Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Extract Preparation
2.3. Antioxidant Activity Assays
2.3.1. ABTS Radical Scavenging Activity
2.3.2. DPPH Radical Scavenging Activity
2.3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4. Determination of Total Polyphenol and Flavonoid Content
2.4.1. Total Polyphenol Content
2.4.2. Total Flavonoid Content
2.5. UPLC-Orbitrap-MS/MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.1.1. ABTS Radical Scavenging Activity
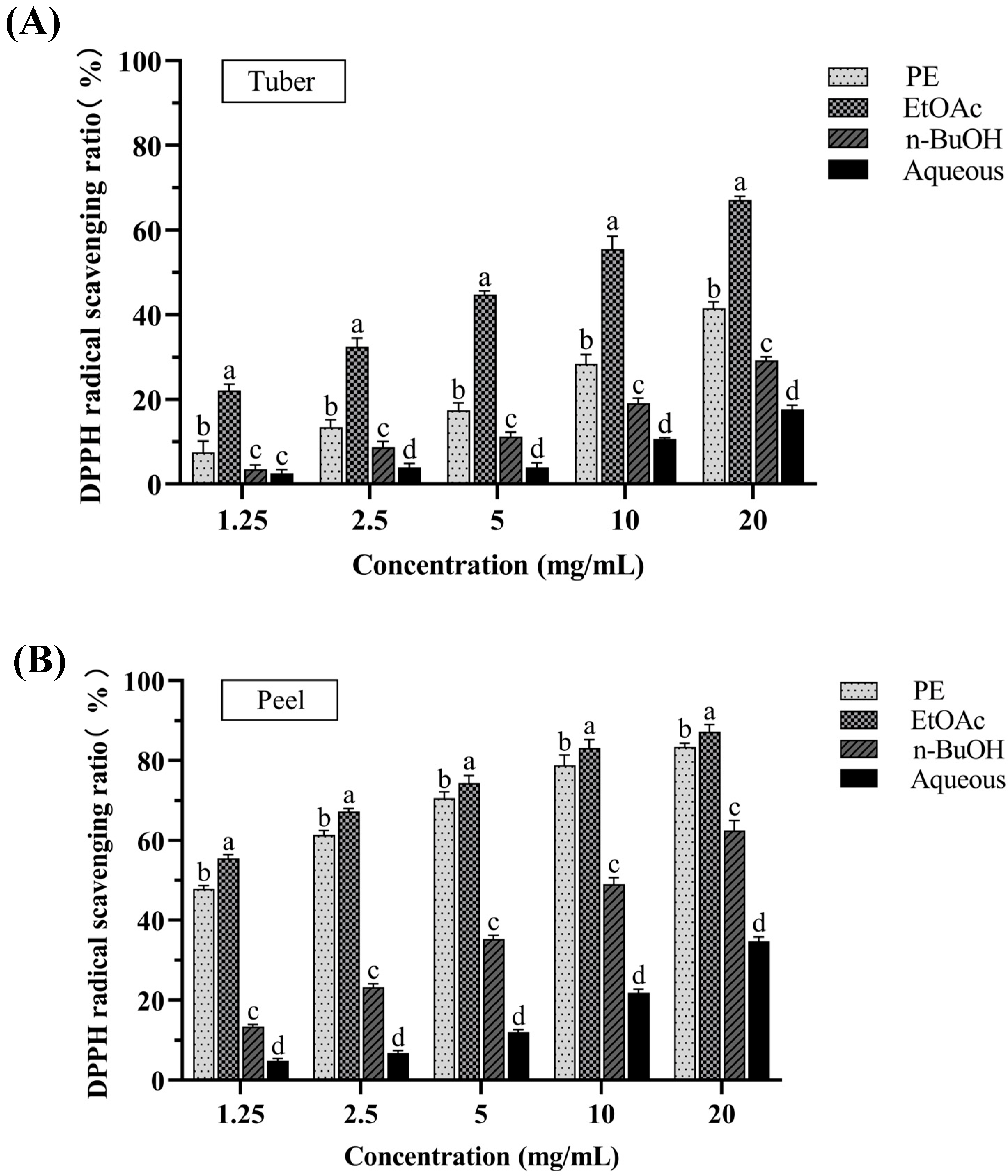
3.1.2. DPPH Radical Scavenging Activity
3.1.3. FRAP Assay
3.2. Determination of Total Polyphenol and Flavonoid Content
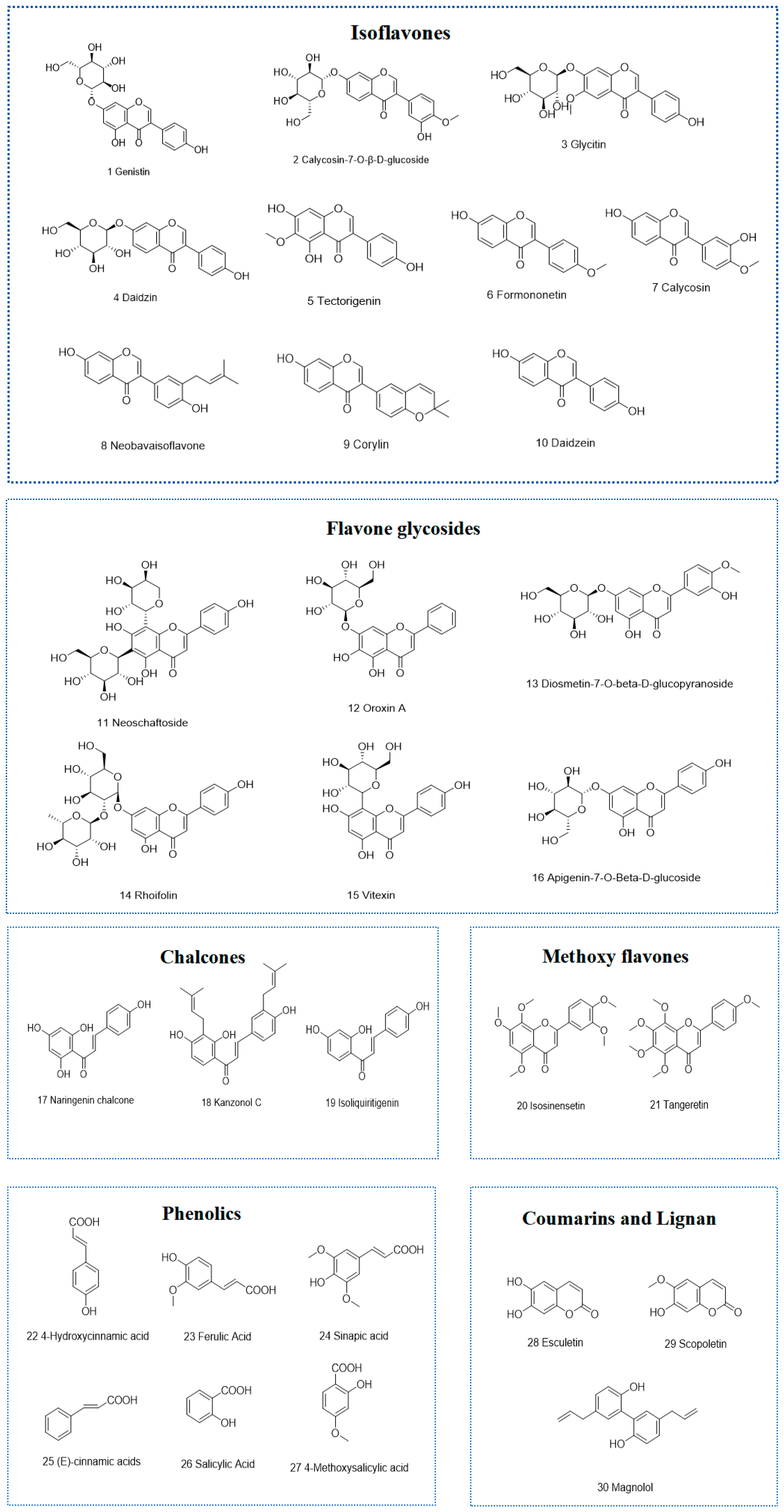
3.3. Identification of Chemical Constituents of the Strong Active Ethyl Acetate Fractions of Tubers and Peels from P. erosus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, Z.X.; Li, M.Q.; Zhang, W.W.; Cai, S.B.; Hu, X.S.; Yi, J.J. Analysis of phenolic compounds in pickled chayote and their effects on antioxidant activities and cell protection. Food Res. Int. 2022, 157, 111325. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhu, M.M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [PubMed]
- Kishore, A.; Patil, R.J.; Singh, A.; Pati, K. Jicama (Pachyrhizus spp.) a nonconventional starch: A review on isolation, composition, structure, properties, modifications and its application. Int. J. Biol. Macromol. 2024, 258, 129095. [Google Scholar]
- Choi, M.H.; Yang, S.H.; Lee, Y.J.; Sohn, J.H.; Lee, K.S.; Shin, H.J. Anti-obesity effect of daidzein derived from Pachyrhizus erosus (L.) Urb. extract via PPAR pathway in MDI-induced 3T3-L1 cell line. Cosmetics 2023, 10, 164. [Google Scholar] [CrossRef]
- Jaiswal, V.; Chauhan, S.; Lee, H.J. The bioactivity and phytochemicals of Pachyrhizus erosus (L.) Urb.: A multifunctional underutilized crop plant. Antioxidants 2021, 11, 58. [Google Scholar] [CrossRef]
- Bhanja, A.; Paikra, S.K.; Sutar, P.P.; Mishra, M. Characterization and identification of inulin from Pachyrhizus erosus and evaluation of its antioxidant and in-vitro prebiotic efficacy. J. Food Sci. Technol. 2023, 60, 328–339. [Google Scholar]
- Laovachirasuwan, P.; Phadungkit, M. Total phenolic and flavonoid contents, anti-tyrosinase and antioxidant activities of Pachyrhizus erosus extracts. Pharmacogn. J. 2023, 15, 839–842. [Google Scholar]
- Siregar, I.D.; Kusuma, H.S.W.; Widowati, W.; Marpaung, H.H.; Ferdinand, S.; Fachrial, E.; Lister, I.N.E. Antioxidant and antityrosinase activities of ethanolic Pachyrhizuserosus peel and tuber extract. MKB. 2019, 51, 75–81. [Google Scholar]
