Lactococcus lactis Subsp. lactis LL-1 and Lacticaseibacillus paracasei LP-16 Influence the Gut Microbiota and Metabolites for Anti-Obesity and Hypolipidemic Effects in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. LAB Strains and Culture Conditions
2.2. Animals, Diets, and Experimental Design
2.3. Determination of Serum Biochemical Indices, Oxidative Stress Level, and Hematoxylin–Eosin Staining of Mice Liver
2.4. Determination of SCFAs
2.5. Genomic DNA Extraction and 16S rRNA High Throughput Sequencing
2.6. Metabolomic Analysis of Gut Contents in Mice
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Body Mass in Mice During Feeding Period
3.2. Serum Biochemical Indices of Mice
3.3. Liver Indices and Oxidative Stress Level of Mice
3.4. Production of SCFAs
3.5. Effect of LAB on Gut Bacterial Diversity Indices in Mice
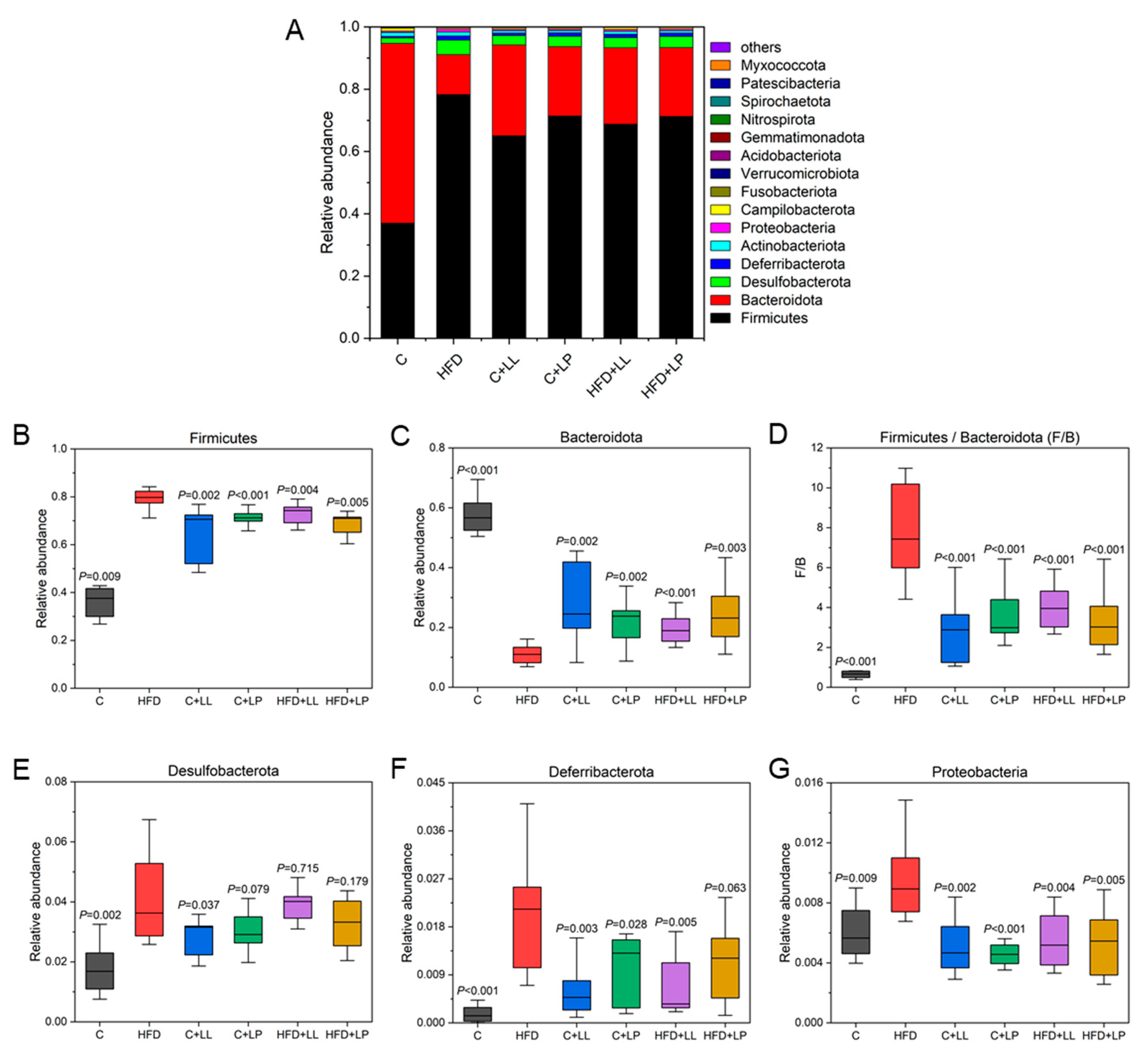
3.6. Effect of LAB on the Gut Bacterial Community Structure in Mice
3.6.1. Species Distribution and Relative Abundance Differences at the Phylum Level
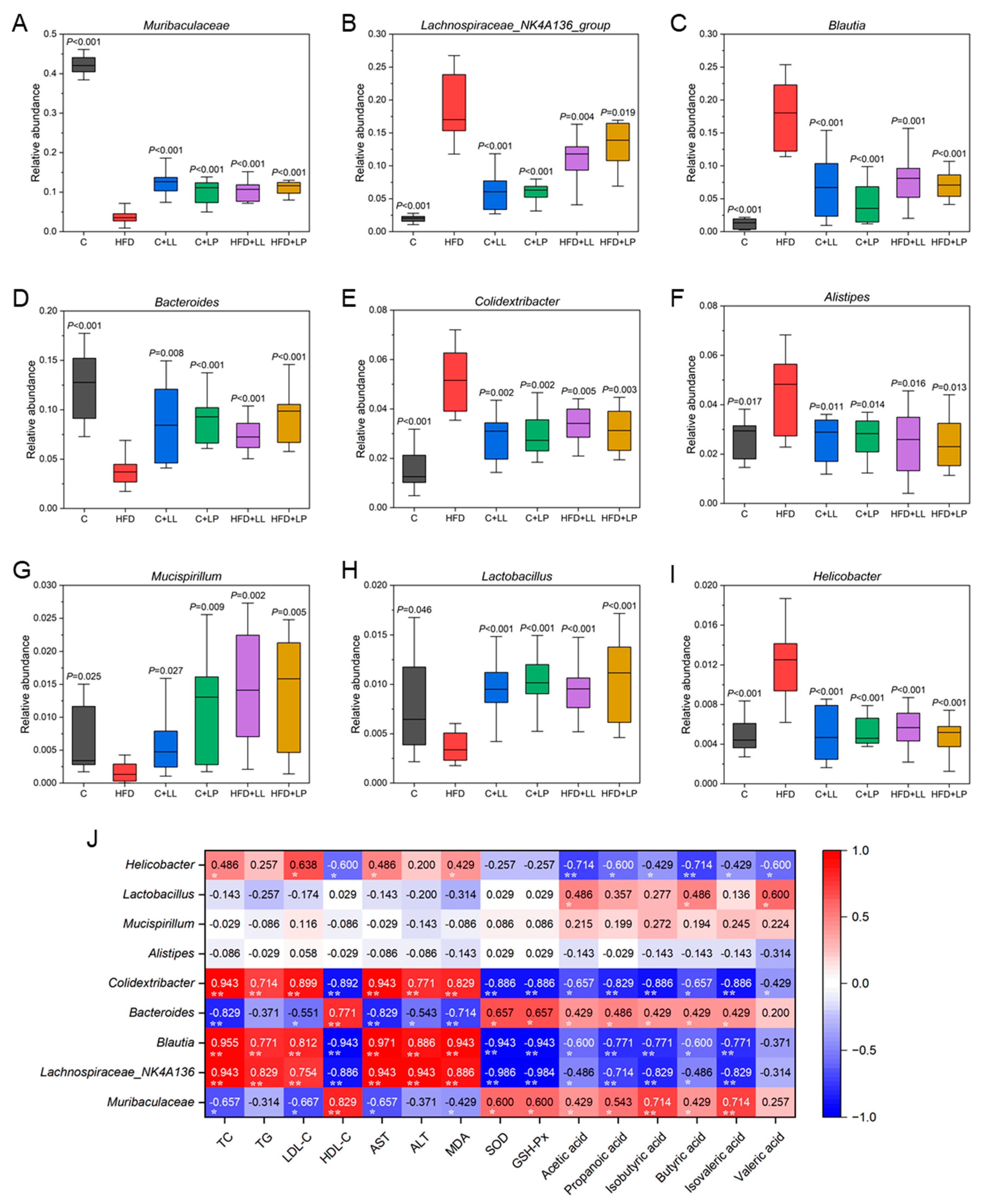
3.6.2. Representative Species Difference at the Genus Level
3.7. Correlations Between the Serum Lipids, Liver Oxidative Stress Levels, Gut SCFAs, and Bacteria Taxa
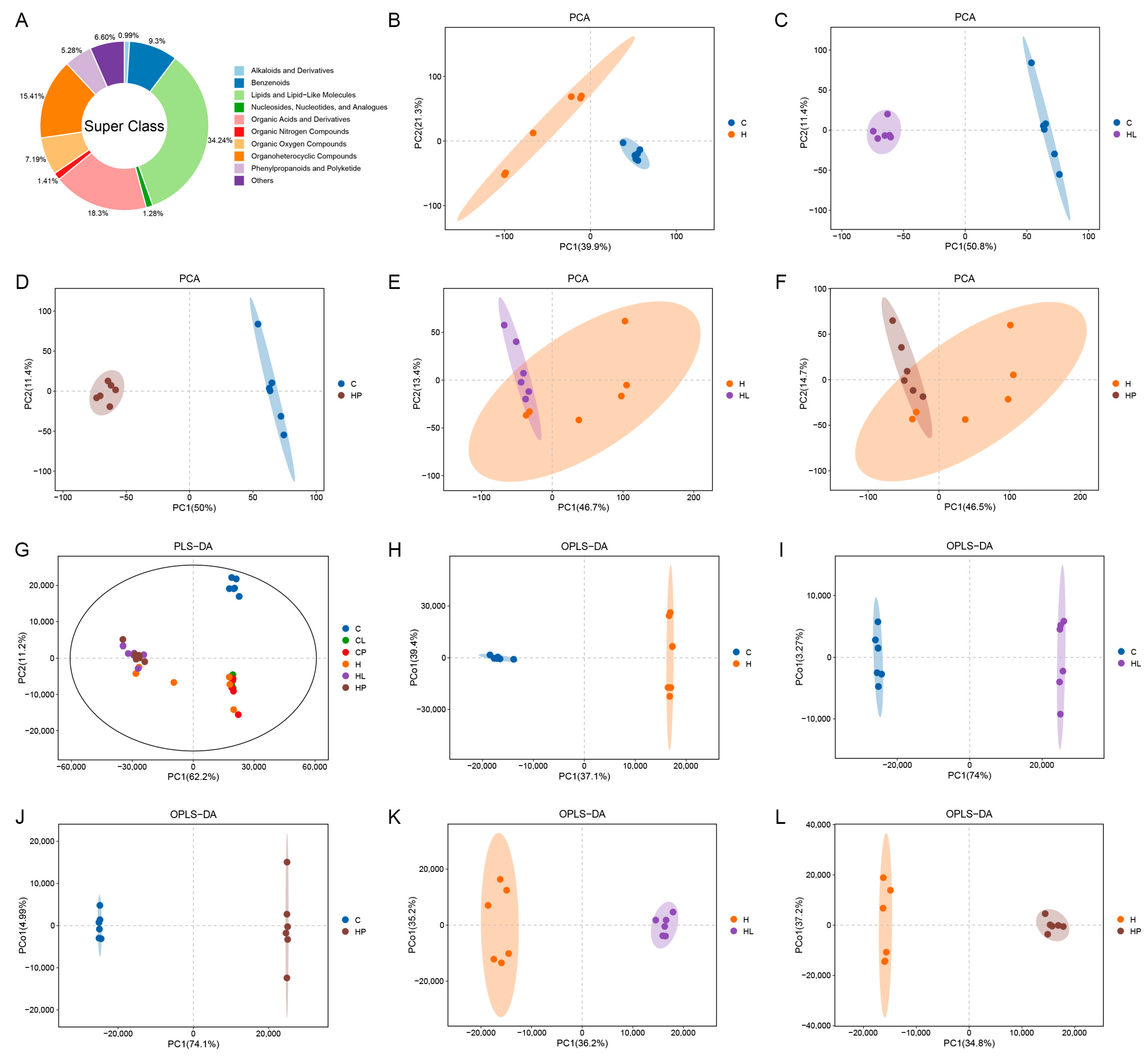
3.8. Multivariate Statistical Analysis of Gut Metabolites in Mice
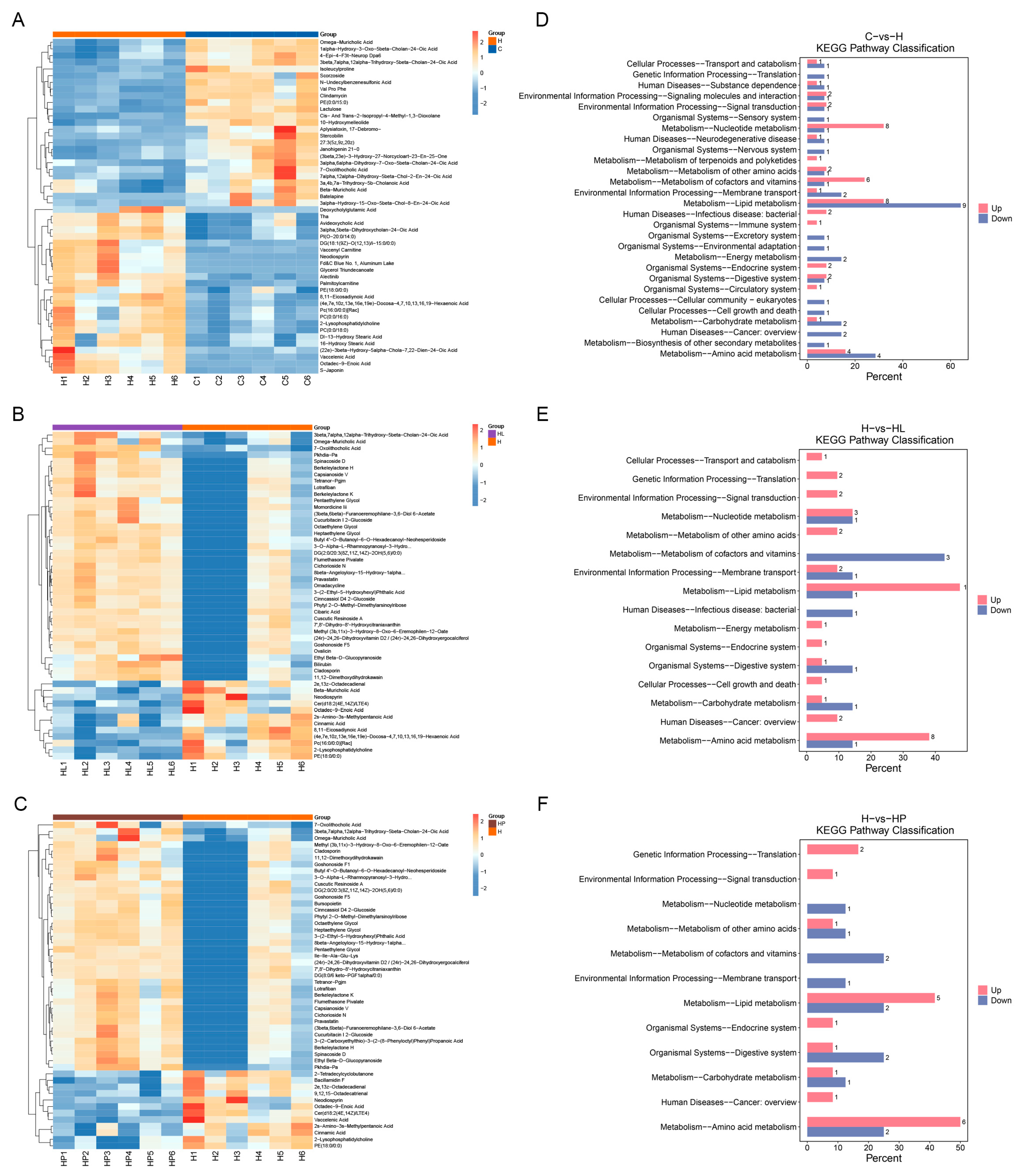
