1. Introduction
Protein scarcity is a pressing global issue, worsened in densely populated regions with limited resources. Numerous sectors, including healthcare, nutrition, and aquaculture, face distinct challenges due to varying protein needs. It is crucial to strategically reduce protein usage across various fields or seek alternative resources, which is essential to alleviate the protein shortage. Expensive and limited protein additives cannot sustainably satisfy the growing demands of animal agriculture, as evidenced by the global deficiency in feed protein, which impacts animal production [
1]. In this context, reducing feed protein levels can help save on feed costs. Research by Li et al. [
2] revealed that pigs on a 13% protein diet exhibited enhanced MyHC-1 and MyHC-lla muscle fiber expression, softer meat, and increased intramuscular fat (IMF) along with monounsaturated fatty acids (MUFAs), contributing to a better meat color and flavor. Wang et al. [
3] reported that in summer, lowering dietary crude protein levels from 12% to 10% while supplementing with restricted amino acids had no negative effects on growth performance indices such as the average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (F:G) compared to a normal protein diet.. Conversely, Morales et al. [
4] demonstrated that increasing the protein level to 21.6% significantly increased the improved ADG and G:F to counteract reduced amino acid intake caused by heat stress, despite a possible rise in body temperature. However, excessive protein intake should be avoided due to its potential negative effects on kidney function and overall health, as too much protein can strain the body [
5,
6]. By selectively adjusting feeding regimens to include high-protein diets, it may be possible to optimize production without compromising health.
Ningxiang pigs are native to Liushahe, Caochong, and other areas within Ningxiang County, Hunan Province, boasting a history of over 1000 years in China. These pigs display an exceptional ability for fat deposition—nearly double that of their leaner counterparts, achieving a rate of 42.3% compared to 21.9% [
7]. Distinguished by their early maturity and considerable fat accumulation capabilities, Ningxiang pigs are recognized as the quintessential semi-fatty breed among various indigenous Chinese pigs bred for both meat and fat production. Given their growth characteristics, Ningxiang pigs require a specialized feeding approach; high-energy, high-protein diets are less effective since they do not improve the feed conversion efficiency and may unnecessarily increase fat storage. On the other hand, an insufficient protein intake can adversely impact growth and lead to excessive fat buildup. Compared to hybrid breeds such as Changbai, Large White, and Duroc, the specific protein and amino acid needs of local Chinese pig breeds like Ningxiang remain inadequately studied. Therefore, developing precise feeding standards is vital to address the growing consumer demand for high-quality pork quality in China. In recent years, researchers have sought to reduce the nutrient levels for Ningxiang pigs during the fattening phase, likely considering their carcass traits and the need to conserve feed resources. In light of the dual pressures from feed resource scarcity, environmental pollution in animal husbandry, and market demand for high-quality animal products, it is crucial to appropriately adjust the diet nutrient level combination and explore its effects on growth performance and carcass traits for the sustainable development of Ningxiang pig germplasm resources.
The objective of this research was to thoroughly assess how five different protein levels impact the growth performance, meat quality, and intestinal metabolites in Ningxiang finishing pigs. This study aims to create a solid experimental foundation to guide the nutritional strategies tailored to the unique dietary requirements of Ningxiang pigs.
3. Results
Table 3 illustrates that when dietary protein was either decreased to 11.09% or increased to 15.09%, both the final body weight and average daily gain significantly diminished compared to the 12.09% protein group
(p < 0.05). Additionally, an increase in dietary protein resulted in a quadratic reduction in both final weight and average daily gain, accompanied by a quadratic rise in the feed-to-gain ratio (
p < 0.01).
Table 4 demonstrated that the redness (a*) and yellowness (b*) values in the
Longissimus dorsi muscle were significantly reduced for protein levels of 13.09%, 14.09%, and 15.09% compared to the baseline 12.09% protein level (
p < 0.05). The intramuscular fat contents for protein levels of 11.09% and 15.09% were significantly increased compared with that for a protein level of 13.09%. Additionally, a linear increase in dietary protein resulted in a linear decline in both the redness and yellowness of the muscle, while the content of intramuscular fat exhibited a quadratic reduction (
p < 0.01).
