Antioxidant and Anti-Inflammatory Benefits of Gymnema inodorum Leaf Extract in Human Umbilical Vein Endothelial Cells Under Peroxynitrite Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Gymnema inodorum Leaf (GiL) Extract
2.2. Quantification of Kaempferol and Quercetin Using High-Performance Liquid Chromatography (HPLC)
2.3. Human Umbilical Vein Endothelial Cell Culture
2.3.1. Cytotoxic Studies of Peroxynitrite and Tested Compounds
2.3.2. Screening for Protective Doses of Tested Compounds
2.4. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR) Analysis
2.5. Statistical Analysis
3. Results
3.1. Cytotoxicity of Peroxynitrite and Tested Compounds
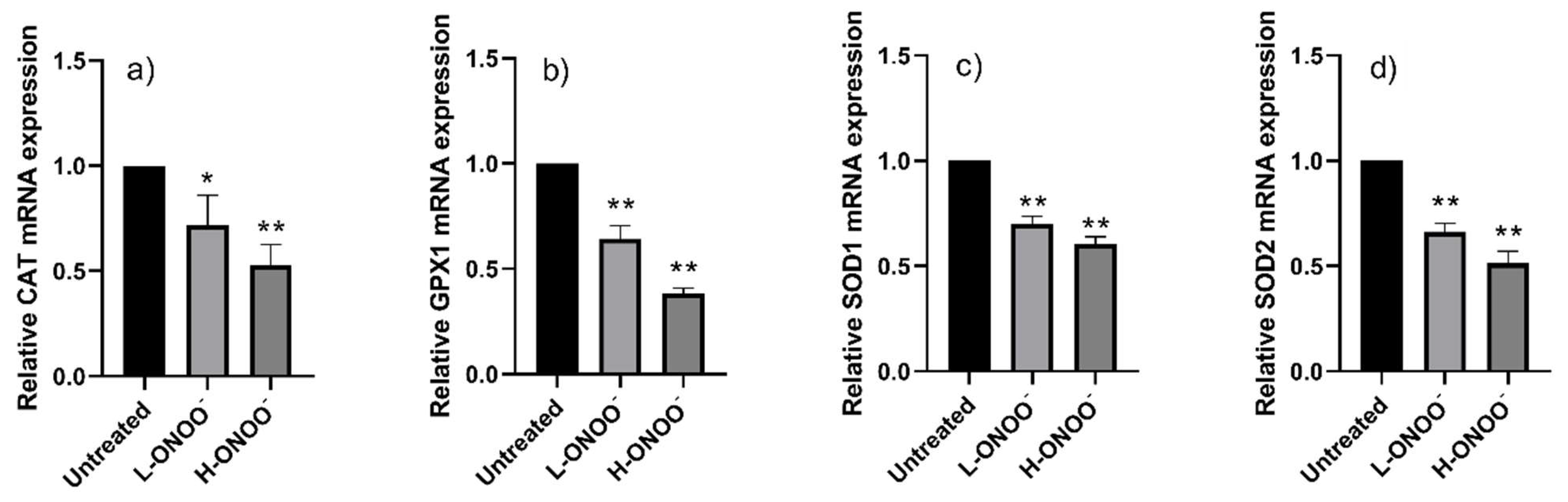
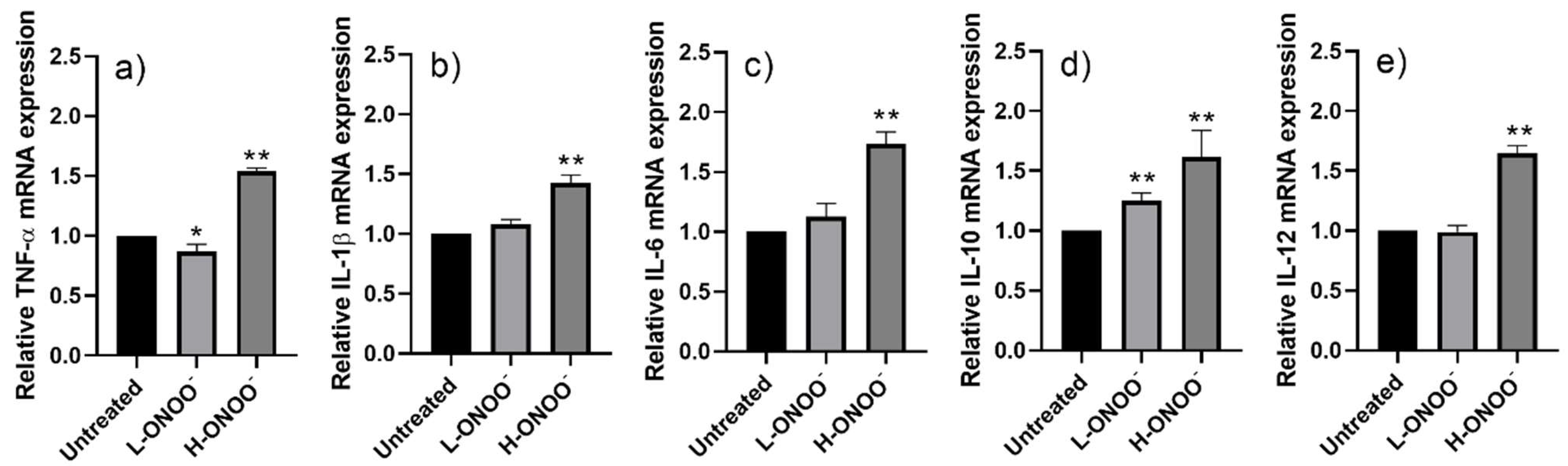
3.2. Effects of Peroxynitrite on HUVECs
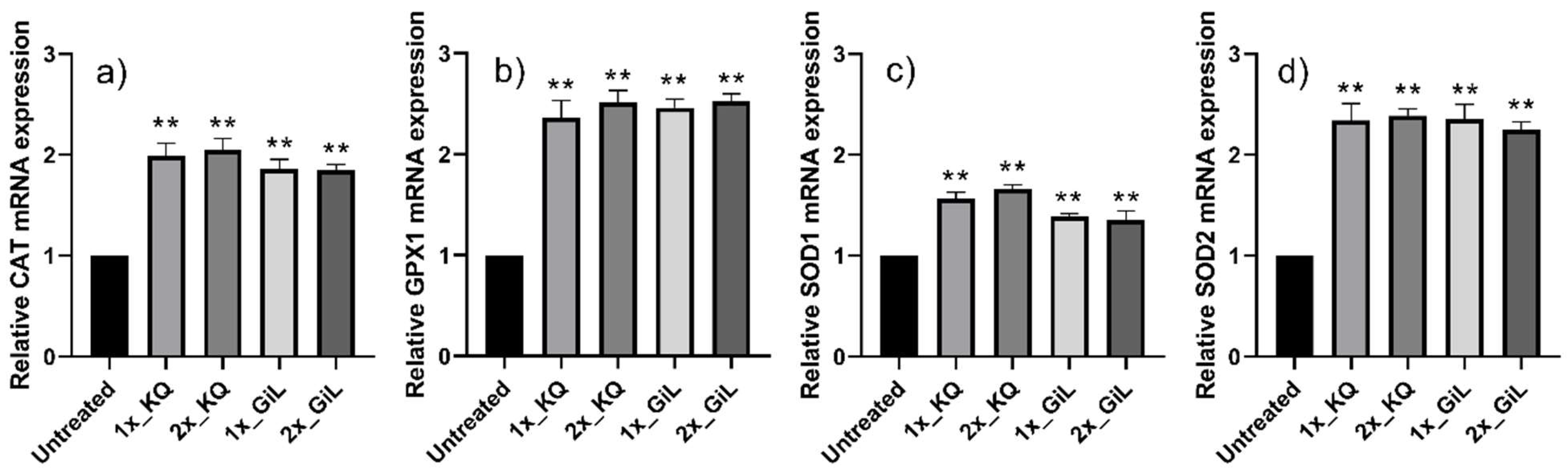
3.3. Effects of Phytochemicals on HUVECs
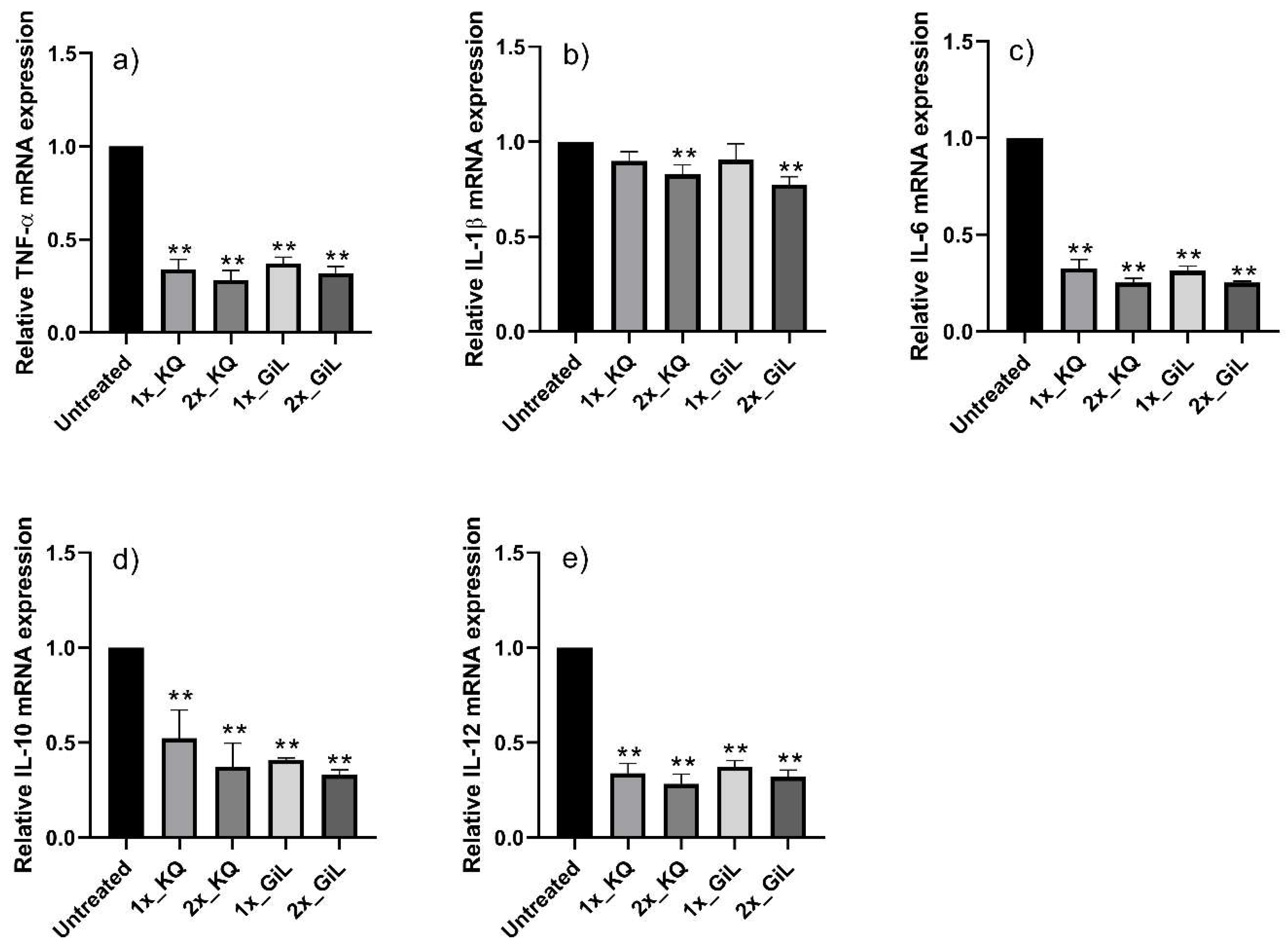
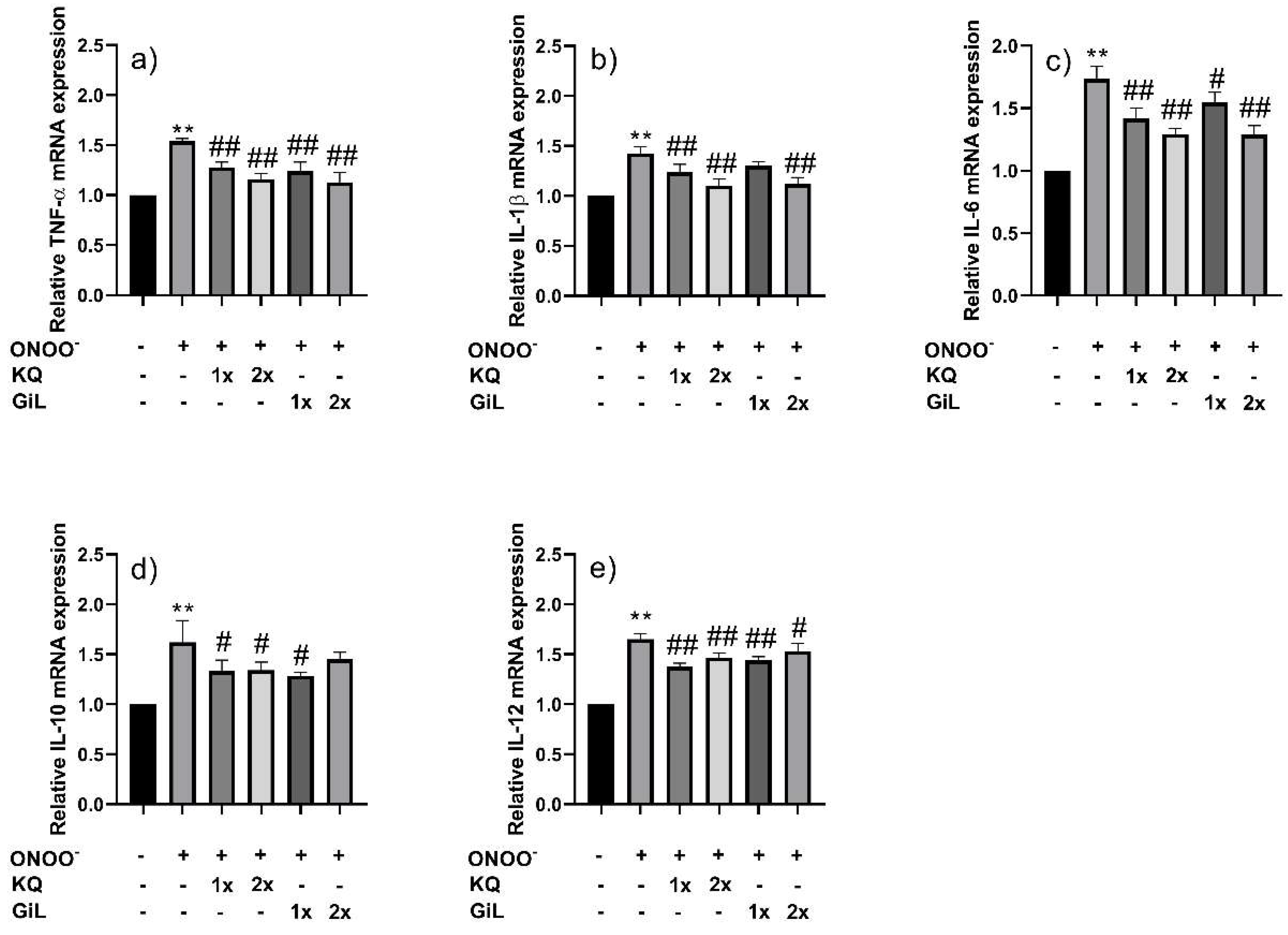
3.4. Protective Effects of Phytochemicals on HUVECs Following Peroxynitrite Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GiL extract | Gymnema inodorum leaf extract |
| HUVECs | Human umbilical vein endothelial cells |
| mRNA | Messenger ribonucleic acid |
| GADPH | Glyceraldehyde 3-phosphate dehydrogenase housekeeping gene |
| CAT | Catalase gene |
| GPX1 | Glutathione peroxidase 1 gene |
| SOD1 | Superoxide dismutase 1 gene |
| SOD2 | Superoxide dismutase 2 gene |
| TNF-α | Tumor necrosis factor-alpha gene |
| IL-1β | Interleukin-1 beta gene |
| IL-6 | Interleukin-6 gene |
| IL-10 | Interleukin-10 gene |
| IL-12 | Interleukin-12 gene |
| NF-κB | DNA transcription factor; nuclear factor-κB |
| T2DM | Type 2 diabetes mellitus |
