Various Cellular Components and Its Signaling Cascades Through the Involvement of Signaling Messengers in Keratinocyte Differentiation
Abstract
1. Introduction
2. Signaling Molecules for Keratinocyte Differentiation
2.1. Phospholipase C and Protein Kinase C
2.2. 1,25-Dihydroxyvitamin D3
2.3. Phosphoprotein Phosphatase 1
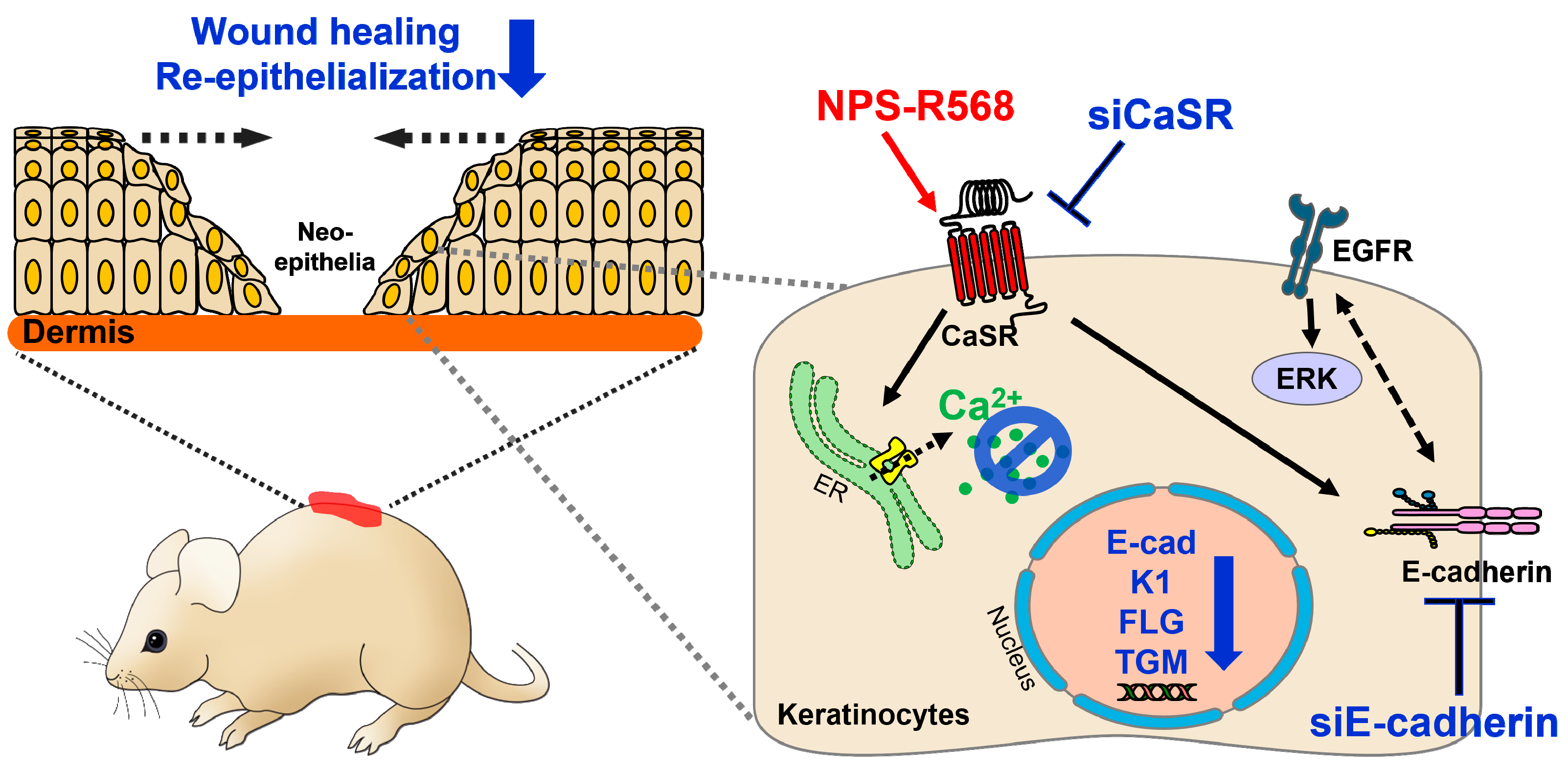
2.4. Calcium-Sensing Receptor and Its Associated Signaling Molecules
2.5. Proline-Rich Protein Tyrosine Kinase 2
2.6. Activator Protein-1
2.7. Thrombomodulin
2.8. CD9
2.9. microRNA-203
2.10. TGF-β-Inducible Gene-h3
2.11. Sphingosine-1-phosphate and Lysophosphatidic Acid
2.12. Serine Protease Inhibitors B7
2.13. Aquaporin 3
2.14. Ephrin-A
2.15. Insulin-like Growth Factor-Binding Protein 7
3. Redox-Sensitive Differentiation Component
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Menon, G.K.; Grayson, S.; Elias, P.M. Ionic calcium reservoirs in mammalian epidermis: Ultrastructural localization by ion-capture cytochemistry. J. Investig. Dermatol. 1985, 84, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Ahn, S.K.; Denda, M.; Brown, B.E.; Crumrine, D.; Kimutai, L.K.; Komuves, L.; Lee, S.H.; Feingold, K.R. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J. Investig. Dermatol. 2002, 119, 1128–1136. [Google Scholar] [CrossRef]
- Menon, G.K.; Elias, P.M. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch. Dermatol. 1991, 127, 57–63. [Google Scholar] [PubMed]
- Berridge, M.J.; Irvine, R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Majerus, P.W.; Connolly, T.M.; Deckmyn, H.; Ross, T.S.; Bross, T.E.; Ishii, H.; Bansal, V.S.; Wilson, D.B. The metabolism of phosphoinositide-derived messenger molecules. Science 1986, 234, 1519–1526. [Google Scholar] [CrossRef]
- Lee, E.; Yuspa, S.H. Changes in inositol phosphate metabolism are associated with terminal differentiation and neoplasia in mouse keratinocytes. Carcinogenesis 1991, 12, 1651–1658. [Google Scholar] [CrossRef]
- Jaken, S.; Yuspa, S.H. Early signals for keratinocyte differentiation: Role of Ca2+-mediated inositol lipid metabolism in normal and neoplastic epidermal cells. Carcinogenesis 1988, 9, 1033–1038. [Google Scholar] [CrossRef]
- Xie, Z.J.; Bikle, D.D. Phospholipase C-γ1 is required for calcium-induced keratinocyte differentiation. J. Biol. Chem. 1999, 274, 20421–20424. [Google Scholar] [CrossRef]
- Xie, Z.; Chang, S.M.; Pennypacker, S.D.; Liao, E.Y.; Bikle, D.D. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol. Biol. Cell 2009, 20, 1695–1704. [Google Scholar] [CrossRef]
- Denning, M.F.; Dlugosz, A.A.; Williams, E.K.; Szallasi, Z.; Blumberg, P.M.; Yuspa, S.H. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995, 6, 149–157. [Google Scholar]
- Dlugosz, A.A.; Yuspa, S.H. Coordinate Changes in Gene-Expression Which Mark the Spinous to Granular-Cell Transition in Epidermis Are Regulated by Protein-Kinase-C. J. Cell Biol. 1993, 120, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Asano, K.; Manabe, A.; Kinouchi, M.; Ishida-Yamamoto, A.; Iizuka, H. The alpha and eta isoforms of protein kinase C stimulate transcription of human involucrin gene. J. Investig. Dermatol. 1998, 110, 218–223. [Google Scholar] [CrossRef]
- Ohba, M.; Ishino, K.; Kashiwagi, M.; Kawabe, S.; Chida, K.; Huh, N.H.; Kuroki, T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol. Cell Biol. 1998, 18, 5199–5207. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yuspa, S.H.; Dlugosz, A.A. Differentiation of cultured human epidermal keratinocytes at high cell densities is mediated by endogenous activation of the protein kinase C signaling pathway. J. Investig. Dermatol. 1998, 111, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Ueda, E.; Ohno, S.; Kuroki, T.; Livneh, E.; Yamada, K.; Yamanishi, K.; Yasuno, H. The eta isoform of protein kinase C mediates transcriptional activation of the human transglutaminase 1 gene. J. Biol. Chem. 1996, 271, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Denning, M.F.; Dlugosz, A.A.; Cheng, C.; Dempsey, P.J.; Coffey, R.J.; Threadgill, D.W.; Magnuson, T.; Yuspa, S.H. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp. Dermatol. 2000, 9, 192–199. [Google Scholar] [CrossRef]
- Denning, M.F.; Dlugosz, A.A.; Threadgill, D.W.; Magnuson, T.; Yuspa, S.H. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J. Biol. Chem. 1996, 271, 5325–5331. [Google Scholar] [CrossRef]
- Xie, Z.; Singleton, P.A.; Bourguignon, L.Y.; Bikle, D.D. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol. Biol. Cell 2005, 16, 3236–3246. [Google Scholar] [CrossRef]
- Xie, Z.J.; Bikle, D.D. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007, 282, 8695–8703. [Google Scholar] [CrossRef]
- Falasca, M.; Logan, S.K.; Lehto, V.P.; Baccante, G.; Lemmon, M.A.; Schlessinger, J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998, 17, 414–422. [Google Scholar] [CrossRef]
- Bae, Y.S.; Cantley, L.G.; Chen, C.S.; Kim, S.R.; Kwon, K.S.; Rhee, S.G. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 4465–4469. [Google Scholar] [CrossRef]
- Rameh, L.E.; Rhee, S.G.; Spokes, K.; Kazlauskas, A.; Cantley, L.C.; Cantley, L.G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J. Biol. Chem. 1998, 273, 23750–23757. [Google Scholar] [CrossRef]
