Biombalance™, an Oligomeric Procyanidins-Enriched Grape Seed Extract, Prevents Inflammation and Microbiota Dysbiosis in a Mice Colitis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition Analysis of OPC-Rich Polyphenolic Extract
2.2. Measure of Biombalance™ Antioxidant Activity
2.3. Experiment Design
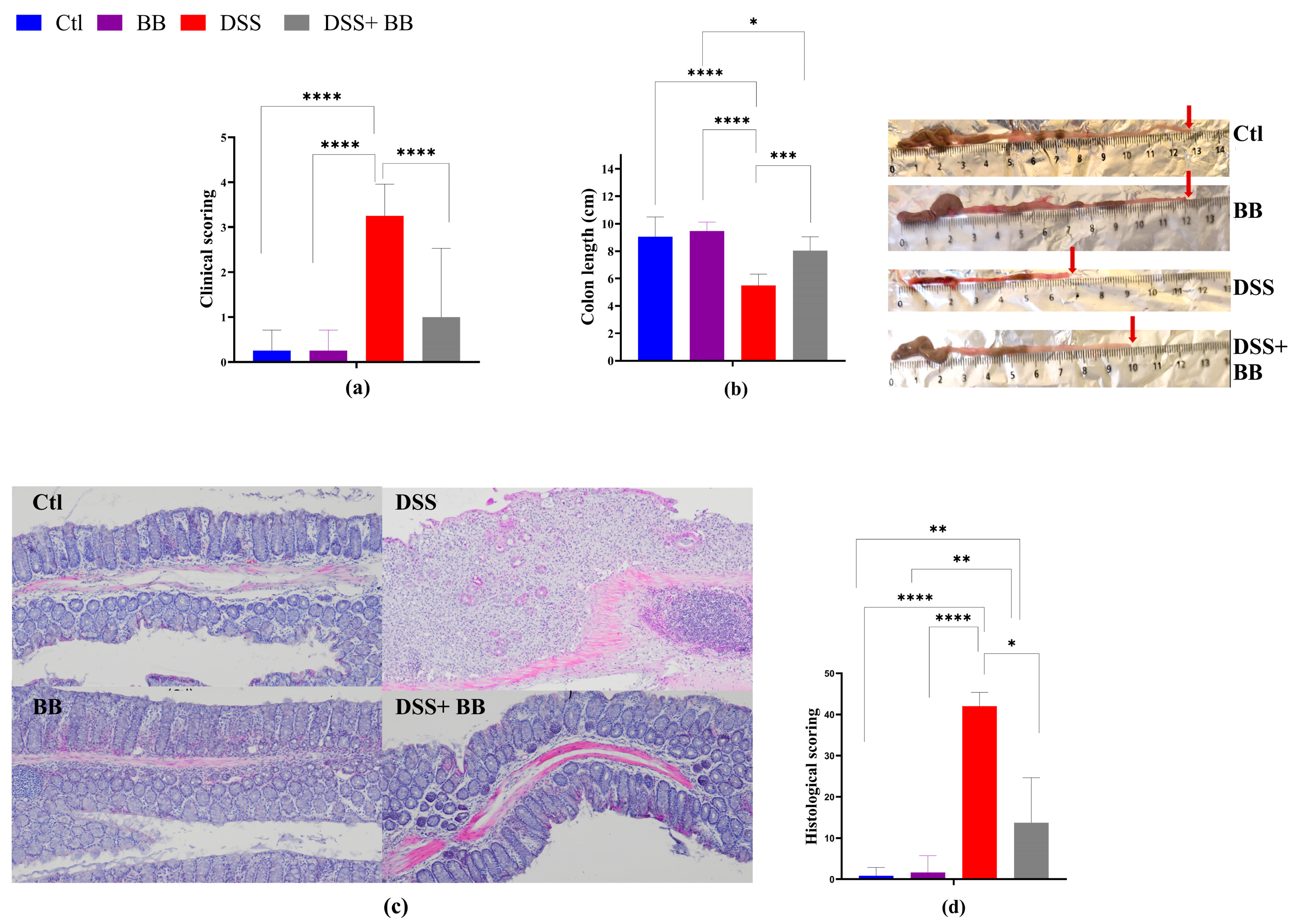
2.4. Clinical Scoring of Murine Colitis
2.5. Histological Evaluation of Colitis
2.6. Tissue Sampling, RNA Extraction and Real-Time PCR (RT-PCR)
2.7. Stool Sampling, Fecal DNA Extraction and 16S rDNA Sequencing
2.8. Short-Chain Fatty Acid (SCFA) Analysis
2.9. PICRUS (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) Analysis
2.10. Statistical Analysis
3. Results
3.1. Characterization of Grape Seed Polyphenolic Extract (OPC)
3.2. Antioxidant Capacity of Biombalance™ Extract
3.3. Biombalance™ Extract Alleviates DSS-Induced Colitis Symptoms in Mice
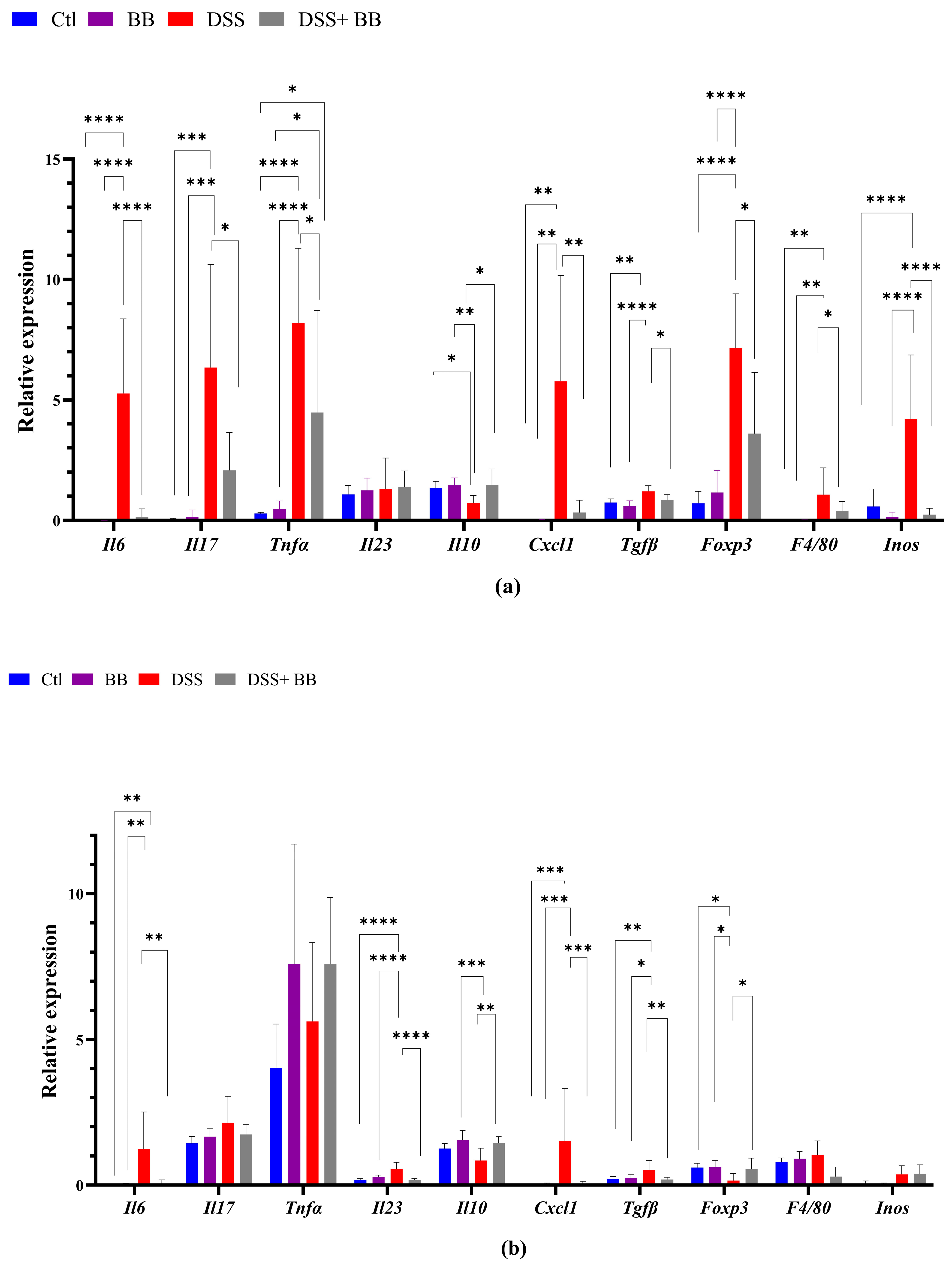
3.4. Biombalance Extract Diminished Production of Proinflammatory Markers in Mice with DSS-Induced Colitis
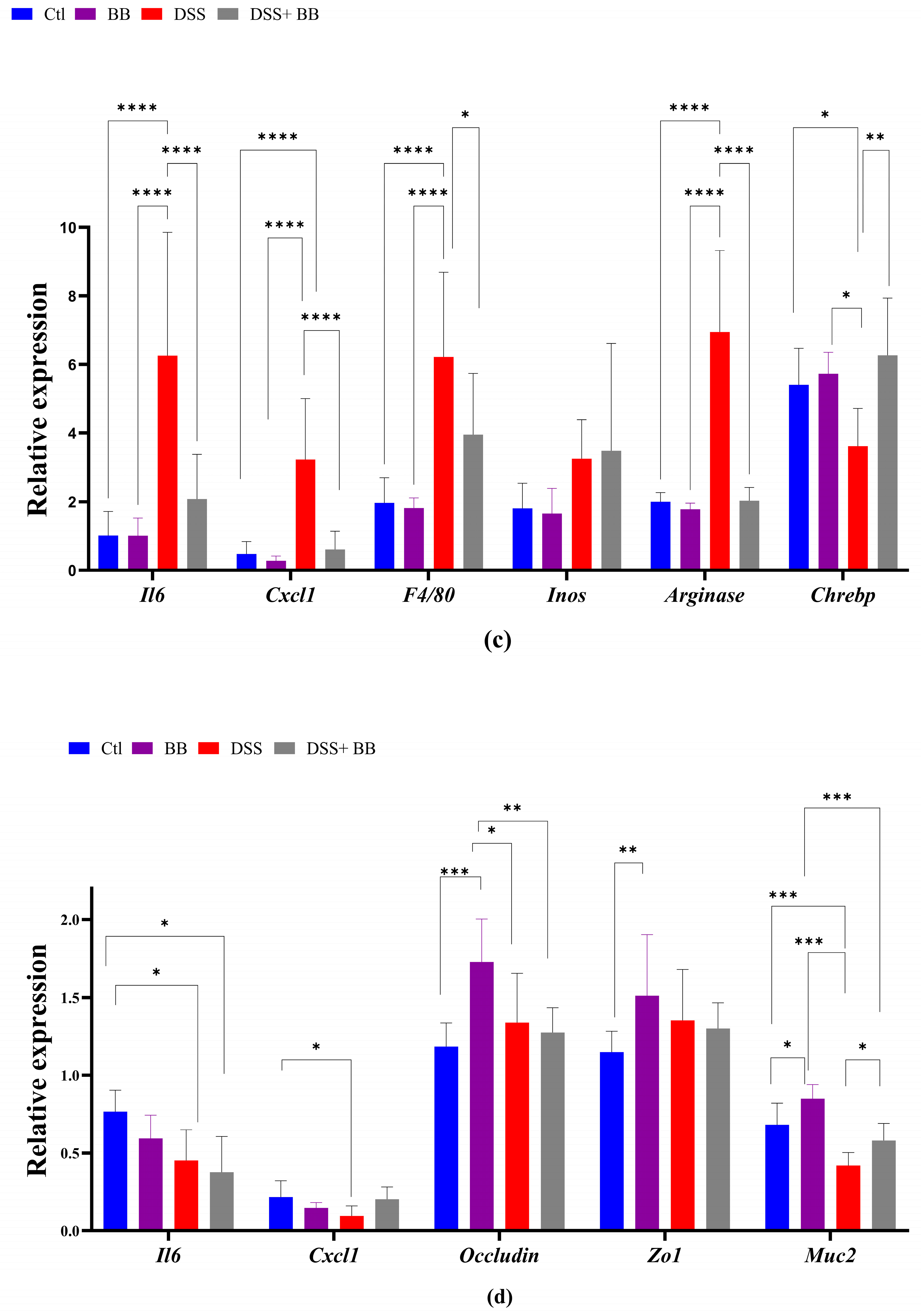
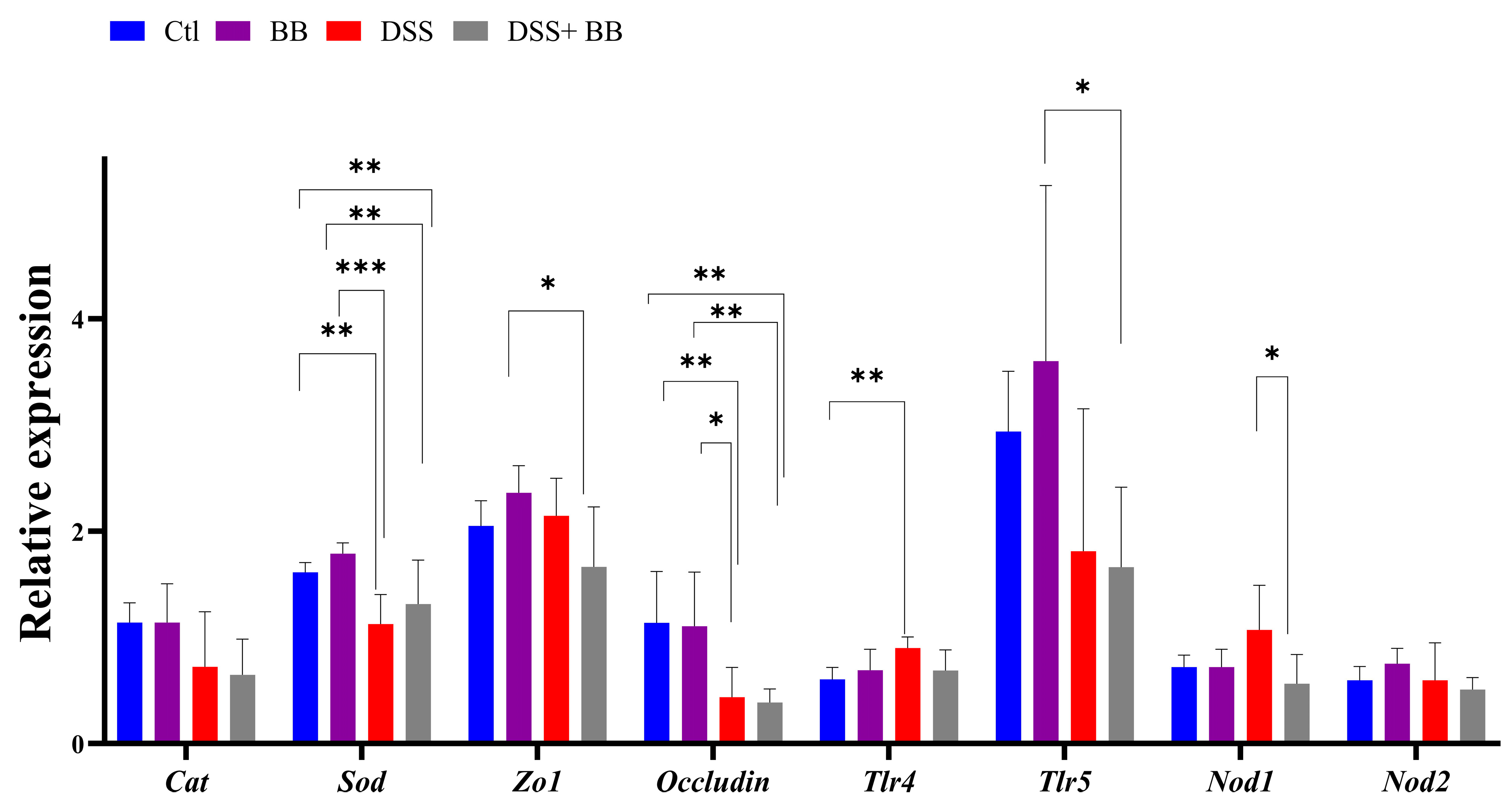
3.5. Involvement of Biombalance™ in Oxidative Stress and Gut Barrier Integrity
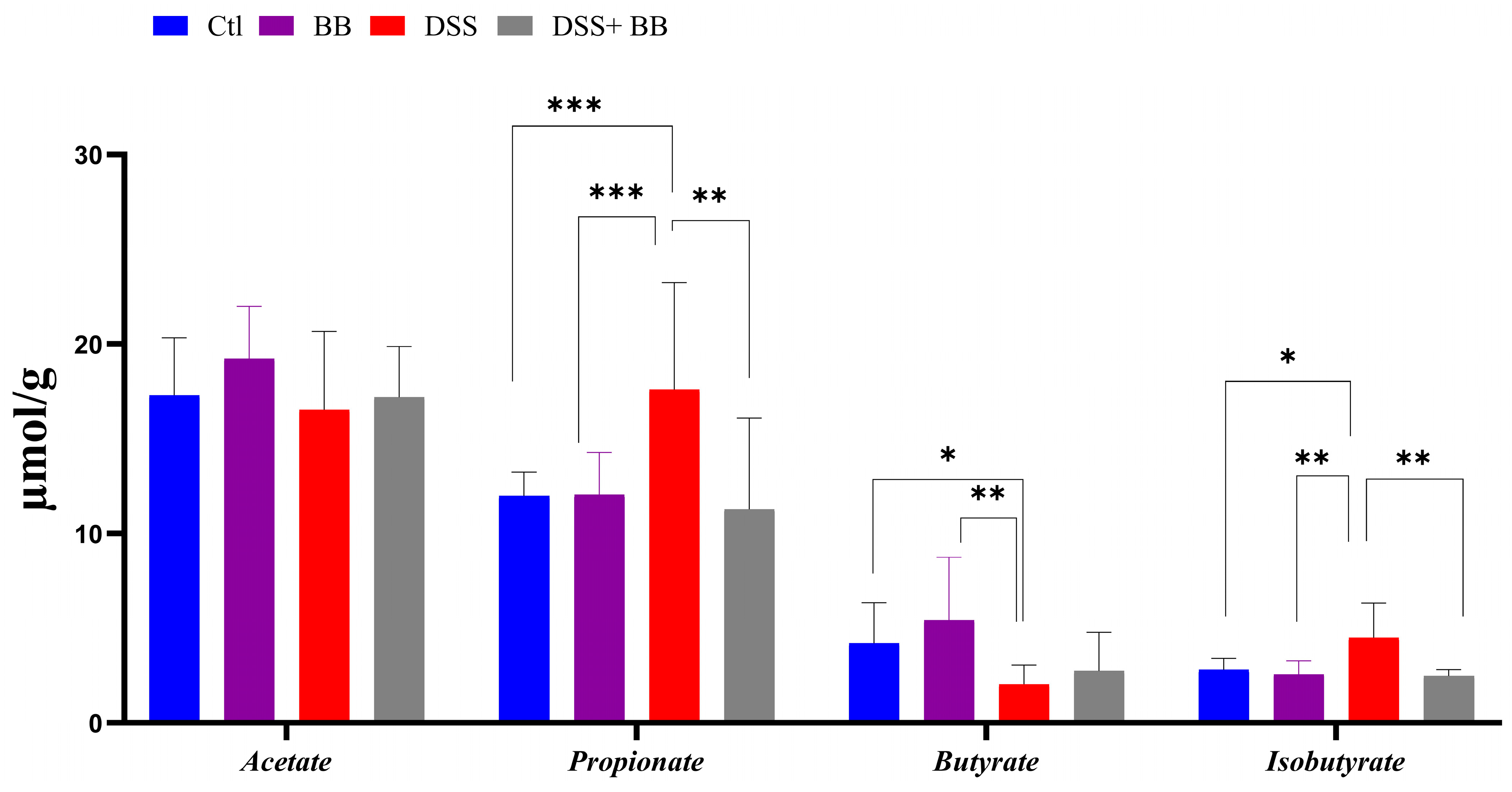
3.6. Biombalance™ Supplementation Influences Caecum SCFA Generation
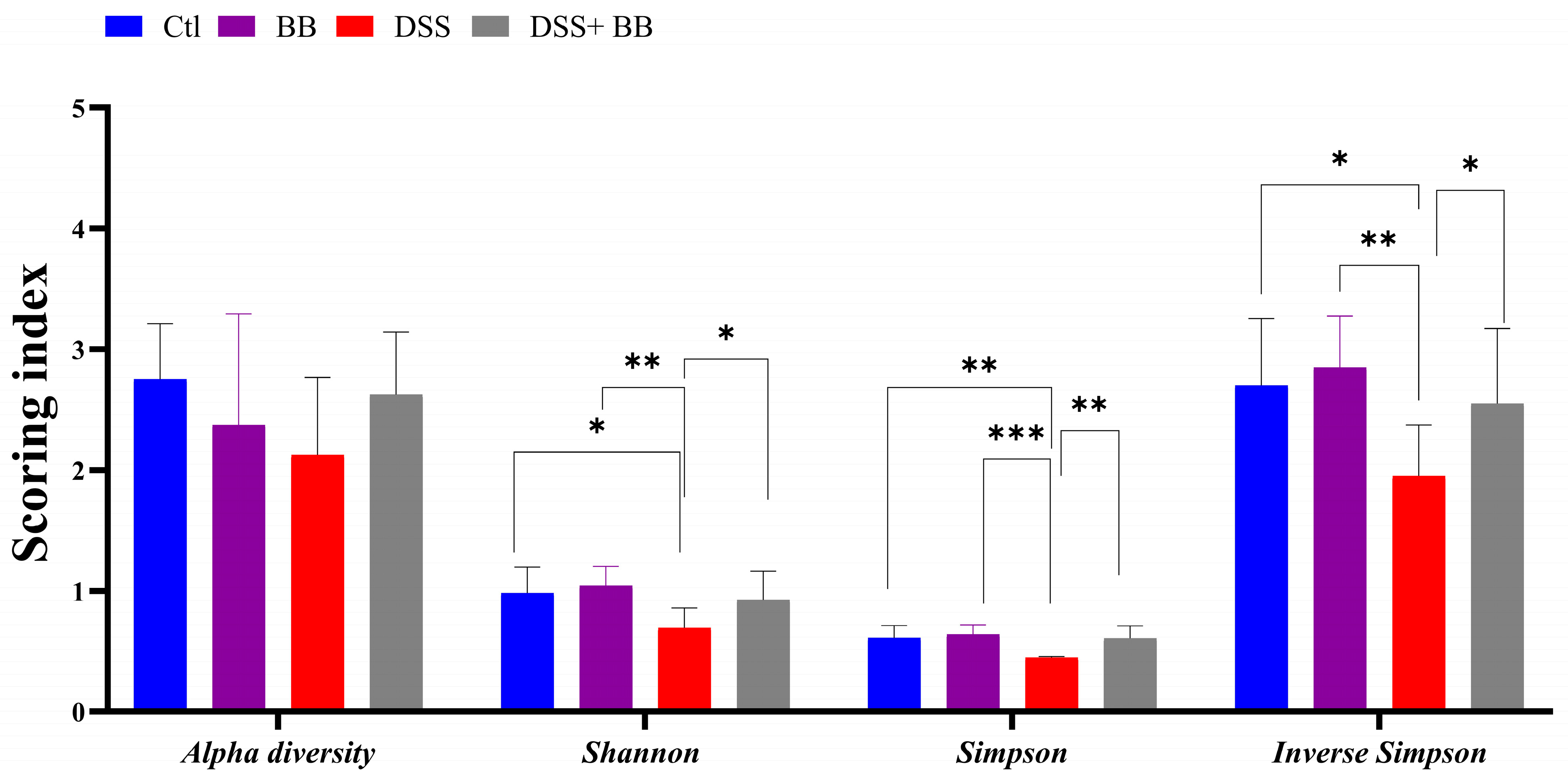
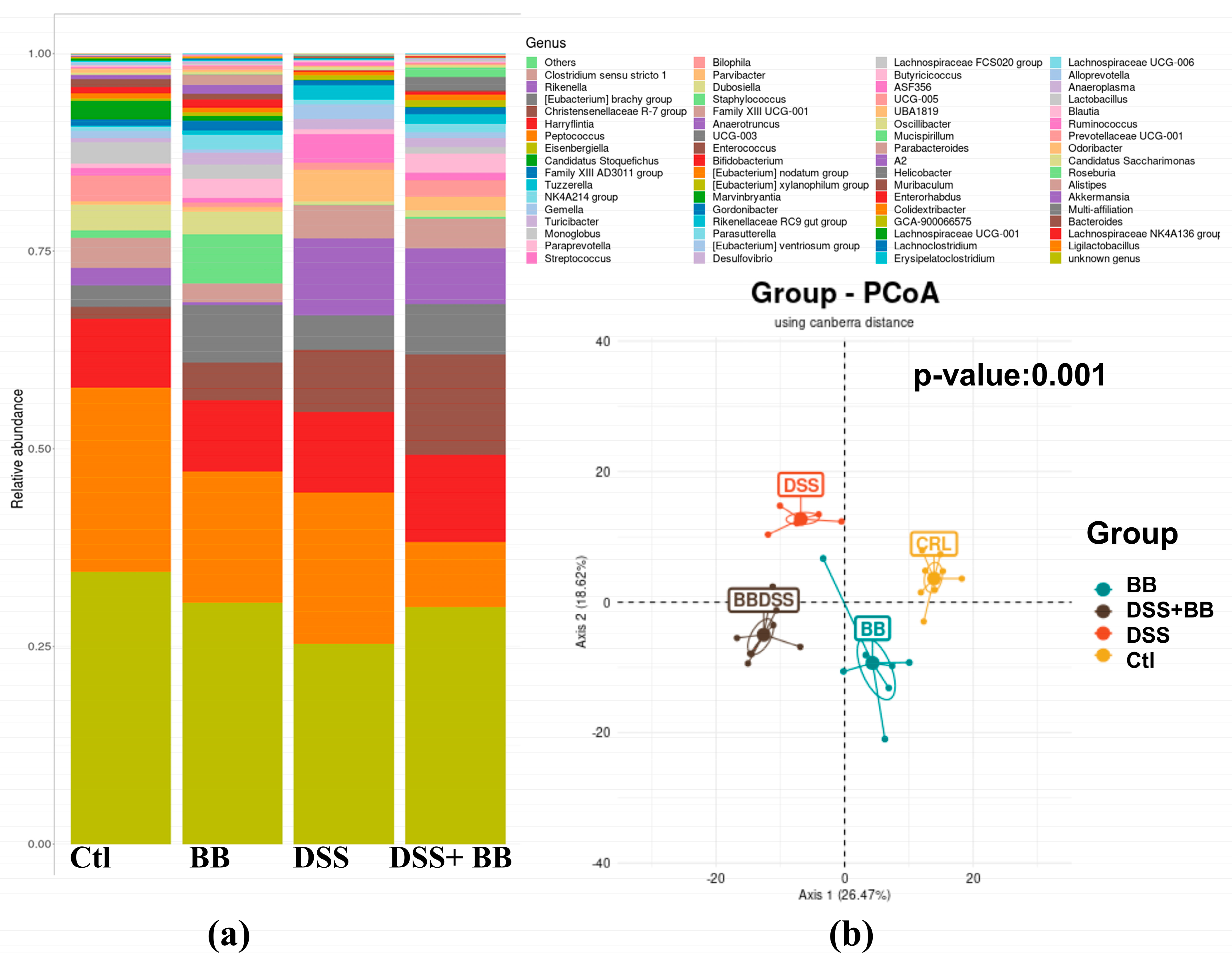
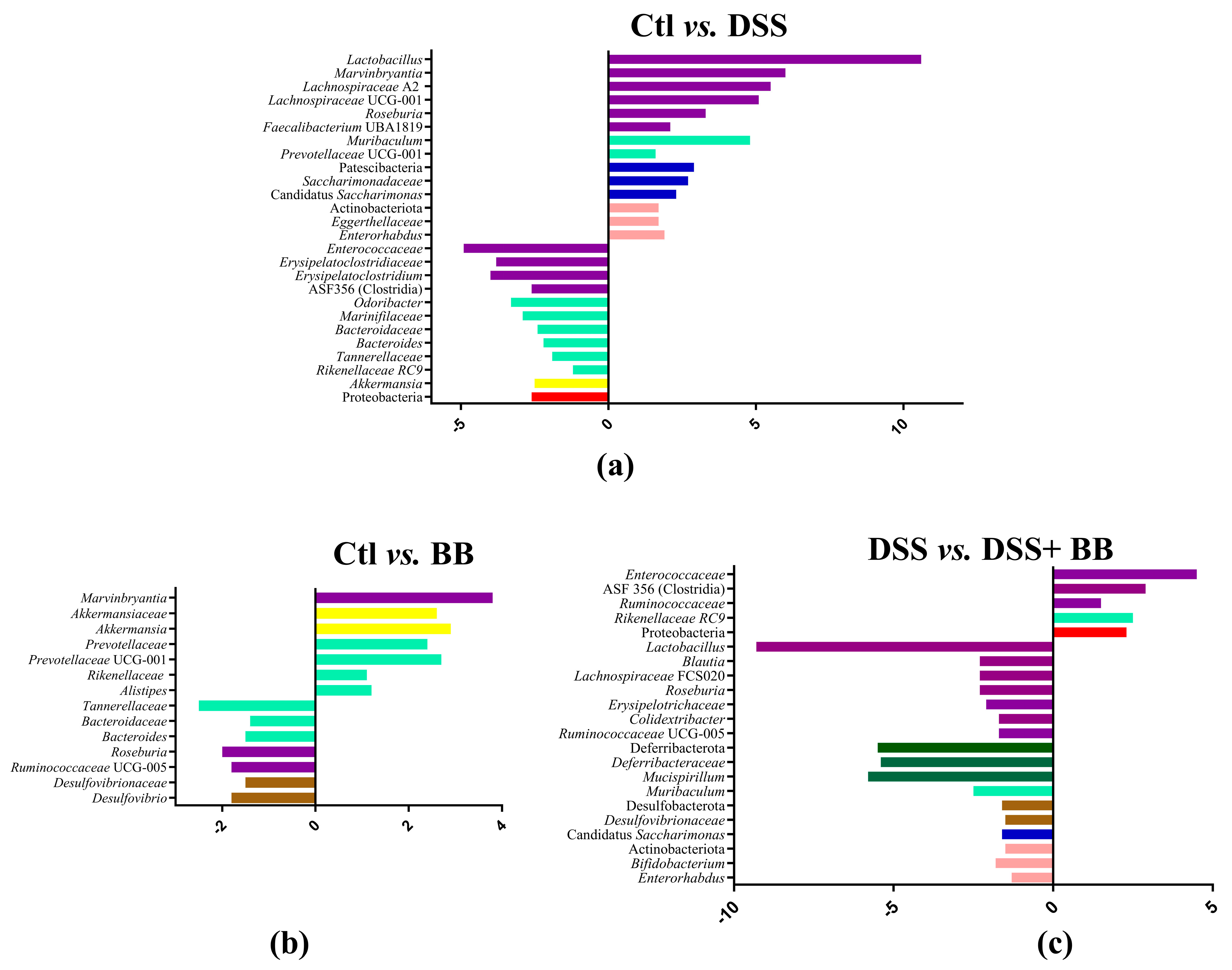
3.7. Effects of Biombalance™ on Gut Microbiota in Mice with DSS-Induced Colitis
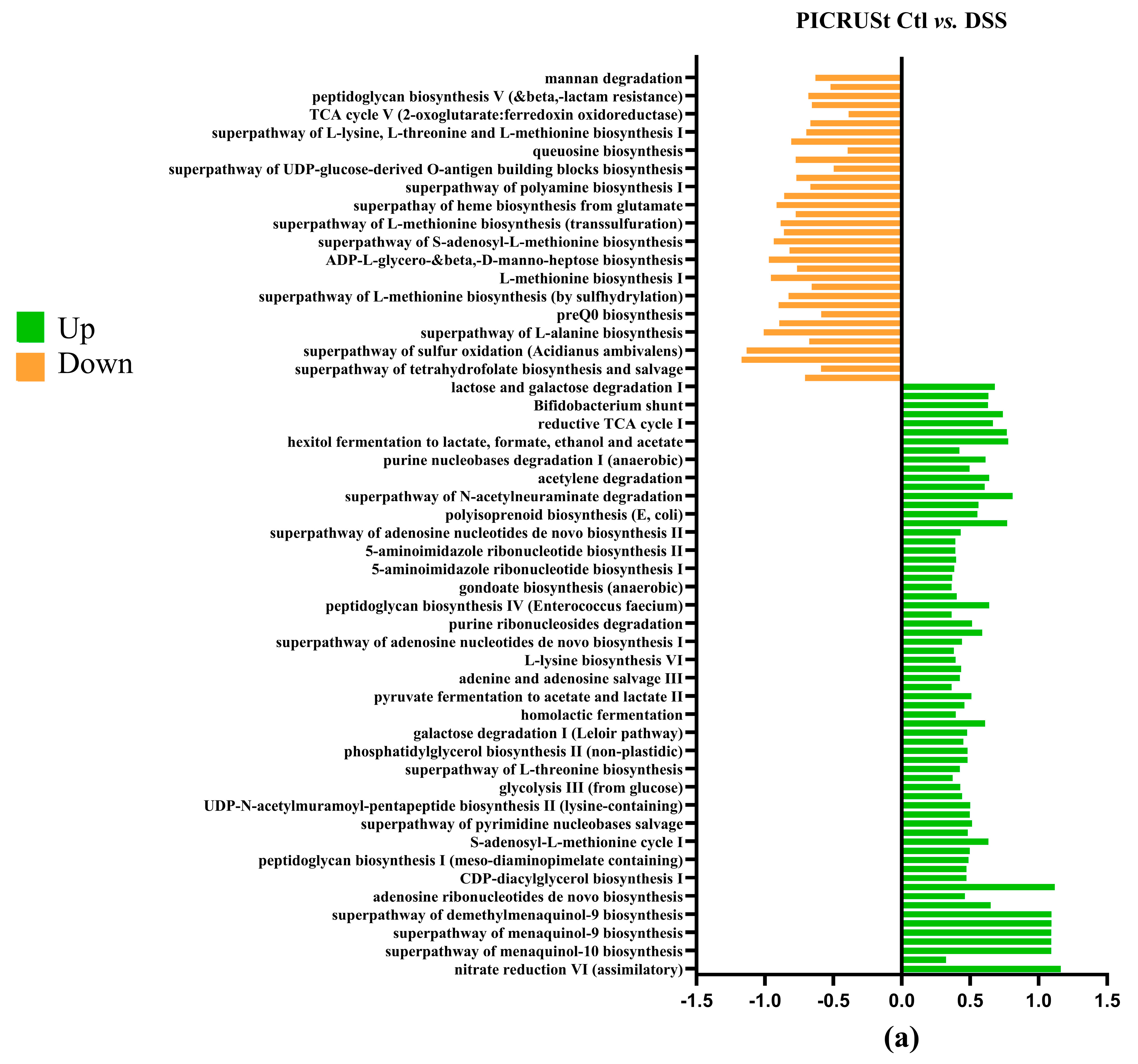
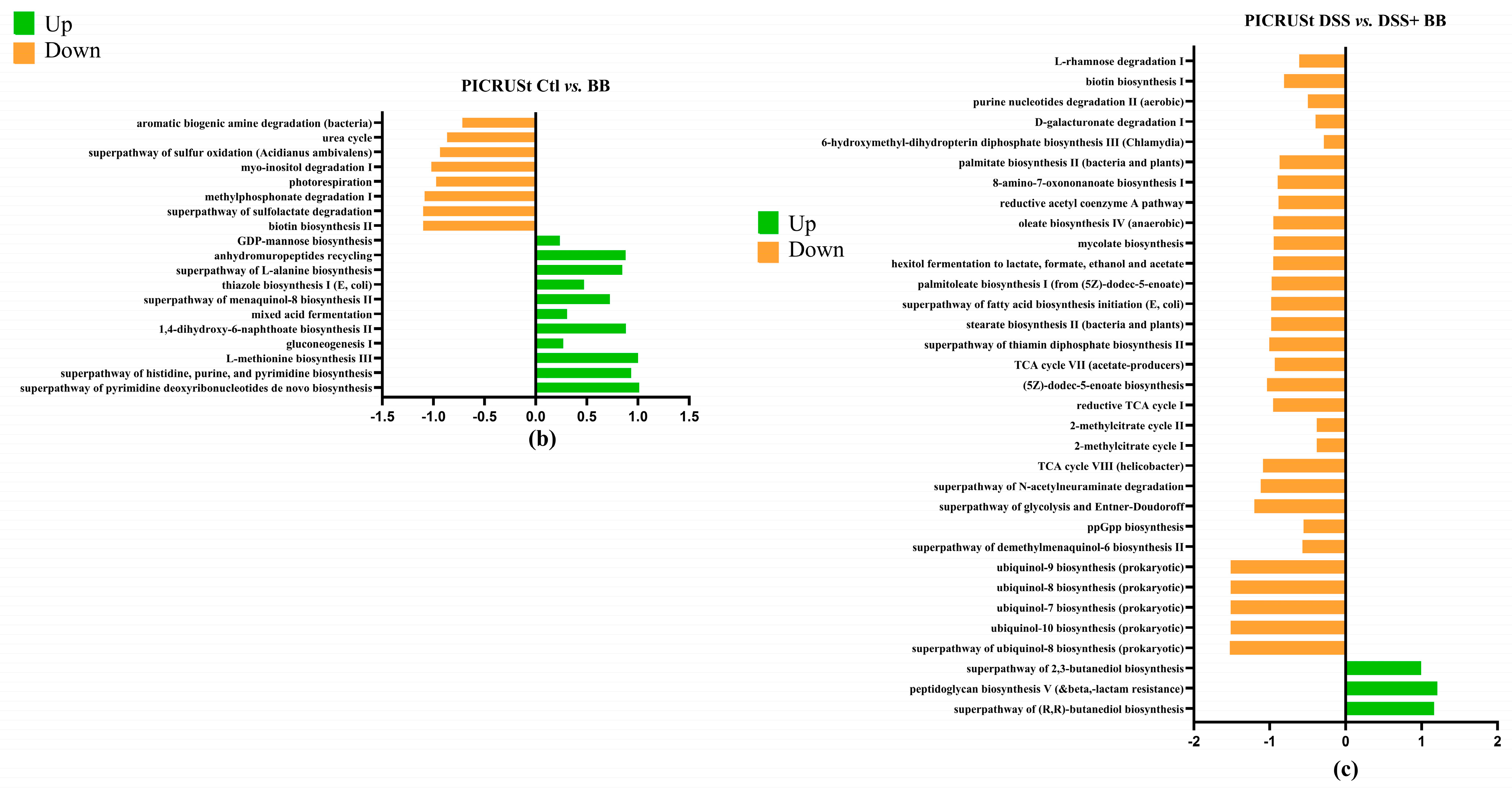
3.8. PICRUSt Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Arg1 | arginase 1 |
| CAT | catalase |
| ChREBP | carbohydrate-responsive element-binding protein |
| CXCL-1 | C-X-C Motif Chemokine Ligand 1 |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSS | Dextran Sulphate Sodium |
| F4/80 | (Adgre1) adhesion G protein-coupled receptor E1 |
| FoxP3 | forkhead box P3 |
| Hprt | hypoxanthine phosphoribosyltransferase |
| IL-6 | interleukin 6 |
| IL-10 | interleukin 10 |
| IL-17 | interleukin 17 |
| IL-23 | interleukin 23 |
| iNOS | inducible nitric oxide synthase |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| MALDI | matrix-assisted laser desorption ionization |
| MUC2 | mucin 2 |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| NOD-1 | nucleotide-binding oligomerization domain-containing protein 1 |
| NOD-2 | nucleotide-binding oligomerization domain-containing protein 2 |
| Ocln | occludin |
| PB1 | procyanidin dimer B1 |
| PB2 | procyanidin dimer B2 |
| PC1 | procyanidin trimer C1 |
| SOD | superoxide dismutase |
| TGF-β | transforming growth factor-beta |
| TLR4 | toll-like receptor 4 |
| TLR5 | toll-like receptor 5 |
| TNF-α | tumour necrosis factor α |
| ZO-1 | zonula occludens-1 |
References