| ROS | Reactive oxygen species |
| AGEs | Advanced glycation end products |
| RNS | Reactive nitrogen species |
| HAT | Hydrogen atom transfer |
| SET | Single-electron transfer |
| PCA | Principal component analysis |
| HCA | Hierarchical cluster analysis |
| LPS | Lipopolysaccharide |
| HPLC | High-performance liquid chromatography |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction |
| L-ONOO− | Low dose of peroxynitrite |
| H-ONOO− | High dose of peroxynitrite |
| KQ | Kaempferol and quercetin mixture |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| ARE | Antioxidant response element |
References
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, S.d.M.; da Fonseca, L.J.S.; Guedes, G.d.S.; Rabelo, L.A.; Goulart, M.O.F.; Vasconcelos, S.M.L. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int. J. Mol. Sci. 2013, 14, 3265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, Y.; Ye, N.; Chen, Y.; Zhang, N.; Sun, Y. Advanced glycation end products-induced mitochondrial energy metabolism dysfunction alters proliferation of human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 15, 2673–2680. [Google Scholar]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (ages): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Vascular nitric oxide resistance in type 2 diabetes. Cell Death Dis. 2023, 14, 1–14. [Google Scholar]
- Scioli, M.G.; Storti, G.; D’amico, F.; Guzmán, R.-R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: Potential diagnostic biomarkers and therapeutic targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus 2023, 15, e45835. [Google Scholar]
