Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vegetable Oil Samples
2.3. Preparation of Oil Blends and Storage Conditions
2.4. Determination of Peroxide Value (PV)
2.5. p-Anisidine Value (AV)
2.6. Conjugated Dienes and Trienes
2.7. EPR Spin Trapping Analysis of Oil Blends: EPR Settings and Spectra Acquisition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of Myrtle Seed Oil (MSO)
3.2. Peroxide Value (PV)
3.3. Dienes and Trienes
3.4. p-Anisidine Value (AV)
3.5. Totox Value (TV)
3.6. Kinetic Evolution of the PBN Adduct Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Vegetable Oils: Global Consumption 2013/14 to 2023/24, by Oil Type. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 5 November 2024).
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Dairi, S.; Galeano-Díaz, T.; Acedo-Valenzuela, M.I.; Godoy-Caballero, M.P.; Dahmoune, F.; Remini, H.; Madani, K. Monitoring oxidative stability and phenolic compounds composition of myrtle-enriched extra virgin olive during heating treatment by flame, oven and microwave using reversed phase dispersive liquid–liquid microextraction (RP-DLLME)-HPLC-DAD-FLD method. Ind. Crops Prod. 2015, 65, 303–314. [Google Scholar] [CrossRef]
- Malheiro, R.; Rodrigues, N.; Manzke, G.; Bento, A.; Pereira, J.A.; Casal, S. The use of olive leaves and tea extracts as effective antioxidants against the oxidation of soybean oil under microwave heating. Ind. Crops Prod. 2013, 44, 37–43. [Google Scholar] [CrossRef]
- Metzner Ungureanu, C.-R.; Poiana, M.-A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to Improve the Thermo-Oxidative Stability of Sunflower Oil by Exploiting the Antioxidant Potential of Blueberries Processing Byproducts. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Smetanska, I.; Ramadan, M.F.; Sarhan, M.A.; Mahmoud, A. Antioxidant potential of sesame (Sesamum indicum) cake extract in stabilization of sunflower and soybean oils. Ind. Crops Prod. 2011, 34, 952–959. [Google Scholar] [CrossRef]
- Odeh, D.; Kraljić, K.; Benussi Skukan, A.; Škevin, D. Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants 2021, 10, 785. [Google Scholar] [CrossRef]
- Poiana, M.-A. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. Int. J. Mol. Sci. 2012, 13, 9240–9259. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef]
- Romanić, R.S.; Lužaić, T.Z.; Radić, B.Đ. Enriched sunflower oil with omega 3 fatty acids from flaxseed oil: Prediction of the nutritive characteristics. LWT 2021, 150, 112064. [Google Scholar] [CrossRef]
- Lakhlifi El Idrissi, Z.; El Guezzane, C.; Boujemaa, I.; El Bernoussi, S.; Sifou, A.; El Moudden, H.; Ullah, R.; Bari, A.; Goh, K.W.; Goh, B.H.; et al. Blending cold-pressed peanut oil with omega-3 fatty acids from walnut oil: Analytical profiling and prediction of nutritive attributes and oxidative stability. Food Chem. X 2024, 22, 101453. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.-T.; Soltani, A.; Hosseini, S.M.H.; Keramat, M. Improving the oxidation kinetics of linseed oil using the blending approach. J. Food Process. Preserv. 2020, 44, e14964. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Blending of Sunflower Oil with Pomegranate Seed Oil from Blanched Seeds: Impact on Functionality, Oxidative Stability, and Antioxidant Properties. Processes 2021, 9, 635. [Google Scholar] [CrossRef]
- Fadda, A.; Montoro, P.; D’Urso, G.; Ravasio, N.; Zaccheria, F.; Sanna, D. Sustainable Extraction Methods Affect Metabolomics and Oxidative Stability of Myrtle Seed Oils Obtained from Myrtle Liqueur By-Products: An Electron Paramagnetic Resonance and Mass Spectrometry Approach. Antioxidants 2023, 12, 154. [Google Scholar] [CrossRef]
- Fadda, A.; Molinu, M.G.; Deiana, P.; Sanna, D. Electron Paramagnetic Resonance Spin Trapping of Sunflower and Olive Oils Subjected to Thermal Treatment: Optimization of Experimental and Fitting Parameters. ACS Food Sci. Technol. 2021, 1, 1294–1303. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Pérez-Gálvez, A.; Mínguez-Mosquera, M.I. A rapid spectrophotometric method for the determination of peroxide value in food lipids with high carotenoid content. J. Am. Oil Chem. Soc. 2001, 78, 1151–1155. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Cd 18-90 p-Anisidine Value. In Official Methods and Recommended Practices of the AOCS, 7th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- International Olive Council. Spectrophotometric Investigation in the Ultraviolet, Method COI/T.20/Doc. No 19/Rev. 5/2019; International Olive Council: Madrid, Spain, 2019. [Google Scholar]
- CXS 210-1999; Standard for Named Vegetable Oils. FAO: Rome, Italy, 2019.
