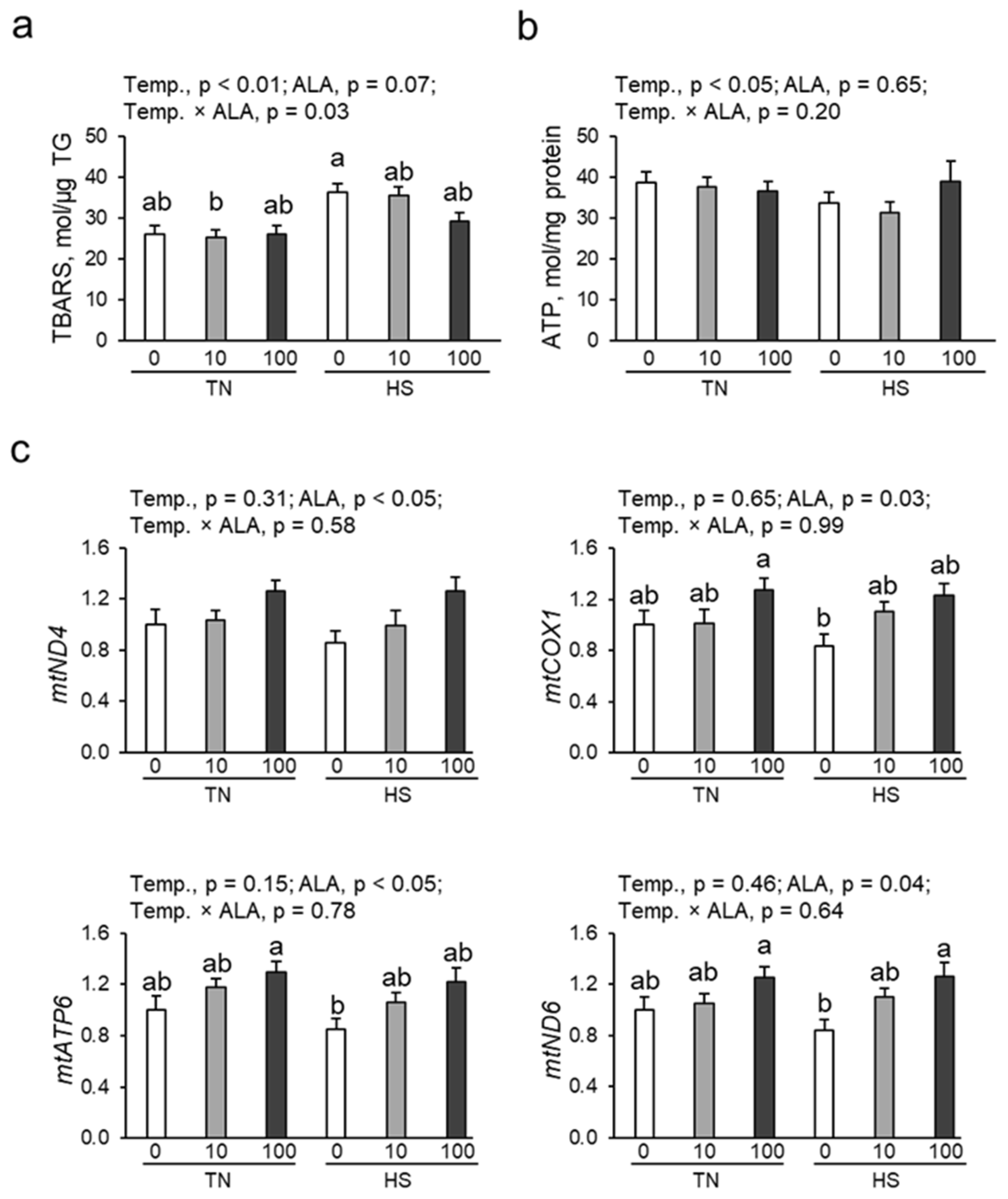
2.6. Determination of 2-Thiobarbituric Acid Reactive Substances (TBARS) Content
2.7. Tissue ATP Content
2.8. Determination of mtDNA Relative Expression and Copy Number
2.9. Statistical Analysis
3. Results
3.1. Laying Performance and Eggshell Quality
3.2. Biochemical Plasmatic Parameters
3.3. Histological Analysis of the Fibrotic Area and Profibrotic mRNA Expression in Renal Tissue
3.4. Oxidative Damage, ATP Content, and mtDNA Copy Number in Renal Tissue
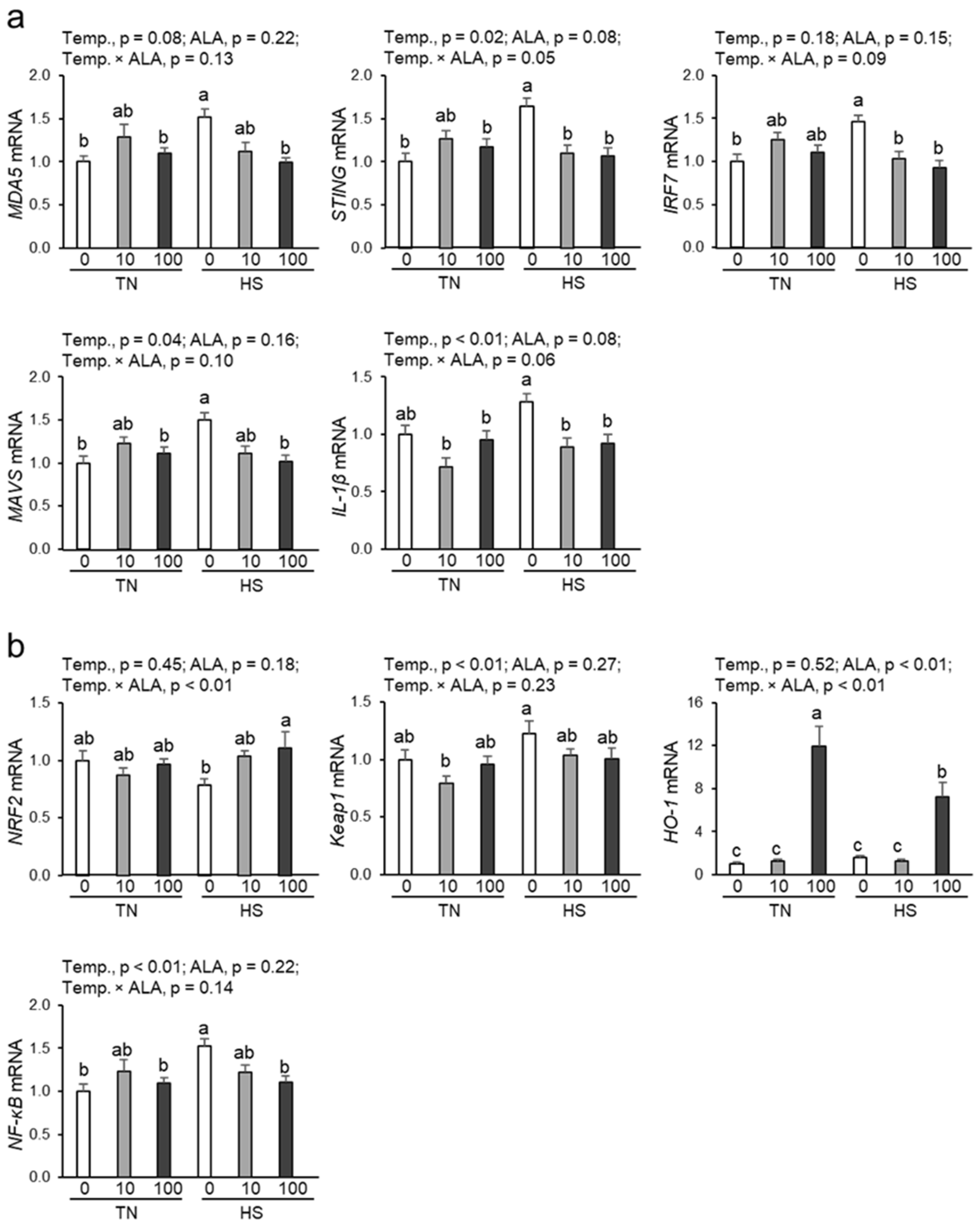
3.5. Expression of Genes Related to the cGAS-STING Pathway in Renal Tissue
3.6. Expression of Genes Related to the NRF2/HO-1 Pathway and NF-kB in Renal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP6 | ATP synthase F0 subunit 6 |
| cGAS | cyclic GMP-AMP synthase |
| COX1 | mitochondrial cytochrome c oxidase 1 |
| HO-1 | heme oxygenase-1 |
| IL-1β | interleukin-1β |
| IRF7 | type I interferon regulatory factor 7 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MAVS | mitochondrial antiviral signaling |
| MDA5 | melanoma differentiation-associated gene 5 |
| mtDNA | mitochondrial DNA copy number |
| ND4 | NADH dehydrogenase subunit 4 |
| ND6 | NADH dehydrogenase subunit 6 |
| NF-ĸB | nuclear factor-κB |
| NRF2 | nuclear factor E2-related factor 2 |
| ROS | reactive oxygen species |
| STING | stimulator of interferon genes |
| TBARS | 2-thiobarbituric acid reactive substances |
| TGF-β | transforming growth factor-β |
| 18srRNA | 18S ribosomal RNA |
References
- Mirzabaev, A.; Bezner Kerr, R.; Hasegawa, T.; Pradhan, P.; Wreford, A.; Cristina Tirado von der Pahlen, M.; Gurney-Smith, H. Severe Climate Change Risks to Food Security and Nutrition. Clim. Risk Manag. 2023, 39, 100473. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain; a Review of the Evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef]
- Allahverdi, A.; Feizi, A.; Takhtfooladi, H.A.; Nikpiran, H. Effects of Heat Stress on Acid-Base Imbalance, Plasma Calcium Concentration, Egg Production and Egg Quality in Commercial Layers. Glob. Vet. 2013, 10, 203–207. [Google Scholar]
- De Baets, R.; Buyse, K.; Antonissen, G.; Delezie, E. Betaine and Feed Restriction as Potential Mitigation Strategies against Heat Stress in Two Strains of Laying Hens. Poult. Sci. 2024, 103, 104104. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Nanto-Hara, F.; Yamazaki, M.; Murakami, H.; Ohtsu, H. Chronic Heat Stress Induces Renal Fibrosis and Mitochondrial Dysfunction in Laying Hens. J. Anim. Sci. Biotechnol. 2023, 14, 81. [Google Scholar] [CrossRef]
