Green Tea Intake Reduces High-Fat Diet-Induced Sensory Neuropathy in Mice by Upregulating the Antioxidant Defense System in the Spinal Cord
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Green Tea
2.2. Animals
2.3. High-Fat Diet (HFD)-Induced Neuropathy Model
2.4. Experimental Design
2.5. Von Frey Test for Assessment of Mechanical Nociceptive Threshold
2.6. Morphological and Morphometric Analyses of Sciatic Nerve
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
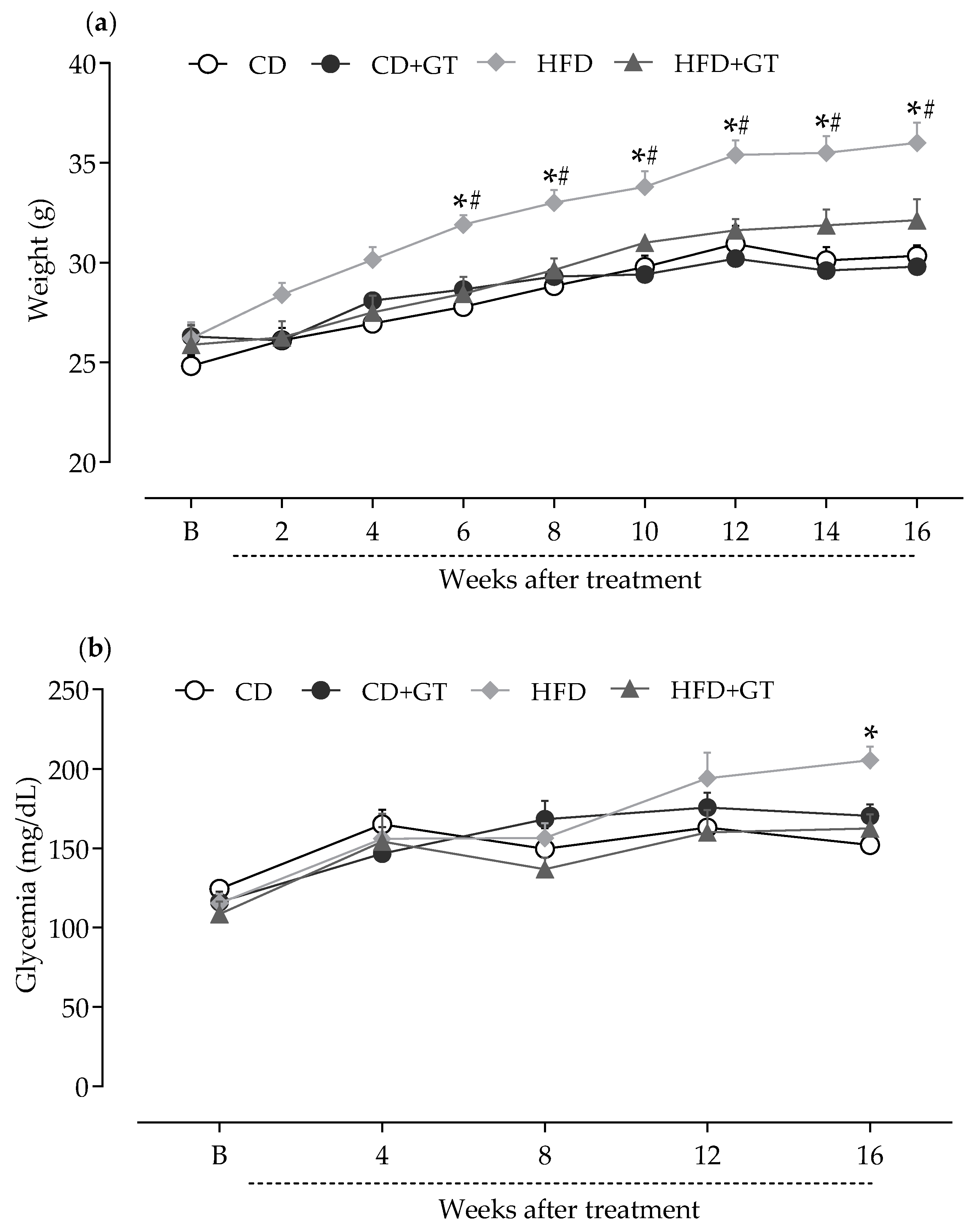
3.1. GT Prevents Weight Gain and Increased Blood Glucose Levels Caused by High-Fat Diet
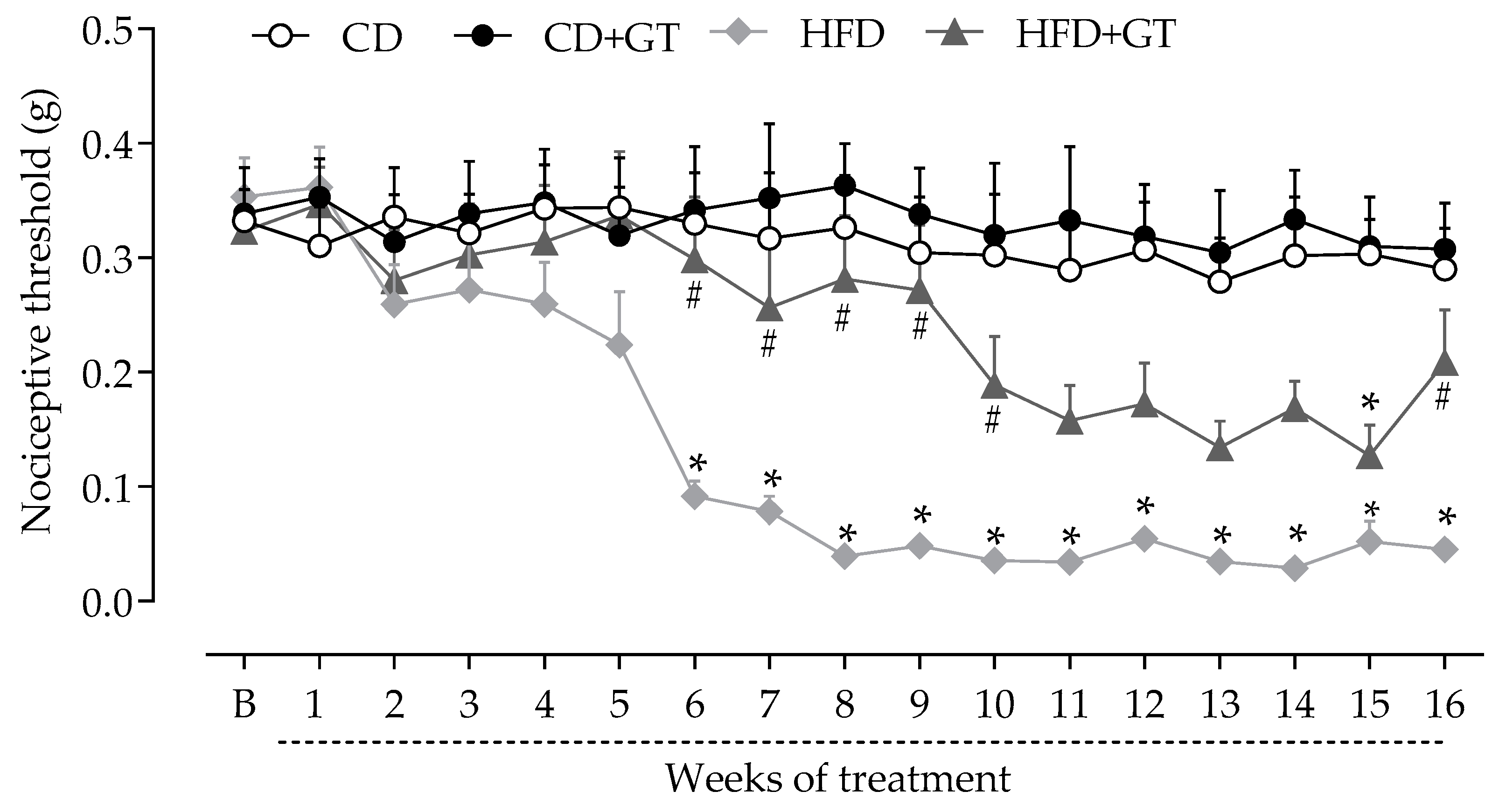
3.2. GT Intake Reduces the Development of Neuropathy in High-Fat Diet -Fed Mice
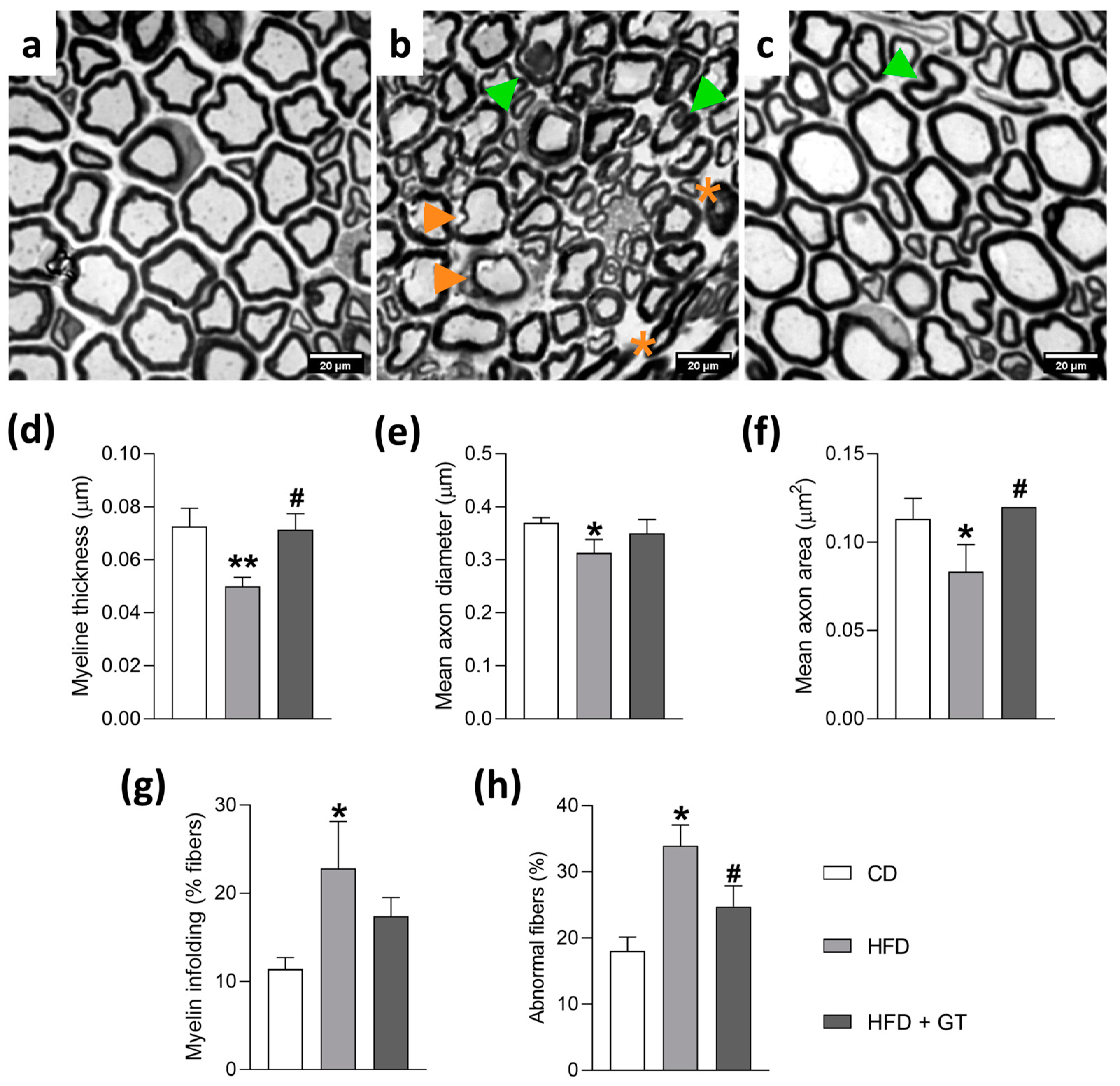
3.3. GT Intake Preserves Morphology of Myelinated Sciatic Nerve Fibers in High-Fat Diet-Induced Neuropathy
3.4. GT Intake Induces Upregulation of Endogenous Antioxidant System Components in the Spinal Cord of Mice in the High-Fat Diet -Induced Neuropathy Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CD | Control diet |
| CNS | Central nervous system |
| GLUT2 | Glucose transporter 2 |
| GPX | Glutathione peroxidase |
| GT | Green tea |
| HFD | High-fat diet |
| NRF-2 | Nuclear factor erythroid 2-related factor 2 |
| PYY | Peptide YY |
| PN | Peripheral neuropathy |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| SD | Standard deviation |
| SGLT-1 | Sodium-glucose transport proteins 1 |
| SOD | Superoxide dismutase |
References
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Primer 2019, 5, 41. [Google Scholar] [CrossRef]