- Talbot, G. 16—The Stability and Shelf Life of Fats and Oils. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 461–503. [Google Scholar]
- Morales, A.; Dobarganes, C.; Márquez-Ruiz, G.; Velasco, J. Quantitation of Hydroperoxy-, Keto- and Hydroxy-Dienes During Oxidation of FAMEs from High-Linoleic and High-Oleic Sunflower Oils. J. Am. Oil Chem. Soc. 2010, 87, 1271–1279. [Google Scholar] [CrossRef]
- Morales, A.; Marmesat, S.; Dobarganes, M.C.; Márquez-Ruiz, G.; Velasco, J. Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vegetable oils. J. Chromatogr. A 2012, 1229, 190–197. [Google Scholar] [CrossRef]
- Khabbaz, E.S.; Jooyandeh, M.; Jaldani, S.; Farhoosh, R. Cut-off conjugated diene values for rejection of vegetable oils. LWT 2024, 192, 115712. [Google Scholar] [CrossRef]
- Marques, L.; Espinosa, M.H.; Andrews, W.; Foster Ii, R.T. Advancing Flavor Stability Improvements in Different Beer Types Using Novel Electron Paramagnetic Resonance Area and Forced Beer Aging Methods. J. Am. Soc. Brew. Chem. 2017, 75, 35–40. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; Andersen, M.L.; Guadix, E.M.; García-Moreno, P.J. Oxidative stability and oxygen permeability of oil-loaded capsules produced by spray-drying or electrospraying measured