Table 5 indicates that the levels of C17:0, C17:1, and C18:3n3 fatty acids in the
Longissimus dorsi muscle were significantly higher in pigs fed a 15.09% protein diet compared to those on a 12.09% protein diet (
p < 0.05). Furthermore, as dietary protein levels increased, the concentrations of C17:0, C17:1, C18:3n3, and C18:3n6 fatty acids in the muscle tissue showed a consistent linear increase (
p < 0.01).
As shown in
Table 6, the contents of glycine (Gly), alanine (Ala), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), arginine (Arg), proline (Pro), non-essential amino acid (NEAA), and total amino acid (TAA) in
Longissimus dorsi muscle were significantly lower in pigs fed 11.09% and 15.09% protein levels compared to those on 13.09% and 14.09% protein levels (
p < 0.05). The contents of threonine (Thr), glutamic acid (Glu), valine (Val), histidine (His), and essential amino acid (EAA) in
Longissimus dorsi muscle were significantly lower in pigs fed a 11.09% protein level compared to those on 13.09% and 14.09% protein levels (
p < 0.05). In addition, with the increase in dietary protein level, the contents of threonine, glutamic acid, glycine, alanine, valine, leucine, tyrosine, phenylalanine, histidine, arginine, proline, essential amino acid, non-essential amino acid, and total amino acid in
Longissimus dorsi muscle exhibited a quadratic curve change (