- Nanto-Hara, F.; Ohtsu, H. In Laying Hens, Chronic Heat Stress-Induced Renal Fibrosis Is Potentially Promoted by Indoxyl Sulfate. Sci. Rep. 2024, 14, 23213. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent Advances in Microbial Production of δ-Aminolevulinic Acid and Vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, K.; Mao, Y.; Ma, H.; Chen, T.; Wang, Z. Natural 5-Aminolevulinic Acid: Sources, Biosynthesis, Detection and Applications. Front. Bioeng. Biotechnol. 2022, 10, 841443. [Google Scholar] [CrossRef]
- Luo, Z.; Pan, F.; Zhu, Y.; Du, S.; Yan, Y.; Wang, R.; Li, S.; Xu, H. Synergistic Improvement of 5-Aminolevulinic Acid Production with Synthetic Scaffolds and System Pathway Engineering. ACS Synth. Biol. 2022, 11, 2766–2778. [Google Scholar] [CrossRef]
- Pu, W.; Chen, J.; Zhou, Y.; Qiu, H.; Shi, T.; Zhou, W.; Guo, X.; Cai, N.; Tan, Z.; Liu, J.; et al. Correction: Systems Metabolic Engineering of Escherichia Coli for Hyper-Production of 5-Aminolevulinic Acid. Biotechnol. Biofuels Bioprod. 2023, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Watanabe, T.; Tanaka, T.; Tanaka, T. Biosynthesis, Biotechnological Production and Applications of 5-Aminolevulinic Acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29. [Google Scholar] [CrossRef]
- Soto, I.C.; Fontanesi, F.; Myers, R.S.; Hamel, P.; Barrientos, A. A Heme-Sensing Mechanism in the Translational Regulation of Mitochondrial Cytochrome c Oxidase Biogenesis. Cell Metab. 2012, 16, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Maruyama, K.; Hagiya, Y.; Sugiyama, Y.; Tsuchiya, K.; Takahashi, K.; Abe, F.; Tabata, K.; Okura, I.; Nakajima, M.; et al. The Effect of 5-Aminolevulinic Acid on Cytochrome c Oxidase Activity in Mouse Liver. BMC Res. Notes 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Ota, U.; Hara, T.; Nakagawa, H.; Tsuru, E.; Tsuda, M.; Kamiya, A.; Kuroda, Y.; Kitajima, Y.; Koda, A.; Ishizuka, M.; et al. 5-Aminolevulinic Acid Combined with Ferrous Ion Reduces Adiposity and Improves Glucose Tolerance in Diet-Induced Obese Mice via Enhancing Mitochondrial Function. BMC Pharmacol. Toxicol. 2017, 18, 7. [Google Scholar] [CrossRef]