- He, Z.Y.; Tao, Y.D.; Zeng, M.M.; Zhang, S.; Tao, G.J.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic changes in phenolic compounds and antioxidant activity in oats (Avena nuda L.) during steeping and germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Approach to optimization of FRAP methodology for studies based on selected monoterpenes. Molecules 2020, 25, 5267. [Google Scholar]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant activity, total phenolic content and total flavonoid content in sweet chestnut (Castanea sativa Mill.) cultivars grown in Northwest Spain under different environmental conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Orsavová, J.; Juríková, T.; Bednaříková, R.; Mlček, J. Total phenolic and total flavonoid content, individual phenolic compounds and antioxidant activity in sweet rowanberry cultivars. Antioxidants 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Saso, L.; D’Angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a modulator of Nrf2, NF-κB, and their crosstalk: Molecular mechanisms and possible clinical applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.Y.; Li, J.L.; Zhu, Y.; Zhou, T.Y.; Wang, H.F.; Zhou, Y.; Su, T.; Zeng, B.B.; Tao, Z.; Chen, Y.; et al. Chemical constituents, pharmacology and safety of isoflavones in Puerariae Lobatae Radix. Pharmacol. Discov. 2024, 4, 21. [Google Scholar]
- Han, L.L.; Song, J.Q.; Yan, C.Q.; Wang, C.Q.; Wang, L.W.; Li, W.; Du, Y.; Li, Q.S.; Liang, T.G. Inhibitory activity and mechanism of calycosin and calycosin-7-O-β-D-glucoside on α-glucosidase: Spectroscopic and molecular docking analyses. Process Biochem. 2022, 118, 227–235. [Google Scholar] [CrossRef]
- Zang, Y.Q.; Igarashi, K.; Yu, C.Q. Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 117–123. [Google Scholar]
- Li, Y.H.; Hu, S.Y.; Chen, Y.Q.; Zhang, X.; Gao, H.C.; Tian, J.; Chen, J. Calycosin inhibits triple-negative breast cancer progression through down-regulation of the novel estrogen receptor-α splice variant ER-α30-mediated PI3K/AKT signaling pathway. Phytomedicine 2023, 118, 154924. [Google Scholar]
- Guo, C.; Tong, L.; Xi, M.M.; Yang, H.F.; Dong, H.L.; Wen, A.D. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar]
- Hao, F.X.; Zeng, M.N.; Cao, B.; Liang, X.W.; Ye, K.L.; Jiao, X.M.; Feng, W.S.; Zheng, X.K. Neobavaisoflavone ameliorates memory deficits and brain damage in Aβ25-35-induced mice by regulating SIRT1. CNS Neurosci. Ther. 2024, 30, e70068. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.C.; Shieh, T.M.; Hsueh, C.; Wang, S.H.; Leu, Y.L.; Lian, J.H.; Wang, T.H. Corylin suppresses hepatocellular carcinoma progression via the inhibition of epithelial-mesenchymal transition, mediated by long noncoding RNA GAS5. Int. J. Mol. Sci. 2018, 19, 380. [Google Scholar] [CrossRef]