p < 0.01).
According to
Table 7, the GSH-Px in the 11.09% dietary protein level group was significantly lower than that in the other four groups, and the serum GSH-Px content was linearly increased with the increase in dietary protein level (
p < 0.05). There were no significant differences in serum SOD, MDA, and T-AOC.
As shown in
Table 8, compared with the 12.09% protein level group, the contents of cademine and spermidine in cecal chyme in the 11.09% protein level group were significantly increased, and spermidine content was significantly decreased (
p < 0.05). Putrescine, spermidine, and cadaverine increased linearly with increasing dietary protein levels (
p < 0.05). On the other hand, spermine was lower, with dietary protein levels greater than 11.09% (
p < 0.05).
As shown in
Table 9, for isobutyric acid, the 14.09% protein group was higher than the 11.09, 12.09, and 13.09% groups (
p < 0.05). For butyric acid, the 14.09% protein group was higher than the 12.09 and 13.09% groups (
p < 0.05). In isovaleric acid, the 14.09% group was higher than the other treatments (
p < 0.05).
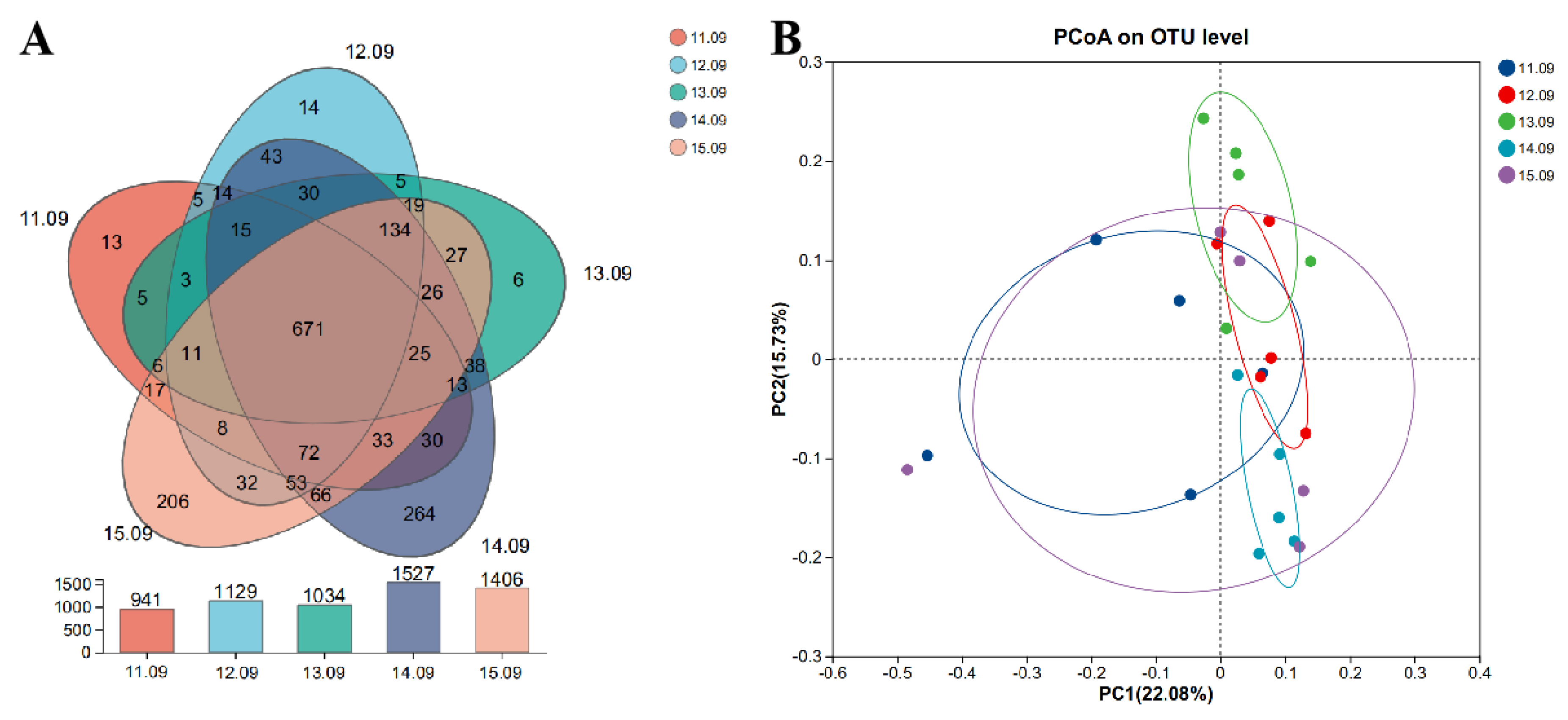
The Venn diagram in
Figure 1 shows the effects of different dietary protein levels on the OTUs of cecal microbiota in Ningxiang pigs at the fattening stage. From low to high dietary protein levels, 941, 1129, 1034, 1527, and 1406 OTUs were detected, respectively, and a total of 671 OTUs were detected in the five groups (