- Pece, S.; Chiariello, M.; Murga, C.; Gutkind, J.S. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J. Biol. Chem. 1999, 274, 19347–19351. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.; Ali, R.G.; McCormack, A.J.; Yap, A.S. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 2002, 277, 6708–6718. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, R.J.; Hodgkin, M.N.; Akhtar, N.; Morse, M.A.; Fuller, K.J.; Saqib, K.; Thompson, N.T.; Wakelam, M.J.O. The p85 subunit of phosphoinositide 3-kinase is associated with β-catenin in the cadherin-based adhesion complex. Biochem. J. 2001, 360, 335–344. [Google Scholar] [CrossRef]
- Pang, J.H.; Kraemer, A.; Stehbens, S.J.; Frame, M.C.; Yap, A.S. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. J. Biol. Chem. 2005, 280, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Calautti, E.; Li, J.; Saoncella, S.; Brissette, J.L.; Goetinck, P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005, 280, 32856–32865. [Google Scholar] [CrossRef]
- Espada, J.; Perez-Moreno, M.; Braga, V.M.; Rodriguez-Viciana, P.; Cano, A. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of beta-catenin in epidermal keratinocytes. J. Cell Biol. 1999, 146, 967–980. [Google Scholar] [CrossRef]
- Davis, M.A.; Ireton, R.C.; Reynolds, A.B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003, 163, 525–534. [Google Scholar] [CrossRef]
- Ireton, R.C.; Davis, M.A.; van Hengel, J.; Mariner, D.J.; Barnes, K.; Thoreson, M.A.; Anastasiadis, P.Z.; Matrisian, L.; Bundy, L.M.; Sealy, L.; et al. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 2002, 159, 465–476. [Google Scholar] [CrossRef]
- Norman, A.W.; Roth, J.; Orci, L. The vitamin D endocrine system: Steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr. Rev. 1982, 3, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 1995, 16, 200–257. [Google Scholar] [CrossRef]
- Bikle, D.D.; Pillai, S. Vitamin D, calcium, and epidermal differentiation. Endocr. Rev. 1993, 14, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; Sar, M.; Reid, F.A.; Tanaka, Y.; DeLuca, H.F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 1979, 206, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef]
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Investig. 1986, 78, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Nemanic, M.K.; Whitney, J.O.; Elias, P.W. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry 1986, 25, 1545–1548. [Google Scholar] [CrossRef]
- Xie, Z.; Bikle, D.D. Inhibition of 1,25-dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-gamma1. J. Investig. Dermatol. 2001, 117, 1250–1254. [Google Scholar] [CrossRef]
- Pillai, S.; Bikle, D.D. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: Differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J. Cell Physiol. 1991, 146, 94–100. [Google Scholar] [CrossRef]
- Pillai, S.; Bikle, D.D.; Su, M.J.; Ratnam, A.; Abe, J. 1,25-Dihydroxyvitamin D3 upregulates the phosphatidylinositol signaling pathway in human keratinocytes by increasing phospholipase C levels. J. Clin. Investig. 1995, 96, 602–609. [Google Scholar] [CrossRef]
- Rhee, S.G.; Choi, K.D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J. Biol. Chem. 1992, 267, 12393–12396. [Google Scholar] [PubMed]
- Li, X.; Wilmanns, M.; Thornton, J.; Kohn, M. Elucidating human phosphatase-substrate networks. Sci. Signal. 2013, 6, rs10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.T. Two isoforms of protein phosphatase 1 may be produced from the same gene. FEBS Lett. 1988, 232, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Shima, H.; Kitagawa, Y.; Irino, S.; Sugimura, T.; Nagao, M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1 alpha gene in rat hepatocellular carcinomas. Jpn. J. Cancer Res. 1990, 81, 1272–1280. [Google Scholar] [CrossRef]
- Kitamura, K.; Mizuno, Y.; Sasaki, A.; Yasui, A.; Tsuiki, S.; Kikuchi, K. Molecular-Cloning and Sequence-Analysis of Cdna for the Catalytic Subunit 1-Alpha of Rat-Kidney Type-1 Protein Phosphatase, and Detection of the Gene-Expression at High-Levels in Hepatoma-Cells and Regenerating Livers as Compared to Rat Livers. J. Biochem. 1991, 109, 307–310. [Google Scholar]
- Shima, H.; Hatano, Y.; Chun, Y.S.; Sugimura, T.; Zhang, Z.J.; Lee, E.Y.C.; Nagao, M. Identification of Pp1 Catalytic Subunit Isotypes Pp1-Gamma-1, Pp1-Delta and Pp1-Alpha in Various Rat-Tissues. Biochem. Biophys. Res. Commun. 1993, 192, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, N.; Mizuno, Y.; Ito, Y.; Kikuchi, K. Tissue distribution of isoforms of type-1 protein phosphatase PP1 in mouse tissues and its diabetic alterations. J. Biochem. 1994, 116, 411–415. [Google Scholar] [CrossRef]
- Depaoli-Roach, A.A.; Park, I.K.; Cerovsky, V.; Csortos, C.; Durbin, S.D.; Kuntz, M.J.; Sitikov, A.; Tang, P.M.; Verin, A.; Zolnierowicz, S. Serine/threonine protein phosphatases in the control of cell function. Adv. Enzym. Regul. 1994, 34, 199–224. [Google Scholar] [CrossRef]
- Shenolikar, S. Protein serine/threonine phosphatases—New avenues for cell regulation. Annu. Rev. Cell Biol. 1994, 10, 55–86. [Google Scholar] [CrossRef]
- Wera, S.; Hemmings, B.A. Serine Threonine Protein Phosphatases. Biochem. J. 1995, 311, 17–29. [Google Scholar] [CrossRef]