- Rumora, A.E.; Guo, K.; Hinder, L.M.; O’Brien, P.D.; Hayes, J.M.; Hur, J.; Feldman, E.L. A High-Fat Diet Disrupts Nerve Lipids and Mitochondrial Function in Murine Models of Neuropathy. Front. Physiol. 2022, 13, 921942. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Xia, R.; Reynolds, E.; Banerjee, M.; Rothberg, A.E.; Burant, C.F.; Villegas-Umana, E.; Pop-Busui, R.; Feldman, E.L. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016, 73, 1468–1476. [Google Scholar] [CrossRef]
- Ozay, R.; Uzar, E.; Aktas, A.; Uyar, M.E.; Gürer, B.; Evliyaoglu, O.; Cetinalp, N.E.; Turkay, C. The Role of Oxidative Stress and Inflammatory Response in High-Fat Diet Induced Peripheral Neuropathy. J. Chem. Neuroanat. 2014, 55, 51–57. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining High-Fat-Diet Rat Models: Metabolic and Molecular Effects of Different Fat Types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, J.G.; Lim, S.S.; Bode, J.C.; Bode, C. A Diet Rich in Fat and Poor in Dietary Fiber Increases the in Vitro Formation of Reactive Oxygen Species in Human Feces. J. Nutr. 1997, 127, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.-X.; Shea, S.; Mayeux, R. Caloric Intake and the Risk of Alzheimer Disease. Arch. Neurol. 2002, 59, 1258–1263. [Google Scholar] [CrossRef]
- Dandona, P.; Mohanty, P.; Ghanim, H.; Aljada, A.; Browne, R.; Hamouda, W.; Prabhala, A.; Afzal, A.; Garg, R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J. Clin. Endocrinol. Metab. 2001, 86, 355–362. [Google Scholar] [CrossRef]