Figure 1A). The β-diversity of cecum flora in each group was characterized by principal axis analysis (
Figure 1B). The group with a dietary protein level of 13.09% showed a significant separation from the other four groups, indicating a significant difference in microbial composition.
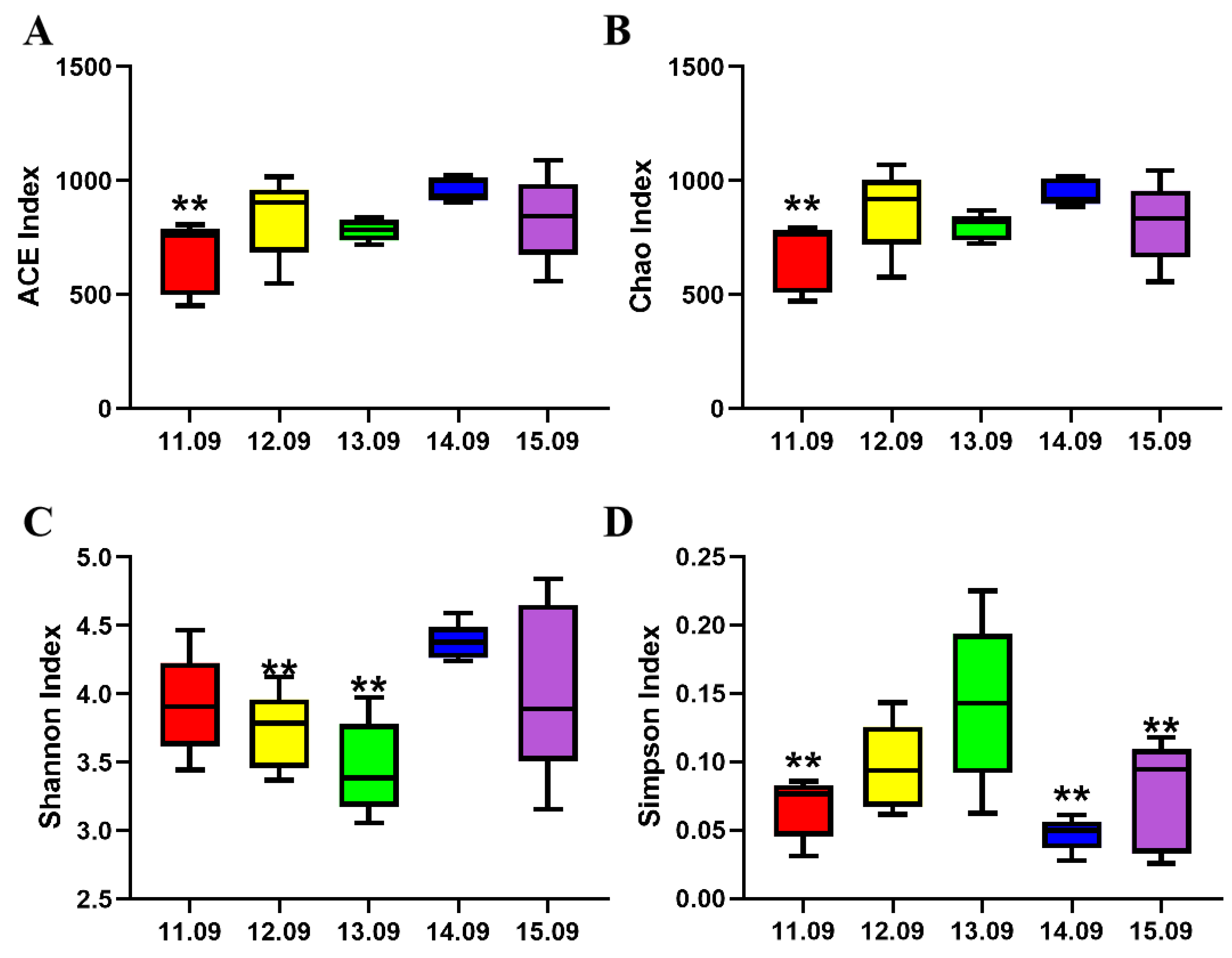
The α-diversity of cecum microbiota is shown in
Figure 2, and the Ace index, Chao index, and Simpson index of the 11.09% dietary protein level group were significantly reduced. The Shannon indexes of the 12.09% dietary protein level group and 13.09% dietary protein level group were significantly decreased.
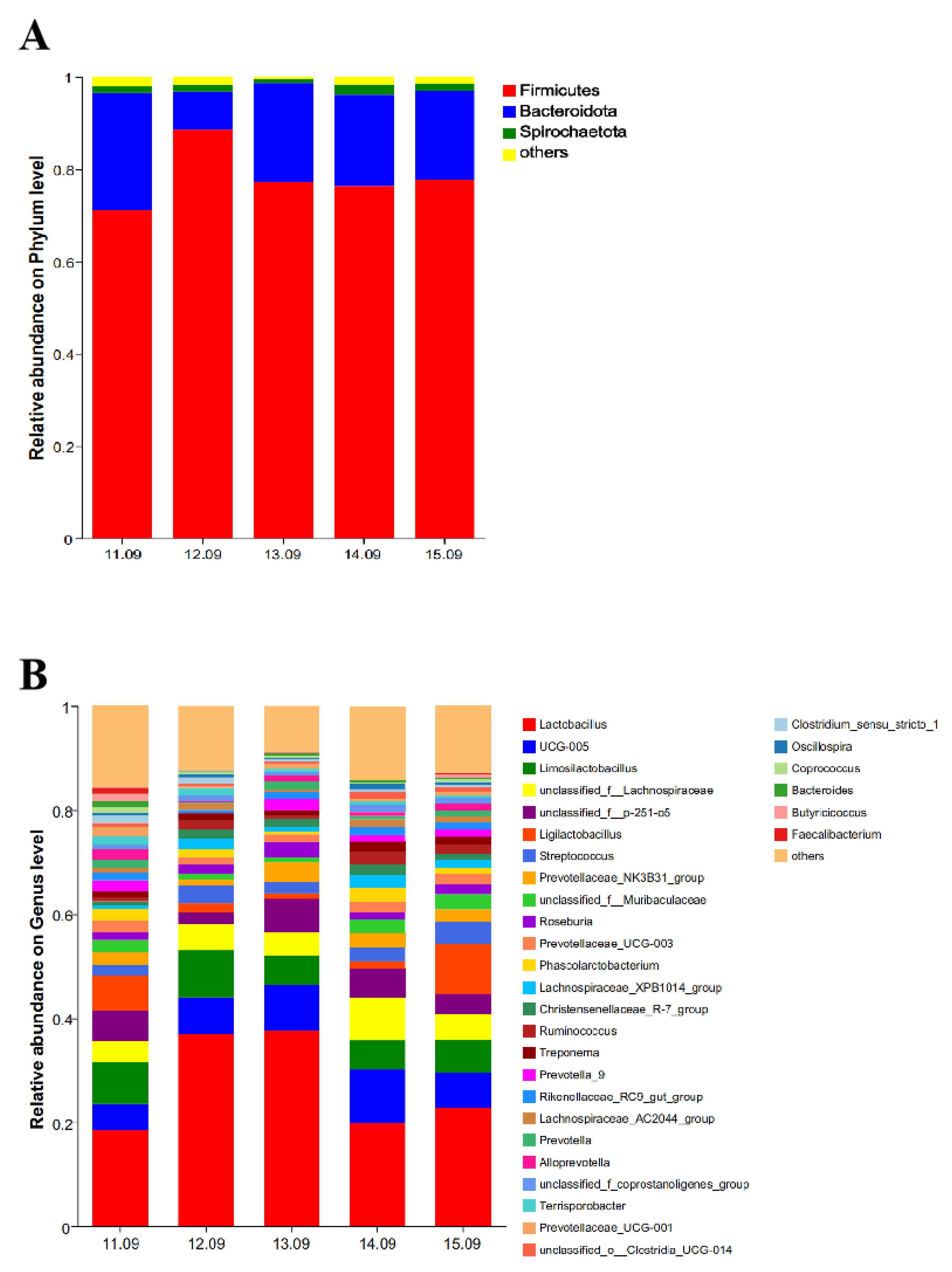
As shown in
Figure 3, at the phylum level, three dominant phyla were detected in the five groups. Firmicutes, Bacteroidota, and Spirochaetota were the dominant phyla, accounting for 71.11, 25.43, and 1.35%, respectively, in the 11.09% dietary protein level group; 88.55, 8.26, and 1.52%, respectively, in the 12.09% dietary protein level group; 77.31, 21.39, and 0.39%, respectively, in the 13.09% dietary protein level group; 76.29, 19.90, and 2.06%, respectively, in the 14.09% dietary protein level group; and 77.72, 19.27, and 1.57%, respectively, in the 15.09% dietary protein level group (
