A Proprietary Punica granatum pericarp Extract, Its Antioxidant Properties Using Multi-Radical Assays and Protection Against UVA-Induced Damages in a Reconstructed Human Skin Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material, Solvents, Chemicals and Standards
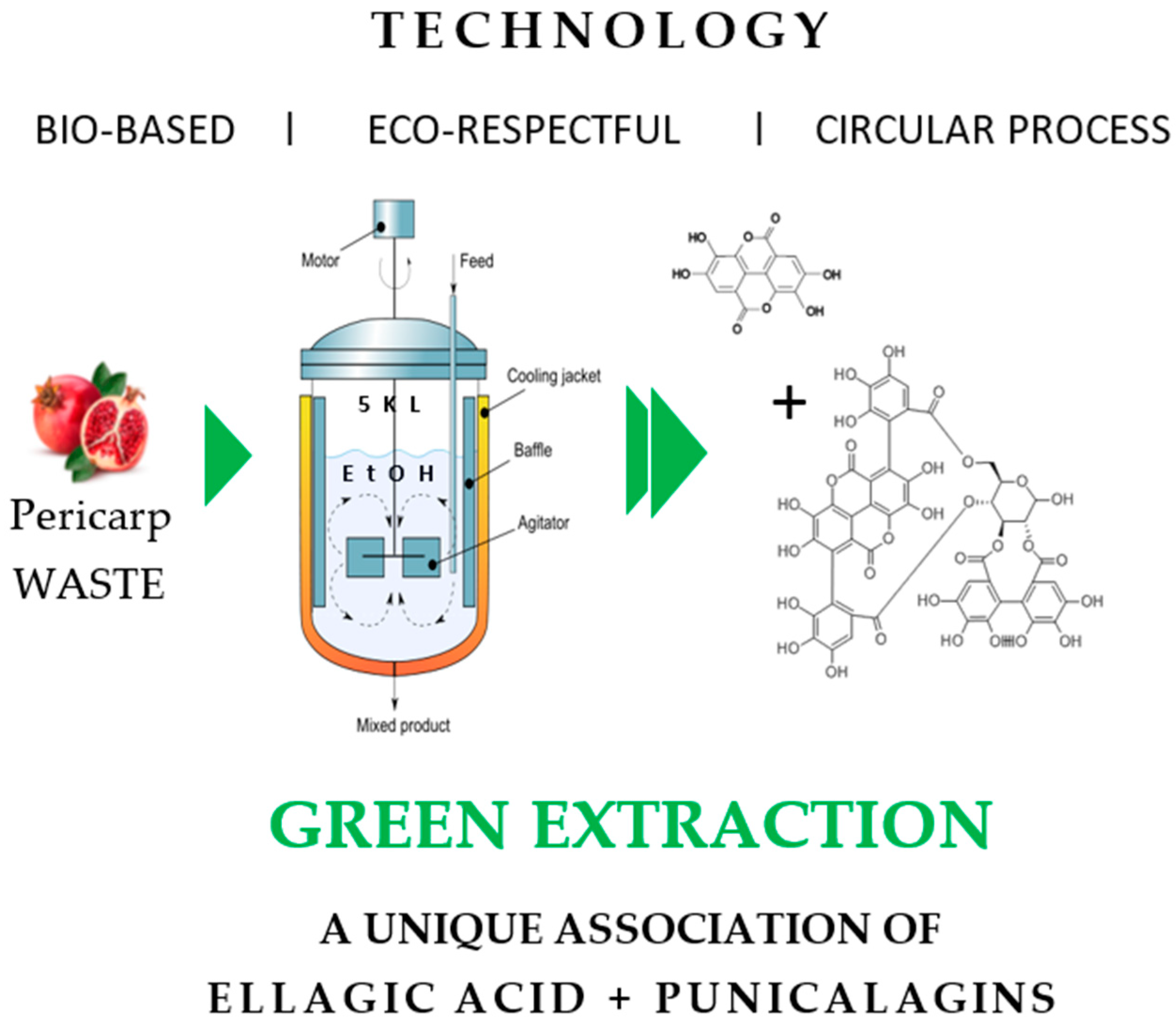
2.2. Extraction Punica granatum pericarp Using One Pot Green Extraction Process
2.3. In Tubo Antioxidant Assays
2.3.1. Oxygen Radical Absorbance Capacity (ORAC)
2.3.2. Superoxide (O2•–) Anion Scavenging Assay (SORAC)
2.3.3. Hydroxyl (HO•) Radical Scavenging Capacity (HORAC)
2.3.4. Singlet Oxygen (1O2) Scavenging Capacity (SOAC)
2.4. Lipid Peroxidation Assay on Human Dermal Fibroblasts
2.4.1. Cell Culture
2.4.2. Cell Viability Assay
2.4.3. 8-Isoprostane Release by Human Dermal Fibroblasts
2.5. Protection Against UVA1-Induced Damage on Reconstructed Full Thickness T-SkinTM Model
- In vitro skin model: T-SkinTM tissue model production
- 2.
