Abstract
Excessive alcohol consumption significantly impacts human health, particularly the brain, due to its susceptibility to oxidative stress, which contributes to neurodegenerative conditions. Alcohol metabolism in the brain occurs primarily via catalase, followed by CYP2E1 pathways. Excess alcohol metabolized by CYP2E1 generates reactive oxygen/nitrogen species (ROS/RNS), leading to cell injury via altering many different pathways. Elevated oxidative stress impairs autophagic processes, increasing post-translational modifications and further exacerbating mitochondrial dysfunction and ER stress, leading to cell death. The literature highlights that alcohol-induced oxidative stress disrupts autophagy and mitophagy, contributing to neuronal damage. Key mechanisms include mitochondrial dysfunction, ER stress, epigenetics, and the accumulation of oxidatively modified proteins, which lead to neuroinflammation and impaired cellular quality control. These processes are exacerbated by chronic alcohol exposure, resulting in the suppression of protective pathways like NRF2-mediated antioxidant responses and increased susceptibility to neurodegenerative changes in the brain. Alcohol-mediated neurotoxicity involves complex interactions between alcohol metabolism, oxidative stress, and autophagy regulation, which are influenced by various factors such as drinking patterns, nutritional status, and genetic/environmental factors, highlighting the need for further molecular studies to unravel these mechanisms and develop targeted interventions.
1. Introduction
Excessive alcohol (ethanol) consumption causes severe consequences for human health, usually negatively affecting multiple organ systems and contributing to a variety of diseases. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), excessive alcohol intake causes more than 200 diseases and accounts for a significant global burden of disease, with millions of individuals affected annually by alcohol-related morbidities and mortalities. In the United States alone, approximately 28.9 million people aged 12 and older reported alcohol use disorder (AUD) in 2023, with annual economic burdens of more than USD 250 billion [1].
Because of its high water solubility, ethanol is distributed to virtually all organs, including the digestive organs, liver, brain, etc., and negatively affects them after heavy drinking. The brain is particularly vulnerable to the toxic effects of alcohol, partly due to having very low levels of antioxidants and antioxidative enzymes and high levels of lipids compared to those in the peripheral tissues such as the liver. Chronic alcohol consumption is associated with various neurodegenerative conditions, including alcohol-related nervous system damage or neurodegeneration, including dementia, Wernicke–Korsakoff syndrome, and fetal alcohol spectrum disorders (FASD) [2,3,4,5]. For example, FASD, a condition resulting from fetal and/or prenatal alcohol exposure, leads to severe developmental and cognitive impairments [6,7,8]. In fact, neuronal cells in gestational periods are much more vulnerable to ethanol-mediated neurotoxicity than adult tissues, eventually leading to FASD [9].
Although the mechanisms underlying alcohol-induced neurodegeneration vary by pattern of alcohol intake such as frequency and amount, nutritional states, gender, genetic makeup, and age, they share several common pathways of toxicity. Key mechanisms include the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS); accumulation of cytotoxic acetaldehyde and other reactive lipid aldehydes, such as 4-hydroxynonenal (4-HNE), malondialdehyde (MDA), and acrolein; induction of endoplasmic reticulum (ER) stress with accumulated misfolded proteins; and impaired lysosomal autophagy or mitophagy upon alcohol exposure [10,11,12]. In addition, alcohol intake alters various signaling pathways including activation of the cell death pathway, elevated inflammation, and mitochondrial dysfunction with depletion of energy supply [13]. Among these, oxidative stress seems to play a central role in initiating inflammation and exacerbating cellular damage, since intake of antioxidants or nutritional support with a balanced diet usually reduces the degree of alcohol-related organ damage in experimental models [14,15,16,17].
Oxidative stress arises from an imbalance between the production of ROS and the cell’s ability to detoxify these reactive molecules [18]. In the context of alcohol metabolism, elevated ROS levels can suppress the function of subcellular organelles, including mitochondria and ER, which results in oxidative modification and inactivation of components in the mitochondrial electron transport chain (ETC), such as complex I (ubiquinone-dependent NADH-oxidoreductase). This modification and inactivation of the ETC produces more electron leakage and ROS, further causing mitochondrial dysfunction and an impaired energy supply, ER stress with elevated levels of misfolded proteins, and eventual cell death. In addition, in the presence of high alcohol concentrations, the ethanol-inducible cytochrome P450-2E1 (CYP2E1) and its isozymes, present in the ER and mitochondria [19,20], also participate in the production of ROS, which causes oxidative modifications to cellular macromolecules, including the proteins, lipids, and DNA. These changes lead to protein post-translational modifications (PTMs), lipid peroxidation products, and DNA-adducts, all of which can potentially contribute to tissue damage, mutagenesis, or carcinogenesis if not treated properly [21]. One notable consequence of oxidative stress is the bi-directional regulations of the lysosomal autophagic pathways [22,23]. Some reports showed that ethanol can activate autophagy, a highly conserved process to remove the damaged proteins or subcellular organelles, while others demonstrated that ethanol can also impair autophagic process through elevated oxidative stress, depending on the patterns of alcohol intake (e.g., chronic versus acute alcohol exposure), cellular contexts, nutritional status, redox balance states, etc. Since autophagy generally serves a protective function by mitigating oxidative damage, its dysregulation can have opposing effects, leading to increased inflammation and cell/tissue injury. In this review, we summarize the current evidence available regarding the involvement of the autophagic pathways in alcohol-related neurotoxicity and their relationship with oxidative stress.
2. Materials and Methods
A literature search was conducted on the impact of alcohol exposure on autophagy and oxidative stress in the brain. Investigators LRL and BJS performed the literature search. We searched PubMed until Nov 21, 2024, using the following search terms: “autophagy” and “oxidative stress” or “redox” or “reactive oxygen species” or “cellular stress” or “antioxidants” and “Wernicke-Korsakoff*” or “behavioral*” or “cognitive*” or “neuro degeneration” or “brain*” and “alcohol*” or “ethanol*.” All articles were in English. The search strategy was supplemented with references found through the snowball technique to obtain information from relevant papers available.
Articles discussing autophagy and oxidative stress in response to alcohol consumption in the brain were selected. Papers with mouse models, cell lines, and human samples were considered. However, studies that did not directly evaluate the effects of alcohol on the brain in respect to the mechanisms of autophagy pathways were excluded. Methodological details and molecular effects on autophagy and oxidative stress of each study were extracted, and the information was summarized in different tables.
3. Literature Review
3.1. Regulation of Autophagy and Lysosomal Protein Degradation
Proteostasis is a normal process to maintain the proper balance of many cellular proteins by regulating the rates of new protein synthesis, adequate protein folding/misfolding, and degradation of cellular proteins [24]. Due to the critical nature of these functions, abnormal proteostasis is frequently associated with many disease states such as alcohol-associated liver disease, immune disorders, cancer, and neurodegeneration [25,26,27]. In mammals, two main protein degradation systems exist to regulate protein homeostasis and maintain their functions and levels. The first one is the ubiquitin-dependent proteasome-dependent degradation system, which is involved in the degradation numerous proteins that are abnormally misfolded in the ER. The other major protein degradation system is autophagic lysosomal proteolysis, which is responsible for the degradation of damaged subcellular organelles and/or aggregated proteins. Thus, disrupted proteostasis often results in accumulation of abnormally misfolded or aggregated proteins, which are potentially toxic to the cells, leading to cell death and various disease states, including alcohol-mediated brain injury and neurodegeneration [27,28].
Autophagy is a highly conserved cellular procedure that removes damaged subcellular organelles and proteins to be re-utilized for promoting cellular homeostasis and survival, especially during insufficient energy supply like fasting [29] or disease states that lead to autophagic flux and autophagic degradation, depending on the nature of disease [30,31,32,33]. Autophagy is usually very low in normal physiological states. However, it can be activated or induced under stressful conditions such as decreased energy supply, hypoxia, ischemia/reperfusion, and viral infections to overcome unfavorable conditions [34], for an excellent review of the autophagy processes, factors, and underlying mechanisms, while it can be suppressed by high levels of oxidative stress and long-term alcohol intake. In fact, autophagy can be regulated by many factors, including oxidative stress, which can cause oxidative DNA damage, alter gene/epigenetic expression, and promote PTMs (Figure 1). Changes in oxidative protein modifications and expression, or single nucleotide polymorphisms of the specific autophagy-related proteins (ATGs) and many associated genes [35], respectively (a few of which are exemplified in Figure 1), can result in different rates of mitophagy, ER stress, and autophagy.
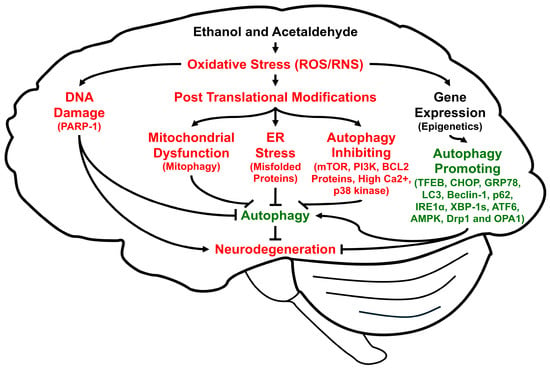
Figure 1.
Schematic diagram of the multiple pathways to show how ethanol and acetaldehyde regulate autophagy through elevated oxidative stress, leading to alcohol-mediated neuronal injury and neurodegeneration. Lines with arrowheads indicate stimulation of the pathway while short perpendicular lines represent inhibition.
It is also known that specific types of autophagy exist in different cell compartments for the proper disposal of cellular debris, aggregated proteins, and damaged subcellular organelles, such as mitochondria (mitophagy), ER (reticulophagy), peroxisomes (pexophagy) [36], and ribosomes (ribophagy) [37], although we only briefly described the functions of mitophagy and reticulophagy in this review. Thus, abnormal changes in the ubiquitin-dependent proteasomal activities and the lysosomal autophagy are frequently associated with many disease states, including alcohol- or nonalcohol-associated chronic liver diseases, cancer, and aging-related neurodegenerative disorders. Alcohol-associated neuronal injury and behavioral and cognitive impairments [38,39,40] are also known to result from elevated oxidative stress and abnormal regulations of autophagy. For instance, it is known that small amounts of ROS activate autophagy or mitophagy to prevent greater amounts from being produced in the mitochondria and thus providing protection from tissue injury. In contrast, large amounts of ROS (e.g., after chronic excessive alcohol intake) are known to impair autophagy process possibly through phosphorylation, Sirt1-dependent deacetylation, and other PTMs of many proteins involved in the autophagy machinery [34].