3.9. Differential Metabolites of Gut Metabolites in Mice
3.10. KEGG Pathway Enrichment Analysis of Differential Metabolites in Mice Gut
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2008, 457, 480–484. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, G.; Li, Q.; Gong, L.; Huang, J.; Sang, Y. Tibet kefir milk decreases fat deposition by regulating the gut microbiota and gene expression of Lpl and Angptl4 in high fat diet-fed rats. Food Res. Int. 2019, 121, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Ishida, Y.; Sawada, D.; Ashida, N.; Sugawara, T.; Sakai, M.; Fujiwara, S. Fragmented lactic acid bacterial cells activate peroxisome proliferator-activated receptors and ameliorate dyslipidemia in obese mice. J. Agric. Food Chem. 2016, 64, 2549–2559. [Google Scholar] [CrossRef]
- Guardamagna, O.; Amaretti, A.; Puddu, P.E.; Raimondi, S.; Abello, F.; Cagliero, P.; Rossi, M. Bifidobacteria supplementation: Effects on plasma lipid profiles in dyslipidemic children. Nutrition 2014, 30, 831–836. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Han, H.; Cui, H.; Peng, M.; Wang, G.; Wang, Z. Effect of probiotics on metabolic profiles in type 2diabetes mellitus. Medicine 2016, 95, e4088. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef]
- National Research Council (US) Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA, 1996. [Google Scholar]