by electron spin resonance. Food Chem. 2024, 430, 136894. [Google Scholar] [CrossRef] [PubMed]
- Falch, E.; Velasco, J.; Aursand, M.; Andersen, M.L. Detection of radical development by ESR spectroscopy techniques for assessment of oxidative susceptibility of fish oils. Eur. Food Res. Technol. 2005, 221, 667–674. [Google Scholar] [CrossRef]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).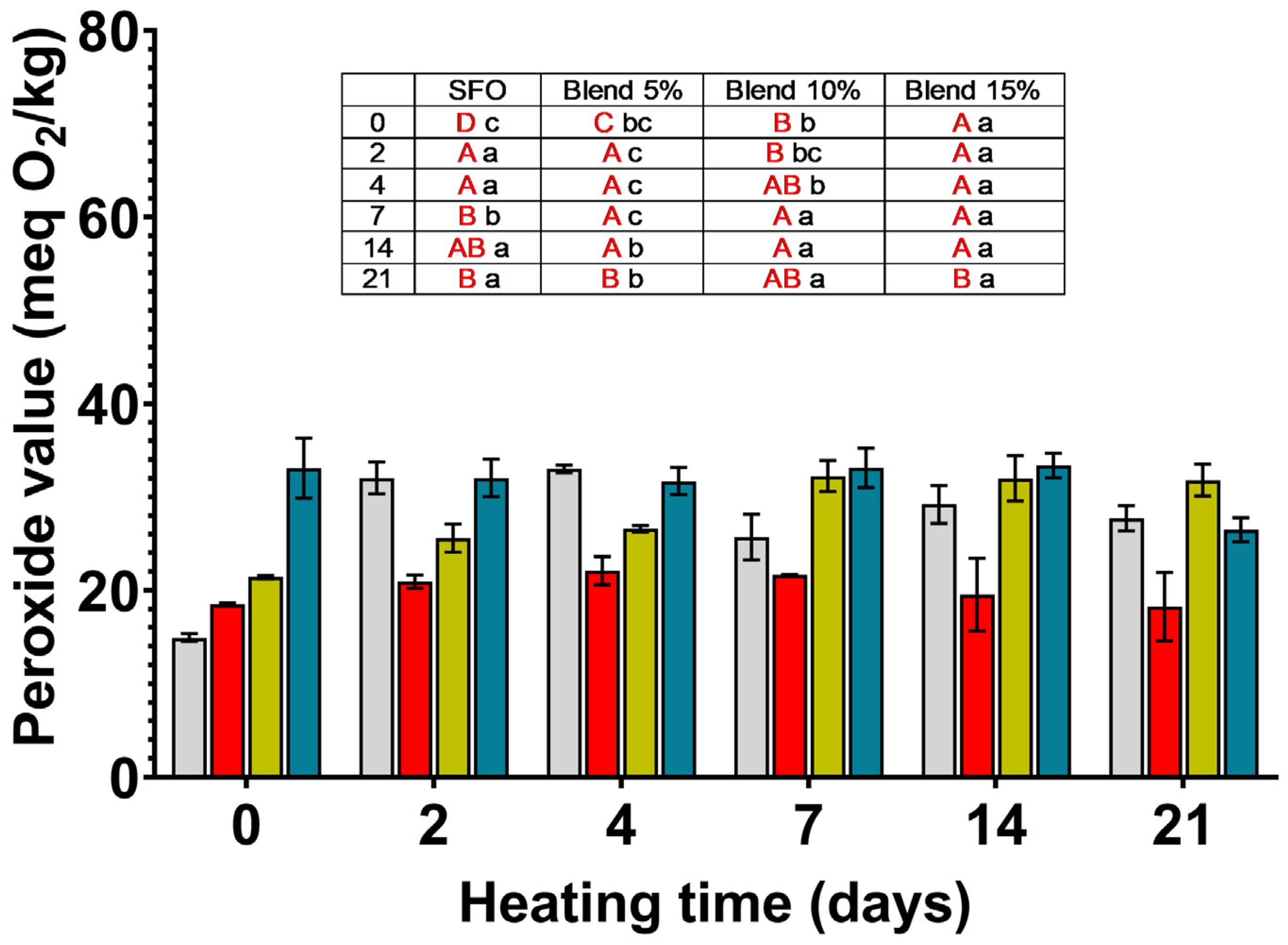
