Pharmacological and Molecular Docking Investigation of Leaves of Eriobotrya japonica: Antioxidant, Enzyme Inhibition, and Anti-Inflammatory Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Preparation of Solvent Extracts
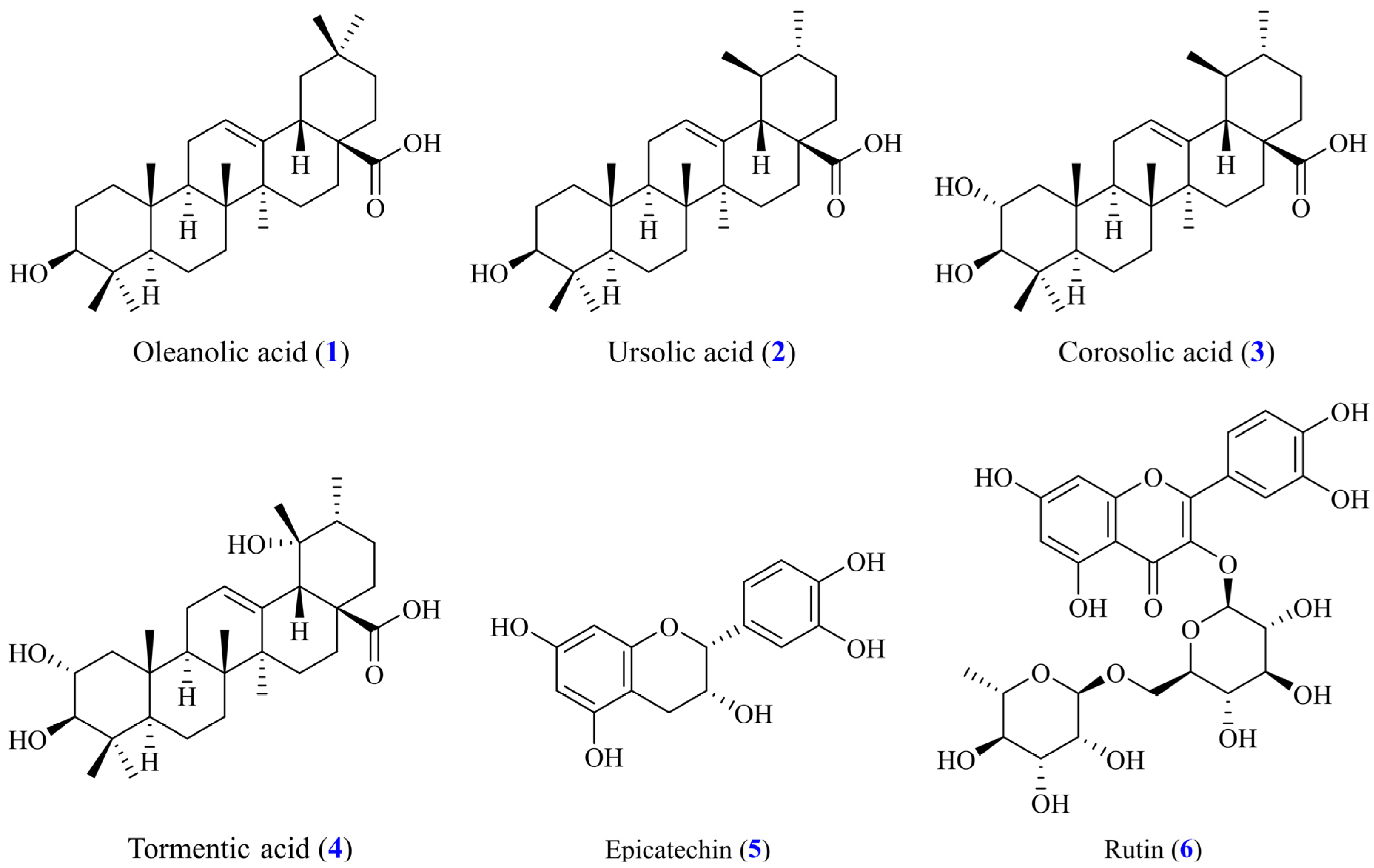
2.3. Isolation of Pure Components
2.4. Total Phenolic Content (TPC) Determination
2.5. Total Flavonoid Content (TFC) Determination
2.6. Radical Scavenging Activity of DPPH
2.7. Radical Scavenging Activity of ABTS
2.8. Radical Scavenging Activity of Superoxide
2.9. Ferric Reducing Antioxidant Power (FRAP)
2.10. Enzyme Inhibition Assay of α-Glucosidase
2.11. Enzyme Inhibition Assay of Acetylcholinesterase (AChE)
2.12. Cell Culture
2.13. Cell Viability Assay
2.14. LPS-Induced NO Inhibition Assay
2.15. Western Blot Analysis
2.16. Molecular Modeling Docking Study
2.17. Statistical Analysis
3. Results
3.1. Determination of TPC, TFC, and Yields of Solvent Extracts
3.2. DPPH Scavenging Capability of Solvent Extracts
3.3. ABTS Scavenging Capability of Solvent Extracts
3.4. Superoxide Scavenging Capability of Solvent Extracts
3.5. Ferric Reducing Antioxidant Power of Solvent Extracts
3.6. α-Glucosidase Inhibition of Solvent Extracts
3.7. Acetylcholinesterase (AChE) Inhibition of Solvent Extracts
3.8. Antioxidant Properties of Isolated Components
3.9. α-Glucosidase Inhibition of Isolated Compounds
3.10. Acetylcholinesterase (AChE) Inhibition of Isolated Compounds
3.11. Anti-Inflammatory Activity of Isolated Compounds
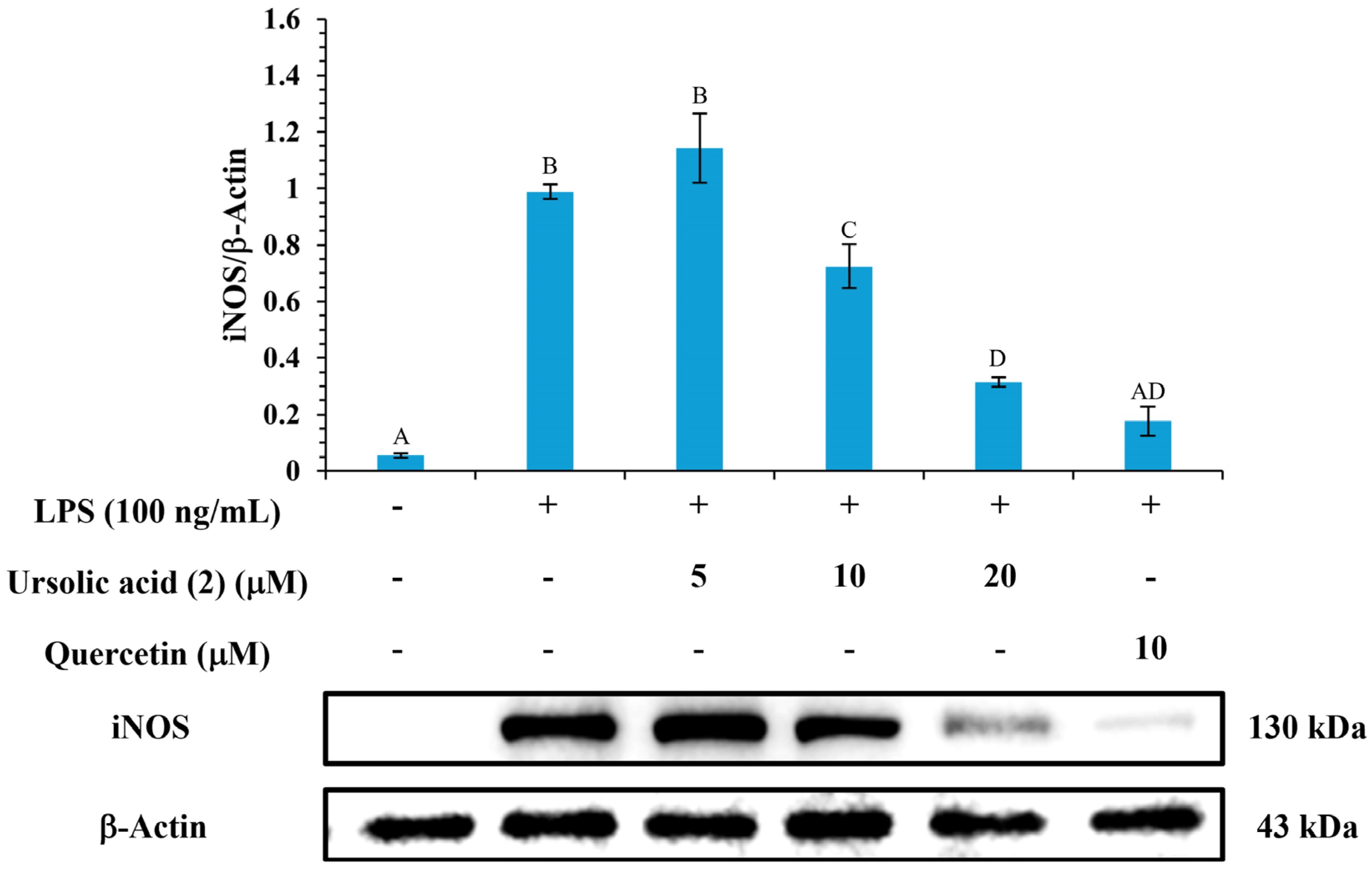
3.12. Western Blot Analysis of iNOS Inhibition
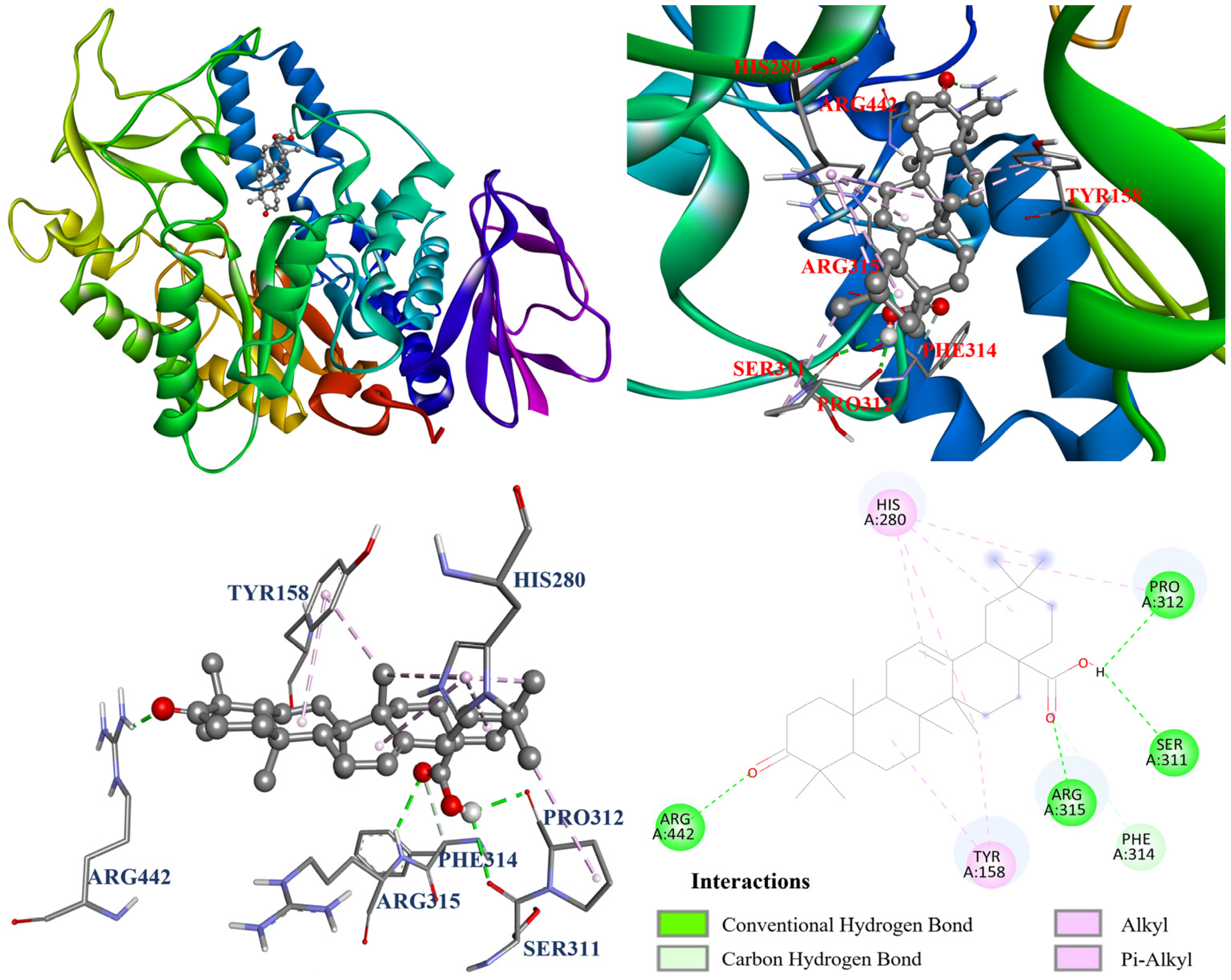
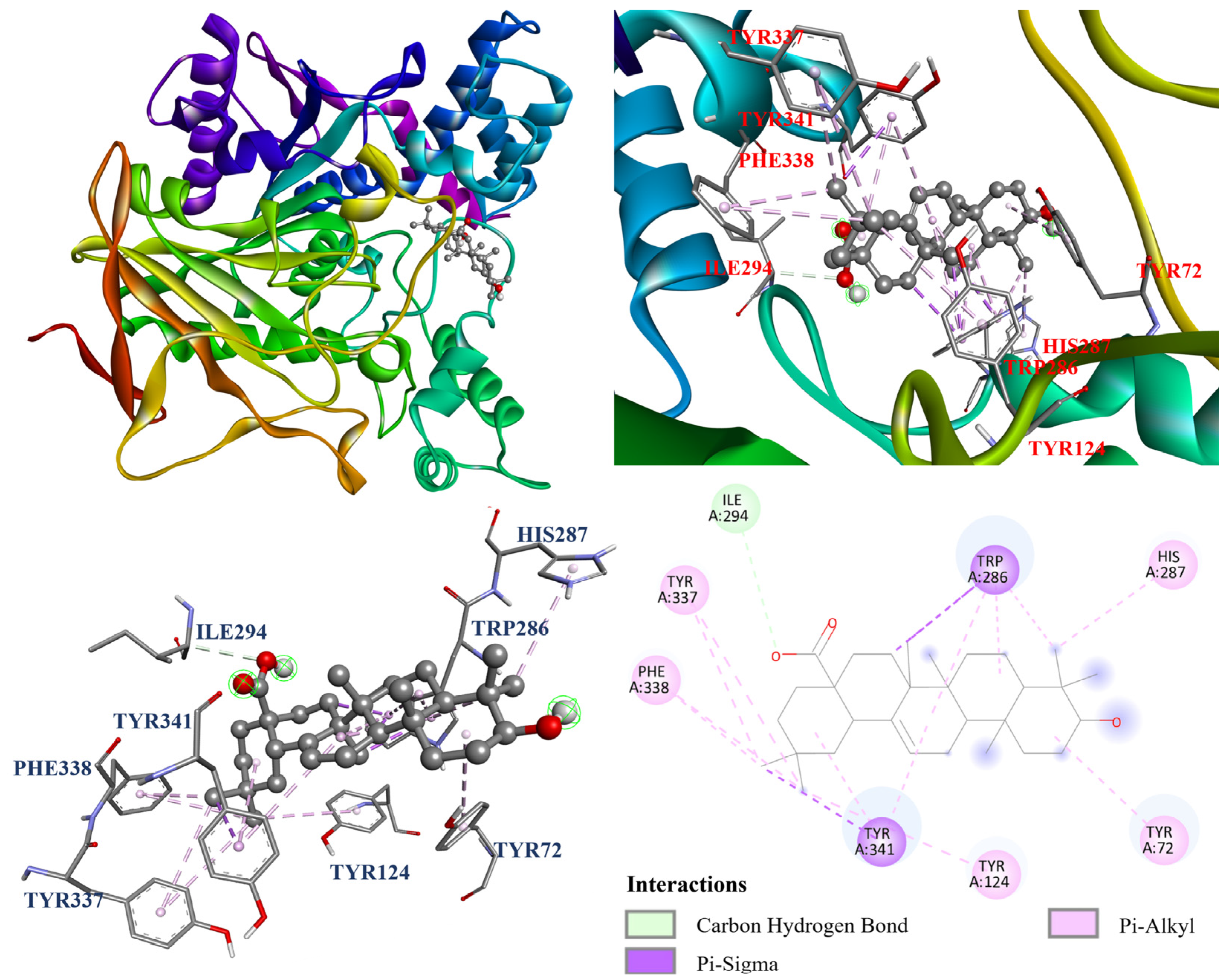
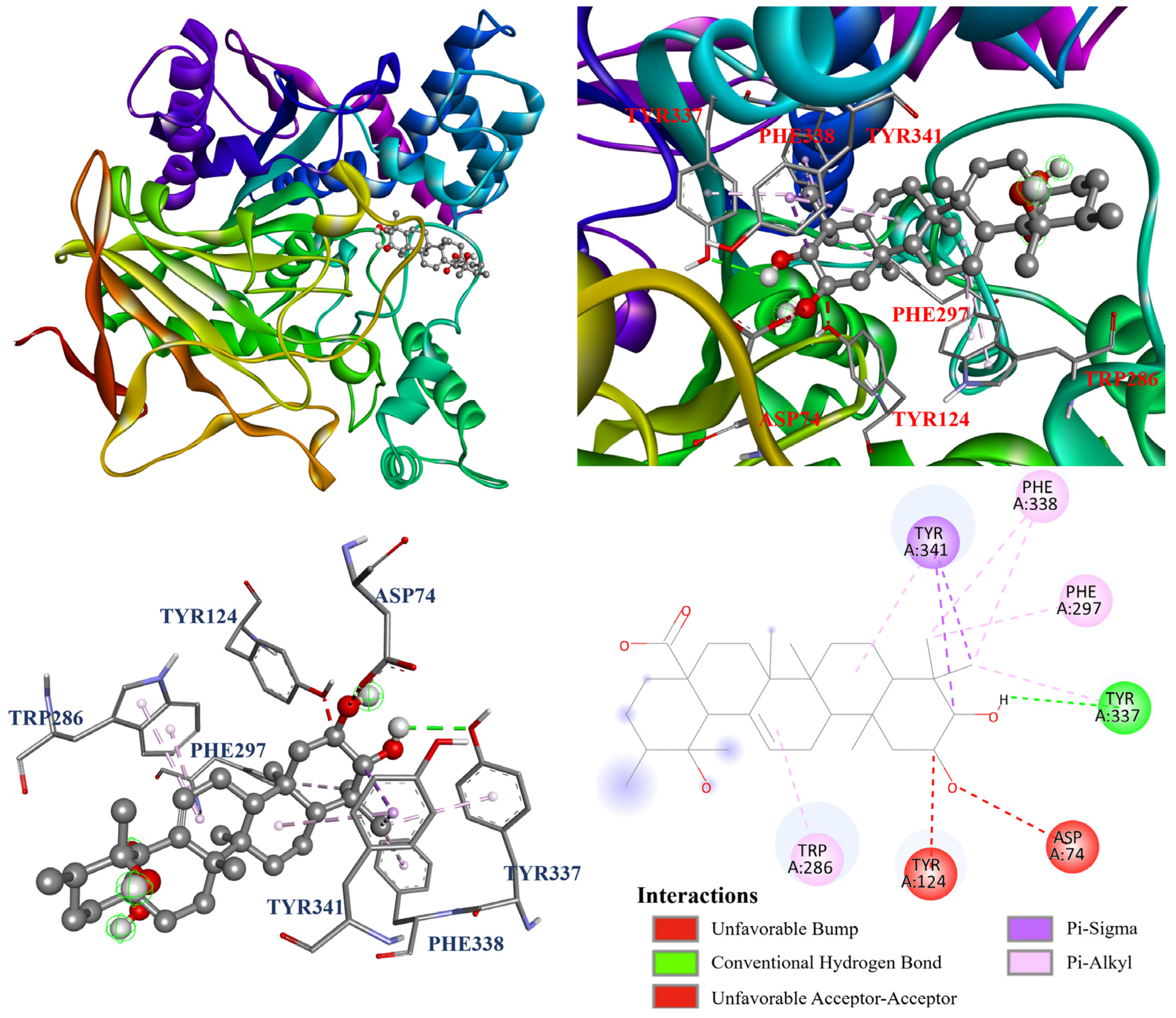
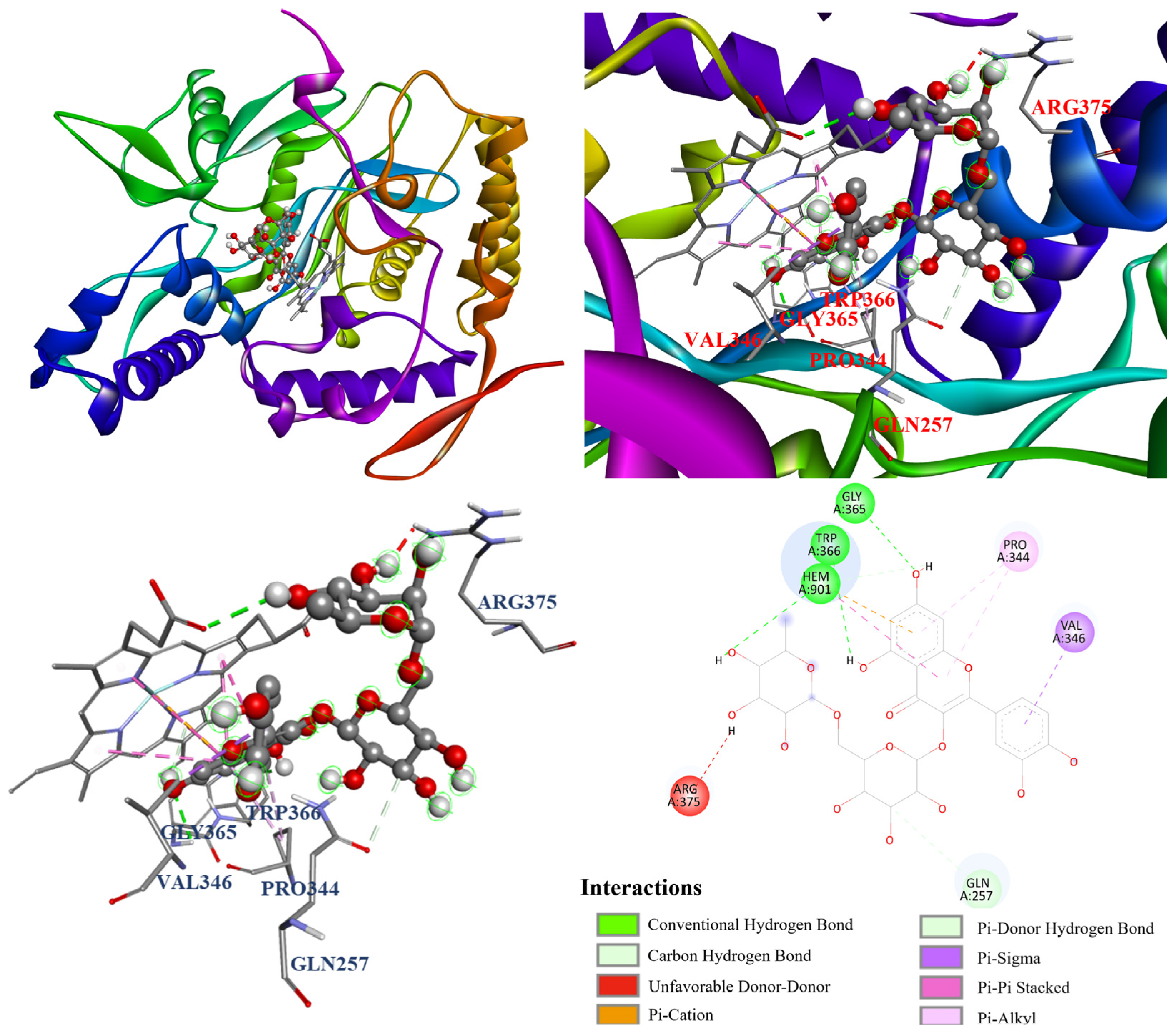
3.13. Molecular Docking Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 1, 1–8. [Google Scholar]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Ahmed, K.; Muniandy, S.; Ismail, I.S. Type 2 diabetes and vascular complications: A pathophysiologic view. Biomed. Res.-India 2010, 21, 147–155. [Google Scholar]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [PubMed]
- McKinley, B.J.; Santiago, M.; Pak, C.; Nguyen, N.; Zhong, Q. Pneumatosis intestinalis induced by alpha-glucosidase inhibitors in patients with diabetes mellitus. J. Clin. Med. 2022, 11, 5918. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar]