- Aoyama, H.; Ikeda, Y.; Miyazaki, Y.; Yoshimura, K.; Nishino, S.; Yamamoto, T.; Yano, M.; Inui, M.; Aoki, H.; Matsuzaki, M. Isoform-specific roles of protein phosphatase 1 catalytic subunits in sarcoplasmic reticulum-mediated Ca2+ cycling. Cardiovasc. Res. 2011, 89, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Devogelaere, B.; Beullens, M.; Sammels, E.; Derua, R.; Waelkens, E.; Van Lint, J.; Parys, J.B.; Missiaen, L.; Bollen, M.; De Smedt, H. Protein phosphatase-1 is a novel regulator of the interaction between IRBIT and the inositol 1,4,5-trisphosphate receptor. Biochem. J. 2007, 407, 303–311. [Google Scholar] [CrossRef]
- Shrestha, C.; Tang, Y.; Fan, H.; Li, L.; Zeng, Q.; Pennypacker, S.D.; Bikle, D.D.; Xie, Z. Phosphoprotein Phosphatase 1 Is Required for Extracellular Calcium-Induced Keratinocyte Differentiation. Biomed. Res. Int. 2016, 2016, 3062765. [Google Scholar] [CrossRef]
- Bouschet, T.; Henley, J.M. Calcium as an extracellular signalling molecule: Perspectives on the Calcium Sensing Receptor in the brain. C. R. Biol. 2005, 328, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Siddiqui, J.A.; Swarnkar, G.; Chattopadhyay, N. Role of calcium-sensing receptor in bone biology. Indian J. Med. Res. 2008, 127, 274–286. [Google Scholar]
- Tu, C.L.; Crumrine, D.A.; Man, M.Q.; Chang, W.; Elalieh, H.; You, M.; Elias, P.M.; Bikle, D.D. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J. Investig. Dermatol. 2012, 132, 2350–2359. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Mithal, A.; Brown, E.M. The calcium-sensing receptor: A window into the physiology and pathophysiology of mineral ion metabolism. Endocr. Rev. 1996, 17, 289–307. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.H.; Bikle, D.D. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J. Biol. Chem. 2001, 276, 41079–41085. [Google Scholar] [CrossRef]
- Komuves, L.; Oda, Y.; Tu, C.L.; Chang, W.H.; Ho-Pao, C.L.; Mauro, T.; Bikle, D.D. Epidermal expression of the full-length extracellular calcium-sensing receptor is required for normal keratinocyte differentiation. J. Cell. Physiol. 2002, 192, 45–54. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, 1074–1083. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Xie, Z.; Bikle, D.D. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J. Biol. Chem. 2008, 283, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert. Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Popp, T.; Steinritz, D.; Breit, A.; Deppe, J.; Egea, V.; Schmidt, A.; Gudermann, T.; Weber, C.; Ries, C. Wnt5a/beta-catenin signaling drives calcium-induced differentiation of human primary keratinocytes. J. Investig. Dermatol. 2014, 134, 2183–2191. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. J. Investig. Dermatol. 2011, 131, 1119–1128. [Google Scholar] [CrossRef]
- Tu, C.L.; Celli, A.; Mauro, T.; Chang, W. Calcium-Sensing Receptor Regulates Epidermal Intracellular Ca2+ Signaling and Re-Epithelialization after Wounding. J. Investig. Dermatol. 2019, 139, 919–929. [Google Scholar] [CrossRef]
- Yang, C.; Rybchyn, M.S.; De Silva, W.G.M.; Matthews, J.; Holland, A.J.A.; Conigrave, A.D.; Mason, R.S. UV-induced DNA Damage in Skin is Reduced by CaSR Inhibition. Photochem. Photobiol. 2022, 98, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.M.; Baumgartner, M.; Gribben, E.M.; Li, L.; Efimova, T. The role of proline-rich protein tyrosine kinase 2 in differentiation-dependent signaling in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, 1094–1106. [Google Scholar] [CrossRef]
- Shi, C.-S.; Kehrl, J.H. PYK2 Links Gqα and G13α Signaling to NF-κB Activation. J. Biol. Chem. 2001, 276, 31845–31850. [Google Scholar] [CrossRef]
- Avraham, H.; Park, S.Y.; Schinkmann, K.; Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000, 12, 123–133. [Google Scholar] [CrossRef]
- Okigaki, M.; Davis, C.; Falasca, I.; Harroch, S.; Felsenfeld, D.P.; Sheetz, M.P.; Schlessinger, J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA 2003, 100, 10740–10745. [Google Scholar] [CrossRef]
- Lev, S.; Moreno, H.; Martinez, R.; Canoll, P.; Peles, E.; Musacchio, J.M.; Plowman, G.D.; Rudy, B.; Schlessinger, J. Protein-Tyrosine Kinase Pyk2 Involved in Ca2+-Induced Regulation of Ion-Channel and Map Kinase Functions. Nature 1995, 376, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Tokiwa, G.; Lev, S.; Courtneidge, S.A.; Schlessinger, J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 1996, 383, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Avraham, S.; Kumar, S.; Nakazawa, A.; Place, A.; Ghanem, L.; Rana, A.; Kumar, V.; Majumder, P.K.; Avraham, H.; et al. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J. Biol. Chem. 1999, 274, 10140–10144. [Google Scholar] [CrossRef]
- Banno, Y.; Ohguchi, K.; Matsumoto, N.; Koda, M.; Ueda, M.; Hara, A.; Dikic, I.; Nozawa, Y. Implication of phospholipase D2 in oxidant-induced phosphoinositide 3-kinase signaling via Pyk2 activation in PC12 cells. J. Biol. Chem. 2005, 280, 16319–16324. [Google Scholar] [CrossRef]
- Yuspa, S.H.; Hennings, H.; Tucker, R.W.; Jaken, S.; Kilkenny, A.E.; Roop, D.R. Signal Transduction for Proliferation and Differentiation in Keratinocytes. Ann. N. Y. Acad. Sci. 1988, 548, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Younus, J.; Gilchrest, B.A.G. Modulation of mRNA levels during human keratinocyte differentiation. J. Cell Physiol. 1992, 152, 232–239. [Google Scholar]
- Eckert, R.L.; Crish, J.F.; Robinson, N.A. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol. Rev. 1997, 77, 397–424. [Google Scholar] [CrossRef]
- Bikle, D.D.; Ng, D.; Tu, C.L.; Oda, Y.; Xie, Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell Endocrinol. 2001, 177, 161–171. [Google Scholar] [CrossRef]