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).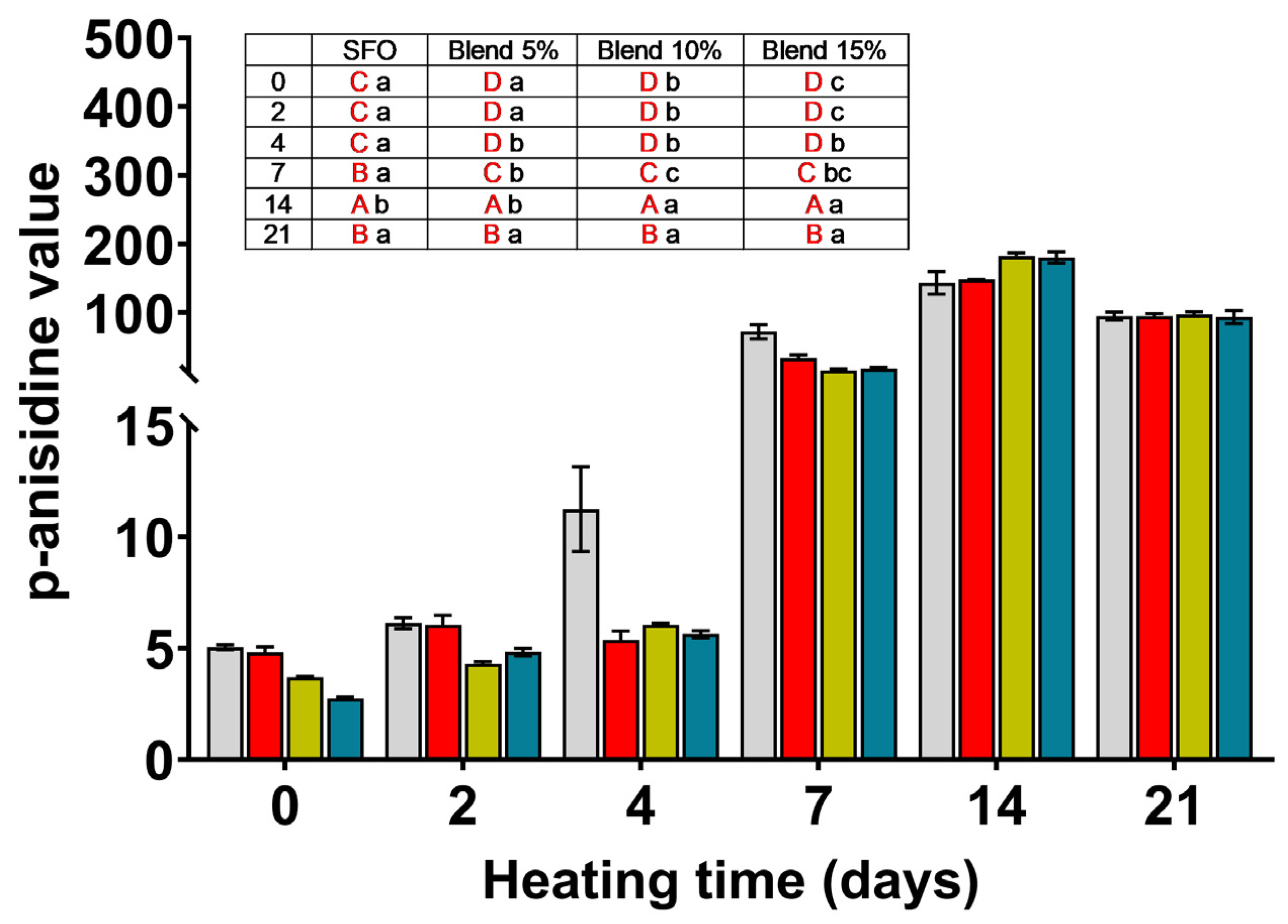
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).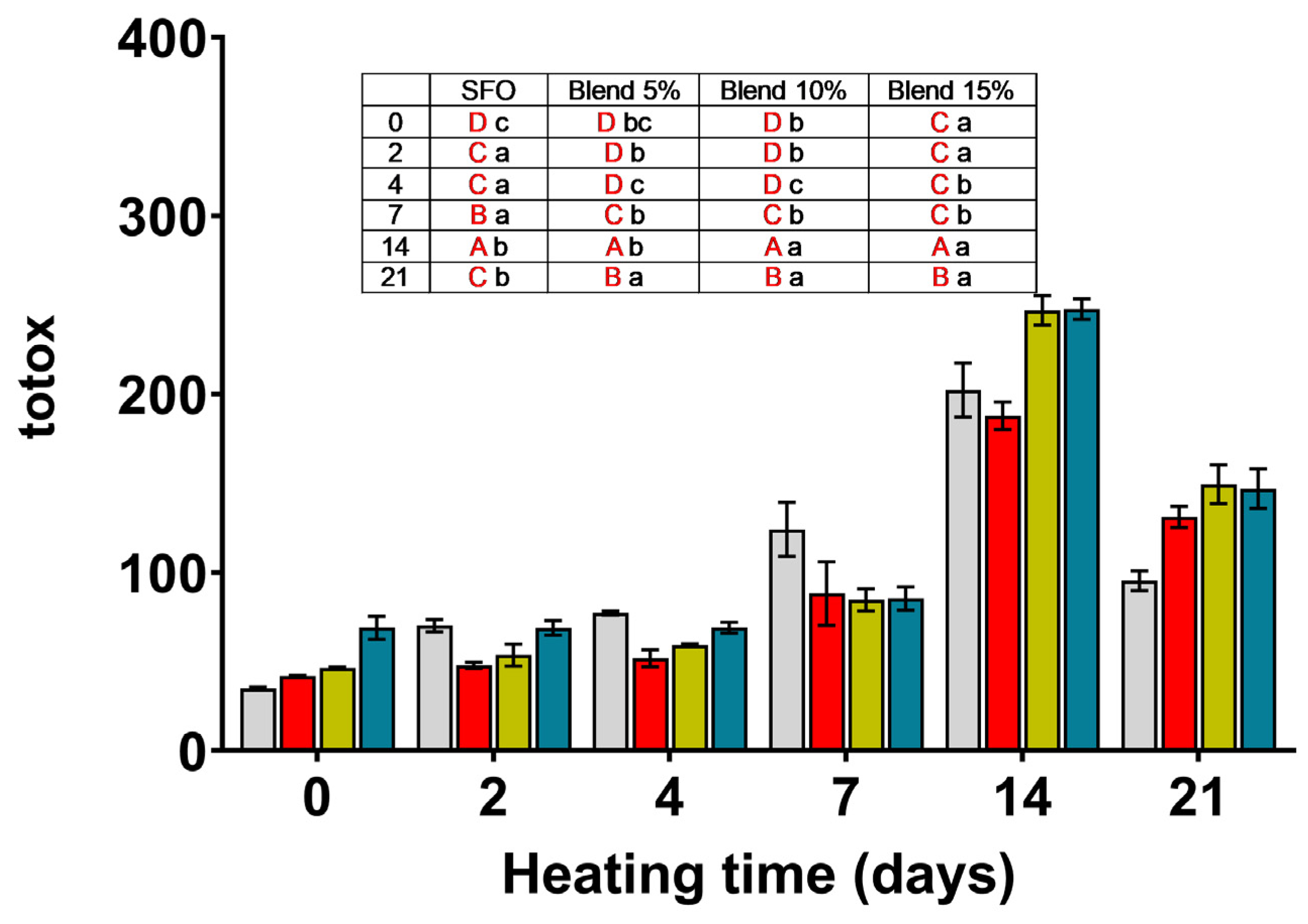
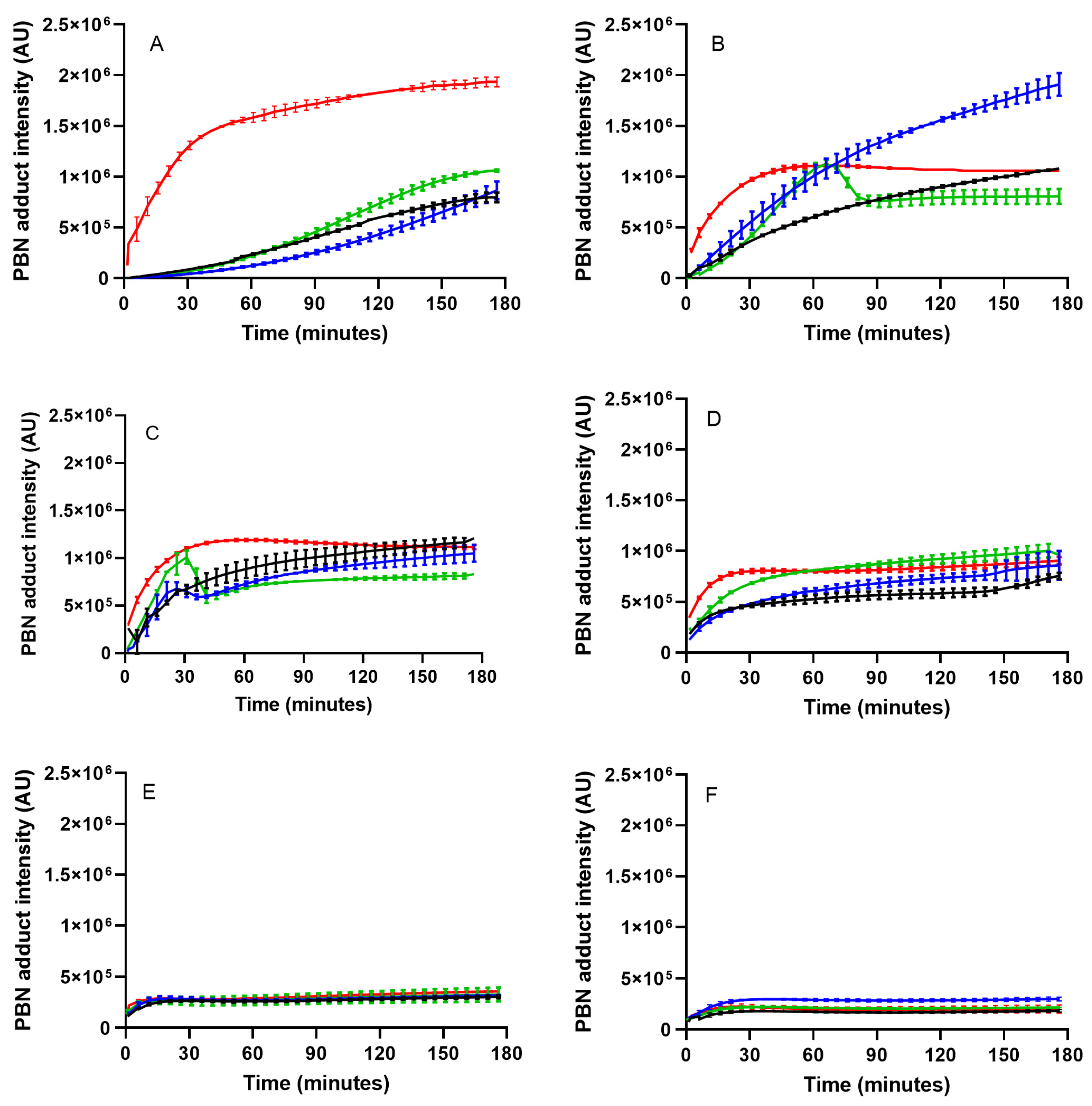
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
 SFO;
SFO;  blend 5%;
blend 5%;  blend 10%;
blend 10%;  blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).