- Huang, Q.; Liao, C.; Ge, F.; Ao, J.; Liu, T. Acetylcholine bidirectionally regulates learning and memory. J. Neurorestoratol. 2022, 10, 100002. [Google Scholar]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Ruangritchankul, S.; Chantharit, P.; Srisuma, S.; Gray, L.C. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: A comprehensive literature review. Ther. Clin. Risk Manag. 2021, 4, 927–949. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. immunol. 2022, 13, 936167. [Google Scholar]
- Casulleras, M.; Zhang, I.W.; López-Vicario, C.; Clària, J. Leukocytes, systemic inflammation and immunopathology in acute-on-chronic liver failure. Cells 2020, 9, 2632. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar]
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate immunity and microbial dysbiosis in hidradenitis suppurativa–vicious cycle of chronic inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar]
- Masoumi, M.; Bashiri, H.; Khorramdelazad, H.; Barzaman, K.; Hashemi, N.; Sereshki, H.A.; Sahebkar, A.; Karami, J. Destructive roles of fibroblast-like synoviocytes in chronic inflammation and joint damage in rheumatoid arthritis. Inflammation 2021, 44, 466–479. [Google Scholar] [PubMed]
- Sojod, B.; Pidorodeski Nagano, C.; Garcia Lopez, G.M.; Zalcberg, A.; Dridi, S.M.; Anagnostou, F. Systemic lupus erythematosus and periodontal disease: A complex clinical and biological interplay. J. Clin. Med. 2021, 10, 1957. [Google Scholar] [CrossRef]
- Lazou, A.; Ikonomidis, I.; Bartekova, M.; Benedek, T.; Makavos, G.; Palioura, D.; Cabrera Fuentes, H.; Andreadou, I. Chronic inflammatory diseases, myocardial function and cardioprotection. Br. J. Pharmacol. 2020, 177, 5357–5374. [Google Scholar]
- Li, X.; Xu, C.; Chen, K. Chapter 16—Nutritional and Composition of Fruit Cultivars: Loquat (Eriobotrya japonica Lindl.). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 371–394. [Google Scholar]
- Costa, B.P.; Ikeda, M.; de Melo, A.M.; Bambirra Alves, F.E.S.; Carpiné, D.; Ribani, R.H. Eriobotrya japonica fruits and its by-products: A promising fruit with bioactive profile and trends in the food application—A bibliometric review. Food Biosci. 2022, 50, 102099. [Google Scholar]