Autophagy also repairs DNA damage, which is elevated by oxidative stress or acetaldehyde [41]. In addition, DNA damage causes autophagy where PARP-1 is involved [42,43]. Thus, if autophagy is inhibited, DNA damage cannot be repaired, and the cells undergo apoptosis (instead of re-utilization of the cellular components via autophagy).
3.2. Increased Oxidative Stress in the Alcohol Metabolism in the Brain
The majority of consumed alcohol (ethanol) is known to be metabolized by oxidative and non-oxidative pathways in the liver as well as the stomach [44,45]. During the oxidative metabolism pathway, alcohol is oxidized by the cytosolic alcohol dehydrogenase (ADH) to acetaldehyde, which is further oxidized to acetate by the mitochondrial aldehyde dehydrogenase-2 (ALDH2) by using NAD+ as a cofactor for both enzymes, resulting in a redox change. In addition, in the presence of a high ethanol concentration through chronic alcohol intake, CYP2E1 present in the endoplasmic reticulum and mitochondria [19,46,47,48] is induced and becomes involved in ethanol oxidation by using NADPH as a cofactor and produce a superoxide anion, which further leads to the production of other reactive oxygen and nitrogen species (ROS/RNS). During the non-oxidative pathway, ethanol is conjugated with various small molecules, such as fatty acids, to produce fatty acid ethyl esters (FAEEs) and phosphatidylethanol [49,50,51].
In general, the oxidative ethanol metabolism increasing the NAD+/NADH ratio and inducing CYP2E1 activity [52] is known to cause oxidative stress and tissue injury through activation of the cell death pathways and elevated production of the cytotoxic acetaldehyde and highly reactive lipid aldehydes such as 4-HNE, MDA, and acrolein-adducts [53]. This organ damage occurs in many peripheral tissues and the central nervous system (CNS). Recent studies also showed that non-oxidative ethanol metabolism is involved in tissue injury through elevated ER stress and cell death pathways in the peripheral tissues, including the adipocytes and liver [54].
Unlike the liver, the brain does not contain the classical oxidative ethanol metabolism pathway starting with the ADH, since all five ADH isozymes, including the major ethanol-metabolizing ADH-II, is absent or very low in the brain tissues [55]. In fact, other scientists reported that the majority of alcohol in the brain is known to be metabolized by catalase (~60%) and partially by CYP2E1 (~20%) and others, although the induction of CYP2E1 occurs after chronic alcohol intake [56,57,58,59,60]. However, it is important to consider the potential impacts of the elevated CYP2E1 on the oxidative cell and brain injury, since CYP2E1 was shown to be induced by 17.5 mM ethanol in primary neuronal cells [61], primary astrocytes [62], and rodent brains after chronic alcohol exposures [63,64,65,66].
In addition to these enzymes, ethanol is also known to be metabolized via the non-oxidative pathway to produce ethanol fatty acid esters, which are also known to cause neuroprotection [67,68] or mitochondrial dysfunction and neurotoxicity [49,50,69], depending on the moiety of the fatty acids and suppression of the oxidative ethanol metabolism enzymes [70]. For instance, FAEEs including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were reported to prevent neurotoxicity or neurodegeneration in animal models of Alzheimer’s disease [71], Parkinson’s disease [67], and Huntington’s disease [72]. On the other hand, ethyl oleate or ethyl palmitate produced through the non-oxidative ethanol metabolism could take place in various tissues, including the pancreas, heart, and brain, where the oxidative ethanol metabolism is weak and causes injury in those tissues [49]. In the latter cases, the amounts of FAEEs positively correlate with the levels of blood alcohol and may promote tissue injury by releasing the free fatty acids, which subsequently suppress the mitochondrial functions, leading to increased organ damage [69].
Finally, most of the ROS produced in the brain could result from the suppressed mitochondrial function and abnormal changes in mitochondrial fission and fusion, which are associated with many chronic disease states, including aging-related neurodegenerative diseases [73,74,75,76]. Excessive amounts of alcohol intake are also known to promote the mitochondrial dysfunction, leading to elevated electron leakage from the mitochondrial ETC to generate ROS and ultimately increased oxidative stress in the peripheral tissues and brain, as reported by many laboratories [77,78,79]. For instance, mitochondrial complexes I–III and IV subunit activities are suppressed by alcohol exposure in rodent models [80]. In addition, alcohol intake is known to alter the intracellular Ca2+ balance, cause changes in the mitochondrial Ca2+ levels, and alter the dynamics of mitochondrial fusion and fission, leading to mitochondrial dysfunction and neuroinflammation [81,82,83]. All these changes eventually result in elevated oxidative stress and subsequent neurotoxicity.
3.3. Effects of Increased Oxidative Stress on Autophagy and Neuronal Damage in Alcohol-Exposed Experimental Models and Individuals with AUD
3.3.1. Effects of Increased Oxidative Stress on Autophagy and Neuronal Damage
In general, the brain usually consumes oxygen at higher rates, possesses high lipid contents, and contains a much smaller amount of antioxidants, including reduced glutathione (GSH), and antioxidative enzymes such as superoxide dismutase (SOD) isozymes and catalase, as well as proteases, compared to those in the liver [84,85,86]. Consequently, various brain cells, including neurons and astroglial cells, are thought to be more sensitive to oxidative injury or cytotoxic agents acetaldehyde and lipid aldehydes, leading to mitochondrial dysfunction and ER stress with misfolded proteins, after exposure to chronic excessive alcohol and other neurotoxic agents compared to the liver hepatocytes. In addition, it is known that an injured liver can regenerate rapidly to fully recover its functions within a short period of time relative to the brain cells [87,88]. The oxidative ethanol metabolism via ethanol-induced CYP2E1, albeit a small amount and with less involvement in the cerebral oxidative ethanol metabolism than catalase, and can cause oxidative stress, neuroinflammation, impairment autophagy, and apoptosis of neuronal cells, leading to brain damage in specific regions, including the hippocampus, cerebellum, and brainstem [61,62,65]. Alcohol-mediated neuronal cell- and/or region-specific damage can result from the unequal distributions of pro-oxidant CYP2E1 [89] and antioxidant ALDH2 [90] in the brain. In addition, hydrogen peroxide (H2O2) can be generated during the turnover of neurotransmitters such as dopamine and serotonin, contributing to oxidative stress, if not properly managed [91]. Additionally, immune-cell-derived NADPH oxidase can contribute to produce oxidative stress in the brain, leading to ER stress, neuroinflammation, and neurodegeneration in the animal models [92,93] and people with AUD [94]. Furthermore, acetaldehyde generated from oxidative ethanol metabolism, mitochondrial dysfunction, and ER stress can produce additional levels of ROS, Ca2+ imbalance, and mitochondrial dynamic changes in the brain, contributing to inhibition of autophagy/mitophagy accompanied by increased neuroinflammation and neurodegeneration in ethanol-exposed cells in rodents [41,95].
Alcohol-mediated oxidative stress is known to cause neurotoxicity and altered autophagy responses possibly through multiple mechanisms as described below. Alcohol-induced changes in autophagic responses seem to be dependent on the different embryonic stages, frequency and patterns of ethanol exposure such as acute and chronic alcohol intake, which causes oxidative stress, various experimental models, nutritional status of the host cells/animals, etc. However, because of the often conflicting results, we have listed many recent reports on the effects of acute and chronic alcohol intake on autophagy regulation in rodent models in Table 1 and Table 2, respectively. In continuation, we have also described the recent reports on the effects of alcohol exposure on autophagy in various brain cell culture models, including neurons, microglia, and astrocytes in Table 3. To provide clinical relevance of these experimental results, we also describe a summary of the effects of ethanol on autophagic flux in the brains of people with AUD (Table 4).

Table 1.
Autophagy in acute alcohol exposure in animal models.

Table 2.
Autophagy in chronic alcohol exposure in animal models.

Table 3.
Autophagy in alcohol-exposed cell culture models—neurons, microglia, or astrocytes.

Table 4.
Autophagy in humans with alcohol use disorder.
3.3.2. Effects of Increased Oxidative Stress on ER Stress and Neuronal Damage
Under increased oxidative and nitrosative stress, we expect that many oxidative PTMs, such as disulfide oxidation, mixed disulfide formation with glutathione, S-nitrosylation, nitration, phosphorylation, acetylation, and protein-adducts, including acetaldehyde-adducts [121,122,123,124], can take place. These oxidative PTMs can take place in virtually all subcellular organelles of the cytoplasm, ER, mitochondria, and nuclei, contributing to accumulation of oxidatively-modified and/or misfolded proteins, ER stress [125], mitochondrial dysfunction, and epigenetic regulations [126], respectively. Increased oxidative/nitrosative stress and subsequent PTMs also cause blood–brain barrier (BBB) destruction, neuroinflammation, and neurotoxicity [127,128,129,130]. For instance, daily ethanol exposures increased the levels of AGE-albumin [131], histone modifications [132], nitration or acrolein-adducts [133], phosphorylated Tau proteins [134], and amyloid beta accumulation with cognitive impairments [134,135,136].
Reticulophagy is a specific autophagy process where damaged ER with misfolded proteins is engulfed and then degraded by lysosomes. The damaged ER with many misfolded proteins under oxidative stress can result from inactivation of many ER chaperone proteins such as Hsp90, Grp78, and protein disulfide isomerase (PDI). Under normal conditions, these chaperone proteins are responsible for various modifications like glycosylation and disulfide formation for proper folding of their client proteins. However, these chaperone proteins can also be oxidatively modified, and their functions or activities become inhibited under oxidative stress conditions after exposure to alcohol or other neurotoxic agents. For instance, nitration of Hsp90 caused its inactivation, leading to death of motor neurons [137]. Phosphorylation of Grp78 became inactivated in the transformed cells, contributing to suppression of glycosylation with decreased binding with its client protein immunoglobulin heavy chains [138], ER stress with protein misfolding, and cellular damage [139,140]. In the case of alcohol exposure, PDI becomes oxidatively modified [141] and inactivated [142] (Moon KH et al. [122], unpublished results), possibly resulting in decreased binding with or misfolding of the substrate proteins, ultimately contributing to chronic liver disease or neurodegeneration, as extensively reviewed [143].