- Qian, Y.; Li, M.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Suo, H. Effects of Lactobacillus Casei YBJ02 on lipid metabolism in hyperlipidemic mice. J. Food Sci. 2019, 84, 3793–3803. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin. Liver Dis. 2018, 22, 23–27. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, X.; Zhao, J.; Zhang, Y.; Hu, J.; Wang, J.; Li, J. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol. Nutr. Food Res. 2017, 61, 1700118. [Google Scholar] [CrossRef]
- Kotsis, V.; Antza, C.; Doundoulakis, G.; Stabouli, S. Obesity, Hypertension, and Dyslipidemia; Sbraccia, P., Finer, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 277. [Google Scholar]
- Heshmati, J.; Farsi, F.; Shokri, F.; Rezaeinejad, M.; Almasi-Hashiani, A.; Vesali, S.; Sepidarkish, M. A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J. Funct. Foods 2018, 46, 66–84. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, H.; Oh, S.Y.; Ji, G.E. Anti-obese effects of two Lactobacilli and two Bifidobacteria on ICR mice fed on a high fat diet. Biochem. Biophys. Res. Commun. 2016, 480, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Yang, J.; Mu, J.; Wang, R.; Zhao, X. Effect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight loss and lipid reduction in mice with obesity induced by a high-fat diet. RSC Adv. 2020, 10, 34276–34289. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-C.; Wu, Q.; Cao, Y.-J.; Lin, Y.-C.; Guo, W.-L.; Rao, P.-F.; Zhang, Y.-Y.; Chen, Y.-T.; Ai, L.-Z.; Ni, L. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition. Food Funct. 2022, 13, 5820–5837. [Google Scholar] [CrossRef]
- Khanna, S.; Walia, S.; Kondepudi, K.K.; Shukla, G. Administration of indigenous probiotics modulate high-fat diet-induced metabolic syndrome in sprague dawley rats. Anton. Leeuw. Int. J. G. 2020, 113, 1345–1359. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and vitamin A contents in biological fluids and tissues of animals as an effect of the diet: A review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Shimizu, H.; Masujima, Y.; Ushiroda, C.; Mizushima, R.; Taira, S.; Ohue-Kitano, R.; Kimura, I. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci. Rep. 2019, 9, 16574. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Tazi, A.; Burlen-Defranoux, O.; Vichier-Guerre, S.; Nigro, G.; Licandro, H.; Demignot, S.; Sansonetti, P.J. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host Microbe 2020, 27, 358–375. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2017, 67, 1269–1279. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Wu, P.; Luo, D.-X.; Sun, Q.; Zheng, H.; Hu, R.; Pandol, S.J.; Li, Q.-F.; Han, Y.-P.; et al. Alternation of gut microbiota in patients with pulmonary tuberculosis. Front. Physiol. 