- He, J.; Du, L.S.; Bao, M.M.; Zhang, B.; Qian, H.X.; Zhou, Q.S.; Cao, Z.F. Oroxin A inhibits breast cancer cell growth by inducing robust endoplasmic reticulum stress and senescence. AntiCancer Drugs 2016, 27, 204–215. [Google Scholar]
- Sun, W.L.; Zhang, B.W.; Yu, X.X.; Zhuang, C.L.; Li, X.; Sun, J.; Xing, Y.; Xiu, Z.; Dong, Y.S. Oroxin A from oroxylum indicum prevents the progression from prediabetes to diabetes in streptozotocin and high-fat diet induced mice. Phytomedicine 2018, 38, 24–34. [Google Scholar] [PubMed]
- Kılıç, C.S.; Kışla, M.M.; Amasya, G.; Şengel-Türk, C.T.; Alagöz, Z.A.; Özkan, A.M.G.; Ates, I.; Gümüşok, S.; Herrera-Bravo, J.; Sharifi-Rad, J.; et al. Rhoifolin: A promising flavonoid with cytotoxic and anticancer properties–molecular mechanisms and therapeutic potential. Excli J. 2025, 24, 289–320. [Google Scholar] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, B.G.; Kim, S.Y.; Jeon, H.; Soh, Y. Vitexin, an HIF-1α inhibitor, has anti-metastatic potential in PC12 cells. Mol. Cells 2006, 22, 291–299. [Google Scholar] [PubMed]
- Can, Ö.D.; Demir Özkay, Ü.; Üçel, U.İ. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar]
- Zhang, S.; Jiang, Z.F.; Pan, Q.; Song, C.Y.; Zhang, W.H. Anti-cancer effect of naringenin chalcone is mediated via the induction of autophagy, apoptosis and activation of PI3K/Akt signalling pathway. Bangladesh J. Pharmacol. 2016, 11, 684–690. [Google Scholar]
- Escribano-Ferrer, E.; Queralt Regué, J.; Garcia-Sala, X.; Boix Montañés, A.; Lamuela-Raventos, R.M. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar]
- Liu, C.; Liu, X.Y.; Ma, Q.; Su, F.Y.; Cai, E.B. Design, synthesis, and antitumor activity of isoliquiritigenin amino acid ester derivatives. Molecules 2024, 29, 2641. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar]
- Qin, Y.W.; Song, D.Z.; Liao, S.J.; Chen, J.C.; Xu, M.L.; Su, Y.G.; Lian, H.Y.; Peng, H.; Wei, L.H.; Chen, K.; et al. Isosinensetin alleviates estrogen deficiency-induced osteoporosis via suppressing ROS-mediated NF-κB/MAPK signaling pathways. Biomed. Pharmacother. 2023, 160, 114347. [Google Scholar]
- Ting, Y.W.; Chiou, Y.S.; Pan, M.H.; Ho, C.T.; Huang, Q.R. In vitro and in vivo anti-cancer activity of tangeretin against colorectal cancer was enhanced by emulsion-based delivery system. J. Funct. Foods 2015, 15, 264–273. [Google Scholar]