- Mansour, A.; Mousa, M.; Abdelmannan, D.; Tay, G.; Hassoun, A.; Alsafar, H. Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Front. Endocrinol. 2023, 14, 1143067. [Google Scholar] [CrossRef]
- Pieme, C.A.; Tatangmo, J.A.; Simo, G.; Nya, P.C.B.; Moor, V.J.A.; Moukette, B.M.; Nzufo, F.T.; Nono, B.L.N.; Sobngwi, E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes 2017, 10, 141. [Google Scholar]
- Jin, Y.; Arroo, R. The protective effects of flavonoids and carotenoids against diabetic complications—A review of in vivo evidence. Front. Nutr. 2023, 10, 1020950. [Google Scholar]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Akpoveso, O.-O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant phytochemicals as potential therapy for diabetic complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.-Y.; Alwasel, S.-H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [PubMed]
- Nuchuchua, O.; Inpan, R.; Srinuanchai, W.; Karinchai, J.; Pitchakarn, P.; Wongnoppavich, A.; Imsumran, A. Phytosome supplements for delivering Gymnema inodorum phytonutrients to prevent inflammation in macrophages and insulin resistance in adipocytes. Foods 2023, 12, 2257. [Google Scholar] [CrossRef]
- Chiabchalard, A.; Tencomnao, T.; Santiyanont, R. Effect of Gymnema inodorum on postprandial peak plasma glucose levels in healthy human. Afr. J. Biotechnol. 2010, 9, 1079–1085. [Google Scholar]
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Medicinal plants in homegardens of four ethnic groups in Thailand. J. Ethnopharmacol. 2019, 239, 111927. [Google Scholar] [PubMed]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Trang, D.T.; Yen, D.T.H.; Cuong, N.T.; Anh, L.T.; Hoai, N.T.; Tai, B.H.; Doan, V.V.; Yen, P.H.; Quang, T.H.; Nhiem, N.X.; et al. Pregnane glycosides from Gymnema inodorum and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2021, 35, 2157–2163. [Google Scholar]