- Terada, Y.; Inoue, K.; Matsumoto, T.; Ishihara, M.; Hamada, K.; Shimamura, Y.; Ogata, K.; Inoue, K.; Taniguchi, Y.; Horino, T.; et al. 5-Aminolevulinic Acid Protects against Cisplatin-Induced Nephrotoxicity without Compromising the Anticancer Efficiency of Cisplatin in Rats In Vitro and In Vivo. PLoS ONE 2013, 8, e80850. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Fujino, M.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhuang, J.; Li, X.-K. 5-Aminolaevulinic Acid (ALA), Enhances Heme Oxygenase (HO)-1 Expression and Attenuates Tubulointerstitial Fibrosis and Renal Apoptosis in Chronic Cyclosporine Nephropathy. Biochem. Biophys. Res. Commun. 2019, 508, 583–589. [Google Scholar] [CrossRef]
- Takeda, T.; Sasai, M.; Adachi, Y.; Ohnishi, K.; Fujisawa, J.; Izawa, S.; Taketani, S. Potential Role of Heme Metabolism in the Inducible Expression of Heme Oxygenase-1. Biochim. Biophys. Acta BBA—Gen. Subj. 2017, 1861, 1813–1824. [Google Scholar] [CrossRef]
- NARO. Japanese Feeding Standard for Poultry, 2011; Japan Livestock Industry Association: Tokyo, Japan, 2012. [Google Scholar]
- Leary, S.; Pharmaceuticals, F.; Underwood, W.; Anthony, R.; Cartner, S.; Johnson, C.L.; Patterson-Kane, E. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Feng, Y.; Hu, Y.; Hou, Z.; Sun, Q.; Jia, Y.; Zhao, R. Chronic Corticosterone Exposure Induces Liver Inflammation and Fibrosis in Association with M6A-Linked Post-Transcriptional Suppression of Heat Shock Proteins in Chicken. Cell Stress Chaperones 2020, 25, 47–56. [Google Scholar] [CrossRef]
- Li, M.; Raheem, M.A.; Han, C.; Yu, F.; Dai, Y.; Imran, M.; Hong, Q.; Zhang, J.; Tan, Y.; Zha, L.; et al. The Fowl Adenovirus Serotype 4 (FAdV-4) Induce Cellular Pathway in Chickens to Produce Interferon and Antigen-Presented Molecules (MHCI/II). Poult. Sci. 2021, 100, 101406. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Chai, Y.; Zhang, H.; Chang, Q.; Li, J.; Zhang, R.; Bao, J. Prolonged Exposure to a Music-Enriched Environment Mitigates Acute Noise-Induced Inflammation and Apoptosis in the Chicken Spleen by Modulating the Keap-1/Nrf2 and NF-ΚB Pathways. Poult. Sci. 2024, 103, 104100. [Google Scholar] [CrossRef]