- Dhiman, A.; Suhag, R.; Thakur, D.; Gupta, V.; Prabhakar, P.K. Current status of loquat (Eriobotrya japonica Lindl.): Bioactive functions, preservation approaches, and processed products. Food Rev. Int. 2022, 38, 286–316. [Google Scholar]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): A review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, P.; Wei, X.; Liu, Q.; Li, X.; Liang, G.; Guo, Q. Effects of triploidization of loquat [Eriobotrya japonica (Thunb.) Lindl.] on flavonoids and phenolics and antioxidant activities in leaves and flower buds. HortScience 2019, 54, 1310–1318. [Google Scholar]
- Jian, T.; Chen, J.; Ding, X.; Lv, H.; Li, J.; Wu, Y.; Ren, B.; Tong, B.; Zuo, Y.; Su, K. Flavonoids isolated from loquat (Eriobotrya japonica) leaves inhibit oxidative stress and inflammation induced by cigarette smoke in COPD mice: The role of TRPV1 signaling pathways. Food Funct. 2020, 11, 3516–3526. [Google Scholar] [CrossRef]
- Kuraoka-Oliveira, Â.M.; Radai, J.A.S.; Leitão, M.M.; Lima Cardoso, C.A.; Silva-Filho, S.E.; Leite Kassuya, C.A. Anti-inflammatory and anti-arthritic activity in extract from the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2020, 249, 112418. [Google Scholar] [PubMed]
- Zhu, X.; Wang, L.; Zhao, T.; Jiang, Q. Traditional uses, phytochemistry, pharmacology, and toxicity of Eriobotrya japonica leaves: A summary. J. Ethnopharmacol. 2022, 298, 115566. [Google Scholar]
- Infante-Rodríguez, D.A.; Aguilar-Méndez, M.J.; Landa-Cansigno, C.; Vásquez-Morales, S.G.; Velázquez-Narváez, A.C.; Valenzuela-González, J.E.; Kiel-Martínez, A.L.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Phytochemical composition of Eriobotrya japonica (Rosaceae) leaves extracts from central Veracruz, Mexico, and its effect on α-glucosidase enzyme inhibition. Bot. Sci. 2024, 102, 1231–1250. [Google Scholar]
- Yao, Z.; Cheng, F.; Ming, T.; Sun, C.; Ran, Q.; Zhang, C.; Shen, C.; Zhang, R.; Peng, C. Eriobotrya japonica (Thunb.) Lindl leaves: Reviewing their specialized metabolites and pharmacology. Biochem. Syst. Ecol. 2023, 110, 104707. [Google Scholar]
- Zhang, L.; Saber, F.R.; Rocchetti, G.; Zengin, G.; Hashem, M.M.; Lucini, L. UHPLC-QTOF-MS based metabolomics and biological activities of different parts of Eriobotrya japonica. Food Res. Int. 2021, 143, 110242. [Google Scholar]