- Srinuanchai, W.; Nooin, R.; Pitchakarn, P.; Karinchai, J.; Suttisansanee, U.; Chansriniyom, C.; Jarussophon, S.; Temviriyanukul, P.; Nuchuchua, O. Inhibitory effects of Gymnema inodorum (Lour.) Decne leaf extracts and its triterpene saponin on carbohydrate digestion and intestinal glucose absorption. J. Ethnopharmacol. 2021, 266, 113398. [Google Scholar]
- Shimizu, K.; Ozeki, M.; Tanaka, K.; Itoh, K.; Nakajyo, S.; Urakawa, N.; Atsuchi, M. Suppression of glucose absorption by extracts from the leaves of Gymnema inodorum. J. Vet. Med. Sci. 1997, 59, 753–757. [Google Scholar] [PubMed]
- An, J.-P.; Park, E.J.; Ryu, B.; Lee, B.W.; Cho, H.M.; Doan, T.P.; Pham, H.T.T.; Oh, W.K. Oleanane triterpenoids from the leaves of Gymnema inodorum and their insulin mimetic activities. J. Nat. Prod. 2020, 83, 1265–1274. [Google Scholar] [PubMed]
- Nuchuchua, O.; Srinuanchai, W.; Chansriniyom, C.; Suttisansanee, U.; Temviriyanukul, P.; Nuengchamnong, N.; Ruktanonchai, U. Relationship of phytochemicals and antioxidant activities in Gymnema inodorum leaf extracts. Heliyon 2023, 10, 23175. [Google Scholar]
- Surinkaew, S.; Sun, D.; Kooltheat, N.; Boonhok, R.; Somsak, V.; Kumphune, S. The cytoprotective effect of Gymnema inodorum leaf extract against hypoxia-induced cardiomyocytes injury. Heliyon 2024, 10, e35846. [Google Scholar]
