Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Source of Chemicals
2.3. Determination of Total Polyphenol and Flavonoid Contents
2.4. Determination of Antioxidant Capacity
2.4.1. DPPH Method
2.4.2. TEAC Protocol
2.4.3. FRAP Assay
2.5. Statistical Evaluation
3. Results
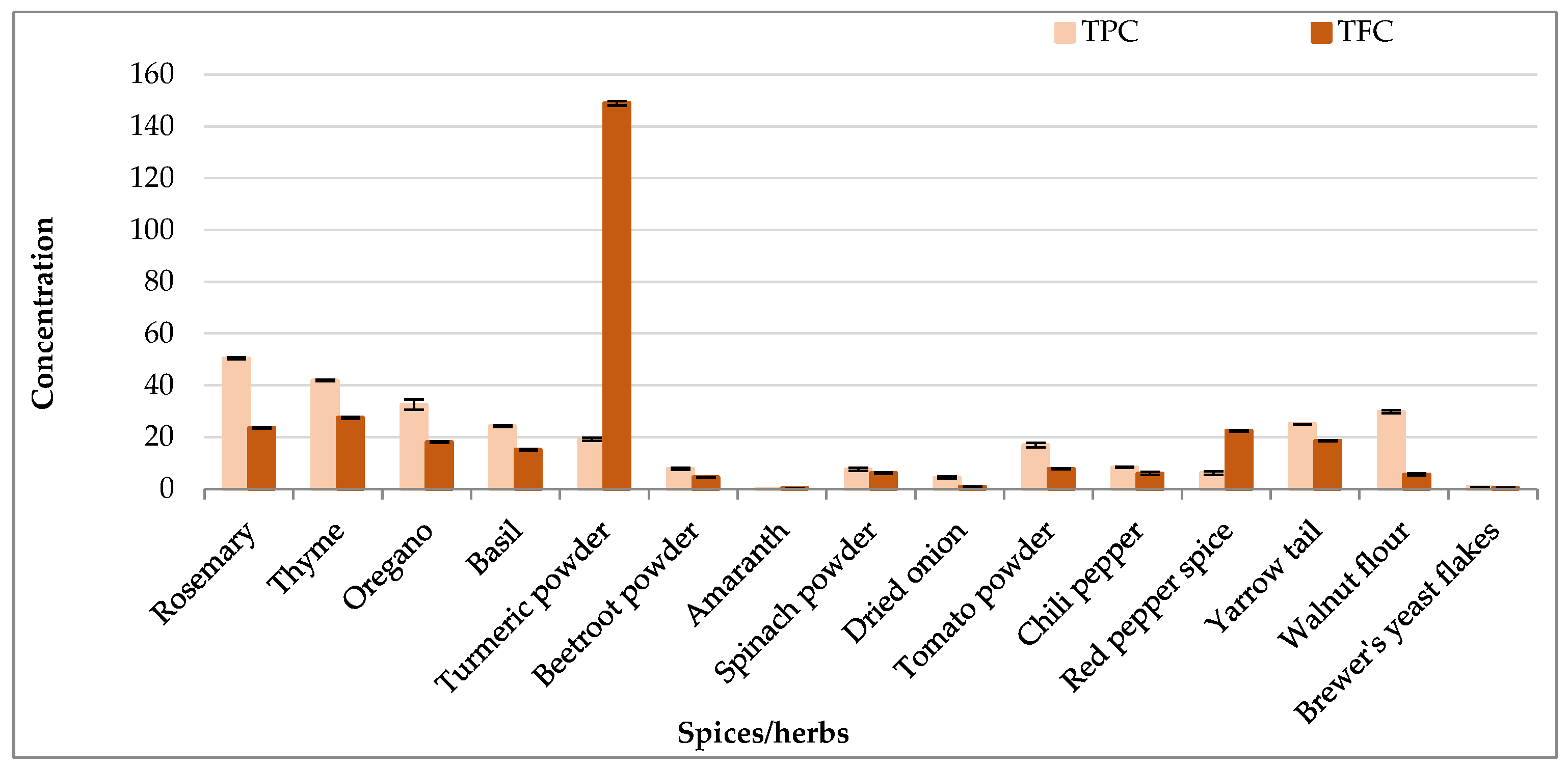
3.1. Total Polyphenol and Flavonoids Contents in Spices, Herbs, and Yeast
3.2. Quantification of Antioxidant Capacity
3.3. Correlation Between Total Polyphenol and Flavonoid Contents and DPPH, TEAC, and FRAP Values
4. Discussion
4.1. The Role of TPC and TFC in Antioxidant Capacity
4.2. Comparative Analysis of TPC and Antioxidant Capacity in TEAC, DPPH, and FRAP Assays
4.3. Correlation Between TFC and Implied Antioxidant Assays
4.4. Comparative Antioxidant Profiles Among Plant Families
4.5. Implications of Antioxidant Assays on Compound Specificity
4.6. Correlation Analysis and Statistical Insights
4.7. Implications for Future Research and Nutritional Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Choi, S.-W. Antioxidants in Food. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 1–53. ISBN 978-0-12-800270-4. [Google Scholar]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 125–178. ISBN 978-0-444-64185-4. [Google Scholar]
- Moshari-Nasirkandi, A.; Alirezalu, A.; Alipour, H.; Amato, J. Screening of 20 Species from Lamiaceae Family Based on Phytochemical Analysis, Antioxidant Activity and HPLC Profiling. Sci. Rep. 2023, 13, 16987. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Alford, A.R.; Niemeyer, E.D. Variation in Phenolic Profiles and Antioxidant Properties among Medicinal and Culinary Herbs of the Lamiaceae Family. Food Meas. 2020, 14, 1720–1732. [Google Scholar] [CrossRef]
- Abdel-Moez, G.; Avula, B.; Sayed, H.; Khalifa, A.; Ross, S.; Katragunta, K.; Khan, I.; Mohamed, S. Phytochemical Profiling of Three Amaranthus Species Using LC-MS/MS Metabolomic Approach and Chemometric Tools. J. Pharm. Biomed. Anal. 2023, 236, 115722. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Adetutu, A.; Olaniyi, T.D. Antioxidant Activity of Amaranthus Species from the Amaranthaceae Family—A Review. S. Afr. J. Bot. 2020, 133, 111–117. [Google Scholar] [CrossRef]
- Alagar Yadav, S.; Sara Koshi, F. Phytochemicals from Solanaceae Family and Their Anticancer Properties. In Medicinal Plants; Kumar, S., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-032-8. [Google Scholar]
- Jan, S.; Iram, S.; Bashir, O.; Shah, S.N.; Kamal, M.A.; Rahman, S.; Kim, J.; Jan, A.T. Unleashed Treasures of Solanaceae: Mechanistic Insights into Phytochemicals with Therapeutic Potential for Combatting Human Diseases. Plants 2024, 13, 724. [Google Scholar] [CrossRef]
- Ambreen, A.; Arooj, A.; Saihah, G. Bactericidal, Antioxidant Activity and in Silico Analysis of Phytochemicals Derived from Selected Plants of Solanaceae Family. Am. Int. J. Biol. Life Sci. 2020, 2, 28–41. [Google Scholar] [CrossRef]