- Ho, H.-y.; Liang, K.-y.; Lin, W.-c.; Kitanaka, S.; Wu, J.-b. Regulation and improvement of triterpene formation in plant cultured cells of Eriobotrya japonica Lindl. J. Biosci. Bioeng. 2010, 110, 588–592. [Google Scholar]
- Khouya, T.; Ramchoun, M.; Elbouny, H.; Hmidani, A.; Bouhlali, E. d. T.; Alem, C. Loquat (Eriobotrya japonica (Thunb) Lindl.): Evaluation of nutritional value, polyphenol composition, antidiabetic effect, and toxicity of leaf aqueous extract. J. Ethnopharmacol. 2022, 296, 115473. [Google Scholar]
- Zhang, J.; Xu, H.-Y.; Wu, Y.-J.; Zhang, X.; Zhang, L.-Q.; Li, Y.-M. Neutrophil elastase inhibitory effects of pentacyclic triterpenoids from Eriobotrya japonica (loquat leaves). J. Ethnopharmacol. 2019, 242, 111713. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-G.; Lee, J.-J.; Kim, A.-R.; Lee, M.-Y. Chemical components and antioxidative effects of Eriobotrya japonica Lindl. leaf. J. Life Sci. 2010, 20, 1625–1633. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacol. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Al Qaraghuli, M.M.; Alsaadi, M.; Alzahrani, A.R.; Niwasabutra, K.; Ferro, V.A. Delivering natural products and biotherapeutics to improve drug efficacy. Ther. Deliv. 2017, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Chen, J.-J.; Duh, C.-Y.; Tsai, I.-L. Cytotoxic lignans from formosan Hernandia nymphaeifolia. Phytochemistry 1997, 45, 991–996. [Google Scholar] [CrossRef]
- Chen, J.-J.; Yang, C.-S.; Peng, C.-F.; Chen, I.-S.; Miaw, C.-L. Dihydroagarofuranoid sesquiterpenes, a lignan derivative, a benzenoid, and antitubercular constituents from the stem of Microtropis japonica. J. Nat. Prod. 2008, 71, 1016–1021. [Google Scholar]
- Chen, J.-J.; Tsai, C.-S.; Hwang, T.-L.; Shieh, P.-C.; Chen, J.-F.; Sung, P.-J. Sesquiterpenes from the rhizome of Curcuma longa with inhibitory activity on superoxide generation and elastase release by neutrophils. Food Chem. 2010, 119, 974–980. [Google Scholar] [CrossRef]
- Yang, P.-S.; Cheng, M.-J.; Peng, C.-F.; Chen, J.-J.; Chen, I.-S. Endiandric acid analogues from the roots of Beilschmiedia erythrophloia. J. Nat. Prod. 2009, 72, 53–58. [Google Scholar] [CrossRef]
- Chou, C.-P.; Huang, N.-C.; Jhuang, S.-J.; Pan, H.-B.; Peng, N.-J.; Cheng, J.-T.; Chen, C.-F.; Chen, J.-J.; Chang, T.-H. Ubiquitin-conjugating enzyme UBE2C is highly expressed in breast microcalcification lesions. PLoS ONE 2014, 9, e93934. [Google Scholar] [CrossRef]