- Wirth, P.J.; Yuspa, S.H.; Thorgeirsson, S.S.; Hennings, H. Induction of common patterns of polypeptide synthesis and phosphorylation by calcium and 12-O-tetradecanoylphorbol-13-acetate in mouse epidermal cell culture. Cancer Res. 1987, 47, 2831–2838. [Google Scholar]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Eckert, R.L.; Welter, J.F. Transcription factor regulation of epidermal keratinocyte gene expression. Mol. Biol. Rep. 1996, 23, 59–70. [Google Scholar] [CrossRef]
- Jochum, W.; Passegue, E.; Wagner, E.F. AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Szabowski, A.; Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 2001, 20, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Rothnagel, J.A.; Longley, M.A.; Tsai, S.Y.; Roop, D.R. Differentiation-Specific Expression of Human Keratin-1 Is Mediated by a Composite Ap-1 Steroid-Hormone Element. J. Biol. Chem. 1994, 269, 7443–7449. [Google Scholar] [PubMed]
- Casatorres, J.; Navarro, J.M.; Blessing, M.; Jorcano, J.L. Analysis of the control of expression and tissue specificity of the keratin 5 gene, characteristic of basal keratinocytes. Fundamental role of an AP-1 element. J. Biol. Chem. 1994, 269, 20489–20496. [Google Scholar]
- Ma, S.; Rao, L.; Freedberg, I.M.; Blumenberg, M. Transcriptional control of K5, K6, K14, and K17 keratin genes by AP-1 and NF-kappaB family members. Gene Expr. 1997, 6, 361–370. [Google Scholar]
- Welter, J.F.; Crish, J.F.; Agarwal, C.; Eckert, R.L. Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J. Biol. Chem. 1995, 270, 12614–12622, Correction in J. Biol. Chem. 1996, 271, 11034. [Google Scholar] [CrossRef]
- DiSepio, D.; Jones, A.; Longley, M.A.; Bundman, D.; Rothnagel, J.A.; Roop, D.R. The proximal promoter of the mouse loricrin gene contains a functional AP-1 element and directs keratinocyte-specific but not differentiation-specific expression. J. Biol. Chem. 1995, 270, 10792–10799. [Google Scholar] [CrossRef]
- Jang, S.I.; Steinert, P.M.; Markova, N.G. Activator protein 1 activity is involved in the regulation of the cell type-specific expression from the proximal promoter of the human profilaggrin gene. J. Biol. Chem. 1996, 271, 24105–24114. [Google Scholar] [CrossRef]
- Ng, D.C.; Shafaee, S.; Lee, D.; Bikle, D.D. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J. Biol. Chem. 2000, 275, 24080–24088. [Google Scholar] [CrossRef]
- Hanley, K.; Wood, L.; Ng, D.C.; He, S.S.; Lau, P.; Moser, A.; Elias, P.M.; Bikle, D.D.; Williams, M.L.; Feingold, K.R. Cholesterol sulfate stimulates involucrin transcription in keratinocytes by increasing Fra-1, Fra-2, and Jun D. J. Lipid Res. 2001, 42, 390–398. [Google Scholar] [PubMed]
- Eckert, R.L.; Crish, J.F.; Efimova, T.; Dashti, S.R.; Deucher, A.; Bone, F.; Adhikary, G.; Huang, G.S.; Gopalakrishnan, R.; Balasubramanian, S. Regulation of involucrin gene expression. J. Investig. Dermatol. 2004, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mehic, D.; Bakiri, L.; Ghannadan, M.; Wagner, E.F.; Tschachler, E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J. Investig. Dermatol. 2005, 124, 212–220. [Google Scholar] [CrossRef]
- Ikebe, D.; Wang, B.; Suzuki, H.; Kato, M. Suppression of keratinocyte stratification by a dominant negative JunB mutant without blocking cell proliferation. Genes Cells 2007, 12, 197–207. [Google Scholar] [CrossRef]
- Zenz, R.; Wagner, E.F. Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors. Int. J. Biochem. Cell Biol. 2006, 38, 1043–1049. [Google Scholar] [CrossRef]
- Sonkoly, E.; Wei, T.; Pavez Lorie, E.; Suzuki, H.; Kato, M.; Torma, H.; Stahle, M.; Pivarcsi, A. Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J. Investig. Dermatol. 2010, 130, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.V.; Rossi, A.; Duranti, G.; Ranalli, M.; Melino, G.; Sabatini, S.; Avigliano, L. Vitamin C recycling is enhanced in the adaptive response to leptin-induced oxidative stress in keratinocytes. J. Investig. Dermatol. 2003, 121, 786–793. [Google Scholar] [CrossRef]
- Brown, P.H.; Chen, T.K.; Birrer, M.J. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene 1994, 9, 791–799. [Google Scholar]
- Brown, P.H.; Kim, S.H.; Wise, S.C.; Sabichi, A.L.; Birrer, M.J. Dominant-negative mutants of cJun inhibit AP-1 activity through multiple mechanisms and with different potencies. Cell Growth Differ. 1996, 7, 1013–1021. [Google Scholar]
- Thompson, E.J.; Gupta, A.; Stratton, M.S.; Bowden, G.T. Mechanism of action of a dominant negative c-jun mutant in inhibiting activator protein-1 activation. Mol. Carcinog. 2002, 35, 157–162. [Google Scholar] [CrossRef]
- Rorke, E.A.; Adhikary, G.; Jans, R.; Crish, J.F.; Eckert, R.L. AP1 factor inactivation in the suprabasal epidermis causes increased epidermal hyperproliferation and hyperkeratosis but reduced carcinogen-dependent tumor formation. Oncogene 2010, 29, 5873–5882. [Google Scholar] [CrossRef]
- Rorke, E.A.; Adhikary, G.; Young, C.A.; Rice, R.H.; Elias, P.M.; Crumrine, D.; Meyer, J.; Blumenberg, M.; Eckert, R.L. Structural and biochemical changes underlying a keratoderma-like phenotype in mice lacking suprabasal AP1 transcription factor function. Cell Death Dis. 2015, 6, e1647. [Google Scholar] [CrossRef]