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Borgo, J.; Wagner, M.S.; Laurella, L.C.; Elso, O.G.; Selener, M.G.; Clavin, M.; Bach, H.; Catalán, C.A.N.; Bivona, A.E.; Sepúlveda, C.S.; et al. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules 2024, 29, 814. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Alavi, M.S.; Fanoudi, S.; Ghasemzadeh Rahbardar, M.; Mehri, S.; Hosseinzadeh, H. An Updated Review of Protective Effects of Rosemary and Its Active Constituents against Natural and Chemical Toxicities. Phytother. Res. 2021, 35, 1313–1328. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Mokhtari, R.; Kazemi Fard, M.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, Antimicrobial Activities, and Characterization of Phenolic Compounds of Thyme (Thymus Vulgaris L.), Sage (Salvia Officinalis L.), and Thyme–Sage Mixture Extracts. J. Food Qual. 2023, 2023, 2602454. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural Diversity in Phenolic Components and Antioxidant Properties of Oregano (Origanum Vulgare L.) Accessions, Grown under the Same Conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Tonin, L.T.D.; De Oliveira, T.F.V.; De Marco, I.G.; Palioto, G.F.; Düsman, E. Bioactive Compounds and Antioxidant, Antimicrobial and Cytotoxic Activities of Extracts of Curcuma Longa. Food Meas. 2021, 15, 3752–3760. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum Basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Ahmad, Z.; Waseem, M.; Mehmood, T.; Javed, M.R.; Ali, M.; Manzoor, M.F.; Goksen, G. Assessment of Beetroot Powder as Nutritional, Antioxidant, and Sensory Evaluation in Candies. J. Agric. Food Res. 2024, 15, 101023. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds of Beetroot and Utilization in Food Processing Industry: A Critical Review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Faujan, N.H.-; Zubairi, S.I.; Ahmad Baker, A.A. Nutritional and Bioactive Constituents of Antioxidant and Antimicrobial Properties in Spinacia Oleracea: A Review. JSM 2023, 52, 2571–2585. [Google Scholar] [CrossRef]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A Review of Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Allium Cepa and Its Main Constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef]
- Jain, R.; Seder-Colomina, M.; Jordan, N.; Dessi, P.; Cosmidis, J.; Van Hullebusch, E.D.; Weiss, S.; Farges, F.; Lens, P.N.L. Entrapped Elemental Selenium Nanoparticles Affect Physicochemical Properties of Selenium Fed Activated Sludge. J. Hazard. Mater. 2015, 295, 193–200. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, H.-W.; Lee, M.-K.; Kim, H.-J.; Kim, J.-B.; Choe, J.-S.; Lee, Y.-M.; Jang, H.-H. Antioxidant and Anti-Inflammatory Activities in Relation to the Flavonoids Composition of Pepper (Capsicum Annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Marksa, M.; Radusiene, J. Comparative Phytoprofiling of Achillea Millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts. Plants 2024, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Silvan, J.M.; Alarcón-Cavero, T.; Villanueva-Bermejo, D.; Jaime, L.; Santoyo, S.; Martinez-Rodriguez, A.J. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea Millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter Pylori. Antioxidants 2022, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.D.; Vu, D.C. A Review on Phytochemical Composition and Potential Health-Promoting Properties of Walnuts. Food Rev. Int. 2023, 39, 397–423. [Google Scholar] [CrossRef]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a Potential Bioactive Food Ingredient from Inner Cellular Content of Brewer’s Spent Yeast. Waste Biomass Valor 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Horn, P.A.; Zeni, A.L.B.; Cavichioli, N.; Winter, E.; Batista, K.Z.S.; Vitali, L.; De Almeida, E.A. Chemical Profile of Craft Brewer’s Spent Yeast and Its Antioxidant and Antiproliferative Activities. Eur. Food Res. Technol. 2023, 249, 2001–2015. [Google Scholar] [CrossRef]
- Mascoloti Spréa, R.; Caleja, C.; Pinela, J.; Finimundy, T.C.; Calhelha, R.C.; Kostić, M.; Sokovic, M.; Prieto, M.A.; Pereira, E.; Amaral, J.S.; et al. Comparative Study on the Phenolic Composition and in Vitro Bioactivity of Medicinal and Aromatic Plants from the Lamiaceae Family. Food Res. Int. 2022, 161, 111875. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and Multivariate Analyses to Investigate Variations in the Polyphenolic Compounds as Well as Antioxidant and Antiglycative Activities of Some Lamiaceae Species Native to Iran. Ind. Crops Prod. 2020, 154, 112640. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Lewoyehu, M.; Amare, M. Comparative Evaluation of Analytical Methods for Determining the Antioxidant Activities of Honey: A Review. Cogent Food Agric. 2019, 5, 1685059. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Marchi, R.C.; Campos, I.A.S.; Santana, V.T.; Carlos, R.M. Chemical Implications and Considerations on Techniques Used to Assess the in Vitro Antioxidant Activity of Coordination Compounds. Coord. Chem. Rev. 2022, 451, 214275. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of Flavonoids, Total Phenols and Antioxidant Properties of Onion Skin: A Comparative Study of Fifteen Indian Cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- El Mannoubi, I. Impact of Different Solvents on Extraction Yield, Phenolic Composition, in Vitro Antioxidant and Antibacterial Activities of Deseeded Opuntia Stricta Fruit. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 176–184. [Google Scholar] [CrossRef]
- Lai, C.; Liang, Y.; Zhang, L.; Huang, J.; Kaliaperumal, K.; Jiang, Y.; Zhang, J. Variations of Bioactive Phytochemicals and Antioxidant Capacity of Navel Orange Peel in Response to Different Drying Methods. Antioxidants 2022, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Rath, S.K.; Logesh, R.; Mishra, S.K.; Devkota, H.P.; Das, N. Capsicum Annuum L. and Its Bioactive Constituents: A Critical Review of a Traditional Culinary Spice in Terms of Its Modern Pharmacological Potentials with Toxicological Issues. Phytother. Res. 2023, 37, 965–1002. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kleiber, T.; Kaczmarek, A. Effect of Increasing Manganese Concentration in Nutrient Solution on the Antioxidant Activity, Vitamin C, Lycopene and Polyphenol Contents of Tomato Fruit. Food Addit. Contam. Part A 2017, 34, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, R.P. Evaluation of Antioxidant Activity in Foods with Special Reference to TEAC Method. Am. J. Food Technol. 2013, 8, 83–101. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, J.-H. Correlation between Solid Content and Antioxidant Activities in Umbelliferae Salad Plants. Prev. Nutr. Food Sci. 2020, 25, 84–92. [Google Scholar] [CrossRef]
- Nićetin, M.; Pezo, L.; Pergal, M.; Lončar, B.; Filipović, V.; Knežević, V.; Demir, H.; Filipović, J.; Manojlović, D. Celery Root Phenols Content, Antioxidant Capacities and Their Correlations after Osmotic Dehydration in Molasses. Foods 2022, 11, 1945. [Google Scholar] [CrossRef]
- Lyu, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic Compounds Profiling and Their Antioxidant Capacity in the Peel, Pulp, and Seed of Australian Grown Avocado. Antioxidants 2023, 12, 185. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 75, pp. 243–266. ISBN 978-0-323-91250-1. [Google Scholar]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and Antioxidant Analysis of Medicinal and Food Plants towards Bioactive Food and Pharmaceutical Resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of Chemical Composition, Antioxidant Activity, and the Effects of Alfalfa Flavonoids on Growth Performance. Oxidative Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent Advances in the Analysis of Rosmarinic Acid from Herbs in the Lamiaceae Family. Nat. Prod. Commun. 2019, 14, 1934578X19864216. [Google Scholar] [CrossRef]
- Bang, J.-H.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.-H.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J.; et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy 2021, 11, 1032. [Google Scholar] [CrossRef]
- Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods 2024, 13, 3379. [Google Scholar] [CrossRef]
- Mitrović, J.; Nikolić, N.; Karabegović, I.; Savić, S.; Petrović, S.; Pešić, M.; Šimurina, O. Evaluation of the Solvent Effect on the Extraction and Antioxidant Activity of Phenolic Compounds from the Nettle (Urtica Dioica L.) Seeds: Application of PCA and Regression Analyses. Food Meas. 2024, 18, 6618–6626. [Google Scholar] [CrossRef]
- Matkovits, A.; Nagy, K.; Fodor, M.; Jókai, Z. Analysis of Polyphenolic Components of Hungarian Acacia (Robinia Pseudoacacia) Honey; Method Development, Statistical Evaluation. J. Food Compos. Anal. 2023, 120, 105336. [Google Scholar] [CrossRef]

| Name | Family | Classification | Major Antioxidant Components | Important Biological Activities | Reference |
|---|---|---|---|---|---|