- Jeytawan, N.; Yadoung, S.; Jeeno, P.; Yana, P.; Sutan, K.; Naksen, W.; Wongkaew, M.; Sommano, S.-R.; Hongsibsong, S. Antioxidant and phytochemical potential of and phytochemicals in Gymnema inodorum (lour.) decne in northern Thailand. Plants 2022, 11, 3498. [Google Scholar] [CrossRef]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Eumkeb, G. Gymnema inodorum (Lour.) Decne. extract alleviates oxidative stress and inflammatory mediators produced by raw264.7 macrophages. Oxid. Med. Cell. Longev. 2021, 2021, 8658314. [Google Scholar]
- Kim, S.B.; Bisson, J.; Friesen, J.B.; Pauli, G.F.; Simmler, C. Selective chlorophyll removal method to “degreen” botanical extracts. J. Nat. Prod. 2020, 83, 1846–1858. [Google Scholar]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of drying processes on phenolics and in vitro health-related activities of indigenous plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839. [Google Scholar]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 2022, 102, 1881–1906. [Google Scholar] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar]
- Petri, S.; Körner, S.; Kiaei, M. Nrf2/ARE Signaling Pathway: Key Mediator in Oxidative Stress and Potential Therapeutic Target in ALS. Neurol. Res Int. 2012, 2012, 878030. [Google Scholar]
- Levrand, S.; Pesse, B.; Feihl, F.; Waeber, B.; Pacher, P.; Rolli, J. Peroxynitite is a potent inhibitor of NF-κ B activation triggered by inflammatory stimuli in cardiac and endothelial cell lines. J. Biol. Chem. 2005, 280, 34878. [Google Scholar]
- Lee, J.; Lee, S.; Zhang, H.; Hill, M.A.; Zhang, C.; Park, Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS ONE 2017, 12, e0187189. [Google Scholar]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [PubMed]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-α-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Investig. Dermatol. 2008, 128, 2606. [Google Scholar]