- Young, C.A.; Rorke, E.A.; Adhikary, G.; Xu, W.; Eckert, R.L. Loss of epidermal AP1 transcription factor function reduces filaggrin level, alters chemokine expression and produces an ichthyosis-related phenotype. Cell Death Dis. 2017, 8, e2840. [Google Scholar] [CrossRef] [PubMed]
- Raife, T.J.; Lager, D.J.; Madison, K.C.; Piette, W.W.; Howard, E.J.; Sturm, M.T.; Chen, Y.; Lentz, S.R. Thrombomodulin expression by human keratinocytes. Induction of cofactor activity during epidermal differentiation. J. Clin. Investig. 1994, 93, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Hanly, A.M.; Redmond, M.; Winter, D.C.; Brophy, S.; Deasy, J.M.; Bouchier-Hayes, D.; Kay, E.W. Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br. J. Cancer 2006, 94, 1320–1325. [Google Scholar] [CrossRef]
- Cheng, T.L.; Wu, Y.T.; Lin, H.Y.; Hsu, F.C.; Liu, S.K.; Chang, B.I.; Chen, W.S.; Lai, C.H.; Shi, G.Y.; Wu, H.L. Functions of rhomboid family protease RHBDL2 and thrombomodulin in wound healing. J. Investig. Dermatol. 2011, 131, 2486–2494. [Google Scholar] [CrossRef]
- Peterson, J.J.; Rayburn, H.B.; Lager, D.J.; Raife, T.J.; Kealey, G.P.; Rosenberg, R.D.; Lentz, S.R. Expression of thrombomodulin and consequences of thrombomodulin deficiency during healing of cutaneous wounds. Am. J. Pathol. 1999, 155, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Wu, Y.T.; Lai, C.H.; Kao, Y.C.; Kuo, C.H.; Liu, S.L.; Hsu, Y.Y.; Chen, P.K.; Cho, C.F.; Wang, K.C.; et al. Thrombomodulin regulates keratinocyte differentiation and promotes wound healing. J. Investig. Dermatol. 2013, 133, 1638–1645. [Google Scholar] [CrossRef]
- Peñas, P.F.; García-Díez, A.; Sánchez-Madrid, F.; Yáñez-Mó, M. Tetraspanins are localized at motility-related structures and involved in normal human keratinocyte wound healing migration. J. Investig. Dermatol. 2000, 114, 1126–1135. [Google Scholar] [CrossRef]
- Inui, S.; Higashiyama, S.; Hashimoto, K.; Higashiyama, M.; Yoshikawa, K.; Taniguchi, N. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J. Cell Physiol. 1997, 171, 291–298. [Google Scholar] [CrossRef]
- Jiang, X.P.; Zhang, D.X.; Teng, M.; Zhang, Q.; Zhang, J.P.; Huang, Y.S. Downregulation of CD9 in keratinocyte contributes to cell migration via upregulation of matrix metalloproteinase-9. PLoS ONE 2013, 8, e77806. [Google Scholar] [CrossRef]
- Jiang, X.; Teng, M.; Ji, R.; Zhang, D.; Zhang, Z.; Lv, Y.; Zhang, Q.; Zhang, J.; Huang, Y. CD9 regulates keratinocyte differentiation and motility by recruiting E-cadherin to the plasma membrane and activating the PI3K/Akt pathway. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118574. [Google Scholar] [CrossRef]
- Charest, J.L.; Jennings, J.M.; King, W.P.; Kowalczyk, A.P.; Garcia, A.J. Cadherin-mediated cell-cell contact regulates keratinocyte differentiation. J. Investig. Dermatol. 2009, 129, 564–572. [Google Scholar] [CrossRef]
- Dehli, J.; Karlsson, C.; Bizelli-Silveira, C.; Jiang, X.; Kraft, D.; Foss, M. E-cadherin mediated cell-biomaterial interaction reduces migration of keratinocytes in-vitro. Colloids Surf. B Biointerfaces 2019, 180, 326–333. [Google Scholar] [CrossRef]
- Tinkle, C.L.; Lechler, T.; Pasolli, H.A.; Fuchs, E. Conditional targeting of E-cadherin in skin: Insights into hyperproliferative and degenerative responses. Proc. Natl. Acad. Sci. USA 2004, 101, 552–557. [Google Scholar] [CrossRef]
- Young, P.; Boussadia, O.; Halfter, H.; Grose, R.; Berger, P.; Leone, D.P.; Robenek, H.; Charnay, P.; Kemler, R.; Suter, U. E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J. 2003, 22, 5723–5733. [Google Scholar] [CrossRef]
- Gazel, A.; Banno, T.; Walsh, R.; Blumenberg, M. Inhibition of JNK promotes differentiation of epidermal keratinocytes. J. Biol. Chem. 2006, 281, 20530–20541. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; O’Carroll, D.; Pasolli, H.A.; Zhang, Z.H.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef]
- Sonkoly, E.; Wei, T.; Janson, P.C.; Saaf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007, 2, e610. [Google Scholar] [CrossRef]
- Yi, R.; Poy, M.N.; Stoffel, M.; Fuchs, E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008, 452, 225–229. [Google Scholar] [CrossRef]
- Skonier, J.; Neubauer, M.; Madisen, L.; Bennett, K.; Plowman, G.D.; Purchio, A.F. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992, 11, 511–522. [Google Scholar] [CrossRef]
- Skonier, J.; Bennett, K.; Rothwell, V.; Kosowski, S.; Plowman, G.; Wallace, P.; Edelhoff, S.; Disteche, C.; Neubauer, M.; Marquardt, H.; et al. Beta-Ig-H3-a Transforming Growth Factor-Beta-Responsive Gene Encoding a Secreted Protein That Inhibits Cell Attachment in-Vitro and Suppresses the Growth of Cho Cells in Nude-Mice. DNA Cell Biol. 1994, 13, 571–584. [Google Scholar] [CrossRef]
- Kim, J.E.; Jeong, H.W.; Nam, J.O.; Lee, B.H.; Choi, J.Y.; Park, R.W.; Park, J.Y.; Kim, I.S. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J. Biol. Chem. 2002, 277, 46159–46165. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.A.; Kumaratilake, J.S.; Cleary, E.G. Immunohistochemical and ultrastructural localization of MP78/70 (betaig-h3) in extracellular matrix of developing and mature bovine tissues. J. Histochem. Cytochem. 1997, 45, 1683–1696. [Google Scholar] [CrossRef]