- Yao, X.L.; Zhu, X.R.; Pan, S.Y.; Fang, Y.P.; Jiang, F.Y.; Phillips, G.O.; Xu, X.Y. Antimicrobial activity of nobiletin and tangeretin against pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.A.; Lee, J.; Seo, H.; Nam, S.J.; Jo, D.G.; Hyun, D.H. 4-Hydroxycinnamic acid attenuates neuronal cell death by inducing expression of plasma membrane redox enzymes and improving mitochondrial functions. Food Sci. Hum. Wellness 2023, 12, 1287–1299. [Google Scholar]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini. Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [PubMed]
- Shi, C.; Zhang, X.R.; Sun, Y.; Yang, M.C.; Song, K.K.; Zheng, Z.W.; Chen, Y.F.; Liu, X.; Jia, Z.Y.; Dong, R.; et al. Antimicrobial activity of ferulic acid against Cronobacter Sakazakii and possible mechanism of action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar]
- Kim, M.S.; Shin, W.C.; Kang, D.K.; Sohn, H.Y. Anti-thrombosis activity of sinapic acid isolated from the lees of bokbunja wine. J. Microbiol. Biotechnol. 2016, 26, 61–65. [Google Scholar]
- Taştemur, Ş.; Hacısüleyman, L.; Karataş, Ö.; Yulak, F.; Ataseven, H. Anticancer activity of sinapic acid by inducing apoptosis in HT-29 human colon cancer cell line. Can. J. Physiol. Pharmacol. 2023, 101, 361–368. [Google Scholar]
- Silambarasan, T.; Manivannan, J.; Krishna Priya, M.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE 2014, 9, e115682. [Google Scholar]
- Ghasemi, M.; Amini-Khoei, H.; Bijad, E.; Rafieian-Kopaei, M.; Sureda, A.; Lorigooini, Z. Sinapinic acid as a potential therapeutic agent for epilepsy through targeting NMDA receptors and nitrite level. Sci. Rep. 2024, 14, 24941–24951. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar]
- Cai, T.; Cai, B. Pharmacological activities of esculin and esculetin: A review. Medicine 2023, 102, e35306. [Google Scholar]
- Firmansyah, A.; Winingsih, W.; Manobi, J.D.Y. Review of scopoletin: Isolation, analysis process, and pharmacological activity. Biointerface Res. Appl. Chem. 2021, 11, 12006–12019. [Google Scholar]
- Cho, S.Y.; Lee, J.H.; Bae, K.H.; Kim, Y.S.; Jeong, C.S. Anti-gastritic effects of magnolol and honokiol from the stem bark of Magnolia obovata. Biomol. Ther. 2008, 16, 270–276. [Google Scholar]
- Chen, Y.H.; Lu, M.H.; Guo, D.S.; Zhai, Y.Y.; Miao, D.; Yue, J.Y.; Yuan, C.H.; Zhao, M.M.; An, D.R. Antifungal effect of magnolol and honokiol from Magnolia officinalis on alternaria alternata causing tobacco brown spot. Molecules 2019, 24, 2140. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef]