- Li, Y.P.; Bang, D.D.; Handberg, K.J.; Jorgensen, P.H.; Man, F.Z. Evaluation of the Suitability of Six Host Genes as Internal Control in Real-Time RT-PCR Assays in Chicken Embryo Cell Cultures Infected with Infectious Bursal Disease Virus. Vet. Microbiol. 2005, 110, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Lu, M.Y.; Wang, W.W.; Qi, G.H.; Xu, L.; Wang, J. Mitochondrial Transcription Factor A Induces the Declined Mitochondrial Biogenesis Correlative with Depigmentation of Brown Eggshell in Aged Laying Hens. Poult. Sci. 2021, 100, 100811. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ji, J.; Wang, H.; Zhu, X.; Du, P.; Zhu, Y.; Huang, Y.; Chen, W. The Distinct Spatiotemporal Distribution and Effect of Feed Restriction on MtDNA Copy Number in Broilers. Sci. Rep. 2020, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.O.; Khattab, M.S.; Sugimura, S.; Sato, K. Effects of 5-Aminolevulinic Acid as a Supplement on Animal Performance, Iron Status, and Immune Response in Farm Animals: A Review. Animals 2020, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Jung, J.H.; Kim, I.H. Effects of Dietary Supplementation with Delta-Aminolevulinic Acid on Growth Performance, Hematological Status, and Immune Responses of Weanling Pigs. Livest. Sci. 2011, 140, 131–135. [Google Scholar] [CrossRef]
- Wang, J.P.; Lee, J.H.; Jang, H.D.; Yan, L.; Cho, J.H.; Kim, I.H. Effects of δ-Aminolevulinic Acid and Vitamin C Supplementation on Iron Status, Production Performance, Blood Characteristics and Egg Quality of Laying Hens: δ-Aminolevulinic Acid and Vitamin C in Laying Hens. J. Anim. Physiol. Anim. Nutr. 2011, 95, 417–423. [Google Scholar] [CrossRef]
- Sato, K.; Matsushita, K.; Takahashi, K.; Aoki, M.; Fuziwara, J.; Miyanari, S.; Kamada, T. Dietary Supplementation with 5-Aminolevulinic Acid Modulates Growth Performance and Inflammatory Responses in Broiler Chickens. Poult. Sci. 2012, 91, 1582–1589. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Shirai, M.; Takeya, H.; Sugimura, S.; Miyanari, S.; Taniguchi, S.; Sato, K. Effects of 5-Aminolevulinic Acid Supplementation on Milk Production, Iron Status, and Immune Response of Dairy Cows. J. Dairy. Sci. 2019, 102, 11009–11015. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific Metabolic Rates of Major Organs and Tissues across Adulthood: Evaluation by Mechanistic Model of Resting Energy Expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/Β-catenin/RAS Signaling Mediates Age-related Renal Fibrosis and Is Associated with Mitochondrial Dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Ho, H.-J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, L.; Hu, P.; Huang, R.; Cao, Y.; Deng, J.; Yuan, W.; Liu, D.; Yang, J.; Gu, H.; et al. Aging-Associated Mitochondrial DNA Mutations Alter Oxidative Phosphorylation Machinery and Cause Mitochondrial Dysfunctions. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2017, 1863, 2266–2273. [Google Scholar] [CrossRef]