2017, 8, 822. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Ma, Y.; Yang, Y.; Cheng, Y.; Ma, H.; Ren, D.; Chen, P. Regulation of viable/inactivated/lysed probiotic Lactobacillus plantarum H6 on intestinal microbiota and metabolites in hypercholesterolemic mice. NPJ Sci. Food 2022, 6, 50. [Google Scholar] [CrossRef]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing reactive oxygen species generation by rebooting gut microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from whole-grain oat alleviated high-fat diet-induced hyperlipidemia via regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef]
- Pan, H.; Yang, S.; Kulyar, M.F.; Ma, H.; Li, K.; Zhang, L.; Mo, Q.; Li, J. Lactobacillus fermentum 016 alleviates mice colitis by modulating oxidative stress, gut microbiota, and microbial metabolism. Nutrients 2025, 17, 452. [Google Scholar] [CrossRef]
- Termite, F.; Archilei, S.; D’Ambrosio, F.; Petrucci, L.; Viceconti, N.; Iaccarino, R.; Liguori, A.; Gasbarrini, A.; Miele, L. Gut microbiota at the crossroad of hepatic oxidative stress and MASLD. Antioxidants 2025, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, Y.; Kim, J.-H.; Zhu, S.; Jung, Y.S.; Han, S.N. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr. Metab. 2020, 17, 44. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, M.; Chen, S.; Wu, S.; Zhang, W. Vitamin D deficiency is associated with dyslipidemia: A cross-sectional study in 3788 subjects. Curr. Med. Res. Opin. 2018, 35, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Alquaiz, A.M.; Kazi, A.; Youssef, R.M.; Alshehri, N.; Alduraywish, S.A. Association between standardized vitamin 25(OH)D and dyslipidemia: A community-based study in Riyadh, Saudi Arabia. Environ. Health Prev. Med. 2020, 25, 4. [Google Scholar] [CrossRef]
- Qin, X.F.; Zhao, L.S.; Chen, W.R.; Yin, D.W.; Wang, H. Effects of vitamin D on plasma lipid profiles in statin-treated patients with hypercholesterolemia: A randomized placebo-controlled trial. Clin. Nutr. 2015, 34, 201–206. [Google Scholar] [CrossRef]
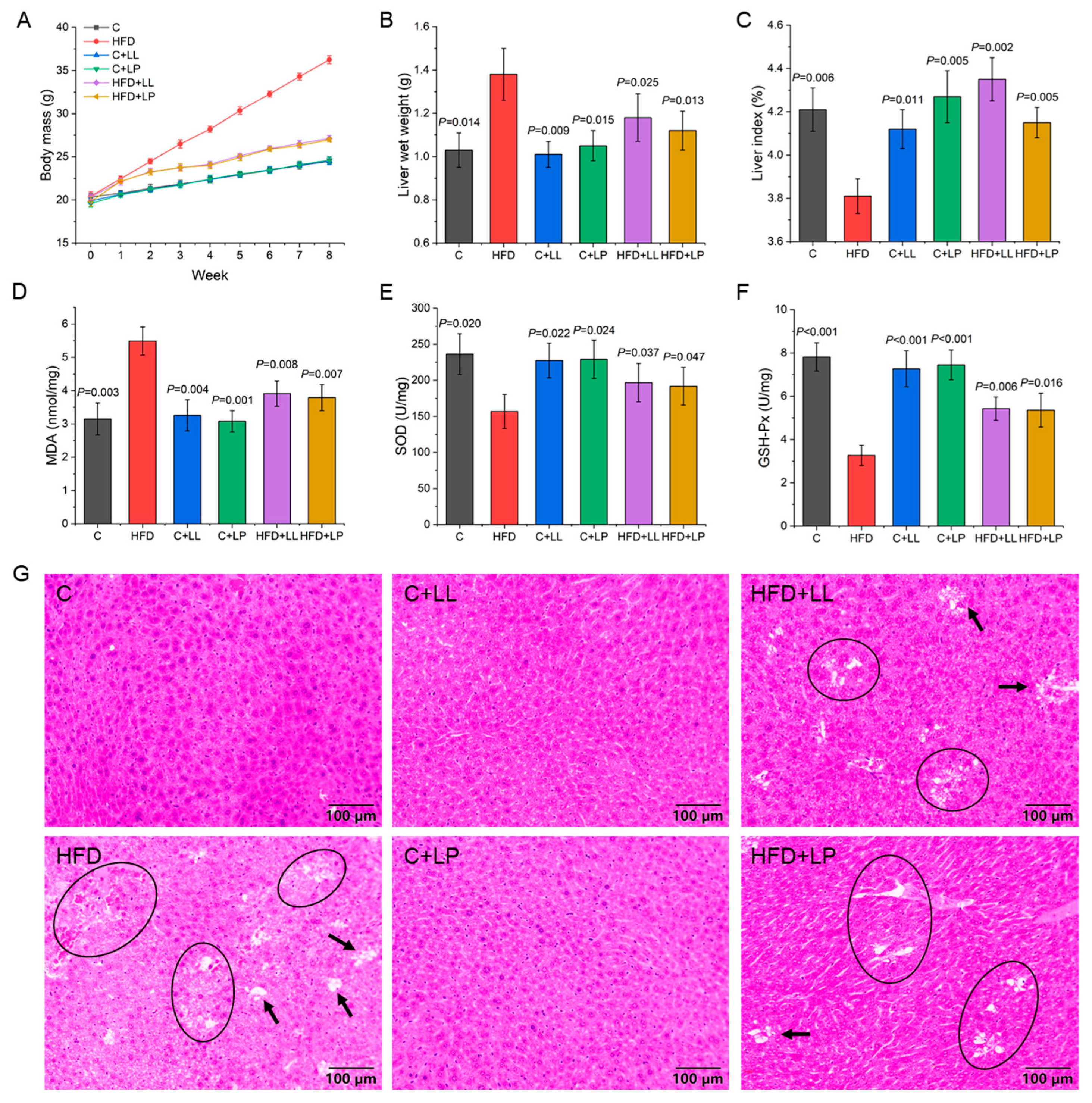

| Group | TC (mmol/L) | p-Value | TG (mmol/L) | p-Value | LDL-C (mmol/L) | p-Value | HDL-C (mmol/L) | p-Value | AST (U/L) | p-Value | ALT (U/L) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 4.10 ± 0.34 | 0.003 | 0.78 ± 0.10 | 0.001 | 0.46 ± 0.03 | <0.001 | 5.08 ± 0.23 | <0.001 | 141.87 ± 8.64 | 0.003 | 40.21 ± 4.25 | <0.001 |
| HFD | 5.78 ± 0.29 | - | 1.49 ± 0.12 | - | 0.88 ± 0.03 | - | 2.69 ± 0.18 | - | 188.90 ± 8.58 | - | 110.49 ± 6.38 | - |
| C+LL | 4.15 ± 0.26 | 0.002 | 0.77 ± 0.08 | <0.001 | 0.42 ± 0.04 | <0.001 | 4.99 ± 0.25 | <0.001 | 146.21 ± 8.41 | 0.004 | 41.27 ± 3.97 | <0.001 |