- Yao, D.S.; Bao, L.X.; Wang, S.C.; Tan, M.; Xu, Y.Y.; Wu, T.X.; Zhang, Z.G.; Gong, K.Z. Isoliquiritigenin alleviates myocardial ischemia-reperfusion injury by regulating the Nrf2/HO-1/SLC7a11/GPX4 axis in mice. Free Radic. Biol. Med. 2024, 221, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Chen, J.B.; Cao, J.P.; Xiao, J.; Li, X.; Sun, C.D. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar]
- Zhu, Z.L.; Wang, X.Y.; Wang, Z.D.; Zhao, Z.; Zhou, P.H.; Gao, X.B. Neobavaisoflavone protects osteoblasts from dexamethasone-induced oxidative stress by upregulating the CRNDE-mediated Nrf2/HO-1 signaling pathway. Drug Dev. Res. 2021, 82, 1044–1054. [Google Scholar]
- Kazmi, Z.; Zeeshan, S.; Khan, A.; Malik, S.; Shehzad, A.; Seo, E.K.; Khan, S. Anti-epileptic activity of daidzin in PTZ-induced mice model by targeting oxidative stress and BDNF/VEGF signaling. Neurotoxicology 2020, 79, 150–163. [Google Scholar]
- Li, M.D.; Huan, Y.Q.; Jiang, T.Q.; He, Y.X.; Gao, Z.X. Rehabilitation training enhanced the therapeutic effect of calycosin on neurological function recovery of rats following spinal cord injury. J. Chem. Neuroanat. 2024, 136, 102384. [Google Scholar]
- Peng, W.S.; Zhou, N.X.; Song, Z.H.; Zhang, H.H.; He, X. Magnolol as a protective antioxidant alleviates rotenone-induced oxidative stress and liver damage through MAPK/mTOR/Nrf2 in broilers. Metabolites 2023, 13, 84. [Google Scholar] [CrossRef]
- Jain, P.G.; Nayse, P.G.; Patil, D.J.; Shinde, S.D.; Surana, S.J. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Futur. J. Pharm. Sci. 2020, 6, 53. [Google Scholar] [CrossRef]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.Y.; Lv, H.Y.; Chen, Y.; Zhang, C.H.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.Y.; Han, L.; Zhong, J.R.; Shu, J.Q.; Cao, Z.L.; Fang, J.Q.; Zhang, X.Y.; Gao, Z.L.; Xiao, F.X. Rhoifolin alleviates alcoholic liver disease in vivo and in vitro via inhibition of the TLR4/ NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 878898. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, S.; Jiang, K.F.; Wang, Y.; Yang, M.; Guo, M.Y. Glycitin alleviates lipopolysaccharide-induced acute lung injury via inhibiting NF-κB and MAPKs pathway activation in mice. Int. Immunopharmacol. 2019, 75, 105749. [Google Scholar] [CrossRef]



| FRAP (mM Fe (II)/g Extract) | PE | EtOAc | n-BuOH | Aqueous | Standard Curve |
|---|---|---|---|---|---|
| Peel | 7.023 ± 0.5591 | 12.56 ± 1.720 | 2.519 ± 0.4109 | 0.6443 ± 0.09023 | Y = 0.4196x + 0.0023, R2 = 0.9997 |
| Tuber | 3.242 ± 0.8131 | 4.113 ± 1.158 | 1.200 ± 0.2784 | 0.3636 ± 0.1828 | Y = 0.4196x + 0.0023, R2 = 0.9997 |