- Lee, F.P.; Chen, Y.C.; Chen, J.J.; Tsai, I.L.; Chen, I.S. Cyclobutanoid amides from Piper arborescens. Helv. Chim. Acta 2004, 87, 463–468. [Google Scholar] [CrossRef]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D.; et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Li, E.-N.; Zhou, G.-D.; Kong, L. Chemical constituents from the leaves of Eriobotrya japonica. Chin. J. Nat. Med 2009, 7, 190–192. [Google Scholar] [CrossRef]
- Wu, J.-B.; Kuo, Y.-H.; Lin, C.-H.; Ho, H.-Y.; Shih, C.-C. Tormentic acid, a major component of suspension cells of Eriobotrya japonica, suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-J.; Nomura, T.; Fukudome, H.; Onjo, M.; Shimada, A.; Samejima, H. Chemical constituents of the leaves of Eriobotrya japonica. Chem. Nat. Compd. 2019, 55, 942–944. [Google Scholar] [CrossRef]
- Ma, T.; Sun, Y.; Wang, L.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. An investigation of the anti-depressive properties of phenylpropanoids and flavonoids in Hemerocallis citrina Baroni. Molecules 2022, 27, 5809. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, A.P. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Sivasothy, Y.; Loo, K.Y.; Leong, K.H.; Litaudon, M.; Awang, K. A potent alpha-glucosidase inhibitor from Myristica cinnamomea King. Phytochemistry 2016, 112, 265–269. [Google Scholar] [CrossRef]
- Tran, T.-D.; Nguyen, T.-C.-V.; Nguyen, N.-S.; Nguyen, D.-M.; Nguyen, T.-T.-H.; Le, M.-T.; Thai, K.-M. Synthesis of novel chalcones as acetylcholinesterase inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef]
- Choudhary, R.; Kumar, P.; Shukla, S.K.; Bhagat, A.; Anal, J.M.H.; Kour, G.; Ahmed, Z. Synthesis and potential anti-inflammatory response of indole and amide derivatives of ursolic acid in LPS-induced RAW 264.7 cells and systemic inflammation mice model: Insights into iNOS, COX2 and NF-κB. Bioorg. Chem. 2025, 155, 108091. [Google Scholar] [CrossRef]
- BIOVIA, Dassault Systèmes. Discovery Studio Client 2021, v.21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Tagami, T.; Yamashita, K.; Okuyama, M.; Mori, H.; Yao, M.; Kimura, A. Molecular basis for the recognition of long-chain substrates by plant α-glucosidase. J. Biol. Chem. 2013, 288, 19296–19303. [Google Scholar] [PubMed]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [PubMed]