- Hu, M.; Caldarelli, G.; Gili, T. Inflammatory Bowel Disease Biomarkers Revealed by the Human Gut Microbiome Network. Sci. Rep. 2023, 13, 19428. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Jin, Y.; Shao, X.; Xu, Y.; Ma, G.; Jiang, Y.; Hu, D. Global, regional, and national burden of inflammatory bowel disease, 1990–2021: In-sights from the global burden of disease 2021. Int. J. Colorectal Dis. 2024, 39, 139. [Google Scholar] [CrossRef] [PubMed]
- Bogale, K.; Maheshwari, P.; Kang, M.; Subhash Gorrepati, V.; Dalessio, S.; Walter, V.; Stuart, A.; Koltun, W.; Bernasko, N.; Tinsley, A.; et al. Symptoms Associated with Healthcare Resource Utilization in the Setting of Inflammatory Bowel Disease. Sci. Rep. 2022, 12, 10577. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Bosso, A.; Cassino, C.; Motta, S.; Panero, L.; Tsolakis, C.; Guaita, M. Polyphenolic Composition and In Vitro Antioxidant Activity of Red Grape Seeds as Byproducts of Short and Medium-Long Fermentative Macerations. Foods 2020, 9, 1451. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds-Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed. Res. Int. 2018, 8584136. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-Rich Extract from Grape and Blueberry Attenuates Cognitive Decline and Improves Neuronal Function in Aged Mice. J. Nutr. Sci. 2018, 7, 10. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef]
- Lessard-Lord, J.; Roussel, C.; Lupien-Meilleur, J.; Généreux, P.; Richard, V.; Guay, V.; Roy, D.; Desjardins, Y. Short Term Supplementation with Cranberry Extract Modulates Gut Microbiota in Human and Displays a Bifidogenic Effect Check for Updates. npj Biofilms Microbiomes 2024, 10, 18. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape Seed Proanthocyanidin Extract Ameliorates Inflammation and Adiposity by Modulating Gut Microbiota in High-Fat Diet Mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-Inflammatory Effect and Mechanism of Proanthocyanidins from Grape Seeds. Acta Pharmacol. Sin. 2001, 1117, 12–22. [Google Scholar]
- Głąbska, D.; Guzek, D.; Gałązka, K.; Lech, G. Therapeutic Potential of Proanthocyanidins in Ulcerative Colitis in Remission. J. Clin. Med. 2020, 9, 771. [Google Scholar] [CrossRef]
- Kangwan, N.; Kongkarnka, S.; Pintha, K.; Saenjum, C.; Suttajit, M. Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model. Biomedicines 2023, 11, 265. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 485842. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, M.; Charradi, K.; Limam, F.; Aouani, E.; Urdaci, M.C. Grape Seed and Skin Extract, a Potential Prebiotic with Anti—Obesity Effect through Gut Microbiota Modulation. Gut Pathog. 2022, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Bolling, B.W. A Review of the Efficacy of Dietary Polyphenols in Experimental Models of Inflammatory Bowel Diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Shen, W.; Wang, Y.; Cao, Y.; Nuerbulati, N.; Chen, W.; Lu, G.; Xiao, W.; Qi, R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology 2020, 105, 9–18. [Google Scholar] [CrossRef]