- Zagozdzon, R.S.; Giermasz, A.; Golab, J.; Stoklosa, T.; Jalili, A.; Jakóbisiak, M. The potentiated antileukemic effects of doxorubicin and interleukin-12 combination are not dependent on nitric oxide production. Cancer Lett. 1999, 147, 67–75. [Google Scholar]
- Xiong, H.; Zhu, C.; Li, F.; Hegazi, R.; He, K.; Babyatsky, M.; Bauer, A.J.; Plevy, S.E. Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol. Chem. 2004, 279, 10776–10783. [Google Scholar] [CrossRef]
- Prolo, C.; Álvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. BioFactors 2014, 40, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Oter, S.; Seyrek, M.; Topal, T. Molecular, genetic and epigenetic pathways of peroxynitrite-induced cellular toxicity. Interdiscip. Toxicol. 2009, 2, 219. [Google Scholar] [PubMed]
- Wen, Y.; Gu, J.; Li, S.-L.; Reddy, M.A.; Natarajan, R.; Nadler, J.L. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 2006, 147, 2518–2525. [Google Scholar] [CrossRef]
- Luo, J.; Ning, T.; Li, X.; Jiang, T.; Tan, S.; Ma, D. Targeting IL-12 family cytokines: A potential strategy for type 1 and type 2 diabetes mellitus. Biomed. Pharmacother. 2024, 170, 115958. [Google Scholar]
- Barreca, M.M.; Alessandro, R.; Corrado, C. Effects of flavonoids on cancer, cardiovascular and neurodegenerative diseases: Role of NF-ĸB signaling pathway. Int. J. Mol. Sci. 2023, 24, 9236. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-H.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, J.; Liu, H. Characterization of the synergistic antioxidant activity of epigallocatechin gallate (EGCG) and kaempferol. Molecules 2023, 28, 5265. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Arias-Santé, M.F.; Fuentes, J. Oxidation of quercetin and kaempferol markedly amplifies their antioxidant, cytoprotective, and anti-inflammatory properties. Antioxidants 2023, 12, 155. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar]
- Yamagata, K. Onion quercetin inhibits vascular endothelial cell dysfunction and prevents hypertension. Eur. Food Res. Technol. 2023, 250, 13. [Google Scholar]
- Ounjaijean, S.; Rattanatham, R.; Somsak, V.; Boonhoh, W.; Surinkaew, S. Gymnema inodorum leaf extract improves cardiac function in experimental mice infected with Plasmodium berghei. J. Evid. Based. Integr. Med. 2023, 8, 2515690X221150526. [Google Scholar]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [PubMed]
- Saiki, P.; Kawano, Y.; Ogi, T.; Klungsupya, P.; Muangman, T.; Phantanaprates, W.; Kongchinda, P.; Pinnak, N.; Miyazaki, K. Purified gymnemic acids from Gymnema inodorum tea inhibit 3T3-L1 cell differentiation into adipocytes. Nutrients 2020, 12, 2851. [Google Scholar] [CrossRef]

| Target Functions | Genes (Accession Numbers) | Primer Sequences (5′-3′) |
|---|---|---|
| Housekeeping gene | GADPH (NM_001289745.3) | F-5′-ACATCGCTCAGACACCAT-3′ R-5′-TGTAGTTGAGGTCAATGAAGGG-3′ |
| Antioxidant enzymes | CAT (NM_001752.3) | F-5′-AGGGGCCTTTGGCTACTTTG-3′ R-5′-ACCCGATTCTCCAGCAACAG-3′ |
| GPX1 (NM_000581.4) | F-5′-CCGGGACTACACCCAGATGA-3′ R-5′-CGTTCTCCTGATGCCCAAAC-3′ | |
| SOD1 (NM_000454.5) | F-5′-AGCATTAAAGGACTGACTGAAGG-3′ R-5′-GTCTCCAACATGCCTCTCTTC-3′ | |
| SOD2 (NM_000636.3) | F-5′-GTTGGGGTTGGCTTGGTTTC-3′ R-5′-ATAAGGCCTGTTGTTCCTTGC-3′ | |
| Inflammatory cytokines | TNF-α (NM_000594.4) | F-5′-CCCCAGGGACCTCTCTCTAA-3′ R-5′-TGAGGTACAGGCCCTCTGAT-3′ |