- Lin, A.H.-M.; Lee, B.-H.; Chang, W.-J. Small intestine mucosal α-glucosidase: A missing feature of in vitro starch digestibility. Food Hydrocoll. 2016, 53, 163–171. [Google Scholar] [CrossRef]
- Xu, Y.; Rashwan, A.K.; Ge, Z.; Li, Y.; Ge, H.; Li, J.; Xie, J.; Liu, S.; Fang, J.; Cheng, K. Identification of a novel α-glucosidase inhibitor from Melastoma dodecandrum Lour. fruits and its effect on regulating postprandial blood glucose. Food Chem. 2023, 399, 133999. [Google Scholar] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Nazzi, C.; Avenanti, A.; Battaglia, S. The involvement of antioxidants in cognitive decline and neurodegeneration: Mens sana in corpore sano. Antioxidants 2024, 13, 701. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, S.-Y. Physiologic functions and therapeutic applications of α7 nicotinic acetylcholine receptor in brain disorders. Pharmaceutics 2022, 15, 31. [Google Scholar] [CrossRef]
- Omena, C.M.B.; Valentim, I.B.; Guedes, G.D.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.H.F.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S.; et al. Antioxidant, anti-acetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seeds of exotic Brazilian fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef]
- Stępnik, K. Biomimetic chromatographic studies combined with the computational approach to investigate the ability of triterpenoid saponins of plant origin to cross the blood–brain barrier. Int. J. Mol. Sci. 2021, 22, 3573. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.; Joseph, X.; Akhil, V.; Mohanan, P. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar]
- Ptaschinski, C.; Lukacs, N.W. Acute and chronic inflammation induces disease pathogenesis. In Molecular Pathology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–43. [Google Scholar]
- Man, M.-Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory role of nitric oxide in cutaneous inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef] [PubMed]






| Extracting Solvents | TPC (mg/g) a (GAE) | TFC (mg/g) b (QE) | Yields (%) c |
|---|---|---|---|
| n-Hexane | 8.11 ± 1.92 A | 64.30 ± 8.42 C | 1.32 |