- Zhang, H.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl Valine Supplementation-Induced Mitigation of Gut Inflammation in a Porcine Model of Colitis. J. Funct. Foods 2016, 24, 558–567. [Google Scholar] [CrossRef]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of Standardized Grape Powder Consumption on the Gut Microbiome of Healthy Subjects: A Pilot Study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Travaglia, F.; Bordiga, M.; Locatelli, M.; Coïsson, J.D.; Arlorio, M. Polymeric Proanthocyanidins in Skins and Seeds of 37 Vitis Vinifera L. Cultivars: A Methodological Comparative Study. J. Food Sci. 2011, 76, C742–C749. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L. Occurrence and Biological Significance of Proanthocyanidins in the American Diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ventura, M.; Ruas-Madiedo, P.; Hidalgo-Cantabrana, C.; Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Martínez-Camblor, P.; Margolles, A.; Gálvez, J. Effect of a Ropy Exopolysaccharide-Producing Bifidobacterium animalis Subsp. lactis Strain Orally Administered on DSS-Induced Colitis Mice Model. Front. Microbiol. 2016, 1, 868. [Google Scholar] [CrossRef]
- Abdelbaqi, M.; Chidlow, J.H.; Matthews, K.M.; Pavlick, K.P.; Barlow, S.C.; Linscott, A.J.; Grisham, M.B.; Fowler, M.R.; Kevil, C.G. Regulation of Dextran Sodium Sulfate Induced Colitis by Leukocyte Beta 2 Integrins. Lab. Investig. 2006, 86, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-Induced Colitis Is Associated with Adipose Tissue Dysfunction and Disrupted Hepatic Lipid Metabolism Leading to Hepatosteatosis and Dyslipidemia in Mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Varon, C.; Dubus, P.; Mazurier, F.; Asencio, C.; Chambonnier, L.; Ferrand, J.; Giese, A.; Senantdugot, N.; Carlotti, M.; Mgraud, F. Helicobacter Pylori Infection Recruits Bone Marrow-Derived Cells That Participate in Gastric Preneoplasia in Mice. Gastroenterology 2012, 142, 281–291. [Google Scholar] [CrossRef]
- Witalison, E.E.; Cui, X.; Causey, C.P.; Thompson, P.R.; Hofseth, L.J. Molecular targeting of protein arginine deiminases to suppress colitis and prevent colon cancer. Oncotarget 2015, 6, 36053–36062. [Google Scholar] [CrossRef]
- Chumanevich, A.A.; Causey, C.P.; Knuckley, B.A.; Jones, J.E.; Poudyal, D.; Chumanevich, A.P.; Davis, T.; Matesic, L.E.; Thompson, P.R.; Hofseth, L.J. Suppression of Colitis in Mice by Cl-Amidine: A Novel Peptidylarginine Deiminase Inhibitor. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 929–938. [Google Scholar] [CrossRef]