- Kobayasi, R.; Akamine, E.H.; Davel, A.P.; Rodrigues, M.A.; Carvalho, C.R.; Rossoni, L.V. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J. Hypertens. 2010, 28, 2111–2119. [Google Scholar] [CrossRef]
- Umbaugh, D.S.; Maciejewski, J.C.; Wooten, J.S.; Guilford, B.L. Neuronal Inflammation is Associated with Changes in Epidermal Innervation in High Fat Fed Mice. Front. Physiol. 2022, 13, 891550. [Google Scholar] [CrossRef]
- Coppey, L.J.; Shevalye, H.; Obrosov, A.; Davidson, E.P.; Yorek, M.A. Determination of Peripheral Neuropathy in High-Fat Diet Fed Low-Dose Streptozotocin-Treated Female C57Bl/6J Mice and Sprague-Dawley Rats. J. Diabetes Investig. 2018, 9, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse Strain-Dependent Variation in Obesity and Glucose Homeostasis in Response to High-Fat Feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Ang, L.; Boulton, A.J.M.; Feldman, E.L.; Marcus, R.L.; Mizokami-Stout, K.; Singleton, J.R.; Ziegler, D. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy; ADA Clinical Compendia Series; American Diabetes Association: Arlington, VA, USA, 2022. [Google Scholar]
- Fornasari, D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017, 6 (Suppl. S1), 25–33. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia Sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Saeed, M.; Naveed, M.; Arain, M.A.; Arif, M.; Tiwari, R.; Khandia, R.; Khurana, S.K.; Karthik, K.; et al. Nutritional Applications and Beneficial Health Applications of Green Tea and L-Theanine in Some Animal Species: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 245–256. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of Green Tea Consumption on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Folgado, S.L.; Rajabpour-Sanati, A.; Khazdair, M.R.; Samarghandian, S. Green Tea Catechins Inhibit Microglial Activation Which Prevents the Development of Neurological Disorders. Neural Regen. Res. 2020, 15, 1792–1798. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.T.; Jeon, E.K.; Won, H.S.; Cho, Y.-S.; Ko, Y.H. Effect of Green Tea Extracts on Oxaliplatin-Induced Peripheral Neuropathy in Rats. BMC Complement. Altern. Med. 2012, 12, 124. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Rohilla, A. Herbal Green Tea Extract Alleviates Neuropathic Pain in Mice. J. Neurol. Sci. 2023, 455, 122709. [Google Scholar] [CrossRef]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The Effects of Green Tea on Lipid Metabolism and Its Potential Applications for Obesity and Related Metabolic Disorders-An Existing Update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef]
- Macêdo, A.P.A.; dos Santos, M.G.; da Neto, O.C.S.; Couto, R.D.; David, J.M. Caracterização Fitoquímica e Estabilidade Química do Extrato do Chá Verde. Rev. Virtual Quím. 2023, 15, 1–6. [Google Scholar] [CrossRef]
- Pereira, J.L.; Souza, P.C.S.; Shinzato, V.I.; Sasso, S.; Santo, B.L.S.d.E.; Santana, L.F.; Restel, T.I.; Freitas, K.D.C. Ganho de peso e alterações metabólicas em camundongos submetidos à dieta hiperlipídica. Ciênc. Saúde 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Home Office. Animals (Scientific Procedures) Act 1986. Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/388535/CoPanimalsWeb.pdf (accessed on 10 March 2025).