| Ethyl acetate | 8.50 ± 1.65 A | 71.42 ± 9.97 C | 4.28 |
| Acetone | 15.06 ± 1.35 AB | 65.01 ± 5.87 C | 5.00 |
| Ethanol | 22.00 ± 1.87 BC | 44.59 ± 7.31 BC | 9.96 |
| Methanol | 35.59 ± 2.82 DE | 32.06 ± 1.03 AB | 15.72 |
| Water | 26.70 ± 2.40 CD | 13.53 ± 0.91 A | 12.16 |
| 100 °C Water | 37.56 ± 1.54 E | 14.57 ± 1.50 A | 14.52 |
| Extracting Solvents | SC50 (μg/mL) a | TE (mM/g) b | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| n-Hexane | 611.84 ± 49.30 C | 247.35 ± 12.89 E | >400 | 156.23 ± 2.31 A |
| Ethyl acetate | 582.60 ± 32.11 C | 162.72 ± 8.71 D | >400 | 358.09 ± 14.20 AB |
| Acetone | 316.92 ± 31.31 B | 77.20 ± 3.94 C | >400 | 151.23 ± 10.15 A |
| Ethanol | 222.44 ± 16.87 AB | 63.59 ± 2.95 BC | >400 | 456.85 ± 23.37 ABC |
| Methanol | 114.11 ± 3.68 A | 35.42 ± 0.60 AB | 191.27 ± 24.04 B | 738.49 ± 33.54 BC |
| Water | 192.74 ± 18.48 AB | 58.80 ± 4.19 ABC | 63.68 ± 5.17 A | 628.09 ± 43.85 BC |
| 100 °C Water | 98.84 ± 10.42 A | 56.22 ± 5.16 ABC | 47.50 ± 5.60 A | 882.65 ± 32.62 C |
| BHT c | 230.05 ± 25.40 AB | 29.95± 1.04 A | — | 3370.27 ± 256.53 D |
| Cynaroside c | — | — | 13.41 ± 2.56 A | — |
| Extracting Solvents | IC50 (μg/mL) a | |
|---|---|---|
| α-Glucosidase | AChE | |
| n-Hexane | >400 | 54.37 ± 3.35 A |
| Ethyl acetate | 23.45 ± 1.84 A | 65.15 ± 5.87 A |
| Acetone | 95.61 ± 0.88 D | 114.14 ± 10.37 BC |
| Ethanol | 28.67 ± 2.24 AB | 106.53 ± 9.02 BC |
| Methanol | 50.40 ± 2.80 C | 76.52 ± 1.46 AB |
| Water | 44.18 ± 3.53 BC | 138.41 ± 5.75 C |
| Water 100 °C | 96.14 ± 7.19 D | 129.36 ± 10.99 C |
| Acarbose b | 197.04 ± 5.70 E | - |
| Chlorogenic acid b | - | 136.93 ± 11.78 C |
| Compounds | SC50 (μM) a | TE (mM/g) b | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| Oleanolic acid (1) | >400 | >400 | >400 | <1 |
| Ursolic acid (2) | >400 | >400 | >400 | <1 |
| Corosolic acid (3) | >400 | >400 | >400 | <1 |
| Tormentic acid (4) | >400 | >400 | >400 | <1 |
| Epicatechin (5) | 56.94 ± 0.91 A | 7.83 ± 0.34 A | 210.27 ± 9.46 C | 1694.43 ± 21.45 A |
| Rutin (6) | 66.30 ± 1.68 A | 20.02 ± 0.30 B | 6.69 ± 0.25 A | 1609.10 ± 25.67 A |
| BHT c | 881.06 ± 70.37 B | 25.87 ± 0.01 C | - | 3082.65 ± 213.69 B |
| Cynaroside c | - | - | 38.33 ± 0.69 B | - |
| Compounds | IC50 (μM) a | |
|---|---|---|
| α-Glucosidase | AChE | |
| Oleanolic acid (1) | 21.10 ± 1.48 A | 306.78 ± 12.14 A |