3.3.3. Effects of Increased Oxidative Stress on Mitophagy and Neuronal Damage
Special autophagy in mitochondria (mitophagy) is known to protect against oxidative stress, mitochondrial dysfunction, inflammation, and aging-related diseases since it not only removes damaged mitochondria but also regulates the rates of neuroinflammation and cognitive deficits [97,112,144,145,146]. Earlier reports showed that Parkin, an E3 ubiquitin ligase, is involved in removing damaged mitochondria [147], while PINK1 (PTEN-induced putative serine/threonine kinase 1) is stabilized on damaged mitochondria to stimulate Parkin, which regulates the cell quality control system by breaking down unneeded or damaged proteins [148]. Thus, PINK1-KO or Parkin-KO mice are thought to have impaired mitophagy compared to the wild-type (WT) mice, and these KO mice become more sensitive to mitochondrial dysfunction and tissue injury caused by alcohol [38,110,149] and other potentially other neurotoxic agents [150]. Additionally, Lin et al. recently showed that chronic alcohol exposure promoted impairment of both receptor-mediated and PINK1-related mitophagy in the medial prefrontal cortex, leading to elevated NLRP3-related neuroinflammation and cognitive decline in C57BL6/J mice through suppression of an antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (NRf2) [40]. In this report, si-RNA mediated silencing of PINK1 or BNIP3 caused mitochondrial dysfunction and alcohol-induced neuroinflammation in BV2 microglial cells. However, treatment with RTA-408, an NRF2 activator, attenuated NLRP3-related neuroinflammation and mitophagy suppression, leading to improvement of alcohol-mediated cognitive dysfunction. These results indicate that alcohol-mediated oxidative stress plays an important role in suppressing mitophagy, which leads to NLRP3 neuroinflammation and cognitive impairment in mice.
In contrast, acetaldehyde induces mitophagic responses with elevated levels of PINK1, Parkin (a member of the E3 ubiquitin ligase, cell quality control system by breaking down unneeded or damaged proteins), resulting in mitochondrial dysfunction and decreased mitochondrial mass, and cytotoxicity in acetaldehyde-exposed SH-SY5Y cells. In this case, the levels of LC3-II, Beclin1, autophagy-related protein Atg5, and Atg16L1 were elevated, while p62 levels were decreased. However, treatment with an autophagy inhibitor such as chloroquine and 3-methyladenine (3-MA), or an antioxidant NAC, prevented decreased mitochondrial mass, suggesting a role of oxidative stress in acetaldehyde-mediated excessive mitophagy [41].
3.3.4. Effects of Increased Oxidative Stress on Neuroinflammation, NETosis, and Neuronal Damage
Excessive alcohol intake also causes neuroinflammation and NETosis in the brain through alterations of the functions of neutrophils. Neutrophils are white blood cells that play a pivotal role in innate and adaptive immunity, wound healing, the resolution of inflammation, and fight against infection from various pathogens [151,152]. The polymorphonuclear neutrophils (PMNs) are involved in several types of non-inflammatory events, e.g., senescence, apoptosis, and efferocytosis [153,154,155], as well as inflammatory processes, e.g., necroptosis, ferroptosis, and necrosis [156,157,158], including NETosis [159], while they defend the host against the inflammatory threat or infection. In many organs, especially in the brain, these types of cell death can contribute to secondary brain damage by causing a high-grade neuroinflammation.
Like other types of cell death, NETosis is also modulated by autophagy/mitophagy [154,155]. It also mediates a few molecular pathways through the oxidative stress, which activates signaling molecules such as NF-κB, NADPH oxidase, Protein Kinase C (PKC), cytokines (IL-1β, IL-8, and HMGB1), anti-neutrophilic cytoplasmic autoantibody (ANCA), all-trans-retinoic acid (ATRA), transient receptor potential channel M2 (TRPM2), etc. [160,161].
More importantly, autophagy regulates the formation of NETs/NETosis. During NETosis, autophagy can manipulate neutrophil cell death to resolve the inflammation in several organs by regulating neutrophil degranulation, differentiation, metabolism, and the formation of neutrophil extracellular traps (NETs) against the pathogens and dreadful stimuli. The active steps and sequence of events in the molecular pathways that occur during the coordination between the autophagy and induction of NETs/NETosis are very crucial in deciding the fate of the cells in which the mTOR/REDD1 (regulation in development and DNA damage response 1) pathway is the central regulator of autophagy in these granulocytes [162]. It has been shown that internal stimuli within the neutrophils activate the protein-arginine deiminase 4 (PAD4) enzyme, which is a common mediator in multiple signaling pathways, controlling the execution of the formation of NETs and occurrence of NETosis. In contrast, inhibition of the PAD4 enzyme significantly blocks the process of NETosis [163]. Alcohol can induce and inhibit autophagy/mitophagy, which modulates the formation of NETs and regulation of NETosis in various organs including the brain, depending on the severity of acute or chronic conditions, amounts of alcohol consumption, and host conditions. The autophagy process in neutrophils regulates not only neutrophil metabolism during granulopoiesis but also the formation of NETs and NETosis, which are closely associated with neuroinflammation and neuronal injury.
This section updates the advancement of the relationship between autophagy and not only intrinsic changes in neutrophil per se but also extrinsic regulation of the events, involving NETs formation/NETosis in response to acute and chronic alcohol consumption. For example, alcohol-exposed neutrophils produce several inflammatory danger molecules in their intracellular space as well as around their microenvironment in the brain. These pro-inflammatory environment prime neutrophils to form NETs/NETosis, which leads to autophagy-mediated generation of ROS and superoxide from NADPH oxidase activity, degranulation, and an increase in calcium levels to activate the PAD4 enzyme, influencing myeloperoxidase/elastase and histone citrullination activities for epigenetic regulation. Thus, understanding the molecular and cellular mechanisms involving the processes of autophagy and the formation of NETs/NETosis will identify potential targets to develop anti-alcohol drugs for clinical application and provide treatment options to patients with alcohol misuse.
3.3.5. Effects of Increased Oxidative Stress on Autophagy and Neuronal Damage by Regulating the Cell Signaling Pathways
As mentioned before, chronic or binge alcohol intake causes oxidative stress, leading to neuroinflammation and organ damage mainly through suppressing antioxidants and defensive enzymes with activation of pro-oxidant proteins and mitochondrial dysfunction possibly by oxidative PTMs. Alcohol-mediated oxidative stress can alter the activities of many protein kinases and phosphatases that are involved in the cell signaling pathways by the regulation of many proteins in ER stress, neuroinflammation, neurogenesis, and neurotoxicity [164,165,166,167,168,169]. For instance, the activities of mTOR kinase, AMPK/mTOR, PI3K/Akt/mTOR, mitogen activated protein kinases, PKC isoforms, and GSK-3β, known to be directly and indirectly involved in the regulation of autophagy-related proteins and genes, including ATG12, LC3-II, Beclin-1, p62, and Bcl-2 (Figure 1), are modulated by alcohol-mediated oxidative stress [101,170,171,172,173,174,175,176]. Furthermore, ethanol exposure can activate the TLR4-NF-κB-cytokines pathway, which can lead to impaired autophagy accompanied by elevated neuroinflammation and neuronal injury [101,111,118,135]. Although the underlying mechanisms by which ethanol-mediated oxidative stress can alter the rates of autophagy and neuronal injury are incompletely understood, the changes in autophagy responses seem to be dependent on the patterns of ethanol exposure such as the frequency, amount, and acute or chronic alcohol intake as well as nutritional status of the host cells/tissues.
3.4. Potential Therapeutic Agents Against Autophagy in Alcohol-Exposed Brains
Alcohol is eliminated from the brain much more slowly than from other organs, and alcohol accumulates in the largest amount in brain tissue compared to other organs [102]. Current medications for AUD are insufficiently effective, highlighting the need for novel highly effective therapies [177,178]. Autophagy plays an important role in neurodegenerative diseases [179], and modulating autophagy by melatonin or its precursor as a therapeutic strategy offers a promising potential against alcohol-related brain damage.
Since autophagy exhibits both a protective mechanism and a damage pathway related to programmed cell death [180], we and others have reported that autophagy can be a double-edged sword in the context of stroke-induced brain injury [180] and aging-related neurodegenerative diseases [179], as well as alcohol-mediated brain damage [181]. On one side, autophagy provides a protective mechanism against alcohol-induced brain damage, and ethanol treatment increased mTOR activity and decreased expression of several ATG genes including Atg12, Atg5, p62/SQSTM1, and LC3 [10]. In this case, autophagy enhancers/inducers likely enhance autophagic flux in alcohol-exposed brains by clearing damaged organelles or misfolded proteins. In contrast, we and others have reported that autophagy acts as a double-edged sword, contributing to brain damage, as seen in the context of stroke-induced brain injury [180,182] and alcohol-related brain damage [181]. Ethanol exposure enhances autophagy markers like Map1lc3-II (LC3-II) and Beclin-1 expression while decreasing SQSTM1 (p62) expression in the brain, liver, and neuroblastoma cells [97,183].
This dual nature of autophagy presents opportunities for targeted therapies. Enhancing autophagy with enhancers/inducers can protect against alcohol-induced brain damage, while inhibitors may mitigate harmful autophagy activation. In addition, targeting molecular pathways such as mTOR, AMPK, and PI3K/Akt/mTOR offers promising therapeutic strategies [102,170,171,172,173,174,175,176]. This review discusses potential therapeutic agents for modulating autophagy in alcohol-exposed brains, as exemplified in Table 5, Table 6 and Table 7.

Table 5.
Autophagy enhancers/inducers/mitophagy activators.

Table 6.
Autophagy inhibitors.

Table 7.
Selective modulators.
In addition, many other naturally occurring polyphenol flavonoids and NRF2 activator sulforaphane can prevent oxidative stress, leading to activation of autophagy and improvement of alcohol-associated liver injury, as extensively reviewed [198]. Since many small-molecule plant-derived polyphenols or flavonoids, like quercetin, luteolin, rutin, berberine, and curcumin, are known to have antioxidant effects and pass through the BBB [199,200,201,202,203,204,205,206,207,208], they can also be used for protecting against alcohol-mediated oxidative stress, impaired autophagy, and neurotoxicity.