| C+LP | 4.13 ± 0.25 | 0.002 | 0.76 ± 0.06 | <0.001 | 0.46 ± 0.03 | <0.001 | 4.87 ± 0.23 | <0.001 | 142.62 ± 8.06 | 0.002 | 39.92 ± 4.06 | <0.001 |
| HFD+LL | 4.73 ± 0.32 | 0.014 | 0.95 ± 0.09 | 0.003 | 0.51 ± 0.03 | <0.001 | 4.73 ± 0.20 | <0.001 | 154.10 ± 8.85 | 0.009 | 70.91 ± 5.66 | 0.001 |
| HFD+LP | 4.66 ± 0.30 | 0.010 | 0.99 ± 0.10 | 0.005 | 0.49 ± 0.04 | <0.001 | 4.80 ± 0.26 | <0.001 | 151.55 ± 8.62 | 0.006 | 71.98 ± 5.89 | 0.002 |
| Group | SCFAs (mmol/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | p-Value | Propanoic Acid | p-Value | Isobutyric Acid | p-Value | Butyric Acid | p-Value | Isovaleric Acid | p-Value | Valeric Acid | p-Value | |
| C | 10.46 ± 0.41 | 0.402 | 7.91 ± 0.22 | <0.001 | 1.36 ± 0.04 | <0.001 | 8.05 ± 0.31 | 0.011 | 1.78 ± 0.06 | <0.001 | 1.25 ± 0.04 | 0.007 |
| HFD | 10.15 ± 0.40 | - | 4.95 ± 0.24 | - | 0.98 ± 0.04 | - | 6.90 ± 0.32 | - | 0.99 ± 0.05 | - | 1.04 ± 0.06 | - |
| C+LL | 15.18 ± 0.67 | <0.001 | 7.60 ± 0.38 | <0.001 | 1.32 ± 0.05 | <0.001 | 9.90 ± 0.50 | <0.001 | 1.62 ± 0.08 | <0.001 | 1.72 ± 0.07 | <0.001 |
| C+LP | 13.53 ± 0.50 | <0.001 | 6.81 ± 0.28 | <0.001 | 1.16 ± 0.03 | 0.003 | 8.72 ± 0.32 | 0.002 | 1.32 ± 0.05 | 0.001 | 1.12 ± 0.04 | 0.127 |
| HFD+LL | 10.43 ± 0.22 | 0.348 | 5.67 ± 0.14 | 0.011 | 1.29 ± 0.03 | <0.001 | 7.31 ± 0.23 | 0.146 | 1.40 ± 0.05 | <0.001 | 1.19 ± 0.05 | 0.029 |