| Ursolic acid (2) | 10.68 ± 0.76 A | 310.82 ± 7.31 A |
| Corosolic acid (3) | 13.83 ± 2.11 A | 282.81 ± 16.97 A |
| Tormentic acid (4) | 319.89 ± 26.07 B | 281.05 ± 8.55 A |
| Epicatechin (5) | >800 | 404.73 ± 26.34 B |
| Rutin (6) | 591.99 ± 10.38 D | 451.76 ± 12.88 B |
| Acarbose b | 419.93 ± 29.15 C | - |
| Chlorogenic acid b | - | 320.25 ± 4.13 A |
| Compounds | NO inhibition IC50 (μM) a |
|---|---|
| Oleanolic acid (1) | >50 |
| Ursolic acid (2) | 20.18 ± 1.46 A |
| Corosolic acid (3) | - c |
| Tormentic acid (4) | >50 |
| Epicatechin (5) | >50 |
| Rutin (6) | 29.47 ± 1.19 A |
| Quercetin b | 17.05 ± 1.63 B |
| Compounds | Affinity (kcal/mol) |
|---|---|
| Oleanolic acid (1) | −8.4 |
| Ursolic acid (2) | −8.7 |
| Corosolic acid (3) | −8.6 |
| Acarbose a | −5.1 |
| Compounds | Affinity (kcal/mol) |
|---|---|
| Oleanolic acid (1) | −8.2 |
| Ursolic acid (2) | −7.8 |
| Corosolic acid (3) | −8.6 |
| Tormentic acid (4) | −8.8 |
| Chlorogenic acid a | −7.8 |
| Compounds | Affinity (kcal/mol) |
|---|---|
| Ursolic acid (2) | −7.5 |
| Rutin (6) | −8.9 |
| Quercetin a | −7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-J.; Chen, L.-T.; Li, S.-M.; Chen, Z.-R.; Chen, J.-J. Pharmacological and Molecular Docking Investigation of Leaves of Eriobotrya japonica: Antioxidant, Enzyme Inhibition, and Anti-Inflammatory Effects. Antioxidants 2025, 14, 413. https://doi.org/10.3390/antiox14040413
Kuo P-J, Chen L-T, Li S-M, Chen Z-R, Chen J-J. Pharmacological and Molecular Docking Investigation of Leaves of Eriobotrya japonica: Antioxidant, Enzyme Inhibition, and Anti-Inflammatory Effects. Antioxidants. 2025; 14(4):413. https://doi.org/10.3390/antiox14040413
Chicago/Turabian StyleKuo, Pao-Jen, Li-Ting Chen, Sin-Min Li, Zih-Rong Chen, and Jih-Jung Chen. 2025. "Pharmacological and Molecular Docking Investigation of Leaves of Eriobotrya japonica: Antioxidant, Enzyme Inhibition, and Anti-Inflammatory Effects" Antioxidants 14, no. 4: 413. https://doi.org/10.3390/antiox14040413
APA StyleKuo, P.-J., Chen, L.-T., Li, S.-M., Chen, Z.-R., & Chen, J.-J. (2025). Pharmacological and Molecular Docking Investigation of Leaves of Eriobotrya japonica: Antioxidant, Enzyme Inhibition, and Anti-Inflammatory Effects. Antioxidants, 14(4), 413. https://doi.org/10.3390/antiox14040413