4. Conclusions
In this review, we have briefly described various types of alcohol-mediated brain injury and neurodegeneration, literature search methods, alcohol metabolism in the brain, sources of oxidative stress, general properties and types of autophagy, and potential translational approaches against alcohol-mediated brain damage by regulating the rates of autophagy. However, as we emphasized, the rates of autophagy or mitophagy are differentially affected, depending on the pattern (binge or chronic) of alcohol intake, nutritional status, and other environmental and genetic factors, all of which affect various cell signaling pathways. Similar factors and underlying mechanisms for impaired autophagy and mitophagy with accumulation of damaged, aggregated proteins, and neuronal damage can be induced by various neurotoxic agents and aging-related neurodegenerative disease states [202,203,204,205,206,207]. A key distinction between alcohol-mediated brain damage and other neurodegenerative diseases could be the selective activation of CYP2E1 by alcohol, since CYP2E1 is not induced by aging-related neurodegeneration. Regardless of distinguished pathophysiological conditions, one common factor could be increased oxidative stress (Figure 1), which will negatively affect the downstream autophagic processes, leading to impaired autophagy and neurodegeneration. Due to the complexity of autophagy regulations, further molecular studies are warranted. For instance, we expect to see additional studies on oxidative PTMs of the individual proteins involved in the different steps of autophagy and their functional alterations. Based on the molecular mechanistic studies on alcohol-mediated autophagy regulations, additional therapeutic agents against alcohol-induced neurotoxicity as well as other brain diseases can be identified and developed for clinical usage in the future.
Author Contributions
Literature search, writing original draft, and revisions, L.R.-L.; writing and editing, M.A.S.K. and X.W.; conceptualization, writing original draft, and revisions, B.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Intramural Research Fund (to BJS; ZIA-AA000036-38) of the National Institute of Alcohol Abuse and Alcoholism (NIAAA). In addition, MASK and XW were supported by R21AA029925 and R21AA030087 grants, respectively, from the NIAAA.
Conflicts of Interest
All authors have declared no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUD | Alcohol use disorder |
| FASD | Fetal alcohol spectrum disorders |
| ROS | Reactive oxygen species |
| 4-HNE | 4-hydroxynonenal |
| MDA | Malondialdehyde |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| CYP2E1 | Cytochrome P450-2E1 |
| PTMs | Post-translational protein modifications |
| ATGs | Autophagy-related proteins |
| ADH | Alcohol dehydrogenase |
| ALDH2 | Aldehyde dehydrogenase-2 |
| FAEEs | Fatty acid ethyl esters |
| CNS | Central nervous system |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| GSH | Glutathione, reduced |
| SOD | Superoxide dismutase |
| H2O2 | Hydrogen peroxide |
| PD# | Postnatal day # |
| WT | Wild-type |
| KO | Knockout |
| PC12 | Pheochromocytoma line 12 cells |
| AMPK | AMP-activated kinase |
| PKC | Protein Kinase C |
| CBD | Cannabidiol |
| BBB | Blood–brain barrier |
| PDI | Protein disulfide isomerase |
| PINK1 | PTEN-induced putative serine/threonine kinase 1 |
| NRf2 | Nuclear factor erythroid 2-related factor 2 |
| 3-MA | 3-methyladenine |
| PMNs | Polymorphonuclear neutrophils |
| ANCA | Anti-neutrophilic cytoplasmic autoantibody |
| ATRA | All-trans-retinoic acid |
| TRPM2 | Transient receptor potential channel M2 |
| REDD1 | Regulated in development and DNA damage response 1 |
| NETs | Neutrophil extracellular traps |
| PAD4 | Protein-arginine deiminase 4 |
| BafA1 | Bafilomycin A1 |
| EPO | Erythropoietin |
| H2S | Hydrogen disulfide |
References
- Alcohol Use Disorder (AUD) in the United States: Age Groups and Demographic Characteristics. Available online: https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics (accessed on 21 November 2024).
- Medical Complications: Common Alcohol-Related Concerns. Available online: https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/medical-complications-common-alcohol-related-concerns (accessed on 21 November 2024).
- Eva, L.; Brehar, F.M.; Florian, I.A.; Covache-Busuioc, R.A.; Costin, H.P.; Dumitrascu, D.I.; Bratu, B.G.; Glavan, L.A.; Ciurea, A.V. Neuropsychiatric and Neuropsychological Aspects of Alcohol-Related Cognitive Disorders: An In-Depth Review of Wernicke’s Encephalopathy and Korsakoff’s Syndrome. J. Clin. Med. 2023, 12, 6101. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.E.; Rutt, L.N.; Kaufman, M.L.; Busquet, N.; Kovacs, E.J.; McCullough, R.L. Binge ethanol exposure in advanced age elevates neuroinflammation and early indicators of neurodegeneration and cognitive impairment in female mice. Brain Behav. Immun. 2024, 116, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Coleman, L.G., Jr.; Macht, V.A.; Vetreno, R.P. Alcohol, HMGB1, and Innate Immune Signaling in the Brain. Alcohol Res. 2024, 44, 4. [Google Scholar] [CrossRef]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Prim. 2023, 9, 11. [Google Scholar] [CrossRef]
- Mattson, S.N.; Bernes, G.A.; Doyle, L.R. Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Understanding Fetal Alcohol Spectrum Disorders. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-fetal-alcohol-spectrum-disorders (accessed on 21 November 2024).
- Luo, J. Autophagy and ethanol neurotoxicity. Autophagy 2014, 10, 2099–2108. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ni, H.M.; Huang, H.; Ding, W.X. Autophagy in alcohol-induced multiorgan injury: Mechanisms and potential therapeutic targets. Biomed Res. Int. 2014, 2014, 498491. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Rodriguez, Y.; Ma, X.; Swerdlow, R.H.; Zhang, J.; Ding, W.X. A perspective on autophagy and transcription factor EB in Alcohol-Associated Alzheimer’s disease. Biochem. Pharmacol. 2023, 213, 115576. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Dlugos, C.A. ATF6 and caspase 12 expression in Purkinje neurons in acute slices from adult, ethanol-fed rats. Brain Res. 2014, 1577, 11–20. [Google Scholar] [CrossRef]
- Comporti, M.; Signorini, C.; Leoncini, S.; Gardi, C.; Ciccoli, L.; Giardini, A.; Vecchio, D.; Arezzini, B. Ethanol-induced oxidative stress: Basic knowledge. Genes Nutr. 2010, 5, 101–109. [Google Scholar] [CrossRef]
- Das, S.K.; Hiran, K.R.; Mukherjee, S.; Vasudevan, D.M. Oxidative stress is the primary event: Effects of ethanol consumption in brain. Indian J. Clin. Biochem. 2007, 22, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Fialova, L.; Mestek, O.; Janebova, M.; Crkovska, J.; Malbohan, I.; Stipek, S.; Mikulikova, L.; Popov, P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J. Biomed. Sci. 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.C. Oxidative stress. JPEN J. Parenter. Enter. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Gonzalez, H.C.; Misare, K.R.; Mendenhall, T.T.; Wolf, B.J.; Mulholland, P.J.; Gordon, K.L.; Hartman, J.H. Transgenic expression of human cytochrome P450 2E1 in C. elegans and rat PC-12 cells sensitizes to ethanol-induced locomotor and mitochondrial effects. Biochem. Biophys. Res. Commun. 2024, 734, 150735. [Google Scholar] [CrossRef]
- Robin, M.A.; Sauvage, I.; Grandperret, T.; Descatoire, V.; Pessayre, D.; Fromenty, B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005, 579, 6895–6902. [Google Scholar] [CrossRef]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.J. Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction. Cell. Mol. Life Sci. 2024, 81, 34. [Google Scholar] [CrossRef]
- Marchetti, B. Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson’s disease. Redox Biol. 2020, 36, 101664. [Google Scholar] [CrossRef]
- Li, H.; Lismont, C.; Revenco, I.; Hussein, M.A.F.; Costa, C.F.; Fransen, M. The Peroxisome-Autophagy Redox Connection: A Double-Edged Sword? Front. Cell Dev. Biol. 2021, 9, 814047. [Google Scholar] [CrossRef]
- Thapa, R.; Ahmad Bhat, A.; Shahwan, M.; Ali, H.; PadmaPriya, G.; Bansal, P.; Rajotiya, S.; Barwal, A.; Siva Prasad, G.V.; Pramanik, A.; et al. Proteostasis disruption and senescence in Alzheimer’s disease pathways to neurodegeneration. Brain Res. 2024, 1845, 149202. [Google Scholar] [CrossRef] [PubMed]
- Ji, C. Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules 2015, 5, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.L.; Quinlan, J.I.; Dhaliwal, A.; Armstrong, M.J.; Elsharkawy, A.M.; Greig, C.A.; Lord, J.M.; Lavery, G.G.; Breen, L. Sarcopenia in chronic liver disease: Mechanisms and countermeasures. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G241–G257. [Google Scholar] [CrossRef]
- Pierson, S.R.; Kolling, L.J.; James, T.D.; Pushpavathi, S.G.; Marcinkiewcz, C.A. Serotonergic dysfunction may mediate the relationship between alcohol consumption and Alzheimer’s disease. Pharmacol. Res. 2024, 203, 107171. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Khaki-Khatibi, F. Proteostasis and neurodegeneration: A closer look at autophagy in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1281338. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, aging, and age-related neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Song, Y.Q.; Tu, J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef]