Figure 3A). At the genus level, a total of 31 genera were detected in the five groups. The dominant genera in the 11.09% dietary protein level group were
Lactobacillus,
Limosilactobacillus,
Ligilactobacillus,
unclassified_f__p-251-o5, and
UCG-005. The proportions were 18.44, 8.11, 6.68, 5.87, and 5.08%, respectively. The dominant strains in the 12.09% dietary protein level group were
Lactobacillus,
Limosilactobacillus,
UCG-005,
unclassified_f__Lachnospiraceae, and
Streptococcus. The proportions were 36.95, 9.09, 7.00, 4.99, and 3.50%, respectively. The dominant genera in the 13.09% dietary protein level group were
Lactobacillus,
UCG-005,
Limosilactobacillus,
unclassified_f__p-251-o5, and
unclassified_f__Lachnospiraceae. The proportions were 37.84, 8.78, 6.22, 5.63, and 4.61%, respectively. The dominant genera in the 14.09% dietary protein level group were
Lactobacillus,
UCG-005,
unclassified_f__Lachnospiraceae,
Limosilactobacillus, and
unclassified_f__p-251-o5. The proportions were 19.84, 10.35, 8.15, 5.60, and 5.57%, respectively. The dominant genera in the 15.09% dietary protein level group were
Lactobacillus,
Ligilactobacillus,
UCG-005,
Limosilactobacillus, and
unclassified_f__Lachnospiraceae. The proportions were 22.86, 9.61, 6.56, 6.45, and 4.98%, respectively (
Figure 3B).