- Mokrani, M.; Elie, A.M.; Jacquot, C.; Kiran, F.; Urdaci, M.C. Encapsulation of a pediocin pa-1 producer Pediococcus acidilactici and its impact on enhanced survival and gut microbiota modulation. Microb. Health Dis. 2024, 6, e996. [Google Scholar] [CrossRef]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- Volant, S.; Lechat, P.; Woringer, P.; Motreff, L.; Campagne, P.; Malabat, C.; Kennedy, S.; Ghozlane, A. SHAMAN: A User-Friendly Website for Metataxonomic Analysis from Raw Reads to Statistical Analysis. BMC Bioinform. 2020, 21, 345. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Jewell, D.P. The IL-23/IL-17 Pathway in Inflammatory Bowel Disease. Expert. Rev. Gastroenterol. Hepatol. 2012, 6, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tu, L.; Bisht, S.; Mao, Y.; Petkovich, D.; Thursby, S.J.; Liang, J.; Patel, N.; Yen, R.W.C.; Largent, T.; et al. Tissue-Location-Specific Transcription Programs Drive Tumor Dependencies in Colon Cancer. Nat. Commun. 2024, 15, 1384. [Google Scholar] [CrossRef]
- Jois, T.; Chen, W.; Howard, V.; Harvey, R.; Youngs, K.; Thalmann, C.; Saha, P.; Chan, L.; Cowley, M.A.; Sleeman, M.W. Deletion of Hepatic Carbohydrate Response Element Binding Protein (ChREBP) Impairs Glucose Homeostasis and Hepatic Insulin Sensitivity in Mice. Mol. Metab. 2017, 6, 1381–1394. [Google Scholar] [CrossRef]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front. Immunol. 2019, 10, 448925. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Wipperman, M.F.; Bhattarai, S.K.; Vorkas, C.K.; Maringati, V.S.; Taur, Y.; Mathurin, L.; McAulay, K.; Vilbrun, S.C.; Francois, D.; Bean, J.; et al. Gastrointestinal Microbiota Composition Predicts Peripheral Inflammatory State during Treatment of Human Tuberculosis. Nat. Commun. 2021, 12, 1141. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.; Wang, Y.; Liang, L.; Zhang, Z.; Tan, X.; Tao, S.; Wu, Z.; Dong, M.; Zheng, J.; et al. Whole Intestinal Microbiota Transplantation Is More Effective than Fecal Microbiota Transplantation in Reducing the Susceptibility of DSS-Induced Germ-Free Mice Colitis. Front. Immunol. 2023, 14, 1143526. [Google Scholar] [CrossRef]
- Marcelino, V.R.; Welsh, C.; Diener, C.; Gulliver, E.L.; Rutten, E.L.; Young, R.B.; Giles, E.M.; Gibbons, S.M.; Greening, C.; Forster, S.C. Disease-Specific Loss of Microbial Cross-Feeding Interactions in the Human Gut. Nat. Commun. 2023, 14, 6546. [Google Scholar] [CrossRef]
- Zhao, L.P.; Wu, J.; Quan, W.; Zhou, Y.; Hong, H.; Niu, G.Y.; Li, T.; Huang, S.B.; Qiao, C.M.; Zhao, W.J.; et al. DSS-Induced Colitis Activates the Kynurenine Pathway in Serum and Brain by Affecting IDO-1 and Gut Microbiota. Front. Immunol. 2023, 13, 1089200. [Google Scholar] [CrossRef]