- Shimura, M.; Nozawa, N.; Ogawa-Tominaga, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Kishita, Y.; Ishii, T.; et al. Effects of 5-Aminolevulinic Acid and Sodium Ferrous Citrate on Fibroblasts from Individuals with Mitochondrial Diseases. Sci. Rep. 2019, 9, 10549. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J.; et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019, 30, 784–799. [Google Scholar] [CrossRef]
- Maekawa, H.; Inoue, T.; Ouchi, H.; Jao, T.M.; Inoue, R.; Nishi, H.; Fujii, R.; Ishidate, F.; Tanaka, T.; Tanaka, Y.; et al. Mitochondrial Damage Causes Inflammation via CGAS-STING Signaling in Acute Kidney Injury. Cell Rep. 2019, 29, 1261–1273. [Google Scholar] [CrossRef]
- Islam, M.A.; Noguchi, Y.; Taniguchi, S.; Yonekura, S. Protective Effects of 5-Aminolevulinic Acid on Heat Stress in Bovine Mammary Epithelial Cells. Anim. Biosci. 2021, 34, 1006–1013. [Google Scholar] [CrossRef]
- Uchida, A.; Kidokoro, K.; Sogawa, Y.; Itano, S.; Nagasu, H.; Satoh, M.; Sasaki, T.; Kashihara, N. 5-Aminolevulinic Acid Exerts Renoprotective Effect via Nrf2 Activation in Murine Rhabdomyolysis-induced Acute Kidney Injury. Nephrology 2019, 24, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic Acid Regulates the Inflammatory Response and Alloimmune Reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637. [Google Scholar] [CrossRef]
- Du, D.; Lv, W.; Su, R.; Yu, C.; Jing, X.; Bai, N.; Hasi, S. Hydrolyzed Camel Whey Protein Alleviated Heat Stress-Induced Hepatocyte Damage by Activated Nrf2/HO-1 Signaling Pathway and Inhibited NF-ΚB/NLRP3 Axis. Cell Stress Chaperones 2021, 26, 387–401. [Google Scholar] [CrossRef]
- Hamada, S.; Mae, Y.; Takata, T.; Hanada, H.; Kubo, M.; Taniguchi, S.; Iyama, T.; Sugihara, T.; Isomoto, H. Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. Int. J. Mol. Sci. 2023, 24, 10151. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Wu, Z.; Gu, H.; Li, C.; Wang, S.; Liu, G. Effects of 5-Aminolevulinic Acid on the Inflammatory Responses and Antioxidative Capacity in Broiler Chickens Challenged with Lipopolysaccharide. Animal 2022, 16, 100575. [Google Scholar] [CrossRef]
| Gene | Primer Sequences (5′–3′) | Accession No. | Source or Reference of the Primer Sequences |