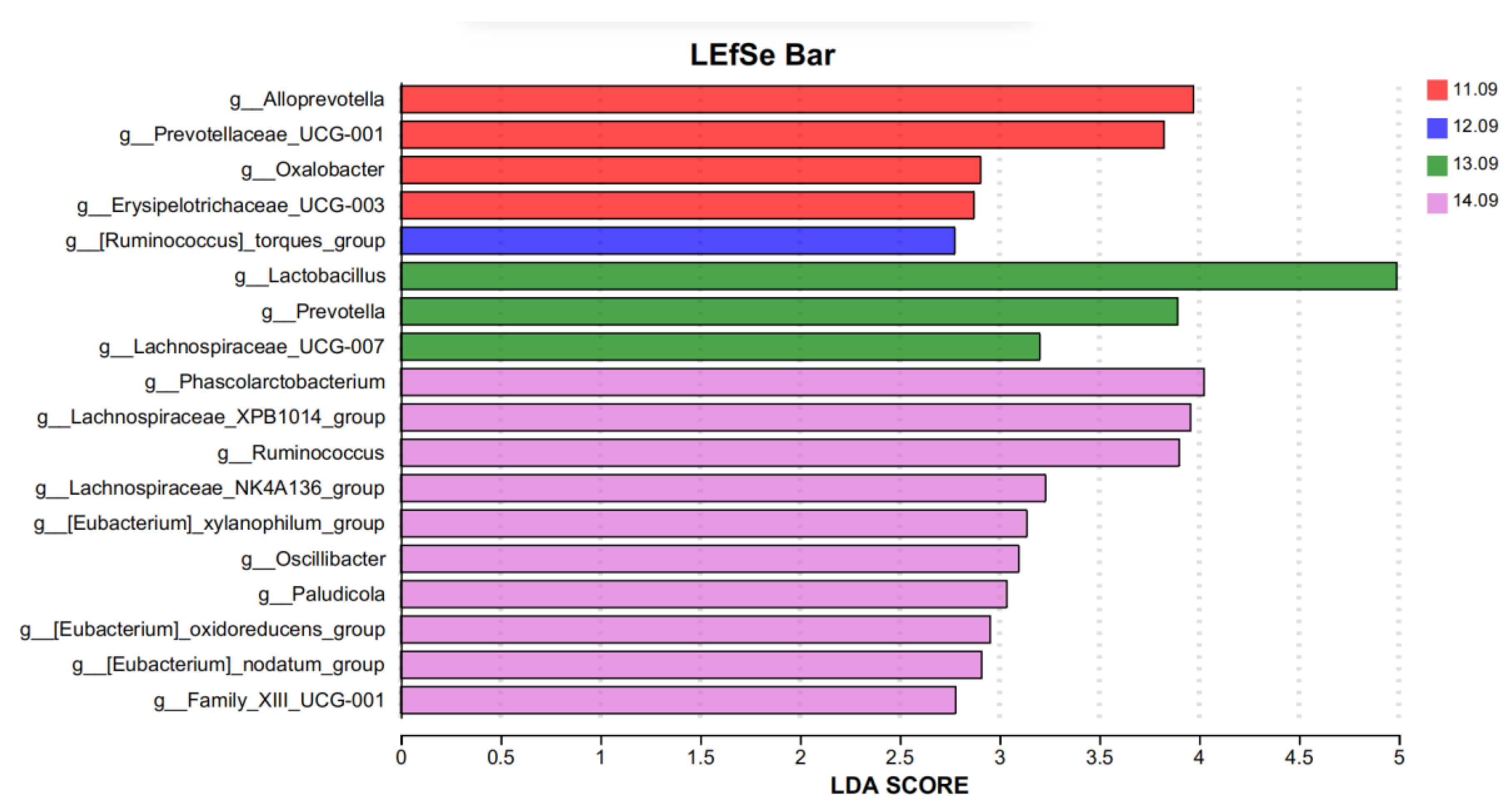
Lefse analysis was used to analyze and identify bacteria with significant differences at the genus level in different protein levels treated in five diets (
Figure 4, LDA = 2.5). Lefse analysis was used to analyze and identify bacteria with significant differences at the genus level in different protein levels treated in the five diets (
Figure 4, LDA = 2.5). Four species of differential bacteria (
Alloprevotella,
Prevotellaceae_UCG-001,
oxalter, and
Erysipelotrichaceae_UCG-003) were significantly enriched at the 11.09% dietary protein level group.
Ruminococcus]_torques_group was significantly enriched in the 12.09% dietary protein level group and three distinct strains (
Lactobacillus,
Prevotella, and
Lachnospiraceae_UCG-007). Ten species of bacteria (
Phascolarctobacterium,
Lachnospiraceae_XPB1014_group,
Xylanophilum_group,
Ruminococcus,
Lachnospiraceae_NK4A136_group, Oscillibacter,
Paludicolag,
Oxidoreducens_group,
Nodatum_group, and
g__Family_XIII_UCG-001) were significantly enriched in the 14.09% diet protein group.