- Preliminary MTT assay
- 3.
- Long UVA photoprotection test on T-SkinTM model:
- Treatment:
- Histology analysis and fibroblast counting:
- MMP-1 and cytokines dosing:
2.5.1. MMP-1 Protein Release
2.5.2. Pro-Inflammatory Mediators Quantification (IL-1a, IL-1ra, IL-6, IL-8, GM-CSF, and TNF-α) Using a Bio-Plex200
3. Results
3.1. In Vitro Antioxidant Assays
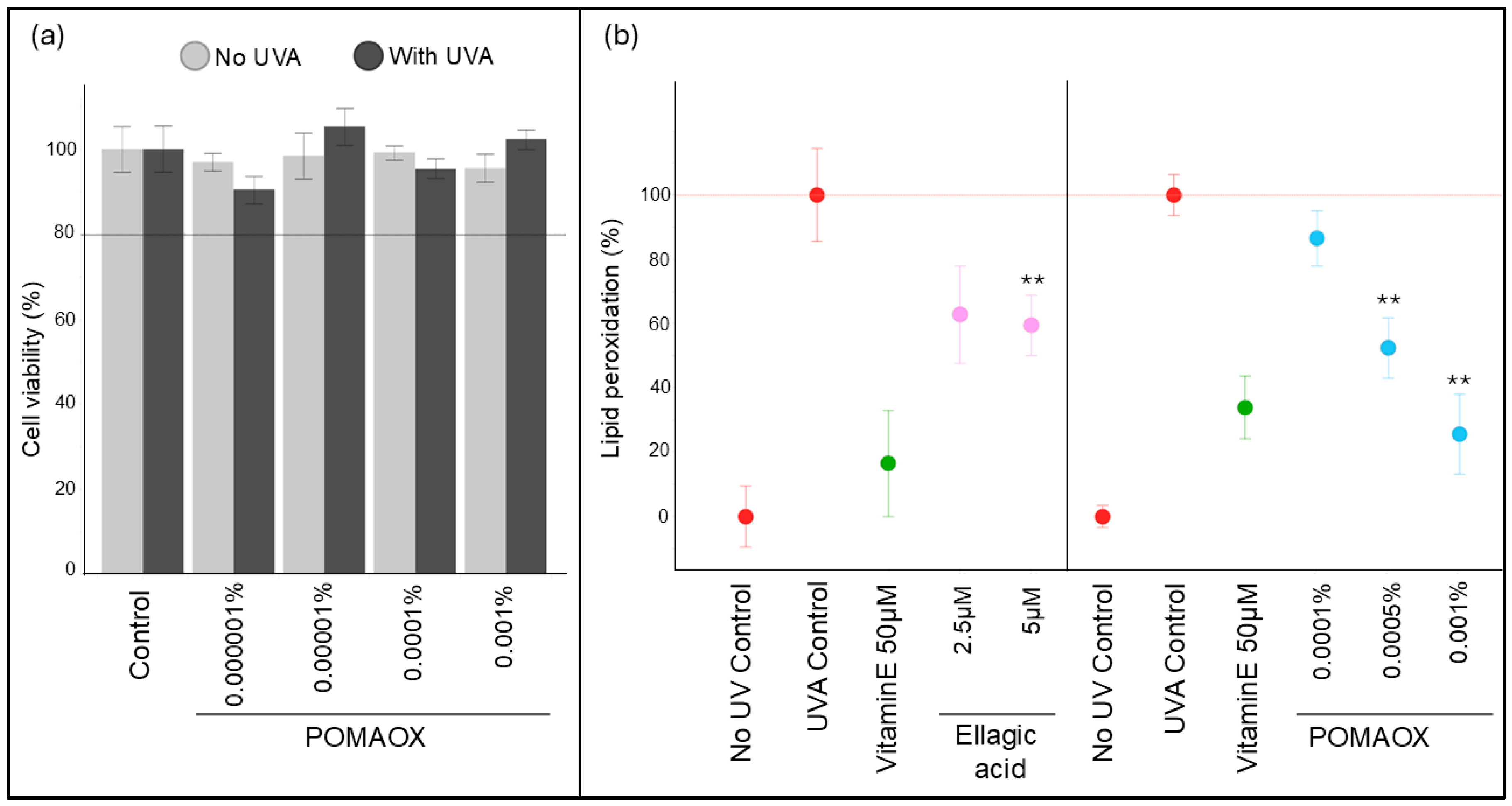
3.2. Protection Against UVA-Induced Lipid Peroxidation in Human Dermal Fibroblasts
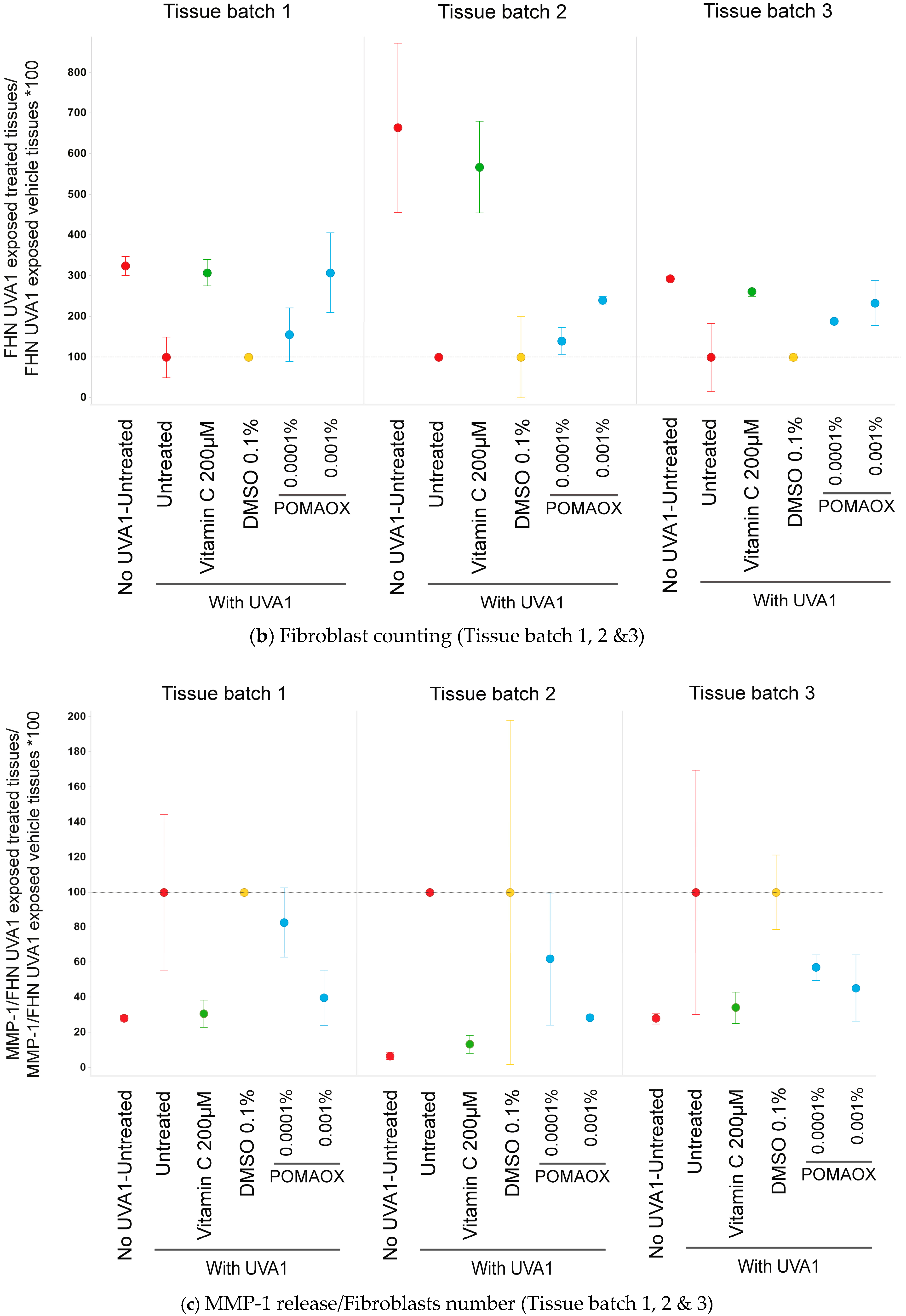
3.3. Protection Against UVA1-Induced Damage on Reconstructed T-SkinTM Model
- Preliminary MTT test
- Histology analysis
- MMP-1 and cytokines quantification
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef] [PubMed]
- Skarupova, D.; Vostalova, J.; Rajnochova Svobodova, A. Ultraviolet A protective potential of plant extracts and phytochemicals. Biomed. Pap. 2020, 164, 1–22. [Google Scholar] [CrossRef]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Pierrard, C.; Golebiewski, C.; Bernerd, F. Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS ONE 2014, 20, e105263. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Horticul. Rev. 2009, 35, 127–191. [Google Scholar]
- Dar, M.; Shrestha, R.; Cochard, R. Plant resource utilization by local inhabitants around Machiara National Park, District. J. Food Agric. Environ. 2012, 10, 1139–1148. [Google Scholar]
- Montes de Oca, M.K.; Pearlman, R.L.; McClees, S.F.; Strickland, R.; Afaq, F. Phytochemicals for the Prevention of Photocarcinogenesis. Photochem. Photobiol. 2017, 93, 956–974. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Park, S.J.; Song, C.H.; Park, J.H.; Lee, Y.J.; Kwang, S. Inhibitory effects of pomegranate concentrated solution on the activities of hyaluronidase, tyrosinase, and metalloproteinase. J. Cosmet. Sci. 2015, 66, 145–159. [Google Scholar] [CrossRef]
- Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Afaq, F.; Mukhtar, H. Photochemopreventive effect of pomegranate fruit extract on UVA-mediated activation of cellular pathways in normal human epidermal keratinocytes. Photochem. Photobiol. 2006, 82, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S. The Role of Topical Antioxidants in Photoprotection; Springer: Greer, SC, USA, 2016; pp. 361–375. [Google Scholar]