| No. | Compound Name | tR (min) | Formula | Mode | Mass Error (ppm) | m/z |
|---|---|---|---|---|---|---|
| Isoflavones | ||||||
| 1 | Genistin | 5.000733333 | C21H20O10 | pos | −1.436209521 | 433.1123027 |
| 2 | Calycosin-7-O-β-D-glucoside | 5.482933333 | C22H22O10 | pos | −1.394990775 | 447.127951 |
| 3 | Glycitin | 5.653616667 | C22H22O10 | pos | −1.516097102 | 447.127897 |
| 4 | Daidzin | 5.684666667 | C21H20O9 | neg | −0.120060599 | 415.1034058 |
| 5 | Tectorigenin | 6.809166667 | C16H12O6 | neg | −0.613314336 | 299.0559276 |
| 6 | Formononetin | 8.593933333 | C16H12O4 | neg | −0.38949262 | 267.066178 |
| 7 | Calycosin | 7.305166667 | C16H12O5 | pos | −2.282324897 | 285.0751016 |
| 8 | Neobavaisoflavone | 9.39485 | C20H18O4 | pos | −2.253131166 | 323.1270597 |
| 9 | Corylin | 10.12973333 | C20H16O4 | neg | −0.747793006 | 319.0973432 |
| 10 | Daidzein | 15.10716667 | C15H10O4 | neg | −0.162841595 | 253.050591 |
| Flavone glycosides | ||||||
| 11 | Neoschaftoside | 5.23475 | C26H28O14 | neg | −0.008722792 | 563.1406242 |
| 12 | Oroxin A | 5.246433333 | C21H20O10 | neg | −0.900136026 | 431.0979815 |
| 13 | Diosmetin-7-O-Beta-D-glucopyranoside | 5.26775 | C22H22O11 | neg | 0.220291863 | 461.1090369 |
| 14 | Rhoifolin | 5.73185 | C27H30O14 | neg | 0.192143435 | 577.1563903 |
| 15 | Vitexin | 5.9234 | C21H20O10 | pos | −1.766953107 | 433.1121598 |
| 16 | Apigenin-7-O-Beta-D-glucoside | 6.586366667 | C21H20O10 | neg | −0.461695408 | 431.0981709 |
| Chalcones | ||||||
| 17 | Naringenin chalcone | 7.83725 | C15H12O5 | pos | −2.643945598 | 273.0750306 |
| 18 | Kanzonol C | 8.081333333 | C25H28O4 | neg | −6.316384301 | 391.1890057 |
| 19 | Isoliquiritigenin | 8.369116667 | C15H12O4 | neg | 0.041119868 | 255.066293 |
| Methoxy flavones | ||||||
| 20 | Isosinensetin | 8.305783333 | C20H20O7 | pos | −2.241569305 | 373.1273453 |
| 21 | Tangeretin | 9.87585 | C20H20O7 | pos | −2.043774215 | 373.1274189 |
| Phenolics | ||||||
| 22 | 4-Hydroxycinnamic acid | 5.785916667 | C9H8O3 | neg | −0.108469542 | 163.0400499 |
| 23 | Ferulic acid | 6.00555 | C10H10O4 | pos | −1.140024779 | 195.064964 |
| 24 | Sinapinic acid | 6.014833333 | C11H12O5 | neg | −0.173561152 | 223.0611582 |
| 25 | (E)-Cinnamic acid | 6.185683333 | C9H8O2 | neg* | −0.734750116 | 193.0505236 |
| 26 | Salicylic acid | 6.251733333 | C7H6O3 | neg | −0.687050513 | 137.0243228 |
| 27 | 4-Methoxysalicylic acid | 7.014166667 | C8H8O4 | neg | −0.327807242 | 167.0349272 |
| Coumarins | ||||||
| 28 | Esculetin | 5.108033333 | C9H6O4 | neg | −0.58725632 | 177.0192277 |