4. Discussion
Protein plays a critical role in animal growth and metabolism. However, an excessive protein level in swine diets is correlated with a decline in [
12]; conversely, judicious restriction of crude protein levels can enhance meat quality, reduce production costs, and mitigate nitrogen pollution [
13]. The findings of the present study indicate that both high-protein and low-protein diets exert inhibitory effects on growth performance. This phenomenon may be attributable to fluctuations in the concentrations of certain non-restrictive amino acids, which disrupt the pre-existing amino acid balance pattern. From the specific effects of protein on pig growth and metabolism, it can be inferred that protein is the main component of pig tissue, and it has an important impact on pig muscle growth, weight gain, and carcass quality. The weight gain rate and feed conversion rate of pigs were affected by the protein level and the ratio of essential amino acids directly. Wang et al. [
14] observed that if crude protein reduction does not exceed 4%, it is essential to supplement the diet with critical amino acids, including lysine, methionine, tryptophan, and threonine. Conversely, reductions surpassing 6% necessitate the supplementation of non-essential amino acids to avert growth impairment. Thus, careful consideration of protein levels in swine diets is imperative for optimizing growth performance and product quality.
Current research has revealed that the specific protein requirements for Ningxiang pigs remain inadequately defined. This lack of clarity may be contributing to the observed slow growth rates within the 11.09% protein group, potentially attributable to an excessive reduction in protein intake, an imbalance in amino acid composition, and inefficient amino acid utilization. Protein deposition exhibits a linear relationship up to a certain threshold, beyond which it becomes contingent upon energy availability [
15,
16]. An overabundance of protein intake can elevate metabolic rates, resulting in increased heat production and insufficient net energy, which in turn may diminish net protein deposition and growth performance [
17].
Furthermore, findings from this study indicate a linear increase in serum GSH-Px content, correlating with higher dietary protein levels, which may reflect the utilization efficiency of dietary amino acid utilization. Despite efforts to maintain a consistent amino acid pattern, discrepancies in dietary intake and utilization persist among Ningxiang pigs, impacting amino acid transformation and leading to the increase in serum GSH-Px levels. Liu et al. [
18] demonstrated that a 14% protein diet reduced plasma GSH-Px activity and the GSH concentration when compared to a 20% protein diet, suggesting that low-protein diets may impair the antioxidant capacity by inhibiting both non-enzymatic and enzymatic antioxidant defense mechanisms. In the work of Wu et al. [
19], it was observed that feeding diets with high protein significantly increased the enhance serum SOD content, yet exhibited no significant effects on the serum CAT, MDA, and T-AOC levels, likely due to insufficient protein nutrients hindering antioxidant enzyme production.
This study also observed a linear decrease in the redness and yellowness parameters of
Longissimus dorsi muscle, which decreased linearly with increasing dietary protein levels. Choi et al. [
20] similarly identified detrimental effects on pork quality associated with both high and low protein levels in growing/finishing pigs. Reports indicate that the L*, a*, and b* values of
Longissimus dorsi muscle in pigs subjected to low-protein diets are elevated [
20,
21], attributed to an increased intramuscular fat (IMF) content [
22]. Notably, our trial recorded significant increases in IMF content across both low- and high-protein diets. Previous investigations have established that implementing low-protein diets during the growth or fattening phases can augment the IMF content of pigs without concomitant increases in back fat [
23,
24], possibly due to the activation of lipogenic enzyme expression within muscle tissues [
25], thus facilitating de novo fatty acid synthesis.