- Clementi, M.E.; Sampaolese, B.; Sciandra, F.; Tringali, G. Punicalagin Protects Human Retinal Pigment Epithelium Cells from Ultraviolet Radiation-Induced Oxidative Damage by Activating Nrf2/HO-1 Signaling Pathway and Reducing Apoptosis. Antioxidants 2020, 2, 473. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, M.S.; Kawakami, C.M.; Pereira, K.C.; Benevenuto, C.G.; Gaspar, L.R. Effects of UV-filter photostabilizers in the photostability and phototoxicity of vitamin A palmitate combined with avobenzone and octyl methoxycinnamate. Photochem. Photobiol. 2021, 97, 700–709. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action. J. AOAC Intern. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Kondo, M.; Fan, E.; Ji, H.; Kou, Y.; Ou, B. Novel high-throughput assay for antioxidant capacity against superoxide anion. J. Agric. Food Chem. 2009, 57, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsh-woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Ou, B.; Zhang, L.; Kondo, M.; Ji, H.; Kou, Y. Method for Assaying the Antioxidant Capacity of a Skin Care Product. US8198091B2, 12 June 2012. [Google Scholar]
- Kazuo, M. Antioxidant Activity of Foods: Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Nutr. Sci. Vitaminol. 2019, 65, 285–302. [Google Scholar]
- Andrés, C.M.C.; Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Lelièvre, D.; Canivet, F.; Thillou, F.; Tricaud, C.; Le Floc’h, C.; Bernerd, F. Use of reconstructed skin model to assess the photoprotection afforded by three sunscreen products having different SPF values against DNA lesions and cellular alterations. J. Photochem. Photobiol. 2024, 19, 100213. [Google Scholar] [CrossRef]