- Girault, V.; Gilard, V.; Marguet, F.; Lesueur, C.; Hauchecorne, M.; Ramdani, Y.; Laquerriere, A.; Marret, S.; Jegou, S.; Gonzalez, B.J.; et al. Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis. 2017, 8, e2610. [Google Scholar] [CrossRef]
- Wang, L.; Cui, J. Palmitoylation promotes chaperone-mediated autophagic degradation of NLRP3 to modulate inflammation. Autophagy 2023, 19, 2821–2823. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Tamargo-Gomez, I.; Fernandez, A.F.; Marino, G. Pathogenic Single Nucleotide Polymorphisms on Autophagy-Related Genes. Int. J. Mol. Sci. 2020, 21, 8196. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Manandhar, L.; Kim, H.; Chhetri, A.; Hwang, J.; Jang, G.; Park, C.; Park, R. Pexophagy and Oxidative Stress: Focus on Peroxisomal Proteins and Reactive Oxygen Species (ROS) Signaling Pathways. Antioxidants 2025, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chen, Y.; Liu, Z.; Wang, Y.; Yi, C. Detection of ribophagy in yeast and mammals. Biophys. Rep. 2024, 10, 82–101. [Google Scholar] [CrossRef]
- Peng, H.; Qin, X.; Chen, S.; Ceylan, A.F.; Dong, M.; Lin, Z.; Ren, J. Parkin deficiency accentuates chronic alcohol intake-induced tissue injury and autophagy defects in brain, liver and skeletal muscle. Acta Biochim. Biophys. Sin. (Shanghai) 2020, 52, 665–674. [Google Scholar] [CrossRef]
- Bian, H.; Wu, Y.; Cui, Z.; Zheng, H.; Li, Y.; Zou, D. Study on the autophagy-related mechanism of puerarin in improving the cognitive impairment induced by alcohol in female mice. Brain Inj. 2022, 36, 137–145. [Google Scholar] [CrossRef]
- Lin, X.; Wang, H.; Zou, L.; Yang, B.; Chen, W.; Rong, X.; Zhang, X.; He, L.; Li, X.; Peng, Y. The NRF2 activator RTA-408 ameliorates chronic alcohol exposure-induced cognitive impairment and NLRP3 inflammasome activation by modulating impaired mitophagy initiation. Free Radic. Biol. Med. 2024, 220, 15–27. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, Y.; Jiang, Z.; Chen, J. Acetaldehyde Induces Cytotoxicity via Triggering Mitochondrial Dysfunction and Overactive Mitophagy. Mol. Neurobiol. 2022, 59, 3933–3946. [Google Scholar] [CrossRef]
- Munoz-Gamez, J.A.; Rodriguez-Vargas, J.M.; Quiles-Perez, R.; Aguilar-Quesada, R.; Martin-Oliva, D.; de Murcia, G.; Menissier de Murcia, J.; Almendros, A.; Ruiz de Almodovar, M.; Oliver, F.J. PARP-1 is involved in autophagy induced by DNA damage. Autophagy 2009, 5, 61–74. [Google Scholar] [CrossRef]
- Xu, X.; Sun, B.; Zhao, C. Poly (ADP-Ribose) polymerase 1 and parthanatos in neurological diseases: From pathogenesis to therapeutic opportunities. Neurobiol. Dis. 2023, 187, 106314. [Google Scholar] [CrossRef]
- Lieber, C.S. Metabolism of alcohol. Clin. Liver Dis. 2005, 9, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Lieber, C.S. Microsomal ethanol-oxidizing system (MEOS): The first 30 years (1968–1998)—A review. Alcohol. Clin. Exp. Res. 1999, 23, 991–1007. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Harjumaki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef] [PubMed]
- Laposata, E.A.; Lange, L.G. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 1986, 231, 497–499. [Google Scholar] [CrossRef]
- Isenberg, K.E.; Bora, P.S.; Zhou, X.; Wu, X.; Moore, B.W.; Lange, L.G. Nonoxidative ethanol metabolism: Expression of fatty acid ethyl ester synthase-III in cultured neural cells. Biochem. Biophys. Res. Commun. 1992, 185, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Saghir, M.; Fernandez-del Castillo, C.; Warshaw, A.L.; Laposata, M. Linkage of oxidative and nonoxidative ethanol metabolism in the pancreas and toxicity of nonoxidative ethanol metabolites for pancreatic acinar cells. Surgery 2001, 129, 736–744. [Google Scholar] [CrossRef]
- Roy, T.S.; Sharma, V.; Seidler, F.J.; Slotkin, T.A. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res. Dev. Brain Res. 2005, 155, 71–80. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Park, S.H.; Seo, W.; Xu, M.J.; Mackowiak, B.; Lin, Y.; He, Y.; Fu, Y.; Hwang, S.; Kim, S.J.; Guan, Y.; et al. Ethanol and its Nonoxidative Metabolites Promote Acute Liver Injury by Inducing ER Stress, Adipocyte Death, and Lipolysis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 281–306. [Google Scholar] [CrossRef]
- Estonius, M.; Svensson, S.; Hoog, J.O. Alcohol dehydrogenase in human tissues: Localisation of transcripts coding for five classes of the enzyme. FEBS Lett. 1996, 397, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Zimatkin, S.M.; Deitrich, R.A. Ethanol metabolism in the brain. Addict. Biol. 1997, 2, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Ziegler, T.L.; Bludeau, P.; Petersen, D.R.; Gonzalez, F.J.; Deitrich, R.A. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenetics Genom. 2006, 16, 51–58. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Pronko, S.P.; Vasiliou, V.; Gonzalez, F.J.; Deitrich, R.A. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol. Clin. Exp. Res. 2006, 30, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Gustafsson, J.A. Effect of ethanol on cytochrome P450 in the rat brain. Proc. Natl. Acad. Sci. USA 1994, 91, 1019–1023. [Google Scholar] [CrossRef]
- Upadhya, S.C.; Tirumalai, P.S.; Boyd, M.R.; Mori, T.; Ravindranath, V. Cytochrome P4502E (CYP2E) in brain: Constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Arch. Biochem. Biophys. 2000, 373, 23–34. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef]
- Montoliu, C.; Sancho-Tello, M.; Azorin, I.; Burgal, M.; Valles, S.; Renau-Piqueras, J.; Guerri, C. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J. Neurochem. 1995, 65, 2561–2570. [Google Scholar] [CrossRef]
- Montoliu, C.; Valles, S.; Renau-Piqueras, J.; Guerri, C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: Effect of chronic alcohol consumption. J. Neurochem. 1994, 63, 1855–1862. [Google Scholar] [CrossRef]
- Tindberg, N.; Ingelman-Sundberg, M. Expression, catalytic activity, and inducibility of cytochrome P450 2E1 (CYP2E1) in the rat central nervous system. J. Neurochem. 1996, 67, 2066–2073. [Google Scholar] [CrossRef]
- Zhong, Y.; Dong, G.; Luo, H.; Cao, J.; Wang, C.; Wu, J.; Feng, Y.Q.; Yue, J. Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology 2012, 302, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Li, J.; Zhang, H.; Li, Y.; Yang, Z.; Zhong, Y.; Dong, G.; Yang, J.; Yue, J. The induction of cytochrome P450 2E1 by ethanol leads to the loss of synaptic proteins via PPARalpha down-regulation. Toxicology 2017, 385, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Meng, Q.; Song, C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 226, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Ding, L.; Zhang, L.; Zhang, T.; Teruyoshi, Y.; Wang, Y.; Xue, C. Eicosapentaenoic Acid-Enriched Phosphatidylcholine Mitigated Abeta1-42-Induced Neurotoxicity via Autophagy-Inflammasome Pathway. J. Agric. Food Chem. 2019, 67, 13767–13774. [Google Scholar] [CrossRef]
- Lange, L.G.; Sobel, B.E. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J. Clin. Investig. 1983, 72, 724–731. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar] [PubMed]
- Xu, Z.J.; Li, Q.; Ding, L.; Shi, H.H.; Xue, C.H.; Mao, X.Z.; Wang, Y.M.; Zhang, T.T. A comparative study of the effects of phosphatidylserine rich in DHA and EPA on Abeta-induced Alzheimer’s disease using cell models. Food Funct. 2021, 12, 4411–4423. [Google Scholar] [CrossRef]
- Puri, B.K.; Bydder, G.M.; Counsell, S.J.; Corridan, B.J.; Richardson, A.J.; Hajnal, J.V.; Appel, C.; McKee, H.M.; Vaddadi, K.S.; Horrobin, D.F. MRI and neuropsychological improvement in Huntington disease following ethyl-EPA treatment. Neuroreport 2002, 13, 123–126. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Flint Beal, M. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013, 63, 1–29. [Google Scholar] [CrossRef]
- Alrouji, M.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Ashour, N.A.; Jabir, M.S.; Negm, W.A.; Batiha, G.E. Metformin role in Parkinson’s disease: A double-sword effect. Mol. Cell. Biochem. 2024, 479, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Hiden, U.; Krstic, J.; Panzitt, K.; Wagner, M.; Enzinger, C.; Khalil, M.; Abdellatif, M.; Malle, E.; Madl, T.; et al. Targeting organ-specific mitochondrial dysfunction to improve biological aging. Pharmacol. Ther. 2024, 262, 108710. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Carvajal, F.J.; Mira, R.G.; Arce, C.; Lerma-Cabrera, J.M.; Orellana, J.A.; Cerpa, W.; Quintanilla, R.A. Adolescent Binge Alcohol Exposure Affects the Brain Function Through Mitochondrial Impairment. Mol. Neurobiol. 2018, 55, 4473–4491. [Google Scholar] [CrossRef]
- Leon, B.E.; Kang, S.; Franca-Solomon, G.; Shang, P.; Choi, D.S. Alcohol-Induced Neuroinflammatory Response and Mitochondrial Dysfunction on Aging and Alzheimer’s Disease. Front. Behav. Neurosci. 2021, 15, 778456. [Google Scholar] [CrossRef]
- Jamali, Z.; Salimi, A.; Garmabi, B.; Khezri, S.; Khaksari, M. Hesperidin Protects Alcohol-Induced Mitochondrial Abnormalities via the Inhibition of Oxidative Stress and MPT Pore Opening in Newborn Male Rats as a Fetal Alcohol Syndrome Model. J. Stud. Alcohol Drugs 2024, 85, 361–370. [Google Scholar] [CrossRef]
- Karadayian, A.G.; Lombardi, P.; Bustamante, J.; Lores-Arnaiz, S. Alcohol hangover effects on brain cortex non-synaptic mitochondria and synaptosomes bioenergetics. Alcohol 2019, 77, 113–123. [Google Scholar] [CrossRef]