|---|---|---|---|
| COL1A1 | F: ACCTCAGCAAGAACCCCAAG | XM_025144131.2 | [21] |
| R: CTCACCGCCGTACTCAAACT | |||
| COL1A2 | F: GCGGTTTCTACTGGATTGA | NM_001079714.2 | [21] |
| R: AGCGAGACGGCTTATTTG | |||
| αSMA | F: AAGCACCACTGAATCCCAAAG | NM_001031229.1 | [21] |
| R: CCAGAGTCAAGCACAATCCCT | |||
| TGF-β | F: GCAAACTGCGTCTGACCG | NM_001318456.1 | [21] |
| R: ACGAAGAAGATGCTGTGGC | |||
| MDA5 | F: CGAATGAAAACCTGGGACAG | AB371640 | [22] |
| R: TGGTTTTGCCACTGCCTGTA | |||
| STING | F: CGGCTGTGACATCTGGGAT | KP893157 | [22] |
| R: CCCGAGTCAGGATGGTCTC | |||
| IRF7 | F: ACAACGCCAGGAAGGATGTC | NM_205372 | [22] |
| R: CCAGCAGCATGAACATGTGA | |||
| MAVS | F: GAACGCAAACCACCTTCAAC | NM_001012893 | [22] |
| R: CCAGGAGCAGCACTCAAATC | |||
| IL-1β | F: GGCCTGAGTCATGCATCGTT | NM_204524.1 | [22] |
| R: ATAAATACCTCCACCCCGACAA | |||
| NRF2 | F: GGACGGTGACACAGGAACAAC | NM_205117.1 | [23] |
| R: CTCCACAGCGGGAAATCAGAAAG | |||
| Keap1 | F: ACTTCGCTGAGGTCTCCAAG | NM_012289.4 | [23] |
| R: CAGTCGTACTGCACCCAGTT | |||
| HO-1 | F: AGCTTCGCACAAGGAGTGTT | NM_205344.2 | [23] |
| R: CTCCGAGTTCTCCCCGAAAG | |||
| 18srRNA | F: TCAGATACCGTCGTAGTTCC | HQ873432.1 | [24] |
| R: TTCCGTCAATTCCTTTAAGTT |
| Gene | Primer Sequences (5′-3′) | Accession No. | Source or Reference of the Primer Sequences |
|---|---|---|---|
| 1 ND4 | F: CGCAGGCTCCATACTACTCG | NC_040970.1 | [26] |
| R: TTAGGGCACCTCATAGGGCT | |||
| 1 COX1 | F: CCATACTACTTACCGACCGCAACC | NC_040970.1 | [27] |
| R: GTGTCTACGTCCATTCCGACTGTG | |||
| 1 ATP6 | F: ATTCTCAAGCCCCTGCCTAC | NC_053523.1 | [27] |
| R: TCAGAGTTGGATGGTGGAGAGG | |||
| 1 ND6 | F: TAACAACAAACCTCACCCAGCC | NC_053523.1 | [27] |
| R: GTGTGTCTTTTGCTCGGTTGGA | |||
| 2 βactin | F: ATCCGGACCCTCCATTGTC | NM_205518.1 | [27] |
| R: AGCCATGCCAATCTCGTCTT |
| TN (24 °C) | HS (33 °C) | TN | HS | Pooled SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA (ppm) | 0 | 10 | 100 | 0 | 10 | 100 | Temp. | ALA | T × A | |||
| Body weight gain (g/d) | 1.62 a | 0.81 a | 0.86 a | −9.42 b | −6.33 b | −6.54 b | 1.10 | −7.43 | 0.714 | <0.01 | 0.74 | 0.27 |
| Feed intake (g/d) | 119.1 a | 116.3 a | 110.0 a | 86.3 b | 94.9 b | 86.7 b | 115.2 | 89.3 | 2.216 | <0.01 | 0.06 | 0.16 |
| 1 Laying rate (%) | 99.6 a | 99.5 a | 96.8 a | 85.9 b | 86.7 b | 91.1 ab | 98.7 | 88.3 | 1.097 | <0.01 | 0.04 | 0.03 |
| 1 Egg weight (g) | 60.6 | 60.2 | 60.2 | 59.0 | 57.9 | 58.7 | 60.3 | 58.5 | 0.416 | <0.05 | 0.66 | 0.64 |