- Billings, P.C.; Herrick, D.J.; Kucich, U.; Engelsberg, E.N.; Abrams, W.R.; Macarak, E.J.; Rosenbloom, J.; Howard, P.S. Extracellular matrix and nuclear localization of βig-h3 in human bladder smooth muscle and fibroblast cells. J. Cell. Biochem. 2000, 79, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Lee, S.H.; Kim, J.E.; Choi, J.Y.; Park, R.W.; Yong Park, J.; Park, H.S.; Sohn, Y.S.; Lee, D.S.; Bae Lee, E.; et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3beta1 integrin. Biochem. Biophys. Res. Commun. 2002, 294, 940–948. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.J.; Lee, B.H.; Park, R.W.; Kim, K.S.; Kim, I.S. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, betaig-h3. J. Biol. Chem. 2000, 275, 30907–30915. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Noshiro, M.; Makihira, S.; Kawamoto, T.; Shen, M.; Yan, W.; Kawashima-Ohya, Y.; Fujimoto, K.; Tanne, K.; Kato, Y. RGD-CAP ((beta)ig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin alpha(1)beta(1). Biochim. Biophys. Acta 1999, 1451, 196–205. [Google Scholar] [CrossRef]
- Lebaron, R.G.; Bezverkov, K.I.; Zimber, M.P.; Pavelec, R.; Skonier, J.; Purchio, A.F. Beta-Ig-H3, a Novel Secretory Protein Inducible by Transforming Growth-Factor-Beta, Is Present in Normal Skin and Promotes the Adhesion and Spreading of Dermal Fibroblasts In-Vitro. J. Investig. Dermatol. 1995, 104, 844–849. [Google Scholar] [CrossRef]
- Oh, J.E.; Kook, J.K.; Min, B.M. βig-h3 induces keratinocyte differentiation via modulation of involucrin and transglutaminase expression through the integrin α3β1 and the phosphatidylinositol 3-kinase/Akt signaling pathway. J. Biol. Chem. 2005, 280, 21629–21637. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Tigyi, G.; Parrill, A.L. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 2003, 42, 498–526. [Google Scholar] [CrossRef]
- Anliker, B.; Chun, J. Lysophospholipid G protein-coupled receptors. J. Biol. Chem. 2004, 279, 20555–20558. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Hla, T. Structural and functional characteristics of S1P receptors. J. Cell Biochem. 2004, 92, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Goetzl, E.J. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat. Rev. Immunol. 2005, 5, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Gardell, S.E.; Dubin, A.E.; Chun, J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol. Med. 2006, 12, 65–75. [Google Scholar] [CrossRef]
- Lichte, K.; Rossi, R.; Danneberg, K.; ter Braak, M.; Kurschner, U.; Jakobs, K.H.; Kleuser, B.; Meyer zu Heringdorf, D. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J. Investig. Dermatol. 2008, 128, 1487–1498. [Google Scholar] [CrossRef]
- Le Stunff, H.; Milstien, S.; Spiegel, S. Generation and metabolism of bioactive sphingosine-1-phosphate. J. Cell Biochem. 2004, 92, 882–899. [Google Scholar] [CrossRef]
- Kohama, T.; Olivera, A.; Edsall, L.; Nagiec, M.M.; Dickson, R.; Spiegel, S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998, 273, 23722–23728. [Google Scholar] [CrossRef]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef]
- Hong, J.H.; Youm, J.K.; Kwon, M.J.; Park, B.D.; Lee, Y.M.; Lee, S.I.; Shin, D.M.; Lee, S.H. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J. Investig. Dermatol. 2008, 128, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Song, J.; Lee, D.; Kim, G.T.; Park, S.H.; Shin, D.Y.; Shin, K.O.; Park, K.; Shim, S.M.; Park, T.S. Inhibition of sphingosine 1-phosphate lyase activates human keratinocyte differentiation and attenuates psoriasis in mice. J. Lipid Res. 2020, 61, 20–32. [Google Scholar] [CrossRef]
- Smith, C.J.; Williams, J.L.; Hall, C.; Casas, J.; Caley, M.P.; O’Toole, E.A.; Prasad, R.; Metherell, L.A. Ichthyosis linked to sphingosine 1-phosphate lyase insufficiency is due to aberrant sphingolipid and calcium regulation. J. Lipid Res. 2023, 64, 100351. [Google Scholar] [PubMed]
- de Veer, S.J.; Furio, L.; Harris, J.M.; Hovnanian, A. Proteases: Common culprits in human skin disorders. Trends Mol. Med. 2014, 20, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Sanrattana, W.; Maas, C.; de Maat, S. SERPINs-From Trap to Treatment. Front. Med. 2019, 6, 25. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, H.; Zhou, H.; Huang, N.; Wei, X.; Liu, X.; Teng, X.; Hu, Z.; Zhang, J.; Zhou, X.; et al. Systematic screening and identification of novel psoriasis-specific genes from the transcriptome of psoriasis-like keratinocytes. Mol. Med. Rep. 2019, 19, 1529–1542. [Google Scholar] [CrossRef]
- Zheng, H.; Gu, L.; Zhao, F.; Zhang, C.; Wang, Z.; Zhou, H.; Hu, Z.; Wei, X.; Liu, X.; Luo, F.; et al. SerpinB7 deficiency contributes to development of psoriasis via calcium-mediated keratinocyte differentiation dysfunction. Cell Death Dis. 2022, 13, 635. [Google Scholar] [CrossRef]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Ren. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef]
- Azad, A.K.; Raihan, T.; Ahmed, J.; Hakim, A.; Emon, T.H.; Chowdhury, P.A. Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases. Front. Genet. 2021, 12, 654865. [Google Scholar] [CrossRef]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.M.; Verkman, A.S. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef] [PubMed]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? Bioessays 2001, 23, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Bollag, W.B. Aquaporin 3 colocates with phospholipase d2 in caveolin-rich membrane microdomains and is downregulated upon keratinocyte differentiation. J. Investig. Dermatol. 2003, 121, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ray, S.; Bollag, W.B. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim. Biophys. Acta 2003, 1643, 25–36. [Google Scholar] [CrossRef]