- Limenitakis, J.; Oppenheim, R.D.; Creek, D.J.; Foth, B.J.; Barrett, M.P.; Soldati-Favre, D. The 2-Methylcitrate Cycle Is Implicated in the Detoxification of Propionate in Toxoplasma Gondii. Mol. Microbiol. 2013, 87, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, S.; Onishi, M.; Tatsuta, T.; Bartsch, T.; Cors, E.; Ried, K.; Lemke, K.; Nolte, H.; Giavalisco, P.; Langer, T. Mitochondria Regulate Intracellular Coenzyme Q Transport and Ferroptotic Resistance via STARD7. Nat. Cell Biol. 2023, 25, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Ewees, M.G.; Messiha, B.A.S.; Abo-Saif, A.A.; Abd El-Latif, H.A.E.T. Is Coenzyme Q10 Effective in Protection against Ulcerative Colitis? An Experimental Study in Rats. Biol. Pharm. Bull. 2016, 39, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free Radicals and Grape Seed Proanthocyanidin Extract: Importance in Human Health and Disease Prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Liu, S.H.; Lu, T.H.; Su, C.C.; Lay, I.S.; Lin, H.Y.; Fang, K.M.; Ho, T.J.; Chen, K.L.; Su, Y.C.; Chiang, W.C.; et al. Lotus Leaf (Nelumbo Nucifera) and Its Active Constituents Prevent Inflammatory Responses in Macrophages via JNK/NF-ΚB Signaling Pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef]
- Yum, H.W.; Zhong, X.; Park, J.; Na, H.K.; Kim, N.; Lee, H.S.; Surh, Y.J. Oligonol Inhibits Dextran Sulfate Sodium-Induced Colitis and Colonic Adenoma Formation in Mice. Antioxid. Redox Signal. 2013, 19, 102–114. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Dimeric Procyanidins Are Inhibitors of NF-ΚB–DNA Binding. Biochem. Pharmacol. 2009, 78, 1252–1262. [Google Scholar] [CrossRef]
- Koláček, M.; Muchová, J.; Dvořáková, M.; Paduchová, Z.; Žitňanová, I.; Čierna, I.; Országhová, Z.; Székyová, D.; Jajcaiová-Zedníčková, N.; Kovács, L.; et al. Effect of Natural Polyphenols (Pycnogenol) on Oxidative Stress Markers in Children Suffering from Crohn’s Disease—A Pilot Study. Free Radic. Res. 2013, 47, 624–634. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, J.; Wu, F.; Lu, X.; Huang, L.; Zhao, M. Expression of arginase I and inducible nitric oxide synthase in the peripheral blood and lymph nodes of HIV-positive patients. Mol. Med. Rep. 2016, 13, 731–743. [Google Scholar] [CrossRef]
- Garate-Carrillo, A.; Navarrete-Yañez, V.; Ortiz-Vilchis, P.; Guevara, G.; Castillo, C.; Mendoza-Lorenzo, P.; Ceballos, G.; Ortiz-Flores, M.; Najera, N.; Bustamante-Pozo, M.M.; et al. Arginase Inhibition by (−)-Epicatechin Reverses Endothelial Cell Aging. Eur. J. Pharmacol. 2020, 885, 173442. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, T.; Bjørndal, B.; Cacabelos, D.; Aasprong, O.G.; Omdal, R.; Svardal, A.; Bohov, P.; Pamplona, R.; Portero-Otin, M.; Berge, R.K.; et al. A Salmon Peptide Diet Alleviates Experimental Colitis as Compared with Fish Oil. J. Nutr. Sci. 2021, 2, e2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, W.; Zhou, X.; Pan, L.; Feng, Y.; Gao, P.; Ji, J.; Zhang, H.; Zhao, K.; Wang, C.; et al. C-X-C Motif Chemokine Ligand 1 Promotes Colitis by Modulating the Gut Microbiota. J. Innate Immun. 2023, 16, 33. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- Ohkawara, T.; Mitsuyama, K.; Takeda, H.; Asaka, M.; Fujiyama, Y.; Nishihira, J. Lack of Macrophage Migration Inhibitory Factor Suppresses Innate Immune Response in Murine Dextran Sulfate Sodium-Induced Colitis. Scand. J. Gastroenterol. 2008, 43, 1497–1504. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F. IL-10 and Macrophages Orchestrate Gut Homeostasis. Immunity 2014, 40, 637–639. [Google Scholar] [CrossRef]
- Ma, X.; Shin, Y.J.; Jang, H.M.; Joo, M.K.; Yoo, J.W.; Kim, D.H. Lactobacillus rhamnosus and Bifidobacterium longum Alleviate Colitis and Cognitive Impairment in Mice by Regulating IFN-γ to IL-10 and TNF-α to IL-10 Expression Ratios. Sci. Rep. 2021, 11, 20659. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; de Acosta, M.B.; Boberg, K.M.; Burisch, J.; Martine, D.V.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-Based Consensus on Extra-Intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Vincent Sarrazy, A.; Sore, S.; Viaud, M.; Guinamard, R.; Gautier, E.L.; Yvan-Charvet Correspondence, L. Maintenance of Macrophage Redox Status by ChREBP Limits Inflammation and Apoptosis and Protects against Advanced Atherosclerotic Lesion Formation. Cell Rep. 2015, 13, 132–144. [Google Scholar] [CrossRef]
- Zeng, K.; Tian, L.; Sirek, A.; Shao, W.; Liu, L.; Chiang, Y.T.; Chernoff, J.; Ng, D.S.; Weng, J.; Jin, T. Pak1 Mediates the Stimulatory Effect of Insulin and Curcumin on Hepatic ChREBP Expression. J. Mol. Cell Biol. 2017, 9, 384–394. [Google Scholar] [CrossRef]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of Reduced Microbial Diversity in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 6951091. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Zhang, H.; Pan, Y.; Jiang, Y.; Chen, M.; Ma, X.; Yu, X.; Ren, D.; Jiang, B. Akkermansia muciniphila ONE Effectively Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis in Mice. npj Sci. Food 2024, 8, 97. [Google Scholar] [CrossRef]
- Shon, H.J.; Kim, Y.M.; Kim, K.S.; Choi, J.O.; Cho, S.H.; An, S.; Park, S.H.; Cho, Y.J.; Park, J.H.; Seo, S.U.; et al. Protective Role of Colitis in Inflammatory Arthritis via Propionate-Producing Bacteroides in the Gut. Front. Immunol. 2023, 14, 1064900. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E. A Meta-Analysis of the Bovine Gastrointestinal Tract Microbiota. FEMS Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Medlock, G.L.; Carey, M.A.; McDuffie, D.G.; Mundy, M.B.; Giallourou, N.; Swann, J.R.; Kolling, G.L.; Papin, J.A. Inferring Metabolic Mechanisms of Interaction within a Defined Gut Microbiota. Cell Syst. 2018, 7, 245-257.e7. [Google Scholar] [CrossRef]
- Graf, J. The Family Rikenellaceae. In The Prokaryotes: Other Major Lineages of Bacteria and The Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 857–859. ISBN 978-3-642-38954-2. [Google Scholar]