| Rosemary (Salvia rosmarinus L.) | Lamiaceae | herb | Phenolics: caffeic acid, rosmarinic acid carnosic acid, carnosol, and hesperidin Flavonoids: diosmin | Antioxidant, anti-inflammatory, antimicrobial, antiviral, anti-metabolic syndrome, anticarcinogenic, antimutagenic, antinociceptive, neuroprotective | [22] |
| Thyme (Thymus vulgaris L.) | Lamiaceae | herb | Phenolics: thymol and carvacrol Flavonoids: apigenin | Antioxidant, antimicrobial, expectorant, spasmolytic, mucolytic, antitussive | [24,25] |
| Oregano (Origanum vulgare L.) | Lamiaceae | herb | Phenolics: rosmarinic, chlorogenic, cinnamic, caffeic, syringic, benzoic, vanillic, gallic, chicoric, and 2,4-dihydroxybenzoic acids Flavonoids: quercetin, apigenin, luteolin, naringenin, and kaempferol | Antioxidant, antifungal, antimicrobial, expectorant, stimulant, carminative, anticancer, antiaging agent | [26] |
| Basil (Ocimum basilicum L.) | Lamiaceae | herb | Phenolics: rosmarinic acid, caffeic acid, chicoric acid, and ferulic acid Flavonoids: apigenin, luteolin, and quercetin | Antioxidant, antibacterial, antifungal, anti-inflammatory, antidiabetic | [27,28] |
| Turmeric powder (Curcuma longa L.) | Zingiberaceae | spice | Phenolics: curcumin and turmerones Flavonoids: quercetin, luteolin, and rutin | Antioxidant, antimicrobial, anti-inflammatory, anticancer, hypoglycemic, anticoagulant | [27] |
| Beetroot powder (Beta vulgaris L.) | Amaranthaceae | spice | Phenolics: chlorogenic acid, caffeic acid, and catechin Flavonoids: betagarin, betavulgarin, quercetin, and rutin Carotenoids | Antioxidant, antiviral, antibacterial, antianemic, anti-inflammatory, antihypertensive | [29,30] |
| Amaranth (Amaranthus spp.) | Amaranthaceae | pseudo-cereal | Phenolics: caffeic acid and ferulic acid Flavonoids: rutin and quercetin | Antioxidant, antimicrobial, anti-inflammatory, antimalarial, antidiabetic, anticarcinogenic, hepatoprotective | [11] |
| Spinach powder (Spinacia oleracea L.) | Amaranthaceae | spice | Phenolics: coumaric acid, ferulic acid, and caffeic acid Flavonoids: patuletin, spinacetin, quercetin, and luteolin Carotenoids | Antioxidant, antimicrobial, anticancer, | [31] |
| Dried onion (Allium cepa L.) | Amaryllidaceae | spice | Phenolics: phenolic acids and anthocyanins Flavonoids: quercetin, rutin, and kaempferol | Antioxidant, anti-inflammatory, immunomodulatory, anticancer | [32,33] |
| Tomato powder (Solanum lycopersicum L.) | Solanaceae | spice | Phenolics: chlorogenic acid, caffeic acid, and vanillic acid Flavonoids: rutin, quercetin, and kaempferol Carotenoids | Antioxidant, anticarcinogenic, cardioprotective, antimicrobial, anti-inflammatory | [34] |
| Chili pepper (Capsicum annuum L.) | Solanaceae | spice | Flavonoids: apigenin, luteolin, and quercetin Carotenoids Capsaicinoids: capsaicin | Antioxidant, anti-inflammatory, antimicrobial, anti-obesity | [35,36] |
| Red pepper (Capsicum annuum L.) | Solanaceae | spice | Phenolics: gallic acid, vanillic acid, caffeic acid, coumaric acid, and chlorogenic acid Flavonoids: myricetin, quercetin, luteolin, and apigenin Capsaicinoids: capsaicin | Antioxidant, immunomodulatory, anticancer, antimutagenic, antiplatelet, antiangiogenic, anti-inflammatory, antiviral | [56] |