- Bataillon, M.; Lelievre, D.; Chapuis, A. Characterization of a New Reconstructed Full Thickness Skin Model, T-Skin™, and its Application for Investigations of Anti-Aging Compounds. Int. J. Mol. Sci. 2019, 20, 2240. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Nouveau, S.; Hourblin, V.; Pillai, K.; Manco, M.; Bastien, P.; Tran, C.; Tricaud, C.; de Lacharrière, O.; Bernerd, F. UVA1-Induced Skin Darkening Is Associated with Molecular Changes Even in Highly Pigmented Skin Individuals. J. Investig. Dermatol. 2017, 137, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tuuli, M.G.; Longtine, M.S.; Shin, J.S.; Lawrence, R.; Inder, T.; Michael Nelson, D. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1142–E1152. [Google Scholar] [CrossRef] [PubMed]
- Zeghad, N.; Ahmed, E.; Khan, M.Z.; Belkhiri, A. Exploring the potential use of pomegranate (Punica granatum L.) and prickly pear (Opuntia ficus indica L.) peels as sources of cosmeceutical sunscreen agent for their antioxidant and photoprotective properties. Pharm. Sci. Asia 2023, 50, 273–280. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Model Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Shin, D.; Habibi, F.; Kim, J.; Sarkhosh, A. Profiling phenolic compounds in juice and peel of fourteen pomegranate (Punica granatum L.) varieties grown in Florida, USA. Food Chem. Adv. 2023, 2, 100225. [Google Scholar] [CrossRef]
- Afaq, F.; Abu Zaid, M.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chou, C.W.; Kumar, K.J.S.; Fu, K.T.; Wang, H.M.; Hsu, L.S.; Kuo, Y.H.; Wu, C.R.; Chen, S.C.; Yang, H.L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermat. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Prior, R.L.; Sintara, M.; Chang, T. Multi-radical (ORAC MR5) antioxidant capacity of selected berries and effects of food processing. J. Berry Res. 2016, 6, 159–173. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) Phenolics Ameliorate Hydrogen Peroxide-Induced Oxidative Stress and Cytotoxicity in Human Keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxid. Med. Cell Longev. 2020, 28, 5194508. [Google Scholar] [CrossRef]
- Kossodo, S.; Wong, W.R.; Simon, G.; Kochevar, I.E. Effects of UVR and UVR-induced cytokines on production of extracellular matrix proteins and proteases by dermal fibroblasts cultured in collagen gels. Photochem. Photobiol. 2004, 79, 86–93. [Google Scholar] [PubMed]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. Eur. J. Dermatol. 2008, 18, 128–135. [Google Scholar]