- Cluis, C.P.; Pinel, D.; Martin, V.J. The Production of Coenzyme Q10 in Microorganisms. Subcell. Biochem. 2012, 64, 303–326. [Google Scholar] [CrossRef]
- Abby, S.S.; Kazemzadeh, K.; Vragniau, C.; Pelosi, L.; Pierrel, F. Advances in Bacterial Pathways for the Biosynthesis of Ubiquinone. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148259. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Coo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Dugas, L.R. Gut Microbiota and Fecal Short Chain Fatty Acids Differ with Adiposity and Country of Origin: The METS-Microbiome Study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef]
- Sun, D.; Chen, P.; Xi, Y.; Sheng, J. From Trash to Treasure: The Role of Bacterial Extracellular Vesicles in Gut Health and Disease. Front. Immunol. 2023, 14, 1274295. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial Extracellular Vesicles and Their Novel Therapeutic Applications in Health and Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef] [PubMed]
- Eraqi, W.A.; El-Sabbagh, W.A.; Aziz, R.K.; Elshahed, M.S.; Youssef, N.H.; Elkenawy, N.M. Gastroprotective and Microbiome-Modulating Effects of Ubiquinol in Rats with Radiation-Induced Enteropathy. Anim. Microbiome 2024, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.Y.; Shirokov, I.V.; Toshchakov, S.V.; Kozlova, A.D.; Obolenskaya, O.N.; Mariasina, S.S.; Ivlev, V.A.; Gartseev, I.B.; Medvedev, O.S. Effects of Coenzyme Q10 on the Biomarkers (Hydrogen, Methane, SCFA and TMA) and Composition of the Gut Microbiome in Rats. Pharmaceuticals 2023, 16, 686. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen Acts as a Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Gaskins, H.R. Contributions of the Microbial Hydrogen Economy to Colonic Homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 504–518. [Google Scholar] [CrossRef]
- Ohta, S. Direct Targets and Subsequent Pathways for Molecular Hydrogen to Exert Multiple Functions: Focusing on Interventions in Radical Reactions. Curr. Pharm. Des. 2021, 27, 595–609. [Google Scholar] [CrossRef]
- Mutuyemungu, E.; Singh, M.; Liu, S.; Rose, D.J. Intestinal Gas Production by the Gut Microbiota: A Review. J. Funct. Foods 2023, 100, 105367. [Google Scholar] [CrossRef]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota. mSystems 2017, 2, e00171-16. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.P.; Flannigan, K.L.; Agbor, T.A.; Beatty, J.K.; Blackler, R.W.; Workentine, M.L.; Da Silva, G.J.; Wang, R.; Buret, A.G.; Wallace, J.L. Hydrogen Sulfide Protects from Colitis and Restores Intestinal Microbiota Biofilm and Mucus Production. Inflamm. Bowel Dis. 2015, 21, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef]
| Time (min) | Weight (Da) | [M − H]− | Fragment Ions in Negative Mode | Structural Hypothesis | Biombalance™ ($) |
|---|---|---|---|---|---|
| 2.0 | 170 | 169 | 125 | Gallic acid * (a) | 1.64 ± 0.01 |