- Mira, R.G.; Lira, M.; Quintanilla, R.A.; Cerpa, W. Alcohol consumption during adolescence alters the hippocampal response to traumatic brain injury. Biochem. Biophys. Res. Commun. 2020, 528, 514–519. [Google Scholar] [CrossRef]
- Alvear, T.F.; Farias-Pasten, A.; Vergara, S.A.; Prieto-Villalobos, J.; Silva-Contreras, A.; Fuenzalida, F.A.; Quintanilla, R.A.; Orellana, J.A. Hemichannels contribute to mitochondrial Ca2+ and morphology alterations evoked by ethanol in astrocytes. Front. Cell Dev. Biol. 2024, 12, 1434381. [Google Scholar] [CrossRef]
- Khatoon, R.; Fick, J.; Elesinnla, A.; Waddell, J.; Kristian, T. Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells. Int. J. Mol. Sci. 2024, 25, 13714. [Google Scholar] [CrossRef]
- Dasuri, K.; Nguyen, A.; Zhang, L.; Fernandez-Kim, O.S.; Bruce-Keller, A.J.; Blalock, B.A.; Cabo, R.D.; Keller, J.N. Comparison of rat liver and brain proteasomes for oxidative stress-induced inactivation: Influence of ageing and dietary restriction. Free Radic. Res. 2009, 43, 28–36. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Temple, S. Neural stem cells: Generating and regenerating the brain. Neuron 2013, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S. Stem Cell-Mediated Regeneration of the Adult Brain. Transfus. Med. Hemother. 2016, 43, 321–326. [Google Scholar] [CrossRef]
- Hansson, T.; Tindberg, N.; Ingelman-Sundberg, M.; Kohler, C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience 1990, 34, 451–463. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Rout, U.K.; Koivusalo, M.; Buhler, R.; Lindros, K.O. Regional distribution of low-Km mitochondrial aldehyde dehydrogenase in the rat central nervous system. Alcohol. Clin. Exp. Res. 1992, 16, 1162–1167. [Google Scholar] [CrossRef]
- Sinet, P.M.; Heikkila, R.E.; Cohen, G. Hydrogen peroxide production by rat brain in vivo. J. Neurochem. 1980, 34, 1421–1428. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 2008, 5, 10. [Google Scholar] [CrossRef]
- Qin, L.; Crews, F.T. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflammation 2012, 9, 5. [Google Scholar] [CrossRef]
- Qin, L.; Vetreno, R.P.; Crews, F.T. NADPH oxidase and endoplasmic reticulum stress is associated with neuronal degeneration in orbitofrontal cortex of individuals with alcohol use disorder. Addict. Biol. 2023, 28, e13262. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca2+ levels. Redox Biol. 2020, 28, 101381. [Google Scholar] [CrossRef] [PubMed]
- Alimov, A.; Wang, H.; Liu, M.; Frank, J.A.; Xu, M.; Ou, X.; Luo, J. Expression of autophagy and UPR genes in the developing brain during ethanol-sensitive and resistant periods. Metab. Brain Dis. 2013, 28, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ke, Z.; Xu, M.; Liao, M.; Wang, X.; Qi, Y.; Zhang, T.; Frank, J.A.; Bower, K.A.; Shi, X.; et al. Autophagy is a protective response to ethanol neurotoxicity. Autophagy 2012, 8, 1577–1589. [Google Scholar] [CrossRef]
- Boschen, K.E.; Steensen, M.C.; Simon, J.M.; Parnell, S.E. Short-term transcriptomic changes in the mouse neural tube induced by an acute alcohol exposure. Alcohol 2023, 106, 1–9. [Google Scholar] [CrossRef]
- Uguz, A.C.; Okan, A.; Doganyigit, Z.; Yilmaz, S.; Ates, S.; Arikan Soylemez, E.S.; Karabulut, S.; Kumru, A.S.; Espino, J. Evaluation of TRPM2 Channel-Mediated Autophagic Signaling Pathway in Hippocampus and Cortex Tissues of Rat Offspring Following Prenatal Exposure to Elevated Alcohol Levels. Environ. Toxicol. 2024, 40, 222–244. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Millan-Esteban, D.; Guerri, C. Binge-like ethanol treatment in adolescence impairs autophagy and hinders synaptic maturation: Role of TLR4. Neurosci. Lett. 2018, 682, 85–91. [Google Scholar] [CrossRef]
- Pascual, M.; Lopez-Hidalgo, R.; Montagud-Romero, S.; Urena-Peralta, J.R.; Rodriguez-Arias, M.; Guerri, C. Role of mTOR-regulated autophagy in spine pruning defects and memory impairments induced by binge-like ethanol treatment in adolescent mice. Brain Pathol. 2021, 31, 174–188. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Tkachenko, H. Melatonin and alcohol-related disorders. Chronobiol. Int. 2020, 37, 781–803. [Google Scholar] [CrossRef]
- Yang, F.; Luo, J. Endoplasmic Reticulum Stress and Ethanol Neurotoxicity. Biomolecules 2015, 5, 2538–2553. [Google Scholar] [CrossRef]
- Fujii, C.; Zorumski, C.F.; Izumi, Y. Ethanol, neurosteroids and cellular stress responses: Impact on central nervous system toxicity, inflammation and autophagy. Neurosci. Biobehav. Rev. 2021, 124, 168–178. [Google Scholar] [CrossRef] [PubMed]
- De Ternay, J.; Naassila, M.; Nourredine, M.; Louvet, A.; Bailly, F.; Sescousse, G.; Maurage, P.; Cottencin, O.; Carrieri, P.M.; Rolland, B. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 2019, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, A.; Ueta, K.; Mauchi, S.; Hirai, K.; Horike, K.; Hikida, T.; Sakurai, T.; Sawa, A.; Tomoda, T. Ulk1 protects against ethanol-induced neuronal stress and cognition-related behavioral deficits. Neurosci. Res. 2017, 117, 54–61. [Google Scholar] [CrossRef]
- Davis-Anderson, K.L.; Wesseling, H.; Siebert, L.M.; Lunde-Young, E.R.; Naik, V.D.; Steen, H.; Ramadoss, J. Fetal regional brain protein signature in FASD rat model. Reprod. Toxicol. 2018, 76, 84–92. [Google Scholar] [CrossRef]
- Nasef, N.A.; Keshk, W.A.; El-Meligy, S.M.; Allah, A.A.A.; Ibrahim, W.M. Modulatory effect of simvastatin on redox status, caspase-3 expression, p-protein kinase B (p-Akt), and brain-derived neurotrophic factor (BDNF) in an ethanol-induced neurodegeneration model. Can. J. Physiol. Pharmacol. 2021, 99, 478–489. [Google Scholar] [CrossRef]
- Lu, N.S.; Chiu, W.C.; Chen, Y.L.; Peng, H.C.; Shirakawa, H.; Yang, S.C. Fish oil up-regulates hepatic autophagy in rats with chronic ethanol consumption. J. Nutr. Biochem. 2020, 77, 108314. [Google Scholar] [CrossRef]
- Hwang, C.J.; Kim, Y.E.; Son, D.J.; Park, M.H.; Choi, D.Y.; Park, P.H.; Hellstrom, M.; Han, S.B.; Oh, K.W.; Park, E.K.; et al. Parkin deficiency exacerbate ethanol-induced dopaminergic neurodegeneration by P38 pathway dependent inhibition of autophagy and mitochondrial function. Redox Biol. 2017, 11, 456–468. [Google Scholar] [CrossRef]
- Pla, A.; Pascual, M.; Renau-Piqueras, J.; Guerri, C. TLR4 mediates the impairment of ubiquitin-proteasome and autophagy-lysosome pathways induced by ethanol treatment in brain. Cell Death Dis. 2014, 5, e1066. [Google Scholar] [CrossRef]
- Chen, H.; Hinz, K.; Zhang, C.; Rodriguez, Y.; Williams, S.N.; Niu, M.; Ma, X.; Chao, X.; Frazier, A.L.; McCarson, K.E.; et al. Late-Life Alcohol Exposure Does Not Exacerbate Age-Dependent Reductions in Mouse Spatial Memory and Brain TFEB Activity. Biomolecules 2024, 14, 1537. [Google Scholar] [CrossRef]
- Wang, L.; Song, L.; Ma, J.; Wang, H.; Li, Y.; Huang, D. Alcohol induces apoptosis and autophagy in microglia BV-2 cells. Food Chem. Toxicol. 2023, 177, 113849. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Zhou, R.; Yang, L.; Cederbaum, A.I. Alcohol steatosis and cytotoxicity: The role of cytochrome P4502E1 and autophagy. Free Radic. Biol. Med. 2012, 53, 1346–1357. [Google Scholar] [CrossRef]
- Flores-Bellver, M.; Bonet-Ponce, L.; Barcia, J.M.; Garcia-Verdugo, J.M.; Martinez-Gil, N.; Saez-Atienzar, S.; Sancho-Pelluz, J.; Jordan, J.; Galindo, M.F.; Romero, F.J. Autophagy and mitochondrial alterations in human retinal pigment epithelial cells induced by ethanol: Implications of 4-hydroxy-nonenal. Cell Death Dis. 2014, 5, e1328. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Ponce, L.; Saez-Atienzar, S.; da Casa, C.; Flores-Bellver, M.; Barcia, J.M.; Sancho-Pelluz, J.; Romero, F.J.; Jordan, J.; Galindo, M.F. On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta 2015, 1852, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Knoops, K.; Berendschot, T.; Benedikter, B.J.; Webers, C.A.B.; Reutelingsperger, C.P.M.; Gorgels, T. PGC-1a mediated mitochondrial biogenesis promotes recovery and survival of neuronal cells from cellular degeneration. Cell Death Discov. 2024, 10, 180. [Google Scholar] [CrossRef]
- Pla, A.; Pascual, M.; Guerri, C. Autophagy Constitutes a Protective Mechanism against Ethanol Toxicity in Mouse Astrocytes and Neurons. PLoS ONE 2016, 11, e0153097. [Google Scholar] [CrossRef]
- Guo, M.L.; Roodsari, S.K.; Cheng, Y.; Dempsey, R.E.; Hu, W. Microglia NLRP3 Inflammasome and Neuroimmune Signaling in Substance Use Disorders. Biomolecules 2023, 13, 922. [Google Scholar] [CrossRef]
- Aki, T.; Funakoshi, T.; Unuma, K.; Uemura, K. Impairment of autophagy: From hereditary disorder to drug intoxication. Toxicology 2013, 311, 205–215. [Google Scholar] [CrossRef]
- Cho, Y.E.; Song, B.J. Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress. Redox Biol. 2018, 18, 266–278. [Google Scholar] [CrossRef]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef]
- Song, B.J.; Akbar, M.; Abdelmegeed, M.A.; Byun, K.; Lee, B.; Yoon, S.K.; Hardwick, J.P. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014, 3, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Behera, J.; Kelly, K.E.; Zhai, Y.; Tyagi, N. Hydrogen sulfide, endoplasmic reticulum stress and alcohol mediated neurotoxicity. Brain Res. Bull. 2017, 130, 251–256. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Ballway, J.W.; Wang, X.; Won, K.J.; Hardwick, J.P.; Song, B.J. Post-translational modifications of histone and non-histone proteins in epigenetic regulation and translational applications in alcohol-associated liver disease: Challenges and research opportunities. Pharmacol. Ther. 2023, 251, 108547. [Google Scholar] [CrossRef]