- Fagot, D.; Asselineau, D.; Bernerd, F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem. Photobiol. 2004, 79, 499–505. [Google Scholar]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Mizutani, T.; Okano, Y.; Masaki, H. The impact of carbonylated proteins on the skin and potential agents to block their effects. Exp. Dermatol. 2019, 28, 32–37. [Google Scholar] [CrossRef]
- Berneburg, M.; Gremmel, T.; Kürten, V.; Schroeder, P.; Hertel, I.; Von Mikecz, A.; Wild, S.; Chen, M.; Declercq, L.; Matsui, M.; et al. Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J. Investig. Dermatol. 2005, 125, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 23, 63. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Hossain, M.E.; Tumpa, A.A.; Abrar, A.G.M.; Ahmed, L.; Rauf, A.; Khalil, A.A.; Al Abdul monem, W.; et al. Pomegranate-specific natural compounds as onco-preventive and onco-therapeutic compounds: Comparison with conventional drugs acting on the same molecular mechanisms. Heliyon 2023, 8, E18090. [Google Scholar] [CrossRef]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef] [PubMed]
- Asselineau, D.; Bernard, B.A.; Bailly, C.; Darmon, M. Epidermal morphogenesis and induction of 67kD keratin polypeptide by culture at the liquid-air interface. Exp. Cell Res. 1985, 159, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Asselineau, D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997, 183, 123–138. [Google Scholar] [CrossRef]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.S. Syringaresinol Inhibits UVA-Induced MMP-1 Expression by Suppression of MAPK/AP-1 Signaling in HaCaT Keratinocytes and Human Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 1, 3981. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Bio. 2017, 996, 15–23. [Google Scholar]
- Watanabe, H.; Shimizu, T.; Nishihira, J.; Abe, R.; Nakayama, T.; Taniguchi, M.; Sabe, H.; Ishibashi, T.; Shimizu, H. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J. Biol. Chem. 2004, 279, 1676–1683. [Google Scholar] [CrossRef]