| 29 | Scopoletin | 6.094333333 | C10H8O4 | neg | 0.026476372 | 191.0349874 |
| Lignan | ||||||
| 30 | Magnolol | 10.99731667 | C18H18O2 | neg | −0.277636992 | 265.1233295 |
| Compound Name | Activity | References |
|---|---|---|
| Genistin (1) | Cardioprotective; Anti-diabetes | [23] |
| Calycosin-7-O-β-D-glucoside (2) | Anti-diabetes | [24] |
| Glycitin (3) | Anti-obese; Anti-diabetes | [25] |
| Daidzin (4) | Anti-epileptic | [23] |
| Tectorigenin (5) | Neuroprotective effect; Anti-diabetic cardiomyopathy | [23] |
| Formononetin (6) | Anti-gastric ulcers; Anti-cancer | [23] |
| Calycosin (7) | Anti-cancer; Neuroprotective effect | [26,27] |
| Neobavaisoflavone (8) | Neuroprotective effect | [28] |
| Corylin (9) | Anti-cancer | [29] |
| Daidzein (10) | Anti-obesity; Neuroprotective effect | [5,23] |
| Oroxin A (12) | Anti-cancer; Anti-diabetes | [30,31] |
| Rhoifolin (14) | Anti-cancer | [32] |
| Vitexin (15) | Anti-cancer; Anti-depressant | [33,34] |
| Naringenin chalcone (17) | Anti-cancer; Anti-allergic | [35,36] |
| Isoliquiritigenin (19) | Anti-cancer; Anti-diabetes | [37,38] |
| Isosinensetin (20) | Anti-Osteoporosis | [39] |
| Tangeretin (21) | Anti-cancer; Anti-microbia | [40,41] |
| 4-Hydroxycinnamic acid (22) | Neuroprotective effect | [42] |
| Ferulic acid (23) | Antimicrobial | [43,44] |
| Sinapinic acid (24) | Anti-thrombosis; Anti-cancer; Cardioprotective; Anti-convulsant | [45,46,47,48] |
| Salicylic acid (26) | Anti-fungal | [49] |
| Esculetin (28) | Anti-tumor; Anti-diabetes | [50] |
| Scopoletin (29) | Anti-bacterial; Anti-fungal | [51] |
| Magnolol (30) | Anti-gastritic; Anti-fungal | [52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Huang, S.; Wu, X.; He, Y.; Shen, H.; Tang, S.; Zhu, F.; Luo, Y. Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus. Antioxidants 2025, 14, 416. https://doi.org/10.3390/antiox14040416
Xiang J, Huang S, Wu X, He Y, Shen H, Tang S, Zhu F, Luo Y. Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus. Antioxidants. 2025; 14(4):416. https://doi.org/10.3390/antiox14040416
Chicago/Turabian StyleXiang, Jing, Shiting Huang, Xingyu Wu, Yixuan He, Haiyan Shen, Shuangyang Tang, Fengyuan Zhu, and Ying Luo. 2025. "Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus" Antioxidants 14, no. 4: 416. https://doi.org/10.3390/antiox14040416
APA StyleXiang, J., Huang, S., Wu, X., He, Y., Shen, H., Tang, S., Zhu, F., & Luo, Y. (2025). Phytochemical Profile and Antioxidant Activity of the Tuber and Peel of Pachyrhizus erosus. Antioxidants, 14(4), 416. https://doi.org/10.3390/antiox14040416