- Novochadlo, M.; Goldim, M.P.; Bonfante, S.; Joaquim, L.; Mathias, K.; Metzker, K.; Machado, R.S.; Lanzzarin, E.; Bernades, G.; Bagio, E.; et al. Folic acid alleviates the blood brain barrier permeability and oxidative stress and prevents cognitive decline in sepsis-surviving rats. Microvasc. Res. 2021, 137, 104193. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, L.; Xu, Y.W.; Liang, H.; Han, J.; Zhao, R.J.; Cheng, Y. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: Inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res. 2012, 1473, 227–235. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, A.Y.; Kim, H.C.; Ryu, D.; Jo, S.A.; Jung, Y.S. Effects of Natural Polyphenols on Oxidative Stress-Mediated Blood-Brain Barrier Dysfunction. Antioxidants 2022, 11, 197. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef]
- Byun, K.; Bayarsaikhan, D.; Bayarsaikhan, E.; Son, M.; Oh, S.; Lee, J.; Son, H.I.; Won, M.H.; Kim, S.U.; Song, B.J.; et al. Microglial AGE-albumin is critical in promoting alcohol-induced neurodegeneration in rats and humans. PLoS ONE 2014, 9, e104699. [Google Scholar] [CrossRef]
- Jarmasz, J.S.; Stirton, H.; Basalah, D.; Davie, J.R.; Clarren, S.K.; Astley, S.J.; Del Bigio, M.R. Global DNA Methylation and Histone Posttranslational Modifications in Human and Nonhuman Primate Brain in Association with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1145–1162. [Google Scholar] [CrossRef]
- Ray, B.; Rungratanawanich, W.; LeFort, K.R.; Chidambaram, S.B.; Song, B.J. Mitochondrial Aldehyde Dehydrogenase 2 (ALDH2) Protects against Binge Alcohol-Mediated Gut and Brain Injury. Cells 2024, 13, 927. [Google Scholar] [CrossRef]
- Bailey, C.S.; Jagielo-Miller, J.E.; Keller, P.S.; Glaser, E.P.; Wilcox, A.L.; Prendergast, M.A. Ethanol sustains phosphorylated tau protein in the cultured neonatal rat hippocampus: Implications for fetal alcohol spectrum disorders. Alcohol 2022, 103, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; David, E.; Rohlman, A.; Nikolova, V.D.; Moy, S.S.; Vetreno, R.P.; Coleman, L.G., Jr. Adolescent Binge Alcohol Enhances Early Alzheimer’s Disease Pathology in Adulthood Through Proinflammatory Neuroimmune Activation. Front. Pharmacol. 2022, 13, 884170. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, Y.; Elharram, A.; Soon-Shiong, R.; Andrew, R.D.; Bennett, B.M. Characterization of Aldh2 (-/-) mice as an age-related model of cognitive impairment and Alzheimer’s disease. Mol. Brain 2015, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Ye, Y.; Refakis, C.A.; Feldman, J.L.; Stokes, A.L.; Basso, M.; Melero Fernandez de Mera, R.M.; Sparrow, N.A.; Calingasan, N.Y.; Kiaei, M.; et al. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. USA 2013, 110, E1102–E1111. [Google Scholar] [CrossRef]
- Satoh, M.; Nakai, A.; Sokawa, Y.; Hirayoshi, K.; Nagata, K. Modulation of the phosphorylation of glucose-regulated protein, GRP78, by transformation and inhibition of glycosylation. Exp. Cell Res. 1993, 205, 76–83. [Google Scholar] [CrossRef]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011, 140, 987–997. [Google Scholar] [CrossRef]
- Wei, J.; Gu, X.; Wang, Y.; Wu, Y.; Yan, C. Two-dimensional separation system by on-line hyphenation of capillary isoelectric focusing with pressurized capillary electrochromatography for peptide and protein mapping. Electrophoresis 2011, 32, 230–237. [Google Scholar] [CrossRef]
- Kim, B.J.; Hood, B.L.; Aragon, R.A.; Hardwick, J.P.; Conrads, T.P.; Veenstra, T.D.; Song, B.J. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics 2006, 6, 1250–1260. [Google Scholar] [CrossRef]
- Carbone, D.L.; Doorn, J.A.; Kiebler, Z.; Petersen, D.R. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 2005, 18, 1324–1331. [Google Scholar] [CrossRef]
- Imaoka, S. Chemical stress on protein disulfide isomerases and inhibition of their functions. Int. Rev. Cell Mol. Biol. 2011, 290, 121–166. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Yin, X.M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Pickrell, A.M. Hidden phenotypes of PINK1/Parkin knockout mice. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129871. [Google Scholar] [CrossRef]
- Huo, S.; Zhang, X.; Xu, J.; Zhang, J.; Du, J.; Li, B.; Song, M.; Shao, B.; Li, Y.; Xu, F. Parkin-mediated mitophagy protects against aluminum trichloride-induced hippocampal apoptosis in mice via the mtROS-NLRP3 pathway. Ecotoxicol. Environ. Saf. 2023, 264, 115459. [Google Scholar] [CrossRef]
- Rane, D.; Patil, T.; More, V.; Patra, S.S.; Bodhale, N.; Dandapat, J.; Sarkar, A. Neutrophils: Interplay between host defense, cellular metabolism and intracellular infection. Cytokine 2018, 112, 44–51. [Google Scholar] [CrossRef]
- Awasthi, D.; Sarode, A. Neutrophils at the Crossroads: Unraveling the Multifaceted Role in the Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 2929. [Google Scholar] [CrossRef]
- Sun, J.K.; Wu, D.; Wong, G.C.; Lau, T.M.; Yang, M.; Hart, R.P.; Kwan, K.M.; Chan, H.Y.E.; Chow, H.M. Chronic alcohol metabolism results in DNA repair infidelity and cell cycle-induced senescence in neurons. Aging Cell 2023, 22, e13772. [Google Scholar] [CrossRef]
- Huang, Y.; Flentke, G.R.; Rivera, O.C.; Saini, N.; Mooney, S.M.; Smith, S.M. Alcohol Exposure Induces Nucleolar Stress and Apoptosis in Mouse Neural Stem Cells and Late-Term Fetal Brain. Cells 2024, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Cho, Y.; Iracheta-Vellve, A.; Saha, B.; Lowe, P.; Adejumo, A.; Furi, I.; Ambade, A.; Gyongyosi, B.; Catalano, D.; et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 2018, 69, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, R.; Wang, Z.; Chen, Z.; Wang, G.; Guan, S.; Lu, J. Melatonin Prevents against Ethanol-Induced Liver Injury by Mitigating Ferroptosis via Targeting Brain and Muscle ARNT-like 1 in Mice Liver and HepG2 Cells. J. Agric. Food Chem. 2022, 70, 12953–12967. [Google Scholar] [CrossRef]
- Tamnanloo, F.; Chen, X.; Oliveira, M.M.; Tremblay, M.; Rose, C.F. Excessive intragastric alcohol administration exacerbates hepatic encephalopathy and provokes neuronal cell death in male rats with chronic liver disease. J. Neurosci. Res. 2024, 102, e25337. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.D.; Pratt, O.E.; Jeyasingham, M.; Shaw, G.K. Alcohol and brain damage. Hum. Toxicol. 1988, 7, 455–463. [Google Scholar] [CrossRef]
- Ortega-Ribera, M.; Zhuang, Y.; Babuta, M.; Brezani, V.; Joshi, R.S.; Zsengeller, Z.; Thevkar Nagesh, P.; Wang, Y.; Bronson, R.; Szabo, G. A Novel Multi-organ Male Model of Alcohol-induced Acute-on-chronic Liver Failure Reveals NET-mediated Hepatocellular Death, Which is Prevented by RIPK3 Inhibition. Cell. Mol. Gastroenterol. Hepatol. 2024, 19, 101446. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Urena-Peralta, J.; Morillo-Bargues, M.J.; Gomez-Pinedo, U.; Guerri, C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem. Res. 2016, 41, 193–209. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Dagil, Y.; Pashenkov, M.; Pinegin, B.; Chernyak, B. Protein kinase C isoforms mediate the formation of neutrophil extracellular traps. Int. Immunopharmacol. 2023, 114, 109448. [Google Scholar] [CrossRef]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Molecular and Signaling Mechanisms for Docosahexaenoic Acid-Derived Neurodevelopment and Neuroprotection. Int. J. Mol. Sci. 2022, 23, 4635. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, R.; Leong Bin Abdullah, M.F.I. The association between neuropsychiatric effects of substance use and occurrence of endoplasmic reticulum and unfolded protein response: A systematic review. Toxicol. Lett. 2024, 391, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Galan-Llario, M.; Rodriguez-Zapata, M.; Gramage, E.; Vicente-Rodriguez, M.; Fontan-Baselga, T.; Ovejero-Benito, M.C.; Perez-Garcia, C.; Carrasco, J.; Moreno-Herradon, M.; Sevillano, J.; et al. Receptor protein tyrosine phosphatase beta/zeta regulates loss of neurogenesis in the mouse hippocampus following adolescent acute ethanol exposure. Neurotoxicology 2023, 94, 98–107. [Google Scholar] [CrossRef]
- Rodriguez-Zapata, M.; Galan-Llario, M.; Caneque-Rufo, H.; Sevillano, J.; Sanchez-Alonso, M.G.; Zapico, J.M.; Ferrer-Alcon, M.; Uribarri, M.; Pascual-Teresa, B.; Ramos-Alvarez, M.D.P.; et al. Implication of the PTN/RPTPbeta/zeta Signaling Pathway in Acute Ethanol Neuroinflammation in Both Sexes: A Comparative Study with LPS. Biomedicines 2023, 11, 1318. [Google Scholar] [CrossRef]
- Galan-Llario, M.; Rodriguez-Zapata, M.; Fontan-Baselga, T.; Gramage, E.; Vicente-Rodriguez, M.; Zapico, J.M.; de Pascual-Teresa, B.; Lasek, A.W.; Herradon, G. Inhibition of RPTPbeta/zeta reduces chronic ethanol intake in adolescent mice and modulates ethanol effects on hippocampal neurogenesis and glial responses in a sex-dependent manner. Neuropharmacology 2023, 227, 109438. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Zhao, Y.; Lv, H.; Li, P.; Zhang, Z.; Qiao, X. BCI Improves Alcohol-Induced Cognitive and Emotional Impairments by Restoring pERK-BDNF. J. Mol. Neurosci. 2024, 74, 59. [Google Scholar] [CrossRef]