- Wenk, J.; Brenneisen, P.; Wlaschek, M.; Poswig, A.; Briviba, K.; Oberley, T.D.; Scharffetter-Kochanek, K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix degrading metalloprotease-1. J. Biol. Chem. 1999, 274, 25869–25876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Dai, H.; Wang, R.; Hsiao, A.; Wang, W.; Betts, R.J.; Marionnet, C.; Bernerd, F.; Qiu, J. Photo-aging evaluation—In vitro biological endpoints combined with collagen density assessment with multi-photon microscopy. J. Dermatol. Sci. 2022, 105, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J. Investig. Dermatol. 2011, 131, 1005–1007. [Google Scholar] [CrossRef]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 20, 6414–6435. [Google Scholar] [CrossRef] [PubMed]


| Test Compounds | Antioxidant Capacity | ||||
|---|---|---|---|---|---|
| ORAC μg (TE/g) | SOAC at 50% Inhibition (ppm) | SORAC (U/mg) | NORAC (ppm) | HORAC (µg/g) | |
| Radical | ROO. | 1O2. | -O2. | -NOO. | HO. |
| Reference | TROLOX | Vitamin E | SOD Enzyme | Vitamin C | Gallic Acid |
| POMAOX | NA | 220 | 500 | 1–3 | 8325 |
| Vitamin C | 1.05 | 0.66 | 7.99 | 1.0 | 0.02 |
| Test Compound | MTT and Treatment Concentrations | Vehicle | Solubility | Cytotoxicity (Viability + Morphology) |
|---|---|---|---|---|
| POMAOX | 0.0001–0.001–0.005% | DMSO 1/1000 | Good | At 0.005% |
| Endpoint | Untreated | Vitamin C 200 μM | POMAOX (PUNICA GRANATUM EXTRACT) | |
|---|---|---|---|---|
| 0.0001% | 0.001% | |||
| FHN | 100 +/− 49 | 379 +/− 157 | 161 +/− 40 | 260 +/− 63 |
| MMP-1 | 100 +/− 41 | 26 +/− 12 | 67 +/− 23 | 38 +/− 14 |
| IL-1a | 100 +/− 55 | 16 +/− 7 | 26 +/− 10 | 16 +/− 9 |
| IL-1ra | 100 +/− 58 | 21 +/− 6 | 27 +/− 11 | 21 +/− 13 |
| IL-6 | 100 +/− 28 | 38 +/− 27 | 77 +/− 36 | 35 +/− 18 |
| IL-8 | 100 +/− 36 | 26 +/− 14 | 67 +/− 30 | 29 +/− 16 |
| GM-CSF | 100 +/− 51 | 19 +/− 14 | 28 +/− 18 | 24 +/− 10 |
| Parameters | Values in % w/w | Method |
|---|---|---|
| Punicalagins | 14.44% | HPLC-UV quantification with Reference standards |
| Ellagic acid | 14.12% | |
| Punicalins | 3.28% | |
| Total polyphenols (punicalins, punicalagins and ellagic acid, gallic acid, epi-catechin gallate, gallagic acid, ellagic acid hexoside/glycoside) | 42% | Folin-Ciocalteu method |