| Yarrow tail (Achillea millefolium L.) | Asteraceae | herb | Phenolics: dicaffeoylquinic acid Flavonoids: apigenin, luteolin, and quercetin | Antioxidant, anti-inflammatory, antiaging, antibacterial, antitumor, antidiabetic | [37,38] |
| Walnut flour (Juglans regia L.) | Juglandaceae | flour | Phenolics: chlorogenic acid and ferulic acid) Flavonoids: catechin and rutin Carotenoids | Antioxidant, anti-inflammatory, antibacterial, anticancer, neuroprotective effects, cholesterol-lowering activity | [39] |
| Brewer’s yeast flakes (Saccharomyces cerevisiae) | Saccharomycetaceae | yeast | Phenolics: gallic acid, protocatechuic acid, catechin, and ferulic acid B-vitamins: biotin and folic acid | Antioxidant, anti-inflammatory, antitumorigenic | [40,41] |
| Sample | Origin of Spice | Plant Part Used to Make Spice | Form of Spice Used to Make Extraction |
|---|---|---|---|
| Rosemary | Hungary | leaves | dry leaves |
| Thyme | Hungary | leaves | dry leaves |
| Oregano | Hungary | leaves | dry leaves |
| Basil | Hungary | leaves | dry leaves |
| Turmeric | India * | rhizomes | powder |
| Beetroot | Hungary | taproot | powder |
| Amaranth | Hungary | seeds | powder |
| Spinach | Hungary | leaves | powder |
| Dried onion | India * | bulbs | granulates |
| Tomato | Hungary | fruits | powder |
| Chili pepper | Hungary | placenta | flakes |
| Red pepper | Hungary | fruits | powder |
| Yarrow tail | Hungary | leaves | dry leaves |
| Walnut | Ukraine * | kernel | flour |
| Brewer’s yeast | Hungary | one cell fungus | flakes |
| TPC | TFC | DPPH | TEAC | |
|---|---|---|---|---|
| TPC | 1 | |||
| TFC | 0.201 | 1 | ||
| DPPH | 0.772 ** | 0.425 | 1 | |
| TEAC | 0.856 ** | 0.499 | 0.845 ** | 1 |
| FRAP | 0.913 ** | 0.355 | 0.844 ** | 0.907 ** |
| TPC | TFC | DPPH | TEAC | |
|---|---|---|---|---|
| TFC | 0.0405 | |||
| DPPH | 0.5686 | 0.1951 | ||
| TEAC | 0.7196 | 0.2777 | 0.6802 | |
| FRAP | 0.7808 | 0.1559 | 0.689 | 0.8061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, A.; Papp, V.A.; Pál, A.; Prokisch, J.; Mirani, S.; Toth, B.E.; Alshaal, T. Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status. Antioxidants 2025, 14, 317. https://doi.org/10.3390/antiox14030317
Kiss A, Papp VA, Pál A, Prokisch J, Mirani S, Toth BE, Alshaal T. Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status. Antioxidants. 2025; 14(3):317. https://doi.org/10.3390/antiox14030317
Chicago/Turabian StyleKiss, Attila, Vivien Anna Papp, Anna Pál, József Prokisch, Sara Mirani, Bela E. Toth, and Tarek Alshaal. 2025. "Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status" Antioxidants 14, no. 3: 317. https://doi.org/10.3390/antiox14030317
APA StyleKiss, A., Papp, V. A., Pál, A., Prokisch, J., Mirani, S., Toth, B. E., & Alshaal, T. (2025). Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status. Antioxidants, 14(3), 317. https://doi.org/10.3390/antiox14030317