- Bailey, L.J.; Choudhary, V.; Bollag, W.B. Possible Role of Phosphatidylglycerol-Activated Protein Kinase C-betaII in Keratinocyte Differentiation. Open Dermatol. J. 2017, 11, 59–71. [Google Scholar] [CrossRef]
- Miao, H.; Wang, B. Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 2009, 41, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Mekala, S.; Dugam, P.; Das, A. Ephrin-Eph receptor tyrosine kinases for potential therapeutics against hepatic pathologies. J. Cell Commun. Signal. 2023, 17, 549–561. [Google Scholar] [CrossRef]
- Zantek, N.D.; Azimi, M.; Fedor-Chaiken, M.; Wang, B.; Brackenbury, R.; Kinch, M.S. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999, 10, 629–638. [Google Scholar]
- Orsulic, S.; Kemler, R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J. Cell Sci. 2000, 113, 1793–1802. [Google Scholar]
- Kim, S.A.; Tai, C.Y.; Mok, L.P.; Mosser, E.A.; Schuman, E.M. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc. Natl. Acad. Sci. USA 2011, 108, 9857–9862. [Google Scholar] [CrossRef]
- Guo, H.; Miao, H.; Gerber, L.; Singh, J.; Denning, M.F.; Gilliam, A.C.; Wang, B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006, 66, 7050–7058. [Google Scholar] [CrossRef]
- Lin, S.; Gordon, K.; Kaplan, N.; Getsios, S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol. Biol. Cell 2010, 21, 3902–3914. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.J.; Briggaman, R.A.; Herman, B. Calcium-induced assembly of adherens junctions in keratinocytes. J. Cell Biol. 1987, 105, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Getsios, S.; Simpson, C.L.; Kojima, S.; Harmon, R.; Sheu, L.J.; Dusek, R.L.; Cornwell, M.; Green, K.J. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 2009, 185, 1243–1258. [Google Scholar] [CrossRef]
- Nousbeck, J.; Sarig, O.; Avidan, N.; Indelman, M.; Bergman, R.; Ramon, M.; Enk, C.D.; Sprecher, E. Insulin-like growth factor-binding protein 7 regulates keratinocyte proliferation, differentiation and apoptosis. J. Investig. Dermatol. 2010, 130, 378–387. [Google Scholar] [CrossRef]
- Neely, E.K.; Morhenn, V.B.; Hintz, R.L.; Wilson, D.M.; Rosenfeld, R.G. Insulin-like growth factors are mitogenic for human keratinocytes and a squamous cell carcinoma. J. Investig. Dermatol. 1991, 96, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.; Spravchikov, N.; Trebicz, M.; Gartsbein, M.; Accili, D.; Avinoah, I.; Nofeh-Moses, S.; Sizyakov, G.; Tennenbaum, T. The regulation of skin proliferation and differentiation in the IR null mouse: Implications for skin complications of diabetes. Endocrinology 2001, 142, 1234–1241. [Google Scholar] [CrossRef]
- Sadagurski, M.; Nofech-Mozes, S.; Weingarten, G.; White, M.F.; Kadowaki, T.; Wertheimer, E. Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology. J. Cell Physiol. 2007, 213, 519–527. [Google Scholar] [CrossRef]
- Wertheimer, E.; Trebicz, M.; Eldar, T.; Gartsbein, M.; Nofeh-Moses, S.; Tennenbaum, T. Differential roles of insulin receptor and insulin-like growth factor-1 receptor in differentiation of murine skin keratinocytes. J. Investig. Dermatol. 2000, 115, 24–29. [Google Scholar] [CrossRef]
- Tamura, K.; Hashimoto, K.; Suzuki, K.; Yoshie, M.; Kutsukake, M.; Sakurai, T. Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular endothelial cell growth factor (VEGF)-induced angiogenesis in human vascular endothelial cells. Eur. J. Pharmacol. 2009, 610, 61–67. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Wilson, E.M.; Rosenfeld, R.G.; Oh, Y. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J. Biol. Chem. 1997, 272, 30729–30734. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Telkoparan-Akillilar, P.; Saso, L. NRF2, a crucial modulator of skin cells protection against vitiligo, psoriasis, and cancer. Biofactors 2023, 49, 228–250. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, J.M.; Jang, S.; Choi, D.K.; Seo, M.S.; Kim, H.R.; Sohn, K.C.; Im, M.; Seo, Y.J.; Lee, J.H.; et al. Role of nuclear factor E2-related factor 2 (Nrf2) in epidermal differentiation. Arch. Dermatol. Res. 2014, 306, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, K.; Hiebert, P.; Werner, S.; Kim, T.G.; Kim, Y.S. Tussilagonone Ameliorates Psoriatic Features in Keratinocytes and Imiquimod-Induced Psoriasis-Like Lesions in Mice via NRF2 Activation. J. Investig. Dermatol. 2020, 140, 1223–1232.e4. [Google Scholar] [CrossRef]
- Helwa, I.; Choudhary, V.; Chen, X.; Kaddour-Djebbar, I.; Bollag, W.B. Anti-Psoriatic Drug Monomethylfumarate Increases Nuclear Factor Erythroid 2-Related Factor 2 Levels and Induces Aquaporin-3 mRNA and Protein Expression. J. Pharmacol. Exp. Ther. 2017, 362, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, H.; Zhao, M.; Li, J.; Hu, Y.; Fu, J.; Pi, J.; Wang, H.; Xu, Y. Nrf2 in keratinocytes protects against skin fibrosis via regulating epidermal lesion and inflammatory response. Biochem. Pharmacol. 2020, 174, 113846. [Google Scholar] [CrossRef]
- Choudhary, V.; Olala, L.O.; Qin, H.; Helwa, I.; Pan, Z.Q.; Tsai, Y.Y.; Frohman, M.A.; Kaddour-Djebbar, I.; Bollag, W.B. Aquaporin-3 re-expression induces differentiation in a phospholipase D2-dependent manner in aquaporin-3-knockout mouse keratinocytes. J. Investig. Dermatol. 2015, 135, 499–507. [Google Scholar] [CrossRef]