| Total fats | 5.6% | Acid hydrolysis method |
| Total protein | 0.73% | Kjeldahl Method |
| Total sugars | 19.1% | Phenol-sulfuric acid method |
| Total saponins | 7.6% | Gravimetric method |
| Moisture | 6.5% | Thermogravimetric approach (Loss on Drying) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannakal, S.T.; Durand, S.; Gizard, J.; Sextius, P.; Planel, E.; Warrick, E.; Lelievre, D.; Lelievre, C.; Eilstein, J.; Beaumard, F.; et al. A Proprietary Punica granatum pericarp Extract, Its Antioxidant Properties Using Multi-Radical Assays and Protection Against UVA-Induced Damages in a Reconstructed Human Skin Model. Antioxidants 2025, 14, 301. https://doi.org/10.3390/antiox14030301
Pannakal ST, Durand S, Gizard J, Sextius P, Planel E, Warrick E, Lelievre D, Lelievre C, Eilstein J, Beaumard F, et al. A Proprietary Punica granatum pericarp Extract, Its Antioxidant Properties Using Multi-Radical Assays and Protection Against UVA-Induced Damages in a Reconstructed Human Skin Model. Antioxidants. 2025; 14(3):301. https://doi.org/10.3390/antiox14030301
Chicago/Turabian StylePannakal, Steve Thomas, Steven Durand, Julie Gizard, Peggy Sextius, Emilie Planel, Emilie Warrick, Damien Lelievre, Celine Lelievre, Joan Eilstein, Floriane Beaumard, and et al. 2025. "A Proprietary Punica granatum pericarp Extract, Its Antioxidant Properties Using Multi-Radical Assays and Protection Against UVA-Induced Damages in a Reconstructed Human Skin Model" Antioxidants 14, no. 3: 301. https://doi.org/10.3390/antiox14030301
APA StylePannakal, S. T., Durand, S., Gizard, J., Sextius, P., Planel, E., Warrick, E., Lelievre, D., Lelievre, C., Eilstein, J., Beaumard, F., Prasad, A., Shetty, S., Duraisamy, A., Gaurav, K., John, S., Benazzouz, A., Fastinger, X., Roy, D., & Sharma, V. (2025). A Proprietary Punica granatum pericarp Extract, Its Antioxidant Properties Using Multi-Radical Assays and Protection Against UVA-Induced Damages in a Reconstructed Human Skin Model. Antioxidants, 14(3), 301. https://doi.org/10.3390/antiox14030301