- NSW Department of Primary Industries and Animal Research Review Panel. Three Rs. Available online: https://www.dpi.nsw.gov.au/dpi/animals/animal-ethics-infolink/three-rs (accessed on 10 March 2025).
- Villarreal, C.F.; Santos, D.S.; Lauria, P.S.S.; Gama, K.B.; Espírito-Santo, R.F.; Juiz, P.J.L.; Alves, C.Q.; David, J.M.; Soares, M.B.P. Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System. Int. J. Mol. Sci. 2020, 21, 4850. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Vannier-Santos, M.A.; de Assis Silva, G.S.; Silva, D.N.; Juiz, P.J.L.; Nonaka, C.K.V.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J. Neuroinflamm. 2018, 15, 189. [Google Scholar] [CrossRef]
- Azcoitia, I.; Leonelli, E.; Magnaghi, V.; Veiga, S.; Garcia-Segura, L.M.; Melcangi, R.C. Progesterone and Its Derivatives Dihydroprogesterone and Tetrahydroprogesterone Reduce Myelin Fiber Morphological Abnormalities and Myelin Fiber Loss in the Sciatic Nerve of Aged Rats. Neurobiol. Aging 2003, 24, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Sameni, H.R.; Panahi, M.; Sarkaki, A.; Saki, G.H.; Makvandi, M. The Neuroprotective Effects of Progesterone on Experimental Diabetic Neuropathy in Rats. Pak. J. Biol. Sci. PJBS 2008, 11, 1994–2000. [Google Scholar] [CrossRef]
- Dos Santos, G.G.L.; Oliveira, A.L.L.; Santos, D.S.; do Espírito Santo, R.F.; Silva, D.N.; Juiz, P.J.L.; Soares, M.B.P.; Villarreal, C.F. Mesenchymal stem cells reduce the oxaliplatin-induced sensory neuropathy through the reestablishment of redox homeostasis in the spinal cord. Life Sci. 2021, 265, 118755–118765. [Google Scholar] [CrossRef]
- Mallet, M.-L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The Role of Oxidative Stress in Peripheral Neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef]
- Jais, A.; Paeger, L.; Sotelo-Hitschfeld, T.; Bremser, S.; Prinzensteiner, M.; Klemm, P.; Mykytiuk, V.; Widdershooven, P.J.M.; Vesting, A.J.; Grzelka, K.; et al. PNOCARC Neurons Promote Hyperphagia and Obesity upon High-Fat-Diet Feeding. Neuron 2020, 106, 1009–1025.e10. [Google Scholar] [CrossRef]
- Sayama, K.; Lin, S.; Zheng, G.; Oguni, I. Effects of Green Tea on Growth, Food Utilization and Lipid Metabolism in Mice. Vivo Athens Greece 2000, 14, 481–484. [Google Scholar]
- Chen, I.-J.; Liu, C.-Y.; Chiu, J.-P.; Hsu, C.-H. Therapeutic Effect of High-Dose Green Tea Extract on Weight Reduction: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin. Nutr. Edinb. Scotl. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The Effects of Green Tea on Weight Loss and Weight Maintenance: A Meta-Analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef]
- Janssens, P.L.H.R.; Hursel, R.; Westerterp-Plantenga, M.S. Nutraceuticals for Body-Weight Management: The Role of Green Tea Catechins. Physiol. Behav. 2016, 162, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Nazaret, C.; Le Gall, M.; Bado, A. Green Tea Decoction Improves Glucose Tolerance and Reduces Weight Gain of Rats Fed Normal and High-Fat Diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A Major Pathway of Intestinal Sugar Absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef]
- Le Gall, M.; Tobin, V.; Stolarczyk, E.; Dalet, V.; Leturque, A.; Brot-Laroche, E. Sugar Sensing by Enterocytes Combines Polarity, Membrane Bound Detectors and Sugar Metabolism. J. Cell. Physiol. 2007, 213, 834–843. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.; Hu, Y.; Li, X.; Wang, J.; Yang, F.; Ji, X.; Wang, S. The Novel Intervention Effect of Cold Green Tea Beverage on High-Fat Diet Induced Obesity in Mice. J. Funct. Foods 2020, 75, 104279. [Google Scholar] [CrossRef]