The relationship between dietary protein levels and amino acids in
Longissimus dorsi muscle reveals significant insights into nutritional biochemistry. Our investigation illustrates that the amino acid composition follows a quadratic trend relative to the dietary protein intake. Notably, both insufficient and excessive protein levels correlate with a diminished amino acid content in the
Longissimus dorsi muscle, aligning with previous findings that denote a positive correlation between dietary protein and muscle protein synthesis [
26,
27]. This observation indicates that an imbalanced amino acid supply may instigate protein turnover, striving to achieve an equilibrium within the amino acid pool of the organism. Furthermore, contrasting reports indicate that the dietary protein level for fattening pigs is reduced from 16% to 14%, and when supplemented with essential amino acids, can enhance the EAA/TAA ratio in muscle [
28]. It follows that a more balanced amino acid supply facilitates a closer alignment of tissue amino acids with the ideal protein pattern [
29]. The amino acid score serves as a vital metric in assessing the nutritional value of meat, where a higher score correlates with enhanced food quality [
30]. In conclusion, discrepancies observed in muscle protein and total hydrolyzed amino acid contents primarily stem from imbalances in dietary amino acid composition, a finding that merits further scrutiny and may be linked to the extent of protein degradation within muscle tissues.
The production of biogenic amines in the posterior intestine is primarily attributed to the action of amino acid decarboxylase, an enzyme found in various intestinal microorganisms, including but not limited to Bacteroides, Clostridium, Bifidobacterium, Enterobacterium, and Streptococcus [
31,
32]. A notable observation within the scope of this study is the inverse relationship between dietary protein levels and the concentrations of putrescine and spermidine within the cecum, aligning with findings from previous research.
Particularly significant is the finding that the spermine content in the group with 11.09% crude protein (CP) was markedly elevated compared to the other experimental groups. Spermidine is recognized for its critical involvement in various physiological processes at the cellular level, particularly in modulating metabolite alterations arising from oxidative stress, which encompasses amino acid metabolism, as well as in fostering the development of the small intestine [
33,
34].
The concentration of biogenic amines present in the gastrointestinal tract is contingent upon the populations of bacteria responsible for their synthesis, as well as those that facilitate their absorption. Notably, both Bacteroides and Clostridium have demonstrated capabilities for the synthesis of putrescine and spermidine, both in vivo and in vitro [
35]. Putrescine serves as a precursor for spermidine, which is subsequently converted into spermidine through the enzymatic action of spermidine synthase [
36].
Moreover, alterations in amino acid metabolism within the organism exert a considerable influence on the biogenic amines present in the hindgut. Intestinal short-chain fatty acids (SCFAs) are typical metabolites resulting from carbohydrate fermentation. Despite the absence of significant changes in total SCFA content during this investigation, specific fatty acids, namely isobutyric acid, butyric acid, and isovaleric acid, exhibited a linear escalation corresponding with increased dietary protein levels. This observation can largely be attributed to the carbohydrate and fiber composition of the diet. Furthermore, it is acknowledged that amino acid fermentation contributes to SCFA production, albeit at a relatively diminished rate [
37]. Previous studies have reported that an elevation in dietary protein correlates with heightened concentrations of branched-chain fatty acids (BCFAs) and SCFAs in digestive samples collected from the cecum and colon, as well as in the feces of growing pigs [
38,
39]. Consequently, it is evident that dietary protein levels significantly influence the production of microbial metabolites within the hindgut.
The manipulation of dietary protein levels significantly influences the structure of cecal microbiota during the fattening phase of Ningxiang pigs. Notably, the Chao and Shannon indices—indicators of microbial abundance and diversity—demonstrated that the cecal floral abundance in the 11.09% dietary protein group was markedly diminished. Conversely, both the 12.09% and 13.09% protein groups exhibited a significant reduction in microbial diversity. While increased microbial diversity typically correlates with enhanced microbiotal stability and pathogen resistance, the current findings suggest that reduced diversity in the latter groups does not necessarily imply increased susceptibility to pathogen invasion [
40]. At both the phylum and genus levels, the relative abundance of Firmicutes and Lactobacillus was elevated in the higher protein groups compared to the other dietary conditions. A heightened Firmicutes to Bacteroides ratio is often indicative of obesity-related metabolic disorders [
41,
42]. Lactobacillus, recognized for its beneficial roles in digestive health, serves to regulate gut microbiota, suppress pathogenic bacteria, and bolster intestinal immunity [
43,
44,
45]. Moreover, a notable increase in Limosilactobacillus abundance was observed in groups with lower protein levels. Although the overall abundance of Lactobacillus in the 11.09% and 15.09% dietary protein groups declined, the presence of beneficial Lactobacillus-related genera, such as Limosilactobacillus and Ligilactobacillus, increased, suggesting a potential advantage in optimizing protein utilization in Ningxiang pigs.