| 3.4 | 154 | 153 | 109 | Protocatechuic acid * (b) | 0.070 ± 0.001 |
| 4.6 | 578 | 577 | 425, 407, 289 | Procyanidin dimer B1 * (c) | 5.55 ± 0.02 |
| 4.9 | 578 | 577 | 425, 407, 289 | Procyanidin dimer B3 * (c) | 2.22 ± 0.03 |
| ok5.2 | 290 | 289 | 245, 205 | Catechin * (d) | 12.49 ± 0.03 |
| 5.4 | 866 | 865 | 577 | Procyanidin trimer ** (c) | 1.14 ± 0.02 |
| 5.5 | 578 | 577 | 425, 407, 289 | Procyanidin dimer ** (c) | 0.97 ± 0.03 |
| 5.8 | 578 | 577 | 425, 407, 289 | Procyanidin dimer B2 * (c) | 4.57 ± 0.04 |
| 6.1 | 290 | 289 | 245, 205 | Epicatechin * (e) | 8.52 ± 0.06 |
| 6.3 | 730 | 729 | 577, 559, 451, 289 | Procyanidin dimer gallate ** (c) | 1.80 ± 0.06 |
| 6.5 | 866 | 865 | 577, 289 | Procyanidin trimer C1 ** (c) | 1.28 ± 0.02 |
| 6.7 | 578 | 577 | 425, 407, 289 | Procyanidin dimer ** (c) | 0.88 ± 0.01 |
| 6.8 | 730 | 729 | 577, 559, 451, 289 | Procyanidin dimer gallate ** (c) | 3.86 ± 0.08 |
| 7.4 | 882 | 881 | 729, 577 | Procyanidin dimer digallate ** (c) | 1.02 ± 0.02 |
| 7.6 | 442 | 441 | 289 | Epicatechin gallate * (f) | 0.84 ± 0.02 |
| Retention Time (min) | Degree of Polymerization (DP) | Contents in mg/100 mg Eq. Epicatechin (n = 3 Repetitions) Biombalance™ |
|---|---|---|
| 7.0 | Monomers (DP1) | 23.39 ± 0.44 |
| 12.3 | Dimers (DP2) | 18.92 ± 0.28 |
| 15.8 | Trimers (DP3) | 6.1 ± 0.15 |
| 18.5 | Tetramers (DP4) | 3.04 ± 0.02 |
| 20.6 | Pentamers (DP5) | 1.05 ± 0.006 |
| 22.3 | Hexamers (DP6) | 0.46 ± 0.01 |
| 23.7 | Heptamers (DP7) | 0.09 ± 0.004 |
| 25.0 | Octamers (DP8) | <LQ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrani, M.; Saad, N.; Nardy, L.; Sifré, E.; Despres, J.; Brochot, A.; Varon, C.; Urdaci, M.C. Biombalance™, an Oligomeric Procyanidins-Enriched Grape Seed Extract, Prevents Inflammation and Microbiota Dysbiosis in a Mice Colitis Model. Antioxidants 2025, 14, 305. https://doi.org/10.3390/antiox14030305
Mokrani M, Saad N, Nardy L, Sifré E, Despres J, Brochot A, Varon C, Urdaci MC. Biombalance™, an Oligomeric Procyanidins-Enriched Grape Seed Extract, Prevents Inflammation and Microbiota Dysbiosis in a Mice Colitis Model. Antioxidants. 2025; 14(3):305. https://doi.org/10.3390/antiox14030305
Chicago/Turabian StyleMokrani, Mohamed, Naima Saad, Ludivine Nardy, Elodie Sifré, Julie Despres, Amandine Brochot, Christine Varon, and Maria C. Urdaci. 2025. "Biombalance™, an Oligomeric Procyanidins-Enriched Grape Seed Extract, Prevents Inflammation and Microbiota Dysbiosis in a Mice Colitis Model" Antioxidants 14, no. 3: 305. https://doi.org/10.3390/antiox14030305
APA StyleMokrani, M., Saad, N., Nardy, L., Sifré, E., Despres, J., Brochot, A., Varon, C., & Urdaci, M. C. (2025). Biombalance™, an Oligomeric Procyanidins-Enriched Grape Seed Extract, Prevents Inflammation and Microbiota Dysbiosis in a Mice Colitis Model. Antioxidants, 14(3), 305. https://doi.org/10.3390/antiox14030305