blend 15%. In the inset table: capital letters in red deal with the comparison between times inside the same blend; lowercase letters deal with the comparison between blends inside the same storage time. Mean differences were calculated according to Tukey’s test (p ≤ 0.05).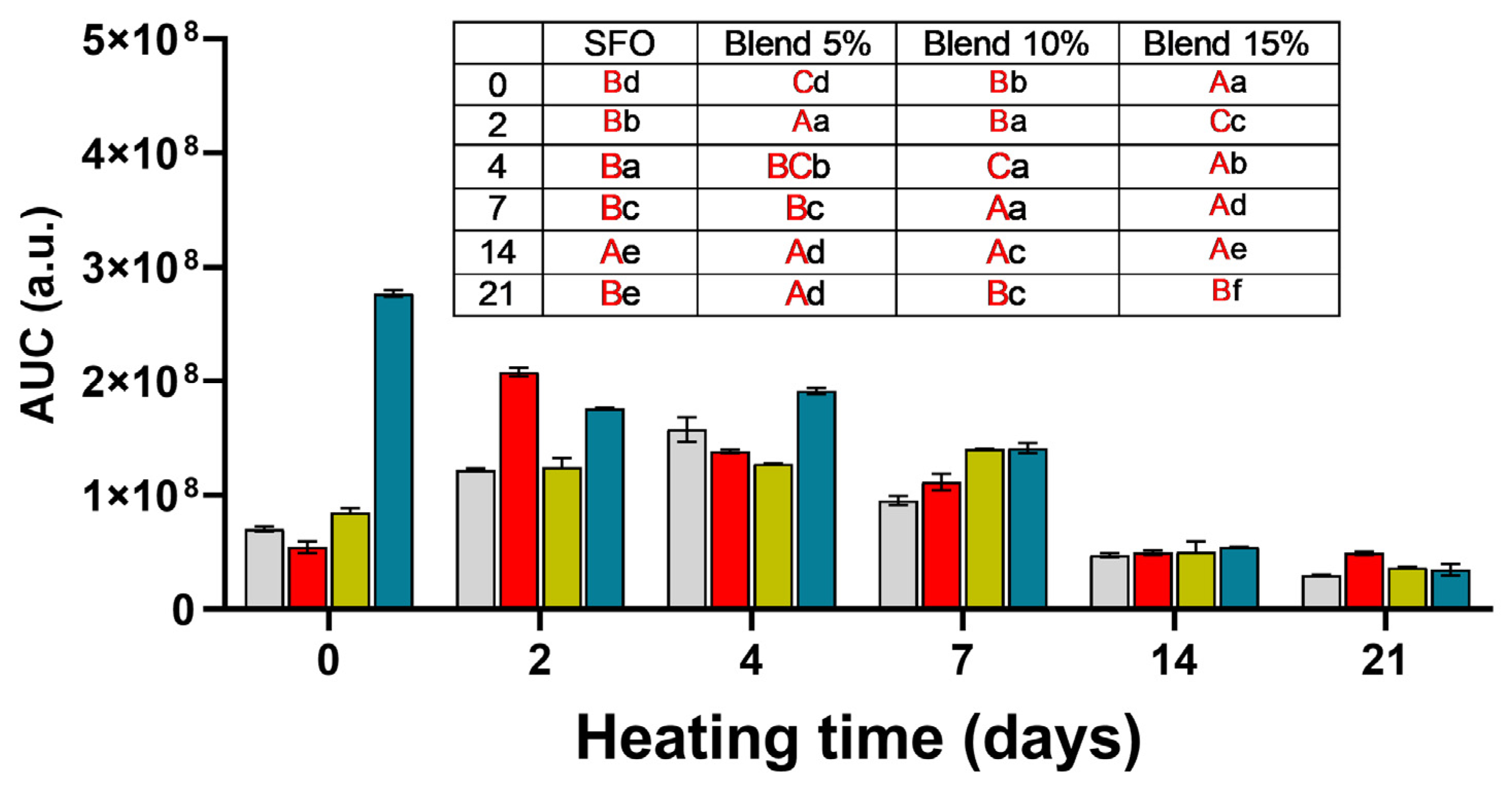
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, D.; Fadda, A. Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product. Antioxidants 2025, 14, 300. https://doi.org/10.3390/antiox14030300
Sanna D, Fadda A. Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product. Antioxidants. 2025; 14(3):300. https://doi.org/10.3390/antiox14030300
Chicago/Turabian StyleSanna, Daniele, and Angela Fadda. 2025. "Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product" Antioxidants 14, no. 3: 300. https://doi.org/10.3390/antiox14030300
APA StyleSanna, D., & Fadda, A. (2025). Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product. Antioxidants, 14(3), 300. https://doi.org/10.3390/antiox14030300