- Schafer, M.; Willrodt, A.H.; Kurinna, S.; Link, A.S.; Farwanah, H.; Geusau, A.; Gruber, F.; Sorg, O.; Huebner, A.J.; Roop, D.R.; et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014, 6, 442–457. [Google Scholar] [CrossRef]



| Signaling Molecules | Functions of Signaling Molecules | Related Physiological States or Diseases for Keratinocyte Differentiation | Refs. |
|---|---|---|---|
| Ca2+/PIP5K1α/PLC-γ1 or Ca2+/PKC-δ | Induction of keratinocyte differentiation | Differentiation of granular layer | [8,9,10,11] |
| Ca2+/PI3K/Akt | Conversion from PIP2 to PIP3, PLC-γ1 activation, involvement in keratinocyte differentiation, and interaction with the E-cadherin complex | Induction of contact regions of cell–cell | [18,19,20,23,24,25,26,27] |
| 1,25(OH)2D3/PLC-γ1 | Induction of activation and expression of PLC isoform, increase in intracellular Ca2+ and IP3, and promotion of keratinocyte differentiation | Maintenance of Ca2+ homeostasis | [31,32,39,40,41] |
| PP1 | Keratinocyte differentiation through the interaction with the E-cadherin–catenin–PIP5K1α complex via the PI3K/PLC/PKC/PP1 signaling pathway | Cell cycle progression and Ca2+ transport | [50,53] |
| Ca2+/CaSR | Ca2+ release from intracellular Ca2+ store and induction of keratinocyte differentiation | Enhanced epidermis proliferation by CaSR knockout | [59,60,61,62,63] |
| CaSR/E-cadherin/EGFR/ERK | Induction of keratinocyte differentiation | Wound healing and re-epithelialization | [27,65] |
| Pyk2 | Activation of Src family tyrosine kinases, MAPK, p70S6K, Rho GTPases, Akt, and NF-κB, and keratinocyte differentiation by Pyk2 overexpression | INV promoter activation | [67,68,71,72,73,74] |
| AP-1 | Involvement of differentiation, proliferation, apoptosis, and oncogenesis | Regulation of skin homeostasis | [81,82,83] |
| TAM67 | Induction of delayed differentiation and increased proliferation | Extensive parakeratosis, hyperkeratosis, aberrant formation of keratin filaments, erythema, Th-1- and -2-associated inflammation | [101,103] |
| TM/p-ERK | Contribution to wound healing and enhanced keratinocyte differentiation | Attenuated cell migration and keratinocyte differentiation by depletion of TM | [106,108] |
| CD9/E-cadherin/PI3K/Akt | Upregulation of cell adhesion and keratinocyte differentiation | Association with keratinocyte motility and growth | [18,19,109,110,112] |
| PKC/miR-203 | Mediation of keratinocyte differentiation | Decrease in epidermal thickness and proliferation | [96,120] |
| β ig-h3 | Enhanced keratinocyte differentiation | Reduced proliferation and mediation of keratinocyte adhesion | [130] |
| S1P/S1P3 receptor or LPA/LPA2 receptor | Increase in keratinocyte differentiation through intracellular Ca2+ spikes and conversion to IP3 | Survival and growth, differentiation, adhesion, cell motility, and the elevation of intracellular Ca2+ levels | [131,132,133,134,135,136,137] |
| K6PC-5 or Ca2+/SphK/S1P | Induction of keratinocyte differentiation through intracellular Ca2+ peaks and suppression of keratinocyte proliferation | Attenuated epidermis hyperplasia | [141,142] |
| SGPL/S1P | Induction of keratinocyte differentiation by inhibition of SGPL | Alleviation of psoriasis symptoms and epidermal thickness through the SGPL inhibitor | [142,143] |
| Serpin B7 | Increased epidermal thickness, inflammatory infiltration, enhanced chemokine expression, and reduced keratinocyte differentiation by serpin B7 depletion | Exacerbated symptoms of psoriasis | [147] |
| AQP3/glycerol or AQP3/PLD2/PG/PKC βII | Reduced glycerol and water transport capacities by AQP3 depletion and promotion of keratinocyte differentiation | Compromised skin elasticity delayed barrier recovery | [150,151,155] |
| Eph A2/desmosomal or Eph A2/ERK/MAPK | Inhibition of keratinocyte proliferation and enhanced keratinocyte stratification and differentiation | Diminished keratinocyte differentiation by desmoglein 1 inhibition | [161,162,163,164] |
| IGFBP7/p-ERK1/2/p-IRS1 or IGFBP7/IGF/insulin | Attenuated keratinocyte differentiation by blocked IGFBP7 expression | Psoriasis | [165,166,167,168,169,170,171] |
| Ca2+/Nrf2, TGN/Nrf2/HO-1 or MMF/Nrf2/AQP3 | Induction of keratinocyte differentiation, alleviation of inflammation, and attenuated ROS level | Psoriasis and skin fibrosis | [173,174,175,176,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Yang, D.; Hong, J.H. Various Cellular Components and Its Signaling Cascades Through the Involvement of Signaling Messengers in Keratinocyte Differentiation. Antioxidants 2025, 14, 426. https://doi.org/10.3390/antiox14040426
Kim HJ, Yang D, Hong JH. Various Cellular Components and Its Signaling Cascades Through the Involvement of Signaling Messengers in Keratinocyte Differentiation. Antioxidants. 2025; 14(4):426. https://doi.org/10.3390/antiox14040426
Chicago/Turabian StyleKim, Hyeong Jae, Dongki Yang, and Jeong Hee Hong. 2025. "Various Cellular Components and Its Signaling Cascades Through the Involvement of Signaling Messengers in Keratinocyte Differentiation" Antioxidants 14, no. 4: 426. https://doi.org/10.3390/antiox14040426
APA StyleKim, H. J., Yang, D., & Hong, J. H. (2025). Various Cellular Components and Its Signaling Cascades Through the Involvement of Signaling Messengers in Keratinocyte Differentiation. Antioxidants, 14(4), 426. https://doi.org/10.3390/antiox14040426