| 1 Daily egg production (g/d) | 60.4 a | 59.9 a | 58.2 ab | 50.7 b | 50.4 b | 53.6 ab | 59.5 | 51.8 | 0.863 | <0.01 | 0.08 | 0.06 |
| 2 Eggshell strength (kg/cm2) | 4.09 a | 3.95 a | 3.9 a | 3.19 b | 3.92 a | 3.61 ab | 3.97 | 3.58 | 0.068 | <0.01 | 0.15 | 0.14 |
| 2 Eggshell weight (g) | 6.96 a | 6.82 a | 6.82 a | 5.71 b | 6.27 b | 6.00 b | 6.67 | 5.99 | 0.084 | <0.01 | 0.38 | 0.35 |
| 2 Eggshell thickness (mm) | 0.43 a | 0.42 a | 0.42 a | 0.37 b | 0.39 b | 0.39 b | 0.42 | 0.39 | 0.004 | <0.01 | 0.56 | 0.48 |
| TN (24 °C) | HS (33 °C) | TN | HS | Pooled SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA (ppm) | 0 | 10 | 100 | 0 | 10 | 100 | Temp. | ALA | T × A | |||
| Creatinin (mg/dL) | 0.04 b | 0.04 b | 0.03 b | 0.06 a | 0.05 ab | 0.05 ab | 0.04 | 0.05 | 0.002 | <0.01 | 0.06 | 0.80 |
| BUN (mg/dL) | 0.78 | 0.83 | 0.88 | 0.73 | 0.76 | 0.80 | 0.83 | 0.77 | 0.026 | 0.45 | 0.63 | 0.77 |
| Albmin (mg/dL) | 2.49 a | 2.45 a | 2.39 a | 2.13 b | 2.24 ab | 2.06 b | 2.44 | 2.14 | 0.033 | <0.01 | 0.26 | 0.81 |
| Calsium (mg/dL) | 28.23 ab | 29.27 a | 30.87 a | 22.37 c | 25.93 bc | 23.9 bc | 29.46 | 24.12 | 0.611 | <0.01 | 0.12 | 0.58 |
| Phosphote (mg/dL) | 4.05 ab | 4.54 a | 4.89 a | 3.03 b | 3.91 ab | 3.27 b | 4.49 | 3.41 | 0.071 | 0.02 | 0.09 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanto-Hara, F.; Ohtsu, H. Dietary 5-Aminolevulinic Acid Alleviates Heat Stress-Induced Renal Injury in Laying Hens by Improving Mitochondrial Quality and Enhancing Antioxidant Activity. Antioxidants 2025, 14, 556. https://doi.org/10.3390/antiox14050556
Nanto-Hara F, Ohtsu H. Dietary 5-Aminolevulinic Acid Alleviates Heat Stress-Induced Renal Injury in Laying Hens by Improving Mitochondrial Quality and Enhancing Antioxidant Activity. Antioxidants. 2025; 14(5):556. https://doi.org/10.3390/antiox14050556
Chicago/Turabian StyleNanto-Hara, Fumika, and Haruhiko Ohtsu. 2025. "Dietary 5-Aminolevulinic Acid Alleviates Heat Stress-Induced Renal Injury in Laying Hens by Improving Mitochondrial Quality and Enhancing Antioxidant Activity" Antioxidants 14, no. 5: 556. https://doi.org/10.3390/antiox14050556
APA StyleNanto-Hara, F., & Ohtsu, H. (2025). Dietary 5-Aminolevulinic Acid Alleviates Heat Stress-Induced Renal Injury in Laying Hens by Improving Mitochondrial Quality and Enhancing Antioxidant Activity. Antioxidants, 14(5), 556. https://doi.org/10.3390/antiox14050556