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J. Peripher. Nerv. Syst. 2015, 20, 24–31. [Google Scholar] [CrossRef]
- Srivastava, B.; Sen, S.; Bhakta, S.; Sen, K. Effect of Caffeine on the Possible Amelioration of Diabetic Neuropathy: A Spectroscopic Study. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2022, 264, 120322. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Abdel-Daim, M.M. Analgesic and Anti-Inflammatory Activities of Epicatechin Gallate from Bauhinia Hookeri. Drug Dev. Res. 2018, 79, 157–164. [Google Scholar] [CrossRef]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiopathology of Neuropathic Pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.R.; Yoon, H.; Kim, H.N.; Choi, C.-I.; Simon, W.; Kleppe, L.; Lanza, I.R.; LeBrasseur, N.K.; Matveyenko, A.; Scarisbrick, I.A. High Fat Diet Consumption Results in Mitochondrial Dysfunction, Oxidative Stress, and Oligodendrocyte Loss in the Central Nervous System. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165630. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Li, H.; Lao, J. Green Tea Polyphenols Promote Functional Recovery from Peripheral Nerve Injury in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923806. [Google Scholar] [CrossRef] [PubMed]
- Jing, B.; Chen, Z.-N.; Si, W.-M.; Zhao, J.-J.; Zhao, G.-P.; Zhang, D. (+)-Catechin Attenuates CCI-Induced Neuropathic Pain in Male Rats by Promoting the Nrf2 Antioxidant Pathway to Inhibit ROS/TLR4/NF-κB-Mediated Activation of the NLRP3 Inflammasome. J. Neurosci. Res. 2024, 102, e25372. [Google Scholar] [CrossRef]
- Islam, F.; Bepary, S.; Nafady, M.H.; Islam, M.R.; Emran, T.B.; Sultana, S.; Huq, M.A.; Mitra, S.; Chopra, H.; Sharma, R.; et al. Polyphenols Targeting Oxidative Stress in Spinal Cord Injury: Current Status and Future Vision. Oxid. Med. Cell. Longev. 2022, 2022, 8741787. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.S.d.A.; Santana, T.d.C.M.; Velozo, A.C.L.; Macêdo, A.P.A.; Gonçalves, M.d.S.; Couto, R.D.; Soares, M.B.P.; Viana, M.D.M.; Villarreal, C.F. Green Tea Intake Reduces High-Fat Diet-Induced Sensory Neuropathy in Mice by Upregulating the Antioxidant Defense System in the Spinal Cord. Antioxidants 2025, 14, 452. https://doi.org/10.3390/antiox14040452
Silva GSdA, Santana TdCM, Velozo ACL, Macêdo APA, Gonçalves MdS, Couto RD, Soares MBP, Viana MDM, Villarreal CF. Green Tea Intake Reduces High-Fat Diet-Induced Sensory Neuropathy in Mice by Upregulating the Antioxidant Defense System in the Spinal Cord. Antioxidants. 2025; 14(4):452. https://doi.org/10.3390/antiox14040452
Chicago/Turabian StyleSilva, Gessica Sabrina de Assis, Thalita da Cruz Monteiro Santana, Ana Carolina Lucchese Velozo, Ana Paula Azevêdo Macêdo, Mariane dos Santos Gonçalves, Ricardo David Couto, Milena Botelho Pereira Soares, Max Denisson Maurício Viana, and Cristiane Flora Villarreal. 2025. "Green Tea Intake Reduces High-Fat Diet-Induced Sensory Neuropathy in Mice by Upregulating the Antioxidant Defense System in the Spinal Cord" Antioxidants 14, no. 4: 452. https://doi.org/10.3390/antiox14040452
APA StyleSilva, G. S. d. A., Santana, T. d. C. M., Velozo, A. C. L., Macêdo, A. P. A., Gonçalves, M. d. S., Couto, R. D., Soares, M. B. P., Viana, M. D. M., & Villarreal, C. F. (2025). Green Tea Intake Reduces High-Fat Diet-Induced Sensory Neuropathy in Mice by Upregulating the Antioxidant Defense System in the Spinal Cord. Antioxidants, 14(4), 452. https://doi.org/10.3390/antiox14040452