| HFD+LP | 11.26 ± 0.40 | 0.027 | 5.94 ± 0.20 | 0.005 | 1.19 ± 0.04 | 0.003 | 8.00 ± 0.30 | 0.012 | 1.21 ± 0.06 | 0.008 | 1.08 ± 0.06 | 0.460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Nie, Y.; Zhao, L.; Zhang, J.; Ge, W. Lactococcus lactis Subsp. lactis LL-1 and Lacticaseibacillus paracasei LP-16 Influence the Gut Microbiota and Metabolites for Anti-Obesity and Hypolipidemic Effects in Mice. Antioxidants 2025, 14, 547. https://doi.org/10.3390/antiox14050547
Gao P, Nie Y, Zhao L, Zhang J, Ge W. Lactococcus lactis Subsp. lactis LL-1 and Lacticaseibacillus paracasei LP-16 Influence the Gut Microbiota and Metabolites for Anti-Obesity and Hypolipidemic Effects in Mice. Antioxidants. 2025; 14(5):547. https://doi.org/10.3390/antiox14050547
Chicago/Turabian StyleGao, Peng, Yuanyang Nie, Lili Zhao, Jing Zhang, and Wupeng Ge. 2025. "Lactococcus lactis Subsp. lactis LL-1 and Lacticaseibacillus paracasei LP-16 Influence the Gut Microbiota and Metabolites for Anti-Obesity and Hypolipidemic Effects in Mice" Antioxidants 14, no. 5: 547. https://doi.org/10.3390/antiox14050547
APA StyleGao, P., Nie, Y., Zhao, L., Zhang, J., & Ge, W. (2025). Lactococcus lactis Subsp. lactis LL-1 and Lacticaseibacillus paracasei LP-16 Influence the Gut Microbiota and Metabolites for Anti-Obesity and Hypolipidemic Effects in Mice. Antioxidants, 14(5), 547. https://doi.org/10.3390/antiox14050547