- Huang, Y.; Flentke, G.R.; Smith, S.M. Alcohol induces p53-mediated apoptosis in neural crest by stimulating an AMPK-mediated suppression of TORC1, S6K, and ribosomal biogenesis. Reprod. Toxicol. 2024, 130, 108747. [Google Scholar] [CrossRef]
- Liu, L.; Sun, T.; Xin, F.; Cui, W.; Guo, J.; Hu, J. Nerve Growth Factor Protects Against Alcohol-Induced Neurotoxicity in PC12 Cells via PI3K/Akt/mTOR Pathway. Alcohol Alcohol. 2017, 52, 12–18. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Miroshnichenko, L.A.; Polyakova, T.Y.; Zhdanov, V.V.; Simanina, E.V.; Stavrova, L.A.; Churin, A.A.; Fomina, T.I. Role of MAPK ERK1/2 and p38 in the Regulation of Secretory Functions of Different Populations of Neuroglia in Ethanol-Induced Neurodegeneration. Bull. Exp. Biol. Med. 2021, 171, 699–703. [Google Scholar] [CrossRef]
- Madaan, P.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Yadav, S.; Kaur, S.; Bhatia, S.; Al-Harrasi, A.; Abdellatif, A.A.H.; et al. Exploring the Therapeutic Potential of Targeting Purinergic and Orexinergic Receptors in Alcoholic Neuropathy. Neurotox. Res. 2022, 40, 646–669. [Google Scholar] [CrossRef]
- Behl, T.; Yadav, H.N.; Sharma, P.L. Alcoholic Neuropathy: Involvement of Multifaceted Signalling Mechanisms. Curr. Mol. Pharmacol. 2021, 14, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Xie, K.Q. CMZ reversed chronic ethanol-induced disturbance of PPAR-alpha possibly by suppressing oxidative stress and PGC-1alpha acetylation, and activating the MAPK and GSK3beta pathway. PLoS ONE 2014, 9, e98658. [Google Scholar] [CrossRef]
- Carabulea, A.L.; Janeski, J.D.; Naik, V.D.; Chen, K.; Mor, G.; Ramadoss, J. A multi-organ analysis of the role of mTOR in fetal alcohol spectrum disorders. FASEB J. 2023, 37, e22897. [Google Scholar] [CrossRef]
- Rolland, B.; Paille, F.; Gillet, C.; Rigaud, A.; Moirand, R.; Dano, C.; Dematteis, M.; Mann, K.; Aubin, H.J. Pharmacotherapy for Alcohol Dependence: The 2015 Recommendations of the French Alcohol Society, Issued in Partnership with the European Federation of Addiction Societies. CNS Neurosci. Ther. 2016, 22, 25–37. [Google Scholar] [CrossRef]
- Soyka, M.; Muller, C.A. Pharmacotherapy of alcoholism—An update on approved and off-label medications. Expert. Opin. Pharmacother. 2017, 18, 1187–1199. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Jiang, J.; Stavrovskaya, I.G.; Li, M.; Li, W.; Wu, Q.; Zhang, X.; Luo, C.; Zhou, S.; et al. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J. Neurosci. 2014, 34, 2967–2978. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Xu, G.; Sun, H. Influence of Chronic Ethanol Consumption on Apoptosis and Autophagy Following Transient Focal Cerebral Ischemia in Male Mice. Sci. Rep. 2020, 10, 6164. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, B.; Zhang, S.; Wang, J.; Yuan, X.; Peled, S.; Chen, W.; Ding, J.; Li, W.; Zhang, A.; et al. The MT1 receptor as the target of ramelteon neuroprotection in ischemic stroke. J. Pineal Res. 2024, 76, e12925. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, C.; Zhang, X.; Liu, Y.; Wang, Z.; Cacciamani, P.; Shi, J.; Cui, Y.; Wang, C.; Sinha, B.; et al. Tartary buckwheat extract alleviates alcohol-induced acute and chronic liver injuries through the inhibition of oxidative stress and mitochondrial cell death pathway. Am. J. Transl. Res. 2020, 12, 70–89. [Google Scholar]
- Lopatynska-Mazurek, M.; Antolak, A.; Grochecki, P.; Gibula-Tarlowska, E.; Bodzon-Kulakowska, A.; Listos, J.; Kedzierska, E.; Suder, P.; Silberring, J.; Kotlinska, J.H. Rapamycin Improves Spatial Learning Deficits, Vulnerability to Alcohol Addiction and Altered Expression of the GluN2B Subunit of the NMDA Receptor in Adult Rats Exposed to Ethanol during the Neonatal Period. Biomolecules 2021, 11, 650. [Google Scholar] [CrossRef]
- Silva, A.A.; Henriques, G.d.M.; Nascimento-Rocha, V.; Dias-Júnior, B.C.; dos Anjos Santos, A.; Lima, A.J.O.; Marinho, E.A.V.; Rubin, M.A.; Mello, C.F. Spermidine prevents the reinstatement of alcohol conditioned place preference. Addict. Neurosci. 2023, 9, 100130. [Google Scholar] [CrossRef]
- Gao, C.; Fang, L.; Zhang, H.; Zhang, W.S.; Li, X.O.; Du, S.Y. Metformin Induces Autophagy via the AMPK-mTOR Signaling Pathway in Human Hepatocellular Carcinoma Cells. Cancer Manag. Res. 2020, 12, 5803–5811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, S.; Fan, Z.; Li, Z.; Zhu, Y.; Shen, T.; Li, K.; Yan, Y.; Tian, J.; Liu, Z.; et al. Metformin attenuates plaque-associated tau pathology and reduces amyloid-beta burden in APP/PS1 mice. Alzheimers Res. Ther. 2021, 13, 40. [Google Scholar] [CrossRef]
- Bergheim, I.; Guo, L.; Davis, M.A.; Lambert, J.C.; Beier, J.I.; Duveau, I.; Luyendyk, J.P.; Roth, R.A.; Arteel, G.E. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology 2006, 130, 2099–2112. [Google Scholar] [CrossRef]
- Redmann, M.; Benavides, G.A.; Berryhill, T.F.; Wani, W.Y.; Ouyang, X.; Johnson, M.S.; Ravi, S.; Barnes, S.; Darley-Usmar, V.M.; Zhang, J. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017, 11, 73–81. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, J.; Xu, F.; Wang, J. Piracetam inhibits ethanol (EtOH)-induced memory deficit by mediating multiple pathways. Brain Res. 2017, 1676, 83–90. [Google Scholar] [CrossRef]
- Nie, X.; Wang, W.; Wang, Q.; Zhu, D.; Song, H. Intranasal erythropoietin ameliorates neurological function impairments and neural pathology in mice with chronic alcoholism by regulating autophagy-related Nrf2 degradation. Mol. Med. Rep. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Z.; Wu, H.; Zhou, W.; Jin, P.; Wei, P.; Zhang, Y.; Zheng, F.; Zhang, J.; Xu, J.; et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy 2014, 10, 2006–2020. [Google Scholar] [CrossRef]
- Qiao, X.; Gai, H.; Su, R.; Deji, C.; Cui, J.; Lai, J.; Zhu, Y. PI3K-AKT-GSK3beta-CREB signaling pathway regulates anxiety-like behavior in rats following alcohol withdrawal. J. Affect. Disord. 2018, 235, 96–104. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 activator RTA-408 attenuates osteoclastogenesis by inhibiting STING dependent NF-kappab signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef] [PubMed]
- Vrechi, T.A.M.; Leao, A.; Morais, I.B.M.; Abilio, V.C.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Bincoletto, C.; Ureshino, R.P.; Smaili, S.S.; et al. Cannabidiol induces autophagy via ERK1/2 activation in neural cells. Sci. Rep. 2021, 11, 5434. [Google Scholar] [CrossRef]
- Read, E.; Zhu, J.; Yang, G. Disrupted H(2)S Signaling by Cigarette Smoking and Alcohol Drinking: Evidence from Cellular, Animal, and Clinical Studies. Antioxidants 2021, 10, 49. [Google Scholar] [CrossRef]
- Mohseni, F.; Bagheri, F.; Rafaiee, R.; Norozi, P.; Khaksari, M. Hydrogen sulfide improves spatial memory impairment via increases of BDNF expression and hippocampal neurogenesis following early postnatal alcohol exposure. Physiol. Behav. 2020, 215, 112784. [Google Scholar] [CrossRef] [PubMed]
- Salete-Granado, D.; Carbonell, C.; Puertas-Miranda, D.; Vega-Rodriguez, V.J.; Garcia-Macia, M.; Herrero, A.B.; Marcos, M. Autophagy, Oxidative Stress, and Alcoholic Liver Disease: A Systematic Review and Potential Clinical Applications. Antioxidants 2023, 12, 1425. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Corsetti, G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef]
- Peng, C.; Ai, Q.; Zhao, F.; Li, H.; Sun, Y.; Tang, K.; Yang, Y.; Chen, N.; Liu, F. Quercetin attenuates cerebral ischemic injury by inhibiting ferroptosis via Nrf2/HO-1 signaling pathway. Eur. J. Pharmacol. 2024, 963, 176264. [Google Scholar] [CrossRef]
- Liu, Y.L.; Guo, T.; Zhang, Y.J.; Tang, S.C.; Zhao, X.M.; He, H.Y.; Yu, C.L.; Deng, Y.H. Berberine Alleviates Ischemic Brain Injury by Enhancing Autophagic Flux via Facilitation of TFEB Nuclear Translocation. Am. J. Chin. Med. 2024, 52, 231–252. [Google Scholar] [CrossRef]
- Singh, A.; Yadawa, A.K.; Rizvi, S.I. Curcumin protects against aging-related stress and dysfunction through autophagy activation in rat brain. Mol. Biol. Rep. 2024, 51, 694. [Google Scholar] [CrossRef]
- Gu, L.; Wang, C.; Liu, J.; Zheng, M.; Tan, Y.; Du, Q.; Li, Q.; Yang, W.; Zhang, X. Unlocking the neuroprotective potential of Ziziphora clinopodioides flavonoids in combating neurodegenerative diseases and other brain injuries. Biomed. Pharmacother. 2025, 182, 117744. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Shang, H.; Li, J.; Chai, H.; Wang, K.; Guo, Z.; Luo, T.; Liu, S.; Liu, Y.; et al. Potential application of natural compounds in ischaemic stroke: Focusing on the mechanisms underlying “lysosomocentric” dysfunction of the autophagy-lysosomal pathway. Pharmacol. Ther. 2024, 263, 108721. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Z.; Liu, M.; Chen, Z. Targeting selective autophagy in CNS disorders by small-molecule compounds. Pharmacol. Ther. 2024, 263, 108729. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Gorantla, V.R.; Mahalakshmi, A.M.; Ghanekar, R.K.; Yang, J.; Sakharkar, M.K.; Chidambaram, S.B. Polyphenols, Autophagy and Neurodegenerative Diseases: A Review. Biomolecules 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.J. Melatonin Prevents Alcohol- and Metabolic Dysfunction- Associated Steatotic Liver Disease by Mitigating Gut Dysbiosis, Intestinal Barrier Dysfunction, and Endotoxemia. Antioxidants 2023, 13, 43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).