| IL-1β (NM_000576.3) | F-5′-TTGAGTCTGCCCAGTTCC-3′ R-5′-TTTCTGCTTGAGAGGTGCT-3′ | |
| IL-6 (NM_00600.5) | F-5′-ACAGGGAGAGGGAGCGATAA-3′ R-5′-GAGAAGGCAACTGGACCGAA-3′ | |
| IL-10 (NM_000572.3) | F-5′-GCCAAGCCTTGTCTGAGATG-3′ R-5′- GGCCTTGCTCTTGTTTTCAC-3′ | |
| IL-12 (NM_002187.2) | F-5′-GTCCTCAGAAGCTAACCATCTCC-3′ R-5′-CCAGAGCCTATGACTCCATGTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuchuchua, O.; Seephan, S.; Srinuanchai, W.; Temviriyanukul, P.; Pongrakhananon, V. Antioxidant and Anti-Inflammatory Benefits of Gymnema inodorum Leaf Extract in Human Umbilical Vein Endothelial Cells Under Peroxynitrite Stress. Antioxidants 2025, 14, 427. https://doi.org/10.3390/antiox14040427
Nuchuchua O, Seephan S, Srinuanchai W, Temviriyanukul P, Pongrakhananon V. Antioxidant and Anti-Inflammatory Benefits of Gymnema inodorum Leaf Extract in Human Umbilical Vein Endothelial Cells Under Peroxynitrite Stress. Antioxidants. 2025; 14(4):427. https://doi.org/10.3390/antiox14040427
Chicago/Turabian StyleNuchuchua, Onanong, Suthasinee Seephan, Wanwisa Srinuanchai, Piya Temviriyanukul, and Varisa Pongrakhananon. 2025. "Antioxidant and Anti-Inflammatory Benefits of Gymnema inodorum Leaf Extract in Human Umbilical Vein Endothelial Cells Under Peroxynitrite Stress" Antioxidants 14, no. 4: 427. https://doi.org/10.3390/antiox14040427
APA StyleNuchuchua, O., Seephan, S., Srinuanchai, W., Temviriyanukul, P., & Pongrakhananon, V. (2025). Antioxidant and Anti-Inflammatory Benefits of Gymnema inodorum Leaf Extract in Human Umbilical Vein Endothelial Cells Under Peroxynitrite Stress. Antioxidants, 14(4), 427. https://doi.org/10.3390/antiox